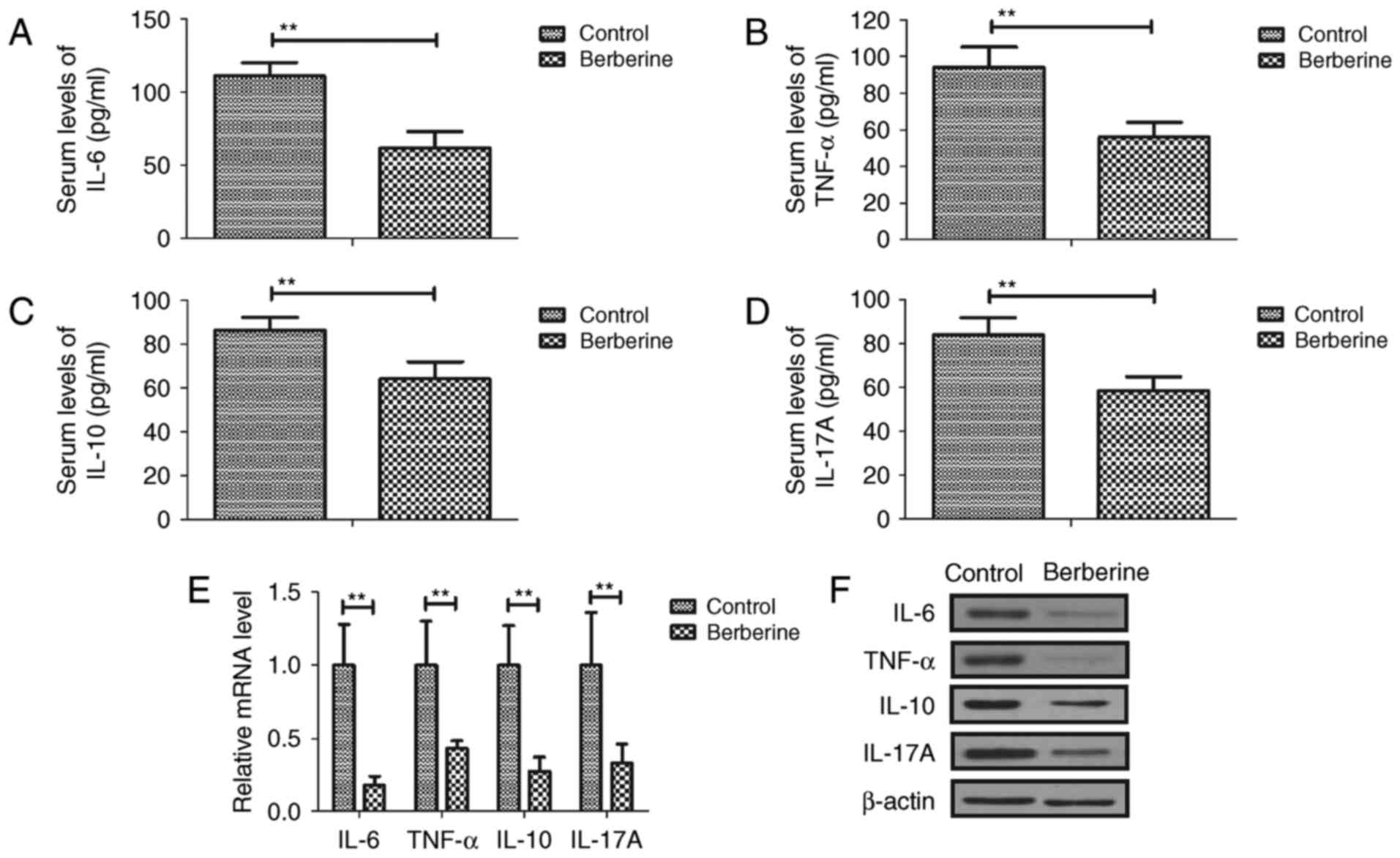
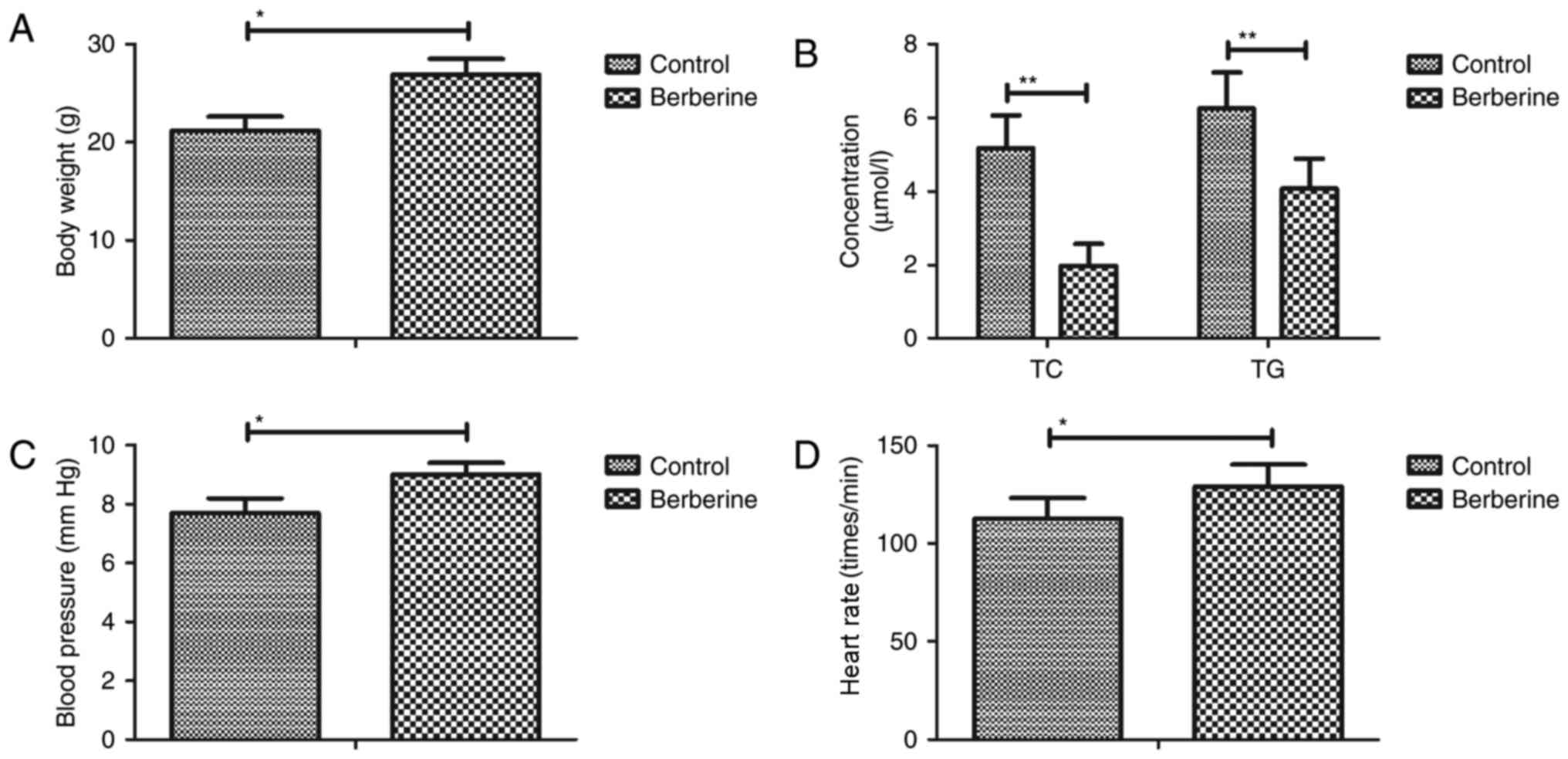
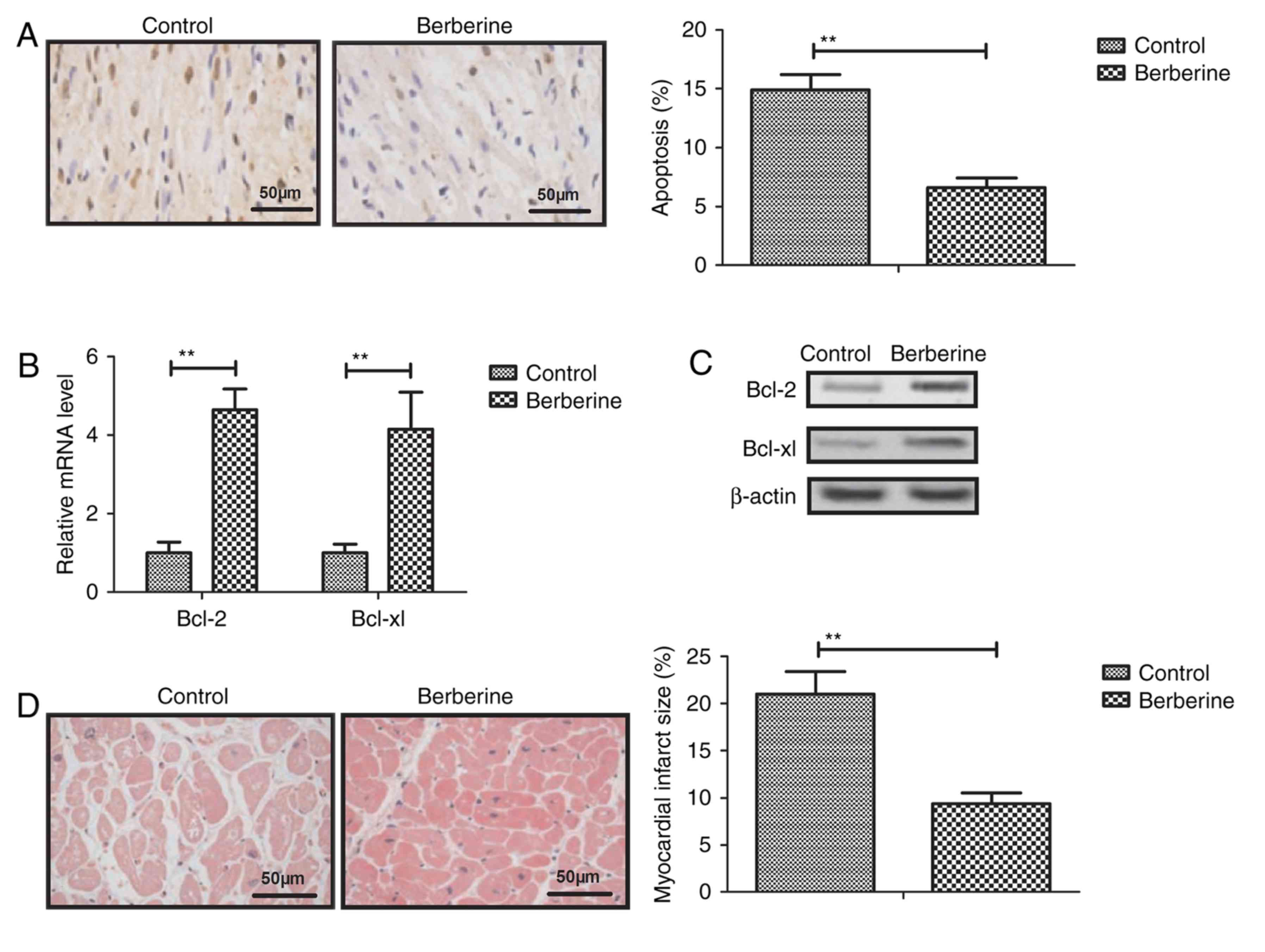
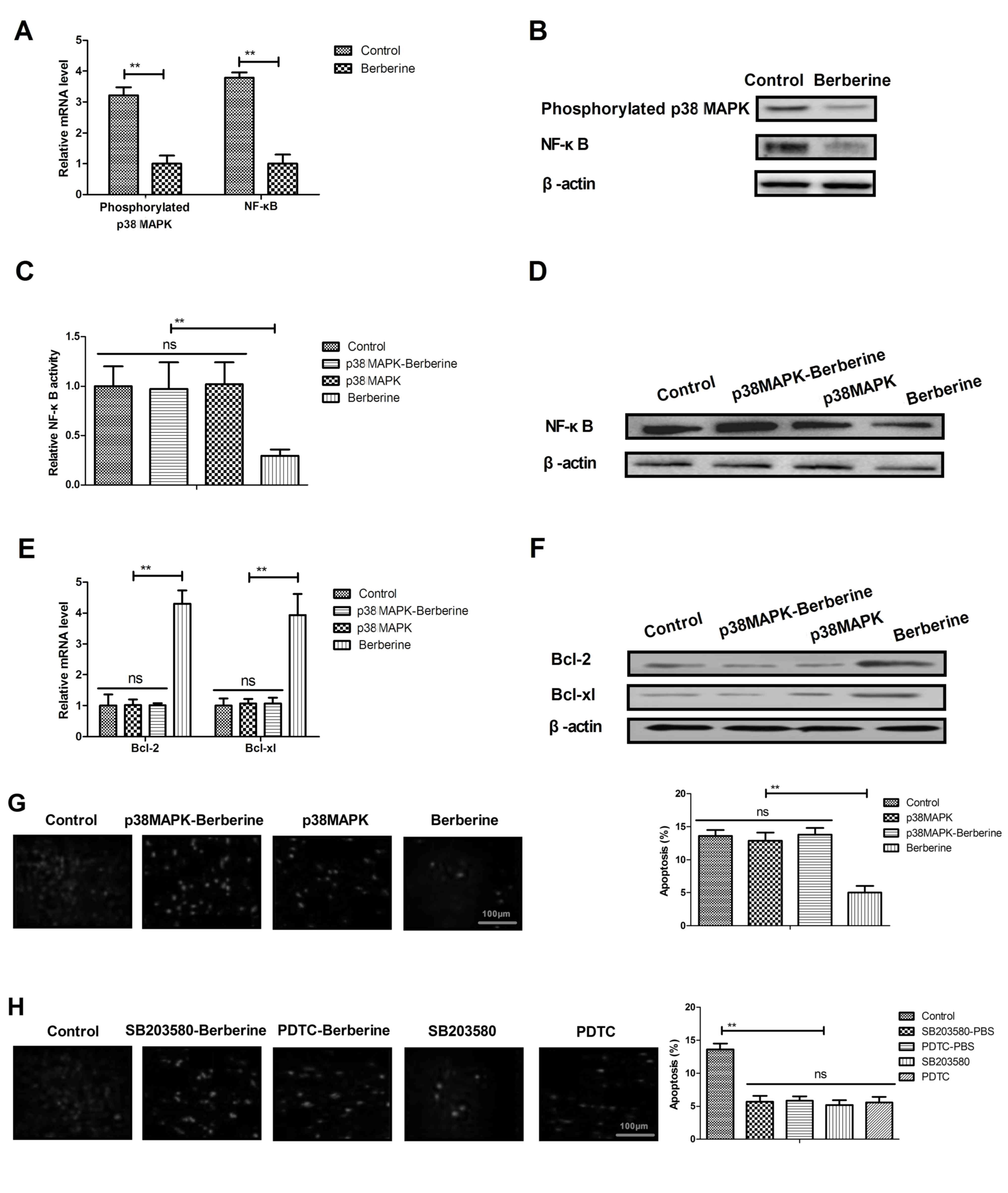
|
1
|
Sankari A, Martin JL and Badr M: A
retrospective review of sleep-disordered breathing, hypertenstion
and cardiovascular diseases in spinal cord injury patients. Spinal
Cord. 53:496–497. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Piepoli MF, Corra U, Abreu A, Cupples M,
Davos C, Doherty P, Höfer S, Garcia-Porrero E, Rauch B, Vigorito C,
et al: Challenges in secondary prevention of cardiovascular
diseases: A review of the current practice. Int J Cardiol.
