Introduction
Lung cancer is the leading cause of cancer mortality
and is the most frequently diagnosed cancer in the world (1). Annually, there are an estimated 1.8
million new lung cancer cases, accounting for about 13% of total
cancer diagnoses (2). Historically,
lung cancer has been classified as small cell lung cancer and
non-small cell lung cancer (NSCLC). NSCLC constitutes 75% of all
lung cancer cases, including adenocarcinoma, squamous cell
carcinoma (SCC) and large cell carcinoma. SCC is the largest subset
of NSCLC (3). Although surgery and
chemoradiotherapy are the primary treatment methods for SCC, the
overall 5-year survival rate remains less than 15% (4). The complex biological characteristics,
recurrence, metastasis and low sensitivity to chemotherapeutic
drugs remain problematic issues in the treatment of SCC (5–7).
Therefore, it is necessary to explore the potential molecular
mechanisms underlying the development and progression of SCC, and
to identify new therapeutic targets.
Transducin (β)-like 1 X-linked receptor 1 (TBL1XR1)
is an F-box/WD-40-containing protein, a subfamily of F-box proteins
that were initially identified as core components of the nuclear
receptor corepressor 1/2 complex; this complex serves a role in
regulating the activation of corepressors (8–11).
Aberrant expression of TBLIXR1 was associated with carcinogenesis
and tumor progression by regulating multiple signaling pathways,
including the nuclear factor-κB, nuclear receptors, Wnt/β-catenin,
and Notch pathways (12–15). Studies have previously demonstrated
that TBL1XR1 was overexpressed in cervical cancer, nasopharyngeal
cancer, breast cancer, hepatocellular carcinoma and esophageal
squamous cell cancer, and TBL1XR1 was associated with tumor
proliferation, migration, invasion and tumor drug resistance
(16–19). Kuang et al (19) found that overexpression of TBL1XR1
was associated with the clinicopathological features and prognosis
of hepatocellular carcinoma, inducing epithelial-mesenchymal
transition (EMT) through the Wnt/β-catenin signaling pathway to
promote tumor progression.
Although previous studies have demonstrated that
TBL1XR1 was highly expressed in human primary lung SCC tissues
(3,20), the biological role of TBL1XR1 and its
molecular mechanism in lung SCC remain to be established. The
present study demonstrated that TBL1XR1 was overexpressed in lung
SCC cells. Furthermore, overexpression of TBL1XR1 promoted cell
growth, migration, invasion and EMT in lung SCC cells through
activation of the TGF-β/Smads pathway. These findings suggested
that TBL1XR1 serves a role in the progression of lung SCC and may
be a potential therapeutic target in lung SCC therapy.
Materials and methods
Cell lines and cell cultures
The human bronchial epithelial cell line 1 (HBE1)
was provided by Xiangya Medical College (Changsha, China) and lung
squamous cell carcinoma (SCC) cell lines (SK-MES-1 and H1703) were
purchased from Cell Bank of the Chinese Academy of Science
(Shanghai, China). Cells were cultured in Dulbecco's modified Eagle
medium (DMEM) supplemented with 10% fetal bovine serum (FBS; both
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100
µg/µl streptomycin and 100 µg/µl penicillin, and maintained at 37°C
in a 5% CO2-humidified incubator.
Plasmids and small interfering RNAs
(siRNAs)
The TBL1XR1 plasmid was purchased from Shanghai
GeneChem Co., Ltd. (Shanghai, China). The corresponding vector was
pEX-1. The TBL1XR1 plasmid and corresponding empty vector were
transfected into SK-MES-1 cells using Lipofectamine®
2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Stably transfected cells
(SK-MES-1-vector, SK-MES-1-TBL1XR1) were selected by puromycin (1
ug/ml; InvivoGen, San Diego, CA, USA). TBL1XR1 siRNA sequences and
negative control sequences were designed and synthesized by
Shanghai GeneChem Co., Ltd. H1703 cells were cultured in six-well
plates and transfected with 400 ng TBL1XR1 small interfering
(si)RNA (si-TBL1XR1-1, 5′-GCAGCAUAAAGGCCCUAUATT-3′; si-TBL1XR1-2,
5′-GCCUGAUGUAGUACAAACATT-3′) using Lipofectamine® 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. Stable cell lines expressing
TBL1XR1-siRNA [negative control, (H1703-NC), H1703-siRNA-1,
H1703-siRNA-2] were generated with 1 ug/ml puromycin. Knockdown and
overexpression of TBL1XR1 were confirmed by western blot analysis.
Western blotting was performed as stated below.
Cell proliferation assay
SK-MES-1-vector, SK-MES-1- TBL1XR1, H1703-NC,
H1703-siRNA-1 and H1703-siRNA-2 cells were seeded in 96-well plates
at a density of 6×103/well and cultured for 12, 24, 36,
48, 60 and 72 h. Cells were incubated with 100 µl of Cell Counting
kit-8 (CCK-8) reagent (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) for 2 h at 37°C. The absorbance was measured at a
wavelength of 450 nm.
