Introduction
The treatment of cardiovascular diseases, especially
ischemic heart disease, has been greatly improved, but heart
failure, as the end-stage manifestation of various cardiovascular
diseases, is the main cause of high mortality and disability rates
of cardiovascular diseases (1).
Despite the great progress made in the treatment of heart failure,
the current treatment still fails to control the progression and
death of heart failure to the greatest extent (2). In recent years, it has been gradually
realized that myocardial cell metabolism plays an important role in
the occurrence and development of congestive heart failure
(3). Studies have found that
myocardial energy metabolism disorder is the main reason for the
occurrence and development of heart failure. In congestive heart
failure, the damage to mitochondrial structure is increased,
mitochondrial dysfunction leads to reduced adenosine triphosphate
(ATP) production, and insufficient energy supply increases
myocardial cell apoptosis. Increased myocardial cell apoptosis is
the main mechanism of myocardial remodeling during congestive heart
failure (4).
Peroxisome proliferator-activated receptor γ
coactivator-1α (PGC-1α) is a co-activator of many transcription
factors in the energy metabolism pathway, which plays a crucial
role in energy metabolic balance (5). Therefore, PGC-1α is regarded as a
molecular switch that regulates mitochondrial energy metabolism.
Studies have shown that PGC-1α plays an important role in adaptive
thermogenesis, mitochondrial generation, β-oxidation of fatty
acids, hepatic gluconeogenesis and other processes (6). The activity of PGC-1α is modified by
multiple post-transcriptional translations, while the regulation of
acetylation level is affected by a variety of sirtuins. Sirt3 is an
energy regulatory molecule expressed in both mitochondria and
nuclei, which, as a member of the sirtuin family, has deacetylation
effect (7). However, some studies
have also found that Sirt3 can not only regulate the ATP production
in myocardial cells, but also hinder the development of myocardial
hypertrophy and heart failure (8).
Phosphodiesterase 5 (PDE5) inhibitor is a
clinically-approved mature drug for the treatment of pulmonary
hypertension and male sexual dysfunction. In recent years, a large
number of clinical and laboratory experiments have been performed
to study the protective effect of PDE5 inhibitor on cardiovascular
diseases. PDE5 inhibitor can fight against myocardial
ischemia-reperfusion injury, and inhibit myocardial remodeling in
heart failure with stress overload (9). Upregulating the expression of PGC-1α in
heart failure with stress overload and improving mitochondrial
function can reduce myocardial cell apoptosis after myocardial
infarction and improve cardiac function (10). The cardiovascular protective effect
of PDE5 inhibitor is achieved by increasing the cyclic guanosine
monophosphate (cGMP) level in cells (11). However, the expression of PGC-1α and
Sirt3 in heart failure after myocardial infarction and the effect
of PDE5 inhibitor on mitochondrial energy metabolism in heart
failure after myocardial infarction remain unclear.
Materials and methods
Experimental materials
Neonatal mice were purchased from Beijing Vital
River Laboratory Animal Technology Co., Ltd. (Beijing, China). Cell
Counting kit-8 (CCK-8), lactate dehydrogenase (LDH), ATP, caspase-3
and JC-1 assay kits were purchased from Beyotime Institute of
Biotechnology (Shanghai, China). Primary rabbit polyclonal PGC-1α
antibody (cat. no. ab54481; dilution, 1:500); rabbit polyclonal
Sirt3 antibody (cat. no. ab86671; dilution, 1:500); rabbit
polyclonal β-actin antibody (cat. no. ab8227; dilution, 1:1,000)
and secondary goat anti-rabbit (HRP) IgG antibody (cat. no. ab6721;
dilution, 1:2,000) were all purchased from Abcam (Cambridge, MA,
USA).
This study was approved by the Animal Ethics
Committee of Shandong Provincial Third Hospital Animal Center
(Jinan, China).
