Introduction
Cardiovascular diseases may affect the heart, brain
and blood vessels and are caused by hyperlipidemia, atherosclerosis
and hypertension (1,2). Cardiovascular diseases also include
cerebrovascular diseases (3).
Cardiovascular disease presents systemic vascular lesion or
systemic vascular lesions in the performance of the heart and
brains, which is the most frequent cause of death in the old
population of economically developed countries (4–6).
Cardiovascular disease also presents the highest mortality in the
world that closely associates with metabolism disorders of the
glucose and lipid metabolism (7,8). At
present, anoxia-reoxygenation injury of the heart represents a
serious threat to human health (9).
As such, developing our understanding of the potential signaling
mechanism of anoxia-reoxygenation injury is essential for the
prevention and treatment of cardiovascular disease.
Anoxia-reoxygenation injury-associated coronary
heart disease has high morbidity and mortality (10). Inflammation is one of the most common
pathogeneses observed and serves an important role in the
progression of anoxia-reoxygenation injury (11,12). It
has previously been reported that berberine is able to attenuate
lipopolysaccharide (LPS)-induced inflammation and extracellular
matrix accumulation via regulating the nuclear factor (NF)-κB
signaling pathway (13). In
addition, berberine attenuates ischemia-reperfusion injury via
regulating adenosine-5′-monophosphate kinase activity in both
non-ischemic and ischemic areas of the rat heart (14). Furthermore, berberine protects the
rat heart from ischemia/reperfusion injury via activating the Janus
kinase 2/signal transducer and activator of transcription 3
signaling pathway and attenuates endoplasmic reticulum stress
(15). However, the potential
molecular signaling pathways mediated by berberine
anoxia-reoxygenation injury remain to be elucidated.
In the present study, the potential signaling
mechanisms mediated by berberine in myocardial injury were
assessed. The therapeutic effects of berberine were also
investigated in a mouse model of anoxia-reoxygenation injury. The
results demonstrated that berberine decreased the expression of
inflammatory cytokines and inhibited myocardial cell apoptosis.
Together, these data indicate that berberine may have potential as
a treatment for anoxia-reoxygenation injury.
Materials and methods
Ethics statement
The present study was conducted in accordance with
the recommendations of the China Guide for the Care and Use of
Laboratory Animals (16). Ethical
approval was granted by the Ethical Committee of Heilongjiang
Provincial Hospital (Harbin, China).
Animal study
A total of 20 male C57BL/6 mice (age, 8 weeks;
weight range, 28–32 g) were purchased from Charles River
Laboratories (Wilmington, MA, USA) and housed at 23±1°C and 50±5%
humidity with a 12 h light/dark cycle and free access to diet and
water. The myocardial injury model was established as previously
described (17).
Anoxia-reoxygenation injury mice were subsequently randomly divided
into two groups (n=10 in each group). Mice were administered with
berberine (10 mg/kg/day; berberine group; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) or an equal volume of PBS (control
group). Cardiac function was then assessed to evaluate the efficacy
of berberine.
Cell culture
Ventriculus sinister myocardial cells were isolated
from mice as previously described (18) and cultured in Dulbecco's modified
Eagle's medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Thermo Fisher Scientific,
Inc.) at 37°C in an atmosphere containing 5% CO2.
Endogenous overexpression of p38
mitogen-activated protein kinase (MAPK)
Myocardial cells were cultured until 90% confluence
was reached, following which the medium was removed. Cells were
subsequently transfected with pedue12.4-p38 mitogen-activated
protein kinase (MAPK; 100 pmol; Invitrogen; Thermo Fisher
Scientific, Inc.) or pedue12.4 empty vector (Control; 100 pmol;
Invitrogen; Thermo Fisher Scientific, Inc.) using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.).
The p38 MAPK-overexpression myocardial cells were used to analyze
the efficacy of berberine on NF-κB signal pathway 72 h following
transfection.
Histological analysis
Cardiac tissues were fixed in 4% paraformaldehyde
for 1 h at room temperature and embedded in paraffin for 2 h at
room temperature. The sections was subjected this section to
Masson's trichrome staining using a staining kit (Sigma-Aldrich;
Merck KGaA) according to the manufacturer's instructions using
light microscope (magnification, ×100). The infarct area was
measured by Masson staining using computer-assisted planimetry
(version 1.2; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
ELISA
Blood samples were obtained from experimental mice
on day 30. Serum interleukin (IL)-6 (cat. no. MBS700340), tumor
necrosis factor (TNF)-α (cat. no. MBS7817), IL-10 (cat. no.
MBS10262) and IL-17A (cat. no. MBS26282; all Thermo Fisher
Scientific, Inc.) levels in mice with anoxia-reoxygenation injury
were measured using ELISA kits according to the manufacturer's
protocol following treatment with berberine or PBS. Serum IL-6,
TNF-α, IL-10 and IL-17A levels were measured using an enzyme
microplate reader at 570 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from myocardial cells using
RNAzol (Thermo Fisher Scientific, Inc.), and DNase RNase-free
(Sigma-Aldrich; Merck KGaA) was used to digest total RNA at 37°C
for 15 min. An RNeasy kit (Thermo Fisher Scientific, Inc.) was then
used to purify RNA and the concentration was adjusted 1 µg/µl. A
total of 2 µg RNA was used as the template to synthesize cDNA at
37°C for 120 min, 99°C for 4 min and 4°C for 3 min. Followed by,
qPCR was performed to amplify the expression of IL-6, TNF-α, IL-10,
IL-17A, B-cell lymphoma (Bcl)-2, P53, p38 MAPK and NF-κB, with
β-actin as a control (Table I).