Transwell invasion assay
Cell invasion assays were performed using 24-well
plates and 8 µm Transwell inserts (Corning Life Sciences, Acton,
MA, USA). Transwell membrane inserts were precoated with
Matrigel® (BD Biosciences, Franklin Lakes, NJ, CA, USA)
before adding the cells. A total of 1×105
SK-MES-1-vector, SK-MES-1-TBL1XR1, H1703-NC, H1703-siRNA-1, and
H1703-siRNA-2 cells in 200 µl serum-free DMEM medium were added to
the upper chamber. DMEM supplemented with 10% FBS (500 µl) was
added to the lower chamber. After incubating the cells at 37°C and
5% CO2 for 48 h of culture, transfected cells remaining
in the upper side of the inserts were removed with cotton swabs.
Cells that had migrated to the lower side of the inserts were fixed
in methanol and stained with 0.1% crystal violet for 10 min. Images
of migrated cells were captured using an inverted microscope at a
magnification, ×200. Six visual fields were randomly selected to
calculate the number of migrated cells.
Wound healing assay
Transfected cells were cultured in six-well plates
until confluent. Straight lines were drawn, in increments of 0.5
cm, on the back of the six-well plates. Cell layers were scratched
with a 20 µl pipette tip and the medium was replaced with 2 ml of
fresh DMEM. Cells were incubated for a further 36 h at 37°C. Images
were captured at 0 and 36 h after the scratches were made using an
inverted microscope at a magnification, ×40. The mean length of the
wound was calculated in ImageJ software (version 1.48U; National
Institutes of Health, Bethesda, MD, USA) by marking six to eight
horizontal lines in the wound.
Western blotting
Total protein was extracted from transfected and
non-transfected HBE1, SK-MES-1, H1703 cells using SDS-lysis buffer
(Thermo Fisher Scientific, Inc.). Total protein was quantified
using the BCA protein assay kit (Thermo Fisher Scientific, Inc.)
and protein (20 ug/lane) separated via SDS-PAGE on 10% gels. The
separated proteins were subsequently transferred onto
polyvinylidene difluoride membranes, and blocked with 5% non-fat
milk for 1 h and incubated overnight at 4°C. Membranes were
incubated with the following primary antibodies at 1:1,000
dilution: Anti-TBL1XR1 (cat. no. ab24550; Abcam, Cambridge, UK),
anti-GAPDH (cat. no. ab32233; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), anti-snail family transcriptional repressor 1
(SNAI1; cat. no. ab3879), anti-zinc finger E-box binding homebox 1
(ZEB1; cat. no. ab3396), anti-E-calcium-dependent adhesion
(E-cadherin; cat. no. ab3195), anti-mothers against decapentaplegic
homolog 2 (Smad2; cat. no. ab5339), anti-Smad3 (cat. no. ab9523),
anti-phosphorylated (p)-Smad2/3; cat. no. ab8685; all Cell
Signaling Technology, Inc., Danvers, MA, USA). Following the
primary incubation, membranes were incubated with corresponding
horseradish peroxidase-conjugated rabbit anti-mouse and goat
anti-rabbit secondary antibodies (1:1,000; cat. no. ab6728 and
ab6721, respectively; Abcam) for 1 h at room temperature. Protein
bands were visualized with using the Enhanced Chemiluminescence
Western Blotting kit (Thermo Fisher Scientific, Inc.) by exposure
to film. Protein expression was quantified using ImageJ software
(version 1.48U).
Statistical analysis
All experiments were repeated at least three times
and all data expressed as the mean ± standard error. All
statistical analyses were carried out using SPSS software (version
19.0; IBM Corp., Armonk, NY, USA). Differences between two groups
were compared using Student's t-tests. For multiple comparisons,
one-way analysis of variance was used followed by Turkey's post hoc
test. A two-tailed value of P<0.05 was considered to indicate a
statistically significant difference.