180:114–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kozlovskaya IL, Bulkina OS, Lopukhova VV,
Chernova NA, Ivanova OV, Kolmakova TE and Karpov YA: Heat and
cardiovascular diseases: A review of epidemiological surveys. Ter
Arkh. 87:84–90. 2015.(In Russian). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klainin-Yobas P, Ng SH, Stephen PDM and
Lau Y: Efficacy of psychosocial interventions on psychological
outcomes among people with cardiovascular diseases: A systematic
review and meta-analysis. Patient Educ Couns. 99:512–521. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang XQ, Pi YL, Chen PJ, Liu Y, Wang R, Li
X, Chen BL, Zhu Y, Yang YJ and Niu ZB: Traditional Chinese exercise
for cardiovascular diseases: Systematic review and meta-analysis of
randomized controlled trials. J Am Heart Assoc. 5:e0025622016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zulli A, Smith RM, Kubatka P, Novak J,
Uehara Y, Loftus H, Qaradakhi T, Pohanka M, Kobyliak N, Zagatina A,
et al: Caffeine and cardiovascular diseases: Critical review of
current research. Eur J Nutr. 55:1331–1343. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baselet B, Rombouts C, Benotmane AM,
Baatout S and Aerts A: Cardiovascular diseases related to ionizing
radiation: The risk of low-dose exposure (Review). Int J Mol Med.
38:1623–1641. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fatema K, Zwar NA, Milton AH, Ali L and
Rahman B: Prevalence of risk factors for cardiovascular diseases in
bangladesh: A systematic review and meta-analysis. PLoS One.
11:e01601802016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang L, Xue Y, Ma H, Shi H, Wang L and Cui
X: Prazosin protects myocardial cells against anoxia-reoxygenation
injury via the extracellular signalregulated kinase signaling
pathway. Mol Med Rep. 17:2145–2152. 2018.PubMed/NCBI
|
|
10
|
Aminde LN and Veerman L: Interventions for
the prevention of cardiovascular diseases: A protocol for a
systematic review of economic evaluations in low-income and
middle-income countries. BMJ Open. 6:e0136682016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
De Lorgeril M: Essential polyunsaturated
fatty acids, inflammation, atherosclerosis and cardiovascular
diseases. Subcell Biochem. 42:283–297. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Candore G, Aquino A, Balistreri CR, Bulati
M, Di Carlo D, Grimaldi MP, Listì F, Orlando V, Vasto S, Caruso M,
et al: Inflammation, longevity, and cardiovascular diseases: Role
of polymorphisms of TLR4. Ann N Y Acad Sci. 1067:282–287. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang Q, Liu P, Wu X, Liu W, Shen X, Lan
T, Xu S, Peng J, Xie X and Huang H: Berberine attenuates
lipopolysaccharide-induced extracelluar matrix accumulation and
inflammation in rat mesangial cells: Involvement of NF-κB signaling
pathway. Mol Cell Endocrinol. 331:34–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang W, Zhang M, Li J, Meng Z, Xiao D,
Wei S, Chen L, Wang C and Hatch GM: Berberine attenuates
ischemia-reperfusion injury via regulation of
adenosine-5′-monophosphate kinase activity in both non-ischemic and
ischemic areas of the rat heart. Cardiovasc Drugs Ther. 26:467–478.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao GL, Yu LM, Gao WL, Duan WX, Jiang B,
Liu XD, Zhang B, Liu ZH, Zhai ME, Jin ZX, et al: Berberine protects
rat heart from ischemia/reperfusion injury via activating
JAK2/STAT3 signaling and attenuating endoplasmic reticulum stress.