Thermocycling conditions were as follows: 95°C for 2 min, followed
by 35 cycles of 95°C for 20 sec, 55.8°C for 30 sec and 72°C for 30
sec, followed by a final extension at 72°C for 5 min. Following
RT-qPCR, agarose electrophoresis with 1% ethidium bromide was used
to check the amplified PCR products. Gene expression was quantified
using TaqMan Gene Expression Assays (Thermo Fisher Scientific,
Inc.). Relative mRNA expression changes were calculated using the
2−ΔΔCq method (19).
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| IL-6 |
TTCCATCCAGTTGCCTTCTTGG |
TTCTCATTTCCACGATTTCCCAG |
| TNF-α |
AGGCGGTGCTTGTTCCTC |
AGGCGAGAAGATGATCTGACTGCC |
| IL-17A |
ATGCACAGCCACCGCGACTT |
CTTCATGACTGCCTCCAAGTAG |
| IL-10 |
CAGTGCAGAAGAGTCGACTGCAAG |
CGCTTGAGATCCTGAAATATA |
| Bcl-2 |
5GATGAAGTACATCCATTATAAGCTGTCACA |
GCGCTCAGCCCTGTGCCACCTGTGGTCCAC |
| Bcl-xl | CCGGAATTCATGGCGA |
CGCGCGGCCGCTCACTTGCTAGCAA |
| p38 MAPK |
TCCCTCAGGAAGCTTGAACCTGAA |
AAACCTAGGGTGTGGATGCCTCTT |
| NF-κB |
TGCTTCCTGATGACGATGTA |
TCCTCGGAGACTGGTAATGG |
| β-actin |
CGGAGTCAACGGATTTGGTC |
AGCCTTCTCCATGGTCGTGA |
Western blotting
Myocardial cells were isolated and lysed in
radioimmunoprecipitation assay buffer (M-PER for cells, T-PER for
tissues; Thermo Fisher Scientific, Inc.) and homogenized at 4°C for
10 min. Protein concentration was measured using a bicinchoninic
acid assay kit (Thermo Fisher Scientific, Inc.). A total of 20 µg
protein was separated by 12.5% SDS-PAGE and transferred to
nitrocellulose membranes. The membranes were incubated in blocking
buffer (5% milk) for 2 h at room temperature, following which they
were incubated with primary antibodies for 12 h at 4°C. The
following primary rabbit anti-mouse antibodies were used: Bcl-2
(1:200; cat. no. ab59348), Bcl-extra large (Bcl-xl; 1:1,000; cat.
no. ab178844), IL-6 (1:1,000; cat. no. ab100712), TNF-α (1:500;
cat. no. ab119139), IL-10 (1:500; cat. no. ab9969), IL-17 (1:500;
cat. no. ab79056), p38 MAPK (1:1,000; cat. no. ab47363), NF-κB
(1:500; cat. no. ab131493) and β-actin (1:500; cat. no. ab8226; all
Abcam, Cambridge, UK). Membranes were subsequently incubated with
horseradish peroxidase-conjugated anti-rabbit IgG (1:5,000; cat.
no. STAR36D649GA; Bio-Rad Laboratories, Inc.) for 2 h at 37°C.
Bands were visualized using a Western Blotting Luminol Reagent and
the band intensities were analyzed by ImageJ software 1.0 (National
Institutes of Health, Bethesda, MD, USA).
TUNEL assay
Myocardial cells (1×106 cells/well) were
cultured in 6-well plates and treated with p38 MAPK inhibitor
SB203580 (1 mg/ml; Sigma-Aldrich; Merck KGaA) or NF-κB inhibitor
PDTC (1 mg/ml; Sigma-Aldrich; Merck KGaA) for 12 h at 37°C.
Apoptosis was analyzed using a TUNEL assay (DeadEnd™ Colorimetric
TUNEL System; Promega Corp., Madison, WI, USA) according to the
manufacturer's protocol. Myocardial tissues were incubated with the
TUNEL reaction mixture for 1 h at 37°C, following which
streptavidin- and DAB-bound biotin was quantified and cells were
counterstained with hemalaun (Merck KGaA) and aquatex (Merck KGaA).
DNA fragmentation was randomly detected by observing three fields
in each tumor section using a confocal microscope at 488 nm
(magnification, ×40).
Statistical analysis
Data are expressed as the mean ± standard deviation
and were analyzed using Student's t-test or one-way analysis of
variance followed by Tukey's post hoc test. All data were analyzed
using SPSS Statistics 19.0 (IBM Corp., Armonk, NY, USA) and
GraphPad Prism version 5.0 (GraphPad Software, Inc., La Jolla, CA,
USA). *P<0.05 was considered to indicate a statistically
significant difference.