Results
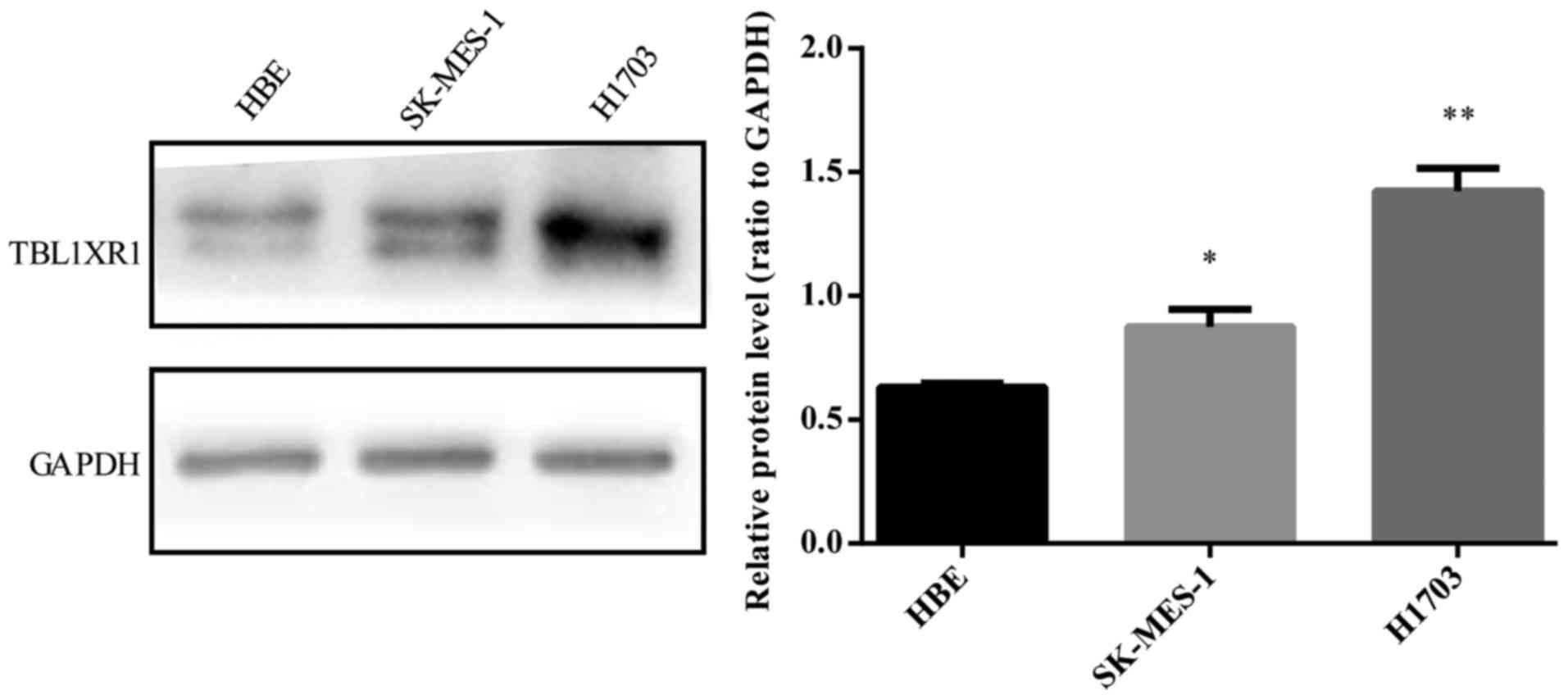
TBL1XR1 is highly expressed in human
lung SCC cells
To determine the biological role of TBL1XR1 in human
lung SCC, TBL1XR1 expression was first examined in lung SCC cell
lines (SK-MES-1 and H1703). TBL1XR1 protein expression was examined
in SCC cell lines and corresponding control, HBE1 cell line.
Protein levels of TBL1XR1 were higher than HBE1 in both SCC cell
lines (Fig. 1).
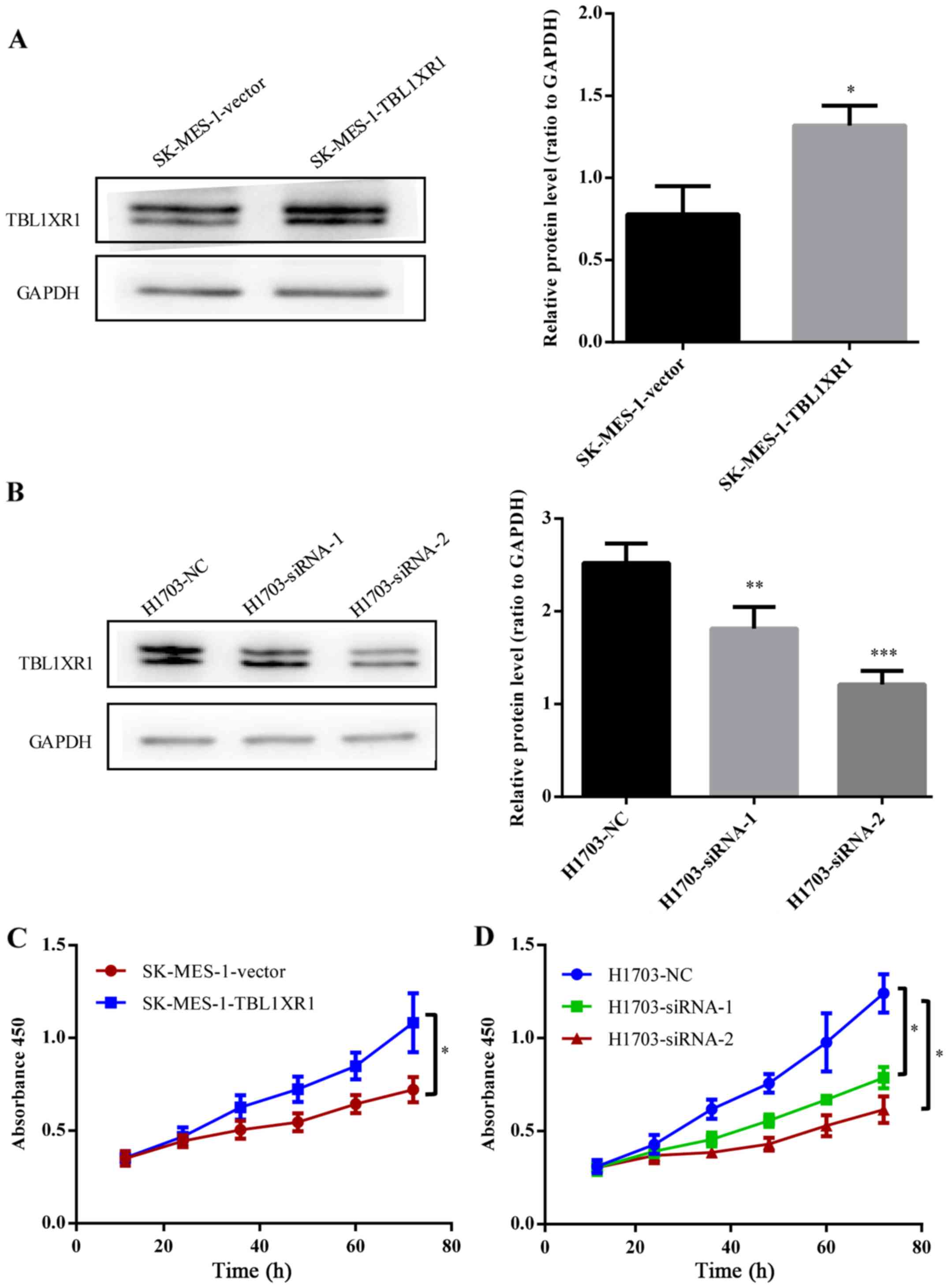
TBL1XR1 promotes lung SCC cell
proliferation in vitro
To further confirm the biological functions of
TBL1XR1 in lung SCC, TBL1XR1 expression in lung SCC cells was
upregulated by transfecting TBL1XR1-expressing vector into SK-MES-1
cells expressing low levels of TBL1XR1 (Fig. 2A). Endogenous TBL1XR1 expression was
silenced by transfecting TBL1XR1-siRNA (siRNA-1, siRNA-2) into