Cell isolation, culture and
treatment
The ventricular tissues of 1-3-day-old C57BL/6
neonatal mice were taken, cut into 0.5–1 mm3 tissue
blocks and digested with trypsin containing ethylene diamine
tetraacetic acid (EDTA). The digestion process was repeated, and
the supernatant was collected into a centrifuge tube after each
digestion. An equal volume of Dulbecco's modified Eagle's medium
(DMEM)/F12 medium containing 5% fetal bovine serum and 10% horse
serum was added to terminate the digestion, followed by
centrifugation at 850 × g at 4°C for 5 min. The supernatant in the
centrifuge tube was removed after centrifugation, and cells were
blown away using DMEM/F12 medium containing 5% fetal bovine serum
and 10% horse serum for standby application. Undigested tissue
blocks were filtered and removed via the 200-mesh nylon mesh, and
cells were inoculated into a 25 cm2 culture flask and
cultured in an incubator with 5% CO2 and saturated humidity at
37°C. Non-myocardial cells were separated via differential adhesion
(culture flask wall) method. Bromodeoxyuridine (BrdU) in a final
concentration of 0.1 mm/l was added into the culture solution to
inhibit fibroblast proliferation, and double antibodies (100 U/ml
penicillin and 100 U/ml streptomycin) were added to prevent
bacterial contamination, followed by culture under 5% CO2 at 37°C
for 3 days. The isolated myocardial cells were taken and added into
serum-free medium for hypoxia for 24 h in a hypoxia tank (the
oxygen partial pressure was <0.1%) to establish the hypoxia
model.
CCK-8 assay
Cells in the logarithmic growth phase were digested,
collected and adjusted into cell suspension at a concentration of
1×105/ml. The suspension was inoculated into a 96-well
plate (100 µl per well), 3 repeated wells were set in the
experiment, and the blank control was also set. After inoculation
overnight, it was confirmed via microscopic observation that cells
adhered well to the wall. After grouping and treatment, 20 µl
methyl thiazolyl tetrazolium (MTT) was added into each well,
followed by culture at 37°C for 4 h. Then the supernatant was
carefully discarded, and 150 µl dimethyl sulphoxide (DMSO) was
added into each well and mixed evenly. The optical density (OD)
value of each well was detected at a wavelength of 570 nm using a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The experiment was repeated 3 times.
LDH assay
Myocardial cells in the logarithmic growth phase
were digested, collected and adjusted into cell suspension at a
concentration of 1×105/ml. The suspension was inoculated
into the 96-well plate (100 µl per well), 3 repeated wells were set
in the experiment, and the blank control was also set. After cell
adherence, the diluted silibinin was added to make the final
concentration 0, 25, 50, 100, 150 and 200 µM. Cells in the culture
plate were incubated in the incubator with 5% CO2 at 37°C for 24 h.
The supernatant was taken (20 µl per well) and added with the
corresponding reagent according to instructions of the kit. The
mixture was mixed evenly and placed at room temperature for 3 min,
followed by zero setting using 440 nm double distilled water and
detection of OD value using the microplate reader (Bio-Rad
Laboratories, Inc.). Unit definition: Αfter 1,000 ml culture
solution reacted with the substrate at 37°C for 15 min, 1
gmolpyruvic acid produced in the reaction system was regarded as 1
unit. The LDH content in the medium was calculated using the
formula.
Flow cytometry
BGC823 (cat. no. BNCC337689; BeNa Culture
Collection, Beijing, China; http://www.bnbio.com/) cells were inoculated into a
6-well plate (5×105/ml) overnight. Silibinin solution at
a final concentration of 0, 50, 100 and 200 µM was added,
respectively, and cells were incubated in the incubator with 5% CO2
and saturated humidity at 37°C for 24 h, digested with trypsin and
collected, followed by centrifugation at 850 × g and 4°C for 4 min.
Then cells were collected, and the medium was abandoned.
Centrifuged cells were washed twice with cold phosphate-buffered
saline (PBS), and resuspended using 200 µl binding buffer at a
concentration of approximately 1×106/ml. A total of 10
µl annexin V-fluorescein isothiocyanate (FITC) was added into the
cell suspension and gently mixed evenly, followed by incubation in
the dark at room temperature for 15 min. A total of 5 µl propidium
iodide (PI) was added and gently mixed evenly, followed by
detection using a flow cytometer within 1 h. MitoProbe™
DiOC2 (3) Assay kit (cat.
no. M34150; ThermoFisher Scientific; Waltham, MA, USA) was used for
flow cytometry. Data were obtained and analyzed using the CellQuest
professional software (Becton, Dickinson and Company, Franklin
Lakes, NJ, USA). Data were obtained and analyzed using the
CellQuest professional software. The experiment was repeated 3
times.
Hoechst staining
The detection of apoptosis was displayed using the
Hoechst staining (KeyGen Biotech Co., Ltd., Nanjing, China). BGC823
cells were seeded in 6-well plates. After hypoxia for 4–6 h, NRVMs
were stained with Hoechst for 15 min and washed by PBS for 5 min 3
times. Paraformaldehyde (4%) (Beyotime Institute of Biotechnology,
Shanghai, China) was used for the fixative for 10 min at 4 °C. Cell
observation used fluorescence microscope (Olympus BX51; Olympus
Corporation, Tokyo, Japan).