Acta Pharmacol Sin. 37:354–367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Davey G and Wu Z: Attitudes in China
toward the use of animals in laboratory research. Altern Lab Anim.
35:313–316. 2007.PubMed/NCBI
|
|
17
|
Jong WM, Ten Cate H, Linnenbank AC, de
Boer OJ, Reitsma PH, de Winter RJ and Zuurbier CJ: Reduced acute
myocardial ischemia-reperfusion injury in IL-6-deficient mice
employing a closed-chest model. Inflamm Res. 65:489–499. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barile L, Chimenti I, Gaetani R, Forte E,
Miraldi F, Frati G, Messina E and Giacomello A: Cardiac stem cells:
Isolation, expansion and experimental use for myocardial
regeneration. Nat Clin Pract Cardiovasc Med. 4 Suppl 1:S9–S14.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin Y and Blikslager AT: Myosin light
chain kinase mediates intestinal barrier dysfunction via occludin
endocytosis during anoxia/reoxygenation injury. Am J Physiol Cell
Physiol. 311:C996–C1004. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang H, Lai S, Wan Q, Qi W and Liu J:
Astragaloside IV protects cardiomyocytes from anoxia/reoxygenation
injury by upregulating the expression of Hes1 protein. Can J
Physiol Pharmacol. 94:542–553. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xia WF, Liu Y, Zhou QS, Tang QZ and Zou
HD: Comparison of the effects of propofol and midazolam on
inflammation and oxidase stress in children with congenital heart
disease undergoing cardiac surgery. Yonsei Med J. 52:326–332. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang R, Zhang YY, Huang XR, Wu Y, Chung
AC, Wu EX, Szalai AJ, Wong BC, Lau CP and Lan HY: C-reactive
protein promotes cardiac fibrosis and inflammation in angiotensin
II-induced hypertensive cardiac disease. Hypertension. 55:953–960.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong SF, Hong Y, Liu M, Hao YZ, Yu HS, Liu
Y and Sun JN: Berberine attenuates cardiac dysfunction in
hyperglycemic and hypercholesterolemic rats. Eur J Pharmacol.
660:368–374. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang YJ, Yang SH, Li MH, Iqbal J,
Bourantas CV, Mi QY, Yu YH, Li JJ, Zhao SL, Tian NL and Chen SL:
Berberine attenuates adverse left ventricular remodeling and
cardiac dysfunction after acute myocardial infarction in rats: Role
of autophagy. Clin Exp Pharmacol Physiol. 41:995–1002. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang Z, Han Z, Ye B, Dai Z, Shan P, Lu Z,
Dai K, Wang C and Huang W: Berberine alleviates cardiac
ischemia/reperfusion injury by inhibiting excessive autophagy in
cardiomyocytes. Eur J Pharmacol. 762:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang YY, Li HM, Wang HD, Peng XM, Wang YP,
Lu DX, Qi RB, Hu CF and Jiang JW: Pretreatment with berberine and
yohimbine protects against LPS-induced myocardial dysfunction via
inhibition of cardiac I-[kappa]B[alpha] phosphorylation and
apoptosis in mice. Shock. 35:322–328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang XD, Ren HM and Liu L: Effects of
different dose berberine on hemodynamic parameters and [Ca2+]i of
cardiac myocytes of diastolic heart failure rat model. Zhongguo
Zhong Yao Za Zhi. 33:818–821. 2008.(In Chinese). PubMed/NCBI
|
|
29
|
Chen H, Zhang RQ, Wei XG, Ren XM and Gao
XQ: Mechanism of TLR-4/NF-κB pathway in myocardial ischemia
reperfusion injury of mouse. Asian Pac J Trop Med. 9:503–507. 2016.
View Article : Google Scholar : PubMed/NCBI
|