H1703 cells expressing high levels of TBL1XR1 (Fig. 2B). CCK-8 assays demonstrated an
increase in the number of TBL1XR1-overexpressing cells
(SK-MES-1-TBL1XR1) compared with empty vector control cells
(SK-MES-1-vector), suggesting that TBL1XR1 overexpression increased
the proliferative capacity of lung SCC cells (P<0.05; Fig. 2C). Conversely, knockdown of TBL1XR1
(H1703-siRNA-1, H1703-siRNA-2) significantly attenuated lung SCC
cell proliferation, leading to a decrease in cell number compared
with H1703-NC (P<0.05; Fig. 2D).
These results indicate that TBL1XR1 promotes lung SCC cell
proliferation in vitro.
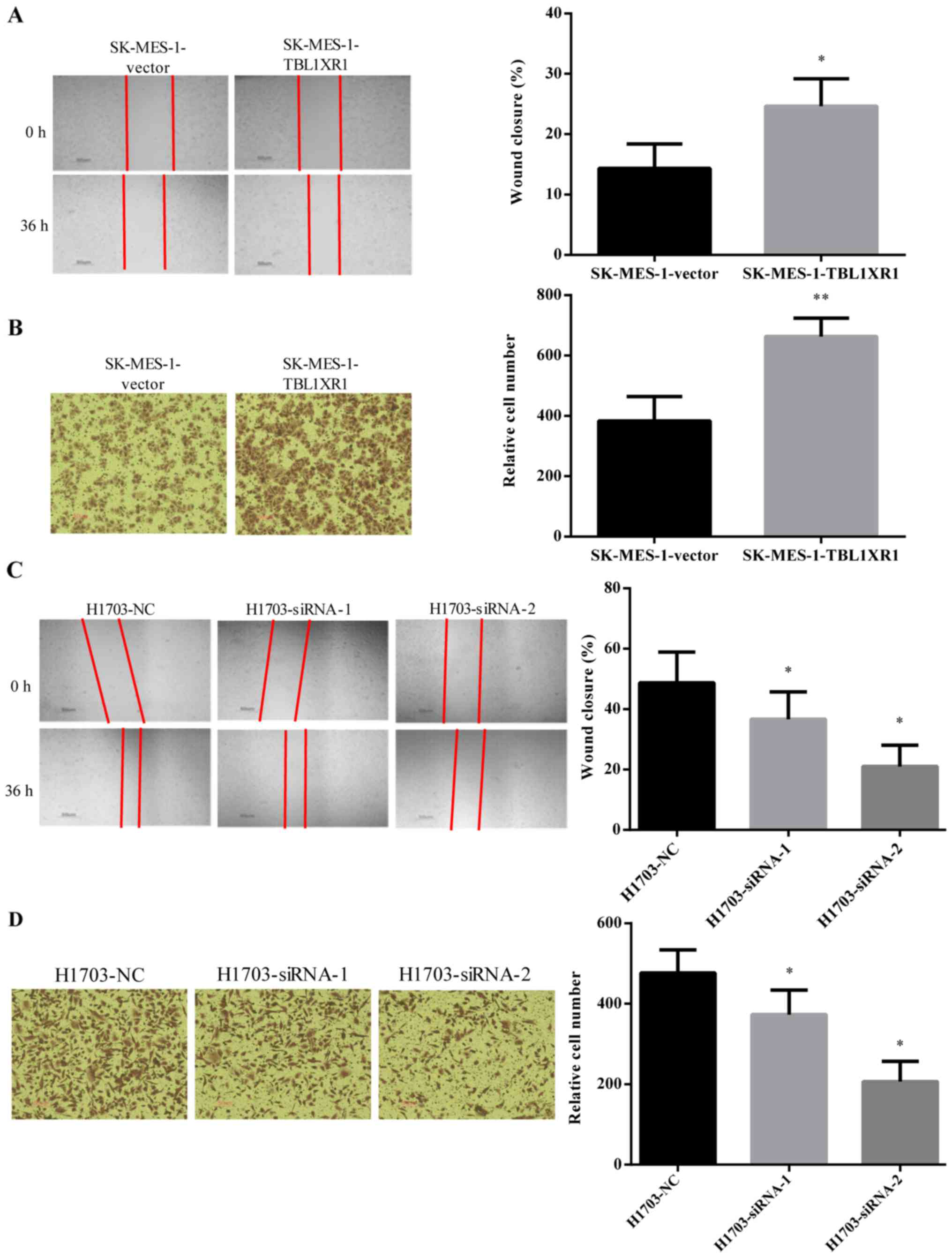
TBL1XR1 promotes the migration and
invasion of lung SCC cells
The effect of TBL1XR1 on cell migration and invasion
ability was analyzed using wound healing and Transwell invasion
assays, respectively. The wounds healed better and faster in