Detection of caspase-3 activity
After gradient dilution of standard sample using the
standard sample diluent, 100 µl standard sample in each
concentration was taken and added into the 96-well plate. The
absorbance value at a wavelength of 405 nm (A405) of each well was
detected using the microplate reader (Bio-Rad Laboratories, Inc.)
and the standard curve was drawn. First, test buffer and protein
sample were added and mixed evenly in the 96-well plate, and
incubated in the incubator at 37°C for 60 min. At the same time, a
small number of protein samples were taken to determine the protein
concentration using the bicinchoninic acid (BCA) method. Five
repeated wells were set for each sample. A405 of each well was
detected using the microplate reader (Bio-Rad Laboratories, Inc.).
According to A405 of standard sample in each concentration, the
standard curve was drawn. The caspase-3 activity in each sample was
calculated according to the standard curve, and the average was
taken.
JC-1 mitochondrial membrane potential
detection
Cells are collected after treatment, and resuspended
in 0.5 ml cell culture medium that could contain serum. A total of
0.5 ml JC-1 staining working solution was added, and mixed several
times evenly, followed by incubation in a dark place in the
incubator at 37°C for 30 min and centrifugation at 500 × g and 4°C.
Then the supernatant was discarded, and cells were washed with 1X
JC-1 staining buffer twice, and resuspended using 1X JC-1 staining
buffer, followed by centrifugation at 500 × g and 4°C and
precipitation, and the supernatant was discarded. The above washing
step was repeated once. The fluorescence intensity of cells was
detected using the flow cytometer.
Fluorescein ATP detection
Cells in each group were lysed on ice for 30 min.
The protein in each group was collected into an Eppendorf (EP) tube
using a cell scraper. After centrifugation at 1,050 × g at 4°C for
10 min, the supernatant was taken to quantify the protein via BCA.
The ATP content was determined according to instructions of the
kit. The sample and reagent were added into the 96-well plate,
fully mixed for 2 sec, and quickly placed into the fluorescence
microplate for fluorescence detection for 10 sec. The relative
level of ATP in each group was calculated based on the fluorescence
amount measured.
Polymerase chain reaction (PCR)
Cells in each group were collected after treatment,
and the total ribonucleic acid (RNA) was extracted using TRIzol.
After the concentration of sample was measured, the reverse
transcription system was added for reverse transcription reaction.
The first 40 cycles were used to synthesize complementary
deoxyribonucleic acid (cDNA), and conditions of reverse
transcription reaction were set for PCR amplification. Fluorescence
signals were collected in real time after each cycle, and
amplification and solubility curves were recorded.
Western blot analysis
Cells in each group were taken and washed twice with
D-Hank's solution, and D-Hank's solution was sucked dry with
absorbent paper. A total of 150 µl pre-cooled lysis buffer was
added in each group and cells were lysed on ice for 30 min. The
protein in each group was collected into an EP tube using the cell
scraper, followed by centrifugation at 10,500 × g at 4°C. The
supernatant was taken and transferred into a new EP tube. After the
protein concentration was determined using the BCA method, 5X
loading buffer was added and mixed evenly, and the protein was
heated at 100°C for 6 min. A total of 30 µl protein was added into
the loading wells of separation gel (10%) and spacer gel (5%),
followed by electrophoresis in the electrophoretic buffer solution
under appropriate voltage. After electrophoresis, gel closely
contacted with the polyvinylidene fluoride (PVDF) membrane,
followed by membrane transfer in transfer buffer at 0°C under
constant voltage at 100 V for 60 min. Then the PVDF membrane was
sealed in 5% skim milk powder at room temperature for 1 h, cut
according to the molecular weight and sealed in the primary
antibody in a refrigerator at 4°C overnight. The next day, the PVDF
membrane was taken, rinsed with Tris-buffered saline with Tween-20
(TBST), and added with the secondary antibody immunoglobulin G
(IgG) (1:5,000) for incubation at room temperature for 1 h. After
incubation, the membrane was rinsed again with TBST, and the image
was developed using the Tanon 5200 immunofluorescence imaging
system (Tanon Science & Technology Co., Ltd., Shanghai, China),
followed by calculation of gray-scale.