Results
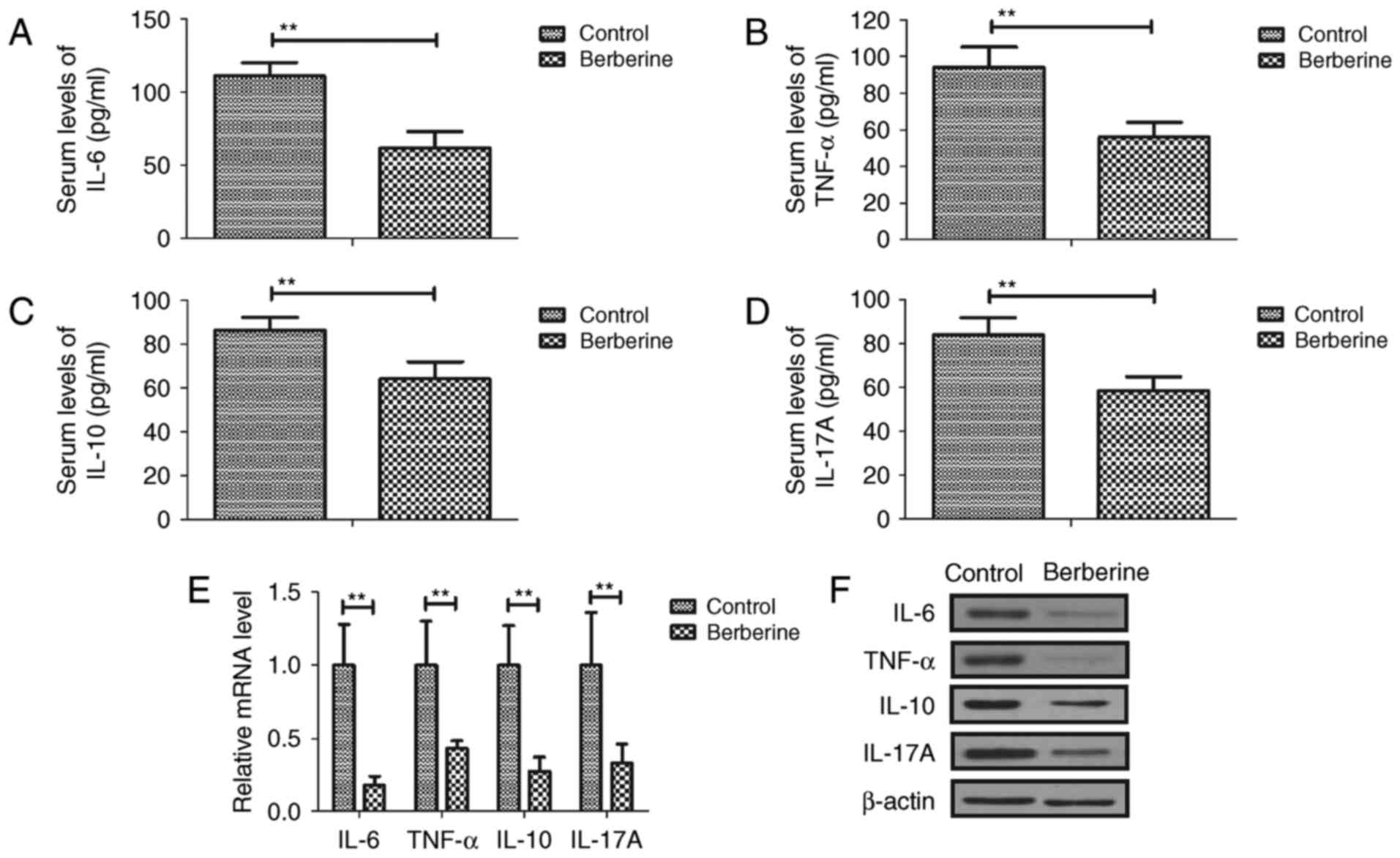
Berberine treatment downregulates the
expression of inflammatory factors in mice with
anoxia-reoxygenation injury
Serum IL-6, TNF-α, IL-10 and IL-17A was
downregulated in the berberine group compared with the control
group (Fig. 1A-D). RT-qPCR results
demonstrated that IL-6, TNF-α, IL-10 and IL-17A mRNA expression was
decreased in myocardial cells from the berberine group compared
with the control group (Fig. 1E).
The results of western blotting also revealed that IL-6, TNF-α,
IL-10 and IL-17A protein expression was downregulated in myocardial
cells from the berberine group compared with the control group
(Fig. 1F). These results suggest
that berberine treatment effectively reduced inflammation in mice
with anoxia-reoxygenation injury.