SK-MES-1-TBL1XR1 cells compared with SK-MES-1-vector cells
(Fig. 3A), suggesting that TBL1XR1
enhanced the migration properties of SK-MES-1 cells. Similar
results were identified in the Transwell invasion assays where
overexpression of TBL1XR1 significantly increased the invasion
ability of SK-MES-1 cells (Fig. 3B).
The wound healing assays and Transwell invasion assays demonstrated
that TBL1XR1 knockdown significantly decreased the migration and
invasion ability of H1703 cells (Fig. 3C
and D). These results suggest that TBL1XR1 may promote lung SCC
cell migration and invasion in vitro.
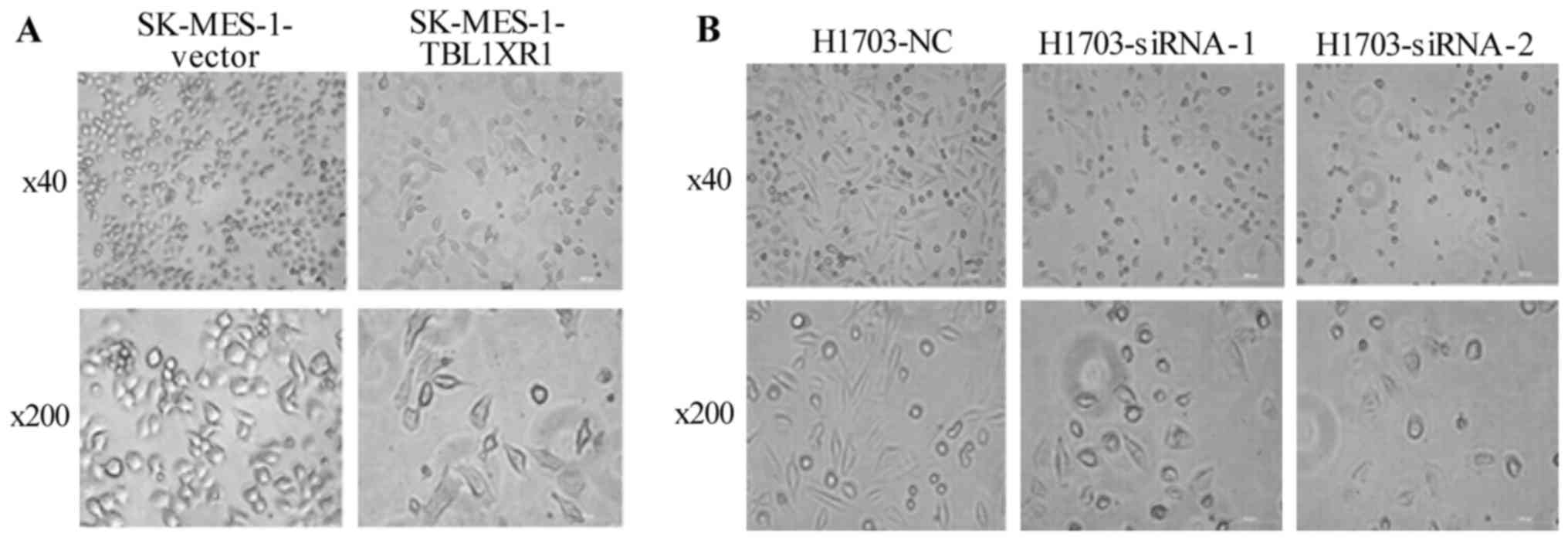
TBL1XR1 promotes EMT in lung SCC
cells
To investigate the molecular mechanism of TBL1XR1 in
the regulation of lung SCC cellular migration and invasion, cell
morphology was analyzed and the levels of EMT markers were tested.