Statistical analysis
Experimental data were presented as mean ± standard
deviation. SPSS 16.0 SPSS, Inc., Chicago, IL, USA) statistical
software was used for statistical analysis. One-way analysis of
variance (ANOVA) followed by post hoc test (Least Significant
Difference) was used for the intergroup comparison, and t-test was
used for the comparison between two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
PDE5 inhibitor reduced cytotoxicity of
hypoxic myocardial cells
In this study, CCK-8 assay was used to detect
changes in cell viability. In the experiment, cells were divided
into blank group, control group and PDE5 inhibitor group. Results
showed that the viability of hypoxic myocardial cells in the
control group was decreased significantly (58.44±4.41%; P<0.05)
compared with that in the blank group, while that in the PDE5
inhibitor group was significantly increased (81.74±4.2%; P<0.05)
compared with that in the control group. The cytotoxic effect was
detected via LDH assay. Results showed that the cytotoxic effect of
hypoxia in the control group was larger, and the LDH release was
significant compared with that in the blank group (P<0.05). The
cytotoxic effect of hypoxia in the PDE5 inhibitor group was
reduced, and the change was significant compared with that in the
control group (P<0.05) (Table
I).
 | Table I.Viability and cytotoxicity of hypoxic
myocardial cells in 2 groups. |
Table I.
Viability and cytotoxicity of hypoxic
myocardial cells in 2 groups.
| Groups | Blank | Control | Sirtuins |
|---|
| Cell viability
(%) | 100% |
58.44±4.41a |
81.74±4.2b |
| LDH (fold of
blank) | 1 | 4.2±0.7a | 2.3±0.4b |
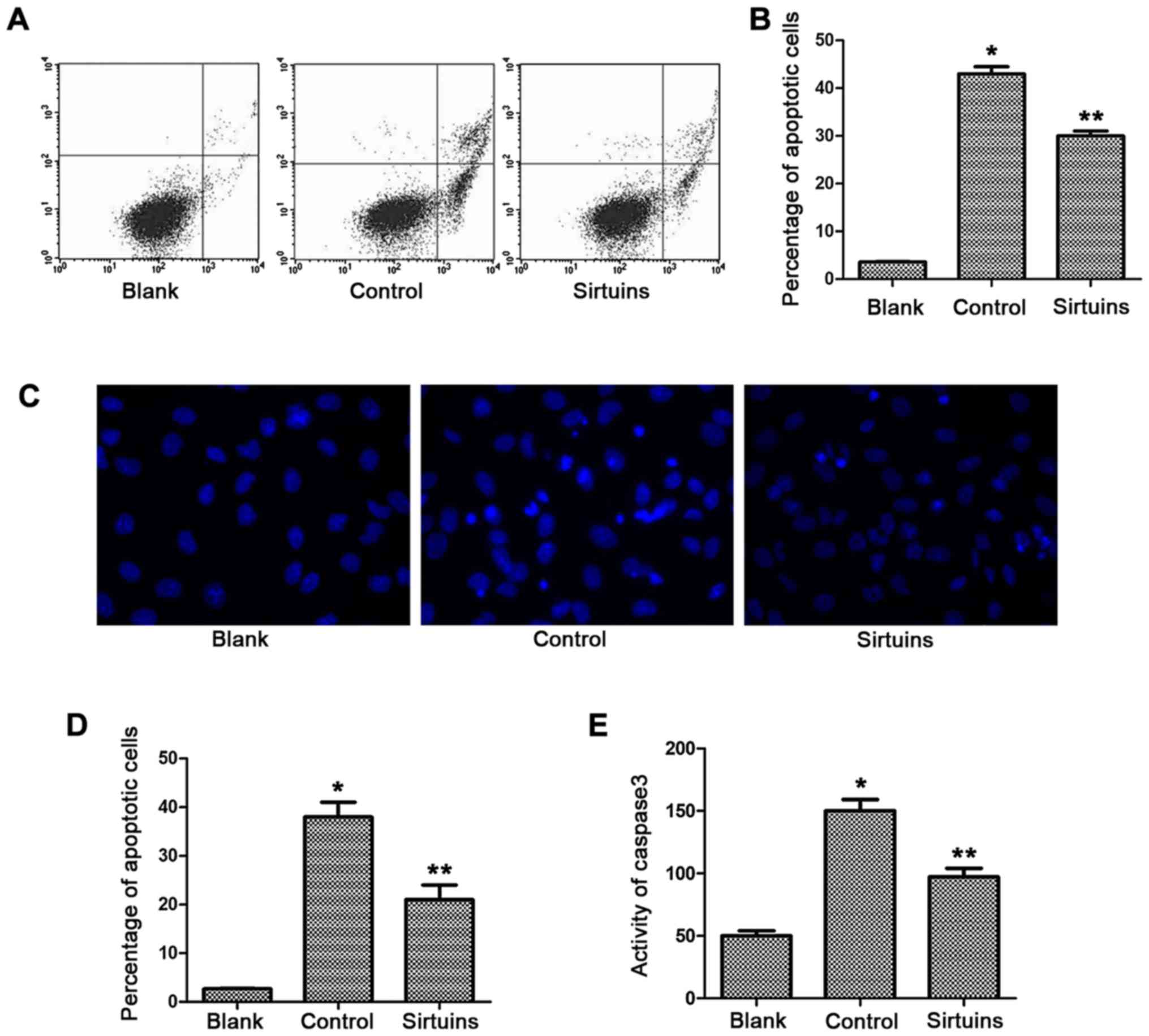
PDE5 inhibitor inhibited apoptosis of
hypoxic myocardial cells
Apoptosis level of myocardial cells was detected via
flow cytometry, Hoechst staining and caspase-3 activity assay.