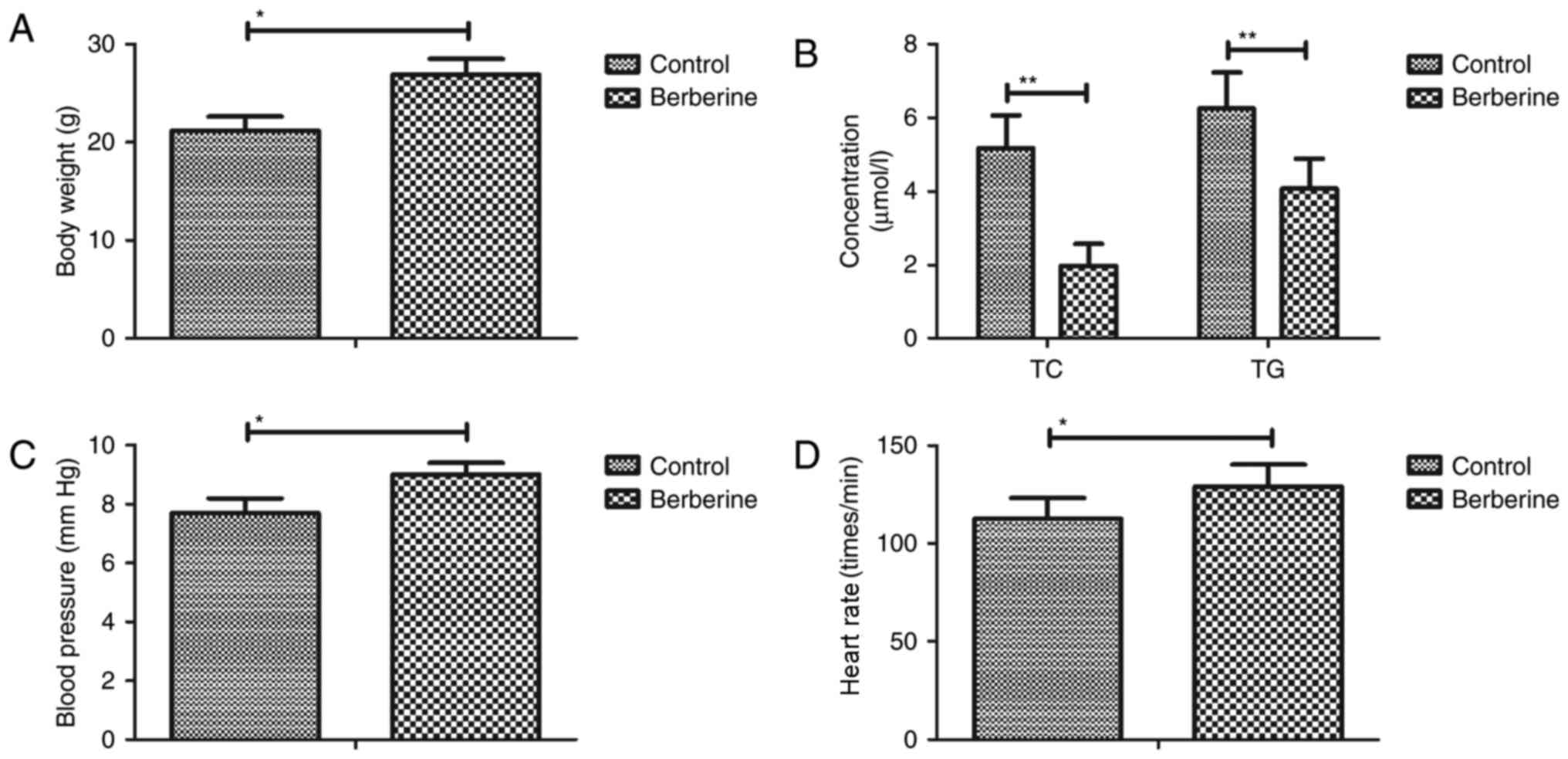
Berberine treatment improves
biochemical parameters in a mouse model of anoxia-reoxygenation
injury
As shown in Fig. 2A,
body weight was increased in the berberine group compared with the
PBS group. Furthermore, blood lipid levels were decreased in the
berberine group compared with the control group (Fig. 2B). The results revealed that blood
pressure and heart rate were increase in the berberine group
compared with the control group (Fig. 2C
and D). These results suggest that berberine treatment improves
biochemical parameters in a mouse model of anoxia-reoxygenation
injury.
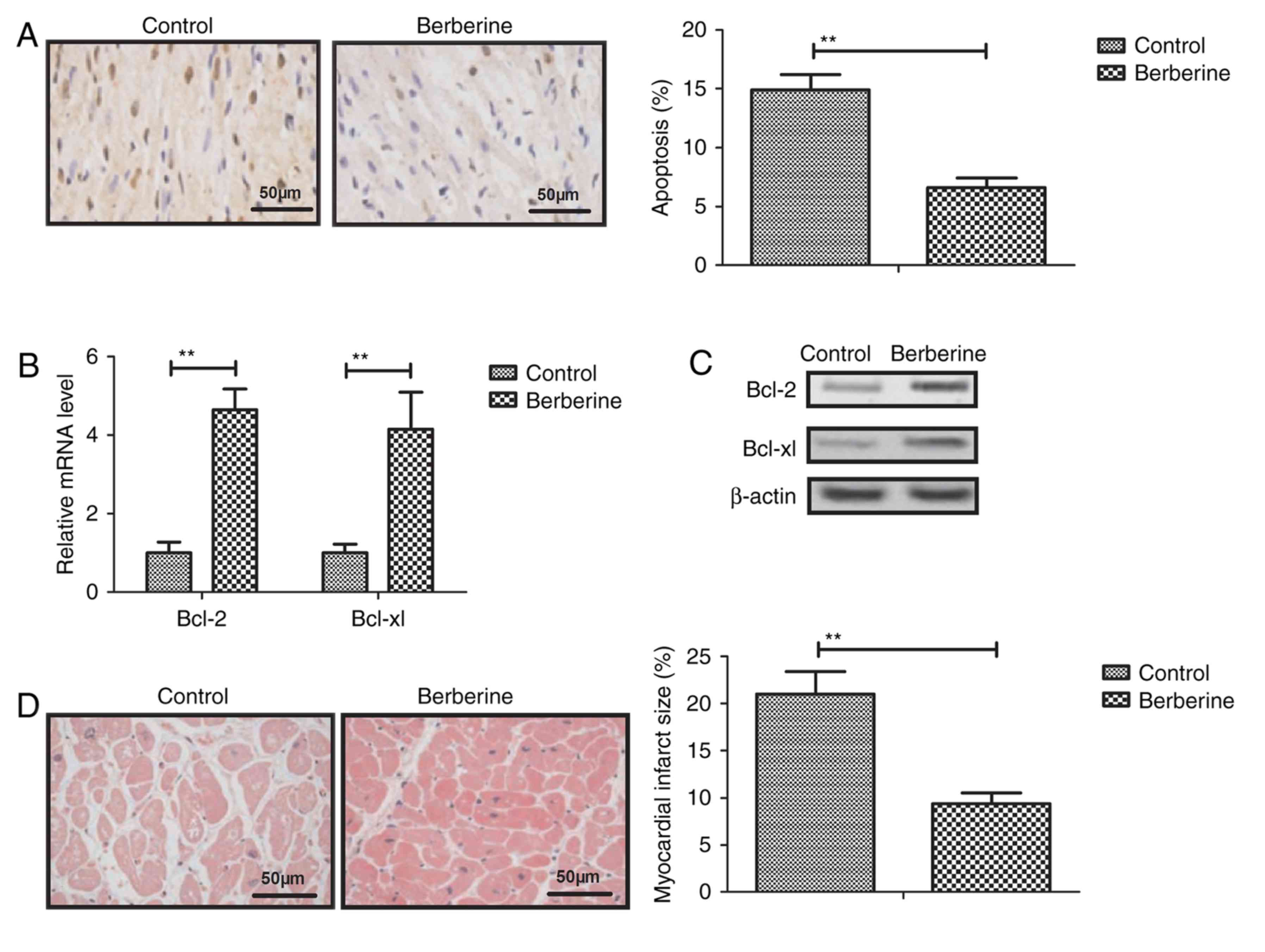
Berberine treatment reduces myocardial
cell apoptosis in a mouse model of anoxia-reoxygenation injury
Berberine treatment was observed to significantly
decrease myocardial apoptosis compare with the control group
(Fig. 3A). The results of RT-qPCR
and western blotting revealed that the expression of Bcl-2 and
Bcl-xl was upregulated in myocardial cells from the berberine group
compared with the control group (Fig. 3B
and C). Furthermore, the myocardial injury area was
significantly decreased following berberine treatment compared with