The morphology of TBL1XR1-transfected lung SCC cells appeared
fusiform with the formation of protuberances associated with a
mesenchymal phenotype (21),
compared with vector control cells (Fig.
4A). TBL1XR1 knockdown reduced the number of irregular branched
structures in both H1703-siRNA cells. The cell morphology appeared
to be short shuttle-like, with some round-shaped cells associated
with an epithelial phenotype, compared with vector control cells
(Fig. 4B). Western blotting revealed
that overexpression of TBL1XR1 significantly decreased the
expression level of the epithelial cell marker E-cadherin and
increased the expression levels of ZEB1 transcription factor
(Fig. 5). SNAI1, a stimulator of EMT
in tumors (22), was elevated in
SK-MES-1-TBL1XR1 cells (Fig. 5A-a, b, c,
g). By contrast, downregulation of TBL1XR1 significantly
increased the expression level of E-cadherin and decreased
expression levels of both ZEB1 and SNAI1 (Fig. 5B-a, b, c, g). Taken together, these
results suggest that TBL1XR1 may induce EMT in lung SCC cells in
vitro.
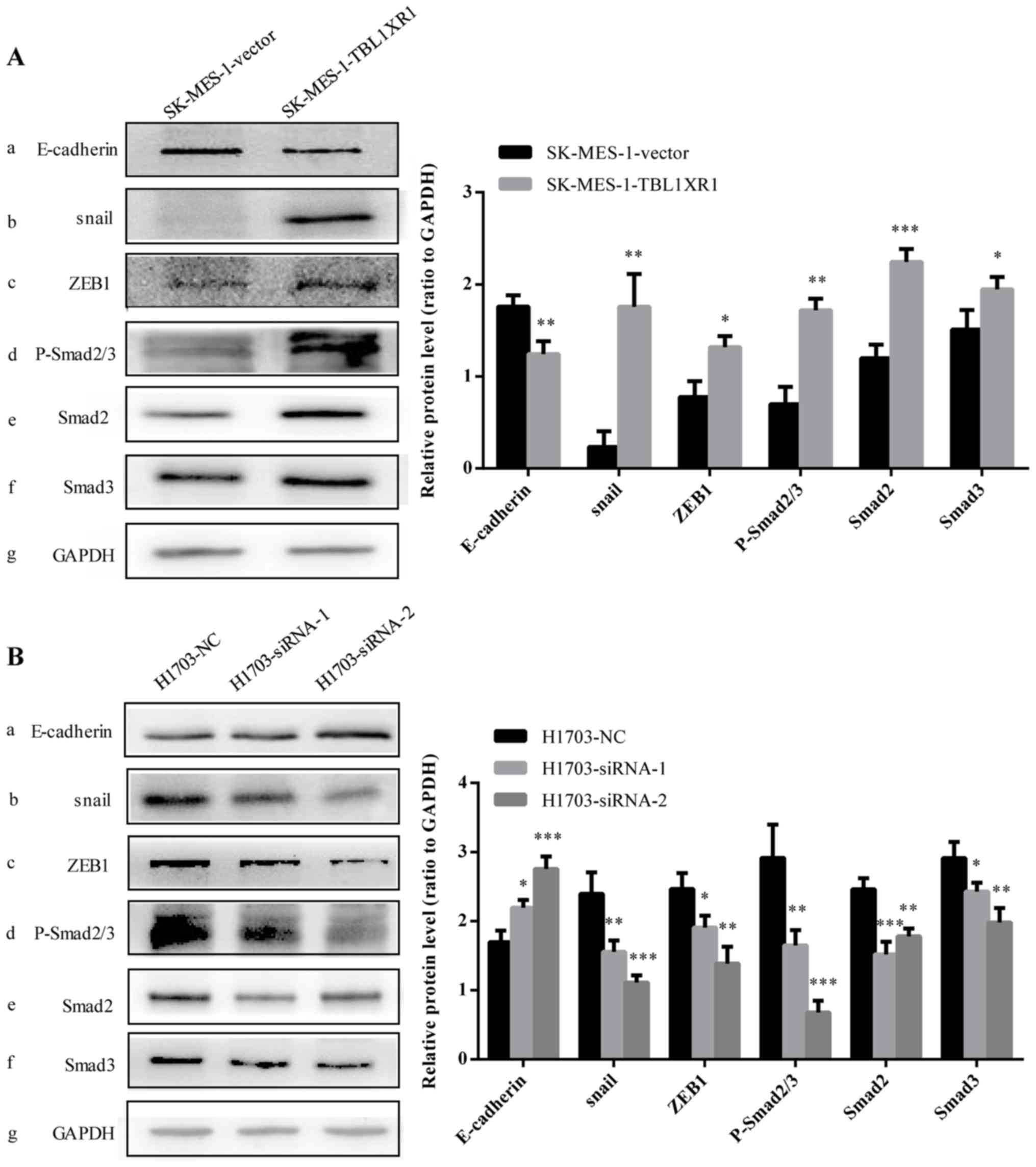 | Figure 5.TBL1XR1-induced EMT is mediated via
the TGF-β/Smad signaling pathway. The protein expression levels of
(A-a) E-cadherin, (A-b) SNAI1, (A-c) ZEB1, (A-d) p-Smad2/3, (A-e)
Smad2 and (A-f) Smad3 were determined using western blot analysis
in SK-MES-1 cells following overexpression of TBL1XR1. GAPDH was
used as the loading control. *P<0.05, **P<0.01 and
***P<0.001 vs. SK-MES-1-vector. The protein expression levels of
(B-a) E-cadherin, (B-b) SNAI1, (B-c) ZEB1, (B-d) p-Smad2/3, (B-e)
Smad2, and (B-f) Smad3 in H1703 cells following TBLIXR1 knockdown.
GAPDH was used as the loading control. *P<0.05, **P<0.01 and
***P<0.001 vs. H1703-NC. EMT, epithelial-mesenchymal transition;
TBL1XR1, transducin (β)-like 1 X-linked receptor 1; TGF-β/Smad,
transforming growth factor β/mothers against decapentaplegic
homolog; p-Smad2/3, phosphorylated Smad2 and Smad3; siRNA, small
interfering RNA; ZEB1, zinc finger E-box binding homeobox 1;
H1703-NC and SK-MES-1-vector, negative transfection controls;
SK-MES-1-TBL1XR1, TBL1XR1-overexpressing SK-MES-1 cells;
H1703-siRNA-1 and H1703-siRNA-2, TBL1XR1 knockdown in H1703
cells. |