Results showed that the proportion of early apoptotic cells in the
control group was significantly increased compared with that in the
blank group (42.8±2.4 vs. 2.6±0.2%; P<0.05) (Fig. 1A and B). Hoechst staining was used to
mark the proportion of apoptotic cells with karyopyknosis and
karyorrhexis, and results revealed that the proportion of apoptotic
myocardial cells after hypoxia was increased compared with that in
the blank group (38.5±3.9 vs. 4.43±0.6%; P<0.05) (Fig. 1C and D). PDE5 inhibitor could reduce
the early apoptosis induced by hypoxia. Results of flow cytometry
showed that there was a significant difference in apoptosis
compared with that in the control group (30.44±2.7 vs. 42.8±2.4%;
P<0.05), and the proportion of cells with karyopyknosis and
karyorrhexis was also significantly decreased (21.8±4.1 vs.
38.5±3.9%; P<0.05) (data not shown). The activity of caspase-3
in the control group was increased compared with that in the blank
group, and the caspase-3 activity was reduced after addition of
PDE5 inhibitor (P<0.05) (Fig.
1E).
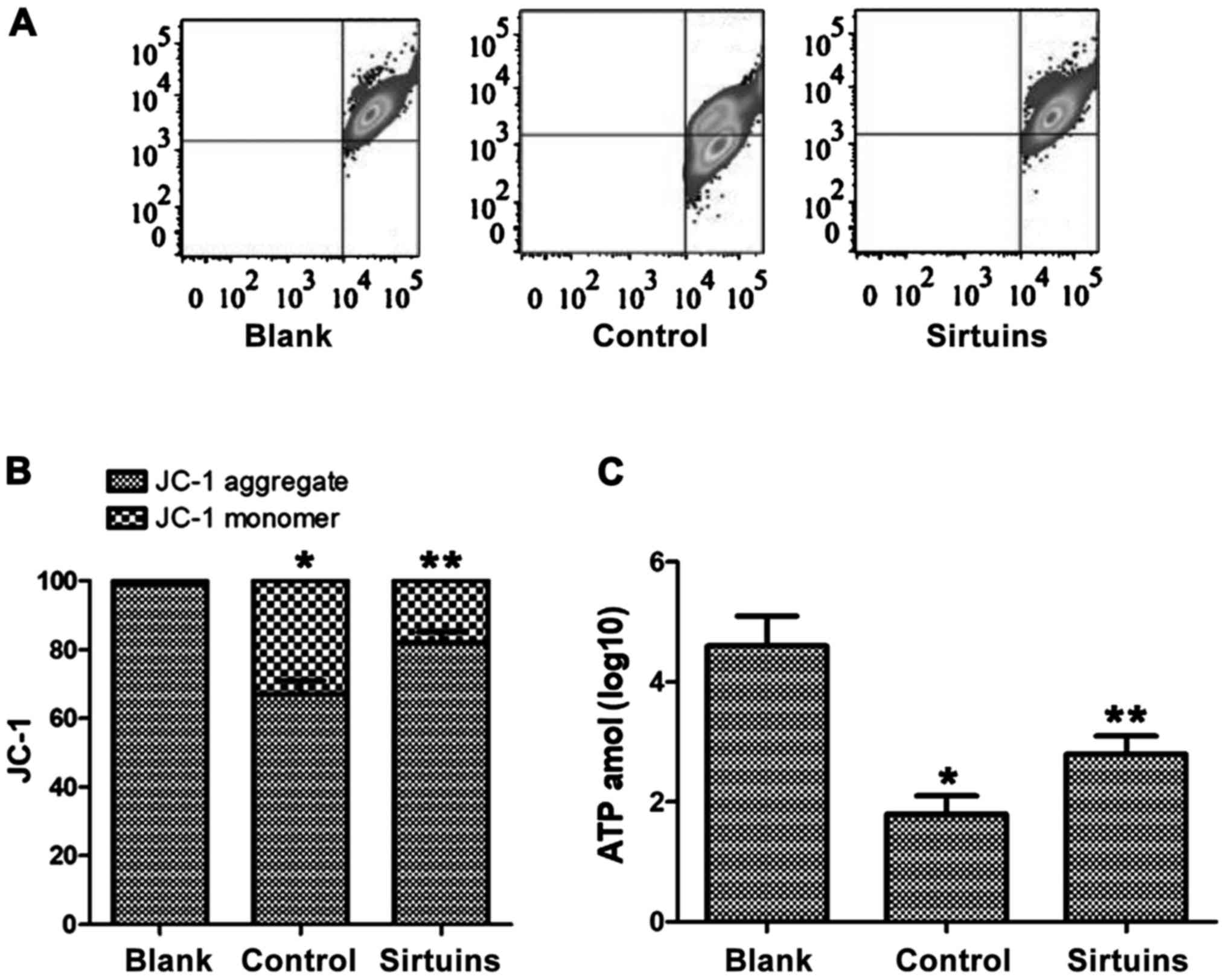
PDE5 inhibitor protected mitochondria
of hypoxic myocardial cells
Changes in mitochondrial membrane potential in each
group of cells were detected via JC-1 staining. Results of JC-1
fluorescence staining showed that the mitochondrial membrane
potential of myocardial cells in the control group was decreased
obviously. ATP assay showed that ATP synthesis was blocked in
myocardial cells after hypoxia, and the ATP content in cells was
decreased significantly compared with that in the blank group
(Fig. 2). PDE5 inhibitor could
protect the mitochondrial membrane potential, ensure the normal