the control group (Fig. 3D). These
results suggest that berberine treatment significantly inhibits
apoptosis anoxia-reoxygenation injury-induced apoptosis in
mice.
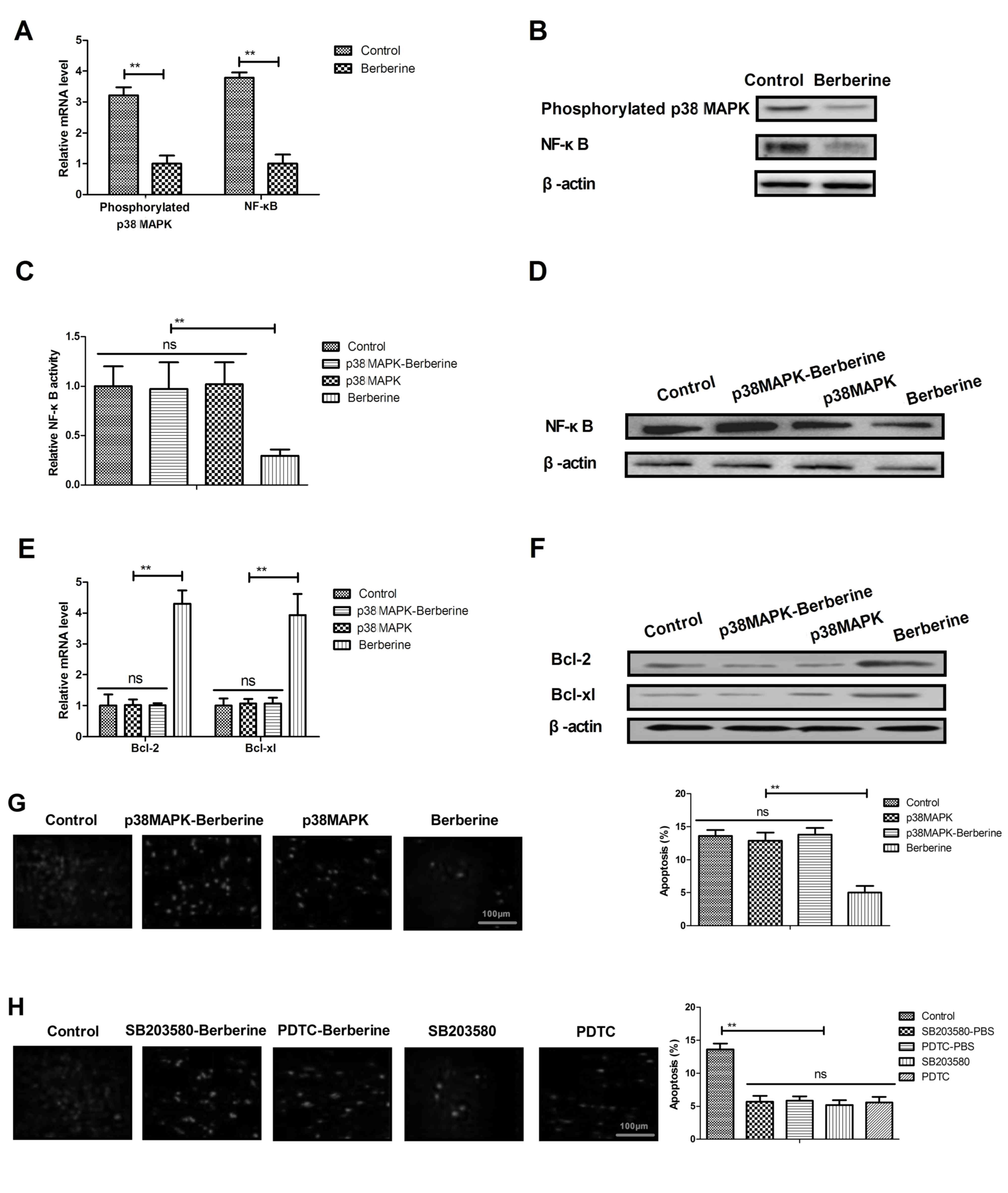
Berberine treatment improves
anoxia-reoxygenation injury via the p38 MAPK-mediated NF-κB
signaling pathway
As shown in Fig. 4A and
B, berberine treatment significantly downregulated the
expression of phosphorylated p38 MAPK and NF-κB in myocardial cells
compared with the control group. It was also demonstrated that p38
MAPK overexpression effectively inhibited the berberine-induced
downregulation of NF-κB activity and expression in myocardial cells
isolated from experimental mice (Fig. 4C
and D). p38 MAPK overexpression treatment also suppressed the
berberine-induced upregulation of Bcl-2 and Bcl-xl in myocardial
cells (Fig. 4E and F). It was
demonstrated that p38 MAPK overexpression abolished
berberine-inhibited myocardial apoptosis in myocardial cells
isolated from mice (Fig. 4G). p38
MAPK or NF-κB inhibitor treatment further decreased apoptosis in
myocardial cells isolated from PBS-treated mice (Fig. 4H). These results suggest that