TBL1XR1 induces EMT the through
TGF-β/Smad signaling pathway
Previous studies revealed that the TGF-β/Smad
signaling pathway served a key role in the process of tumor cell
migration and invasion via the induction of EMT (23–25).
Another study demonstrated that TGF-β-induced EMT in non-small cell
lung cancer (26). The present study
evaluated whether activation of the TGF-β/Smad pathway was involved
in TBL1XR1-induced EMT in lung SCC. Western blot analysis revealed
that expression levels of p-Smad2/3, Smad2 and Smad3 were
significantly increased in SK-MES-1-TBL1XR1 cells whilst these
expression levels significantly decreased following the knockdown
of TBL1XR1 in lung SCC cells (H1703-siRNA-1, H1703-siRNA-2),
compared with the H1702-NC group respectively (Fig. 5A-d, e, f, g and B-d, e, f, g).
Therefore, TBL1XR1 appears to regulate EMT to induce lung SCC cell
proliferation and invasion via the TGF-β/Smad signaling
pathway.
Discussion
TBL1XR1 is a transcriptional cofactor involved in
controlling the switch between gene activation and repression in
transcriptional regulation (12).
Abnormal TBLIXR1 expression is associated with the occurrence and
development of malignant tumors (27,28).
TBLIXR1 inhibited the growth of prostate cancer by selectively
activating androgen receptor target genes (27). Furthermore, one study indicated that
TBLIXR1 promoted proliferation and tumorigenicity in breast cancer
by activating genes downstream of β-catenin (17). Previous studies demonstrated that
TBL1XR1 was overexpressed in human primary lung SCC cells (20). However, the exact role of TBL1XR1 in
lung SCC remained unexplored. Consistent with previous studies, the
data presented in the current study demonstrated that TBL1XR1
protein expression was higher in lung SCC cells compared with human
bronchial epithelial cell lines. Furthermore, overexpression of
TBL1XR1 promoted the proliferation, invasion and migration of lung
SCC cells in vitro, while knockdown of TBL1XR1 significantly
inhibited tumorigenicity, by promoting invasion and migration, and
proliferation in lung SCC cells. Taken together, the data presented
in this study suggested an oncogenic function of TBL1XR1 in the
development and progression of lung SCC.