function of mitochondria and increase the ATP content in cells,
showing significant differences from the control group (Fig. 2).
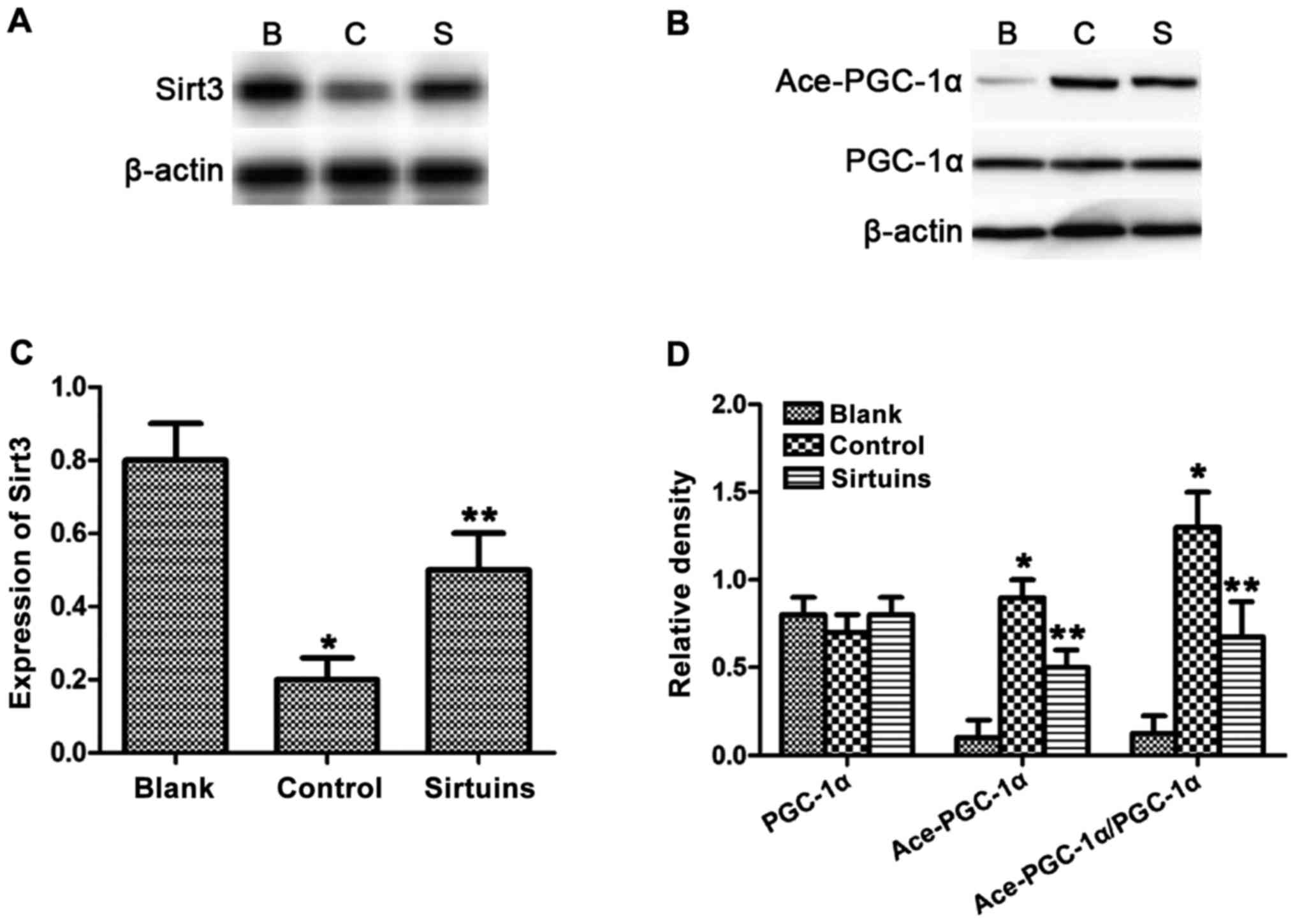
PDE5 inhibitor could regulate Sirt3
and PGC-1α acetylation
The content of PDE5a, Sirt3, PGC-1α and acetylated
PGC-1α in each group of cells was detected via western blot
analysis. Results revealed that the expression of Sirt3 was
decreased in myocardial cells after hypoxia, whereas PDE5 inhibitor
could reverse such a change induced by hypoxia (Fig. 3A and C). The expression level of
acetylated PGC-1α was increased compared with that in the blank
group, whereas PDE5 inhibitor could significantly reduce PGC-1α
acetylation (Fig. 3B and D).
Discussion
After myocardial ischemia, mitochondrial dysfunction
and myocardial energy metabolism disorder are the main factors
leading to myocardial cell apoptosis, as well as the main factors
of myocardial remodeling after myocardial ischemia. Myocardial
remodeling can result in further decline in ischemic cardiac
function, and constant decrease.
PGC-1α is a co-activator of many transcription
factors in the energy metabolism pathway, which plays a crucial
role in energy metabolic balance. Moreover, PGC-1α can promote
expression of related genes in mitochondrial biogenesis and
mitochondrial respiratory function through increasing the
capacities of mitochondrial fatty acid oxidation and oxidative
phosphorylation (12). PGC-1α is
highly expressed in the heart and exerts an important protective
effect on the cardiovascular system. When PGC-1α is acetylated, it
will be transformed into a protein molecule that lacks activity.