berberine treatment is able to improve anoxia-reoxygenation injury
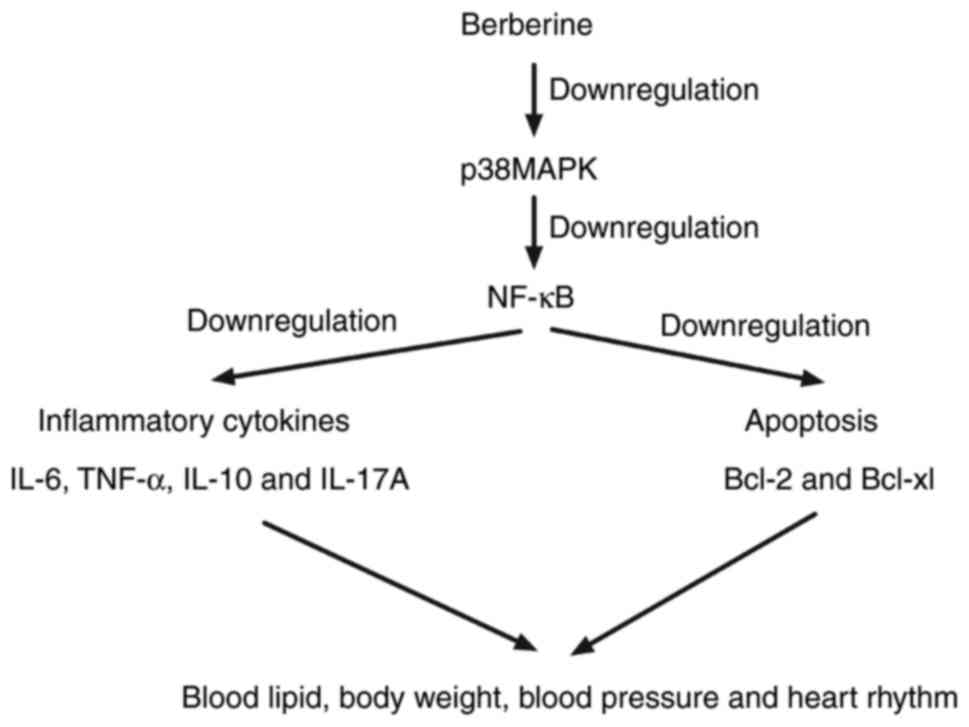
via upregulating p38 MAPK-mediated NF-κB signaling (Fig. 5).
Discussion
Anoxia-reoxygenation injury is the most common form
of cardiovascular disease and typically occurs during myocardial
infarction, cardiopulmonary bypass surgery, heart attack or heart
transplantation (20,21). Inflammation serves an important role
in myocardial ischemia-reperfusion injury (22,23) and
it has been suggested that berberine treatment is able to attenuate
cardiac dysfunction in hyperglycemic and hypercholesterolemic rats
by alleviating cardiac lipid accumulation and promoting glucose
transport (24). The results of the
present study demonstrate that berberine treatment decreases the
expression of inflammatory cytokines and improves the biochemical
parameters of myocardial cells in a mouse model of
anoxia-reoxygenation injury. Furthermore, it was revealed that
berberine decreases myocardial cell apoptosis via upregulating
Bcl-2 and Bcl-xl expression. Together, these results suggest that
berberine treatment may attenuate anoxia-reoxygenation injury via
the p38 MAPK-mediated NF-κB signaling pathway.