EMT, a process in which epithelial cells
differentiate into mesenchymal cells in response to a number of
physiological and pathological conditions, serves an important role
in tumor invasion and metastasis (29). In the EMT process, migratory and
invasive capabilities are acquired through the loss of polarity and
cell-cell adhesion of the epithelial cells, and as a result, cell
morphology is transformed from the low-invasive epithelial
phenotype to the high-invasive mesenchymal phenotype (30). Cell surface markers change
correspondingly, with the downregulation of the epithelial marker
E-cadherin and the upregulation of the mesenchymal marker
N-cadherin (31). Various
transcription factors, including SNAI1, twist-related protein 1 and
ZEB1 are also upregulated (30,31–33). The
present study revealed that TBL1XR1-induced lung SCC cells undergo
morphological alterations caused by decreased expression of
E-cadherin and increased expression of SNAI1 and ZEB1. These
findings suggested that TBL1XR1 may promote lung SCC aggressiveness
by inducing EMT, and, therefore, TBL1XR1 may be a potential
therapeutic target in lung SCC therapy.
It has been reported that EMT occurs by activation
of several signaling pathways, including phosphoinositide
3-kinase/protein kinase B (Akt), TGFβ/Smad, integrin-linked protein
kinase/Akt, and Wnt-β-catenin, which activate E-cadherin repressors
of the Snai1 family (34). TGFβ-Smad
is one of the most important signaling pathways contributing to
EMT, which involves the binding of TGF-β to its receptor
(TGF-βRI/TGF-βRII) and the subsequent phosphorylation, leading to
Smad2/3 activation (35,36). Activated Smad2/3 forms complexes with
Smad4 in the cytoplasm, moving into the nucleus where transcription
factors regulate the transcription of target genes (37). SNAI1 is a TGF-β/Smad signaling
pathway-mediated gene that promotes EMT by repressing E-cadherin
expression and increasing invasion and metastasis of tumor cells
(25). The current study
demonstrated that overexpression of TBL1XR1 increased the
expression of SNAI1 and ZEB1 transcription factors, and p-Smad2/3,
Smad2 and Smad3 proteins. Downregulation of TBL1XR1 gave the
opposite results. The present study indicated that TBL1XR1 may
induce EMT of lung SCC cells through activation of the TGF-β/Smad
signaling pathway to promote the development of lung SCC.
Although the present study provided some
understanding of TBL1XR1, certain limitations should be noted.
TBL1XR1 was detected at a cellular level, and, therefore, an
additional histological study would be required to validate the
results. Furthermore, the current study indicated that TBL1XR1 may
induce EMT of lung SCC cells by activating the TGF-β/Smad signaling
pathway. Due to a lack of TGF-β inhibitor treatment group, definite
conclusions cannot be drawn and further investigation is needed to
verify this effect.
In conclusion, this preliminary study identified a
biological role for TBL1XR1 and possible molecular mechanisms in
lung SCC. First, TBL1XR1 expression was elevated in lung SCC cells.
Second, TBL1XR1 promoted proliferation, invasion and migration of
lung SCC cells in vitro. Finally, TBL1XR1 may induce EMT of
lung SCC cells through activation of the TGF-β/Smad signaling
pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in the published article.
Authors' contributions
YZ, HL, and MG performed experimental work. YZ and
JJ analyzed the data. YZ wrote the manuscript. XL designed the
study and revised the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Huashan Hospital of Fudan University (Shanghai,
China) and informed consent was taken from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tanoue LT, Tanner NT, Gould MK and
Silvestri GA: Lung cancer screening. Am J Respir Crit Care Med.
191:19–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
An Q, Pacyna-Gengelbach M, Schlüns K,
Deutschmann N, Guo S, Gao Y, Zhang J, Cheng S and Petersen I:
Identification of differentially expressed genes in immortalized
human bronchial epithelial cell line as a model for in vitro study
of lung carcinogenesis. Int J Cancer. 103:194–204. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng TD, Cramb SM, Baade PD, Youlden DR,
Nwogu C and Reid ME: The international epidemiology of lung cancer:
Latest trends, disparities, and tumor characteristics. J Thorac
Oncol. 11:1653–1671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liao BC, Shao YY, Chen HM, Shau WY, Lin
ZZ, Kuo RN, Lai CL, Chen KH, Cheng AL, Yang JC and Lai MS:
Comparative effectiveness of first-line platinum-based chemotherapy
regimens for advanced lung squamous cell carcinoma. Clin Lung
Cancer. 16:137–143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Travis WD: Pathology of lung cancer. Clin
Chest Med. 32:669–692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mizoguchi K, Nakamura Y, Sano K, Sato S,
Ikegami Y, Motoshima K, Takemoto S, Ogawara D, Senju H, Sugasaki N,
et al: Pharmacokinetic parameters of gefitinib predict efficacy and
toxicity in patients with advanced non-small cell lung cancer
harboring EGFR mutations. Cancer Chemother Pharmacol. 78:377–382.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Dormady SP and Basch RS:
Identification of four human cDNAs that are differentially
expressed by early hematopoietic progenitors. Exp Hematol.
28:1286–1296. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Andersson S, Wallin KL, Hellström AC,
Morrison LE, Hjerpe A, Auer G, Ried T, Larsson C and
Heselmeyer-Haddad K: Frequent gain of the human telomerase gene
TERC at 3q26 in cervical adenocarcinomas. Br J