Sirtuin family is a protein family, and Sirt3, as a member of the
sirtuin family, is the only protein associated with longevity
(13).
Recent studies have shown that PDE5 is highly
expressed in patients with heart failure, and its expression in
myocardium of left ventricle of patients with heart failure is 4–5
times that in normal myocardium (14). PDE5a-specific inhibitor sildenafil
can inhibit the decomposition of cGMP, so that a variety of growth
pathways are inactivated, thus preventing the development of heart
failure and myocardial hypertrophy caused by pressure overload
(15). In animal models, sildenafil
can alleviate the myocardial ischemia-reperfusion injury and reduce
the myocardial infarct area.
In this study, it was found that after hypoxia of
myocardial cells, the cell viability was significantly decreased,
the cytotoxic effect was enhanced, the proportion of early
apoptotic cells was increased, the number of cells with
karyopyknosis and karyorrhexis was significantly increased, the
activity of apoptosis-related protein caspase-3 was increased, the
mitochondrial function was decreased obviously, the Sirt3
expression was decreased significantly, and the PGC-1α acetylation
level was increased. After addition of PDE5 inhibitor, cell injury
caused by hypoxia was significantly alleviated, apoptosis was
reduced, mitochondrial function was protected, Sirt3 protein
expression was induced and PGC-1α acetylation level was decreased.
Results suggested that PDE5 inhibitor can increase Sirt3
expression, reduce PGE-1α acetylation, and protect mitochondrial
function, thus protecting hypoxic myocardial cells from
apoptosis.