A previous study suggested that berberine attenuates
adverse left ventricular remodeling and cardiac dysfunction in rats
following acute myocardial infarction and that this is mediated via
inhibition of the p38 MAPK pathway and activation of the pAKT
pathway (25). Huang et al
(26) reported that berberine
treatment could alleviate cardiac ischemia/reperfusion injury by
inhibiting excessive autophagy in cardiomyocytes. The results of
the present study demonstrate that berberine treatment
significantly decreases myocardial infarction by inhibiting
myocardial cell apoptosis in a mouse model of anoxia-reoxygenation
injury. Furthermore, pretreatment with berberine has been observed
to protect the heart against LPS-induced myocardial dysfunction via
inhibiting cardiac IκBα phosphorylation and apoptosis in mice
(27). In the present study,
berberine treatment attenuated the p38 MAPK-mediated NF-κB
signaling pathway in a mouse model of anoxia-reoxygenation injury,
suggesting that p38 MAPK may be a potential treatment target for
anoxia-reoxygenation injury.
The effects of berberine on hemodynamic parameters
and Ca2+ have been investigated in cardiac myocytes
harvested from rats with diastolic heart failure and it was
suggested that berberine may be an effective dose-dependent
treatment for symptomatic relief of heart failure (28). In the present study, it was
demonstrated that the protective effect of berberine in myocardial
anoxia-reperfusion injury may be regulated by the p38 MAPK-mediated
NF-κB signaling pathway in myocardial cells. The NF-κB pathway is
associated with myocardial anoxia-reperfusion injury and may
trigger the release of inflammatory cytokines (29). The results herein suggest that
berberine treatment inhibits the p38 MAPK-mediated NF-κB signal
pathway, which in turn downregulates the expression of inflammatory
cytokines IL-6, TNF-α, IL-10 and IL-17A in mice with
anoxia-reoxygenation injury.
In summary, the results of the present study
indicate that berberine treatment downregulates inflammatory
cytokine expression and improves biochemical parameters, including
body weight, blood lipid levels, blood pressure and heart rate, in
a mouse model of anoxia-reoxygenation injury. Berberine is able to
regulate anoxia-reoxygenation injury via downregulating the p38
MAPK-mediated NF-κB signaling pathway, which may contribute to
decreased inflammation and apoptosis in myocardial cells. These
results may provide a basis for the clinical use of berberine as a
therapeutic treatment for anoxia-reoxygenation injury.
Acknowledgements
Not applicable.
Funding
The present study was supported by a study on
macrophage's action mechanism in post-acute myocardial infarction
disposing from by the Natural Science Foundation of Heilongjiang
Province (grant no. 2016-499).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
XT performed the experiments. GL, KW, YX and YQ
prepared and analyzed experimental data. YZ designed the study and
experiments. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Ethical approval was granted by the Ethical
Committee of Heilongjiang Provincial Hospital (Harbin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests.
References
|
1
|
Sankari A, Martin JL and Badr M: A
retrospective review of sleep-disordered breathing, hypertenstion
and cardiovascular diseases in spinal cord injury patients. Spinal
Cord. 53:496–497. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Piepoli MF, Corra U, Abreu A, Cupples M,
Davos C, Doherty P, Höfer S, Garcia-Porrero E, Rauch B, Vigorito C,
et al: Challenges in secondary prevention of cardiovascular
diseases: A review of the current practice. Int J Cardiol.
180:114–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kozlovskaya IL, Bulkina OS, Lopukhova VV,
Chernova NA, Ivanova OV, Kolmakova TE and Karpov YA: Heat and
cardiovascular diseases: A review of epidemiological surveys. Ter
Arkh. 87:84–90. 2015.(In Russian). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klainin-Yobas P, Ng SH, Stephen PDM and
Lau Y: Efficacy of psychosocial interventions on psychological
outcomes among people with cardiovascular diseases: A systematic
review and meta-analysis. Patient Educ Couns. 99:512–521. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang XQ, Pi YL, Chen PJ, Liu Y, Wang R, Li
X, Chen BL, Zhu Y, Yang YJ and Niu ZB: Traditional Chinese exercise
for cardiovascular diseases: Systematic review and meta-analysis of
randomized controlled trials. J Am Heart Assoc. 5:e0025622016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zulli A, Smith RM, Kubatka P, Novak J,
Uehara Y, Loftus H, Qaradakhi T, Pohanka M, Kobyliak N, Zagatina A,
et al: Caffeine and cardiovascular diseases: Critical review of
current research. Eur J Nutr. 55:1331–1343. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baselet B, Rombouts C, Benotmane AM,
Baatout S and Aerts A: Cardiovascular diseases related to ionizing
radiation: The risk of low-dose exposure (Review). Int J Mol Med.
38:1623–1641. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fatema K, Zwar NA, Milton AH, Ali L and
Rahman B: Prevalence of risk factors for cardiovascular diseases in
bangladesh: A systematic review and meta-analysis. PLoS One.
11:e01601802016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang L, Xue Y, Ma H, Shi H, Wang L and Cui
X: Prazosin protects myocardial cells against anoxia-reoxygenation
injury via the extracellular signalregulated kinase signaling
pathway. Mol Med Rep. 17:2145–2152. 2018.PubMed/NCBI
|
|
10
|
Aminde LN and Veerman L: Interventions for
the prevention of cardiovascular diseases: A protocol for a
systematic review of economic evaluations in low-income and
middle-income countries. BMJ Open. 6:e0136682016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
De Lorgeril M: Essential polyunsaturated
fatty acids, inflammation, atherosclerosis and cardiovascular
diseases. Subcell Biochem. 42:283–297. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Candore G, Aquino A, Balistreri CR, Bulati
M, Di Carlo D, Grimaldi MP, Listì F, Orlando V, Vasto S, Caruso M,
et al: Inflammation, longevity, and cardiovascular diseases: Role
of polymorphisms of TLR4. Ann N Y Acad Sci. 1067:282–287. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang Q, Liu P, Wu X, Liu W, Shen X, Lan
T, Xu S, Peng J, Xie X and Huang H: Berberine attenuates
lipopolysaccharide-induced extracelluar matrix accumulation and
inflammation in rat mesangial cells: Involvement of NF-κB signaling
pathway. Mol Cell Endocrinol. 331:34–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang W, Zhang M, Li J, Meng Z, Xiao D,
Wei S, Chen L, Wang C and Hatch GM: Berberine attenuates
ischemia-reperfusion injury via regulation of
adenosine-5′-monophosphate kinase activity in both non-ischemic and
ischemic areas of the rat heart. Cardiovasc Drugs Ther. 26:467–478.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao GL, Yu LM, Gao WL, Duan WX, Jiang B,
Liu XD, Zhang B, Liu ZH, Zhai ME, Jin ZX, et al: Berberine protects
rat heart from ischemia/reperfusion injury via activating
JAK2/STAT3 signaling and attenuating endoplasmic reticulum stress.
Acta Pharmacol Sin. 37:354–367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Davey G and Wu Z: Attitudes in China
toward the use of animals in laboratory research. Altern Lab Anim.
35:313–316. 2007.PubMed/NCBI
|
|
17
|
Jong WM, Ten Cate H, Linnenbank AC, de
Boer OJ, Reitsma PH, de Winter RJ and Zuurbier CJ: Reduced acute
myocardial ischemia-reperfusion injury in IL-6-deficient mice
employing a closed-chest model. Inflamm Res. 65:489–499. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barile L, Chimenti I, Gaetani R, Forte E,
Miraldi F, Frati G, Messina E and Giacomello A: Cardiac stem cells:
Isolation, expansion and experimental use for myocardial
regeneration. Nat Clin Pract Cardiovasc Med. 4 Suppl 1:S9–S14.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin Y and Blikslager AT: Myosin light
chain kinase mediates intestinal barrier dysfunction via occludin
endocytosis during anoxia/reoxygenation injury. Am J Physiol Cell
Physiol. 311:C996–C1004. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang H, Lai S, Wan Q, Qi W and Liu J:
Astragaloside IV protects cardiomyocytes from anoxia/reoxygenation
injury by upregulating the expression of Hes1 protein. Can J
Physiol Pharmacol. 94:542–553. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xia WF, Liu Y, Zhou QS, Tang QZ and Zou
HD: Comparison of the effects of propofol and midazolam on
inflammation and oxidase stress in children with congenital heart
disease undergoing cardiac surgery. Yonsei Med J. 52:326–332. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang R, Zhang YY, Huang XR, Wu Y, Chung
AC, Wu EX, Szalai AJ, Wong BC, Lau CP and Lan HY: C-reactive
protein promotes cardiac fibrosis and inflammation in angiotensin
II-induced hypertensive cardiac disease. Hypertension. 55:953–960.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong SF, Hong Y, Liu M, Hao YZ, Yu HS, Liu
Y and Sun JN: Berberine attenuates cardiac dysfunction in
hyperglycemic and hypercholesterolemic rats. Eur J Pharmacol.
660:368–374. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang YJ, Yang SH, Li MH, Iqbal J,
Bourantas CV, Mi QY, Yu YH, Li JJ, Zhao SL, Tian NL and Chen SL:
Berberine attenuates adverse left ventricular remodeling and
cardiac dysfunction after acute myocardial infarction in rats: Role
of autophagy. Clin Exp Pharmacol Physiol. 41:995–1002. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang Z, Han Z, Ye B, Dai Z, Shan P, Lu Z,
Dai K, Wang C and Huang W: Berberine alleviates cardiac
ischemia/reperfusion injury by inhibiting excessive autophagy in
cardiomyocytes. Eur J Pharmacol. 762:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang YY, Li HM, Wang HD, Peng XM, Wang YP,
Lu DX, Qi RB, Hu CF and Jiang JW: Pretreatment with berberine and
yohimbine protects against LPS-induced myocardial dysfunction via
inhibition of cardiac I-[kappa]B[alpha] phosphorylation and
apoptosis in mice. Shock. 35:322–328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang XD, Ren HM and Liu L: Effects of
different dose berberine on hemodynamic parameters and [Ca2+]i of
cardiac myocytes of diastolic heart failure rat model. Zhongguo
Zhong Yao Za Zhi. 33:818–821. 2008.(In Chinese). PubMed/NCBI
|
|
29
|
Chen H, Zhang RQ, Wei XG, Ren XM and Gao
XQ: Mechanism of TLR-4/NF-κB pathway in myocardial ischemia
reperfusion injury of mouse. Asian Pac J Trop Med. 9:503–507. 2016.
View Article : Google Scholar : PubMed/NCBI
|