In conclusion, PDE5 inhibitor inhibits PGC-1α
acetylation and protects mitochondrial function via increasing
Sirt3 expression, thus inhibiting apoptosis of hypoxic myocardial
cells.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HJ and YY designed the study and performed the
experiments, HJ and ZG raised the animals, HJ and ZG collected the
data, HJ and YY analyzed the data, HJ and YY prepared the
manuscript. All authors read and approved the final study.
Ethics approval and consent to
participate
This study was approved by the Animal Ethics
Committee of Shandong Provincial Third Hospital Animal Center
(Jinan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nanchen D, Leening MJ, Locatelli I, Cornuz
J, Kors JA, Heeringa J, Deckers JW, Hofman A, Franco OH, Stricker
BH, et al: Resting heart rate and the risk of heart failure in
healthy adults: The Rotterdam Study. Circ Heart Fail. 6:403–410.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cowie MR, Wood DA, Coats AJ, Thompson SG,
Suresh V, Poole-Wilson PA and Sutton GC: Survival of patients with
a new diagnosis of heart failure: A population based study. Heart.
83:505–510. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Bilsen M, Smeets PJ, Gilde AJ and van
der Vusse GJ: Metabolic remodelling of the failing heart: The
cardiac burn-out syndrome? Cardiovasc Res. 61:218–226. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu YF, Chu YY, Zhang XZ, Zhang M, Xie FG,
Zhou M, Wen HH and Shu AH: TGFβ1 protects myocardium from apoptosis
and oxidative damage after ischemia reperfusion. Eur Rev Med
Pharmacol Sci. 21:1551–1558. 2017.PubMed/NCBI
|
|
5
|
Lin J, Handschin C and Spiegelman BM:
Metabolic control through the PGC-1 family of transcription
coactivators. Cell Metab. 1:361–370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huss JM, Torra IP, Staels B, Giguère V and
Kelly DP: Estrogen-related receptor alpha directs peroxisome
proliferator-activated receptor alpha signaling in the
transcriptional control of energy metabolism in cardiac and
skeletal muscle. Mol Cell Biol. 24:9079–9091. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Zhang S, Blander G, Tse JG, Krieger
M and Guarente L: SIRT1 deacetylates and positively regulates the
nuclear receptor LXR. Mol Cell. 28:91–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sundaresan NR, Gupta M, Kim G, Rajamohan
SB, Isbatan A and Gupta MP: Sirt3 blocks the cardiac hypertrophic
response by augmenting Foxo3a-dependent antioxidant defense
mechanisms in mice. J Clin Invest. 119:2758–2771. 2009.PubMed/NCBI
|
|
9
|
Kukreja RC, Salloum FN, Das A, Koka S,
Ockaili RA and Xi L: Emerging new uses of phosphodiesterase-5
inhibitors in cardiovascular diseases. Exp Clin Cardiol.
16:e30–e35. 2011.PubMed/NCBI
|
|
10
|
Das A, Xi L and Kukreja RC: Protein kinase
G-dependent cardioprotective mechanism of phosphodiesterase-5
inhibition involves phosphorylation of ERK and GSK3beta. J Biol
Chem. 283:29572–29585. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
De Toni L, Strapazzon G, Gianesello L,
Caretta N, Pilon C, Bruttocao A and Foresta C: Effects of type
5-phosphodiesterase inhibition on energy metabolism and
mitochondrial biogenesis in human adipose tissue ex vivo. J
Endocrinol Invest. 34:738–741. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ventura-Clapier R, Garnier A and Veksler
V: Transcriptional control of mitochondrial biogenesis: The central
role of PGC-1alpha. Cardiovasc Res. 79:208–217. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bellizzi D, Rose G, Cavalcante P, Covello
G, Dato S, De Rango F, Greco V, Maggiolini M, Feraco E, Mari V, et
al: A novel VNTR enhancer within the SIRT3 gene, a human homologue
of SIR2, is associated with survival at oldest ages. Genomics.
85:258–263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu Z, Xu X, Hu X, Lee S, Traverse JH, Zhu
G, Fassett J, Tao Y, Zhang P, dos Remedios C, et al: Oxidative
stress regulates left ventricular PDE5 expression in the failing
heart. Circulation. 121:1474–1483. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Das A, Durrant D, Salloum FN, Xi L and
Kukreja RC: PDE5 inhibitors as therapeutics for heart disease,
diabetes and cancer. Pharmacol Ther. 147:12–21. 2015. View Article : Google Scholar : PubMed/NCBI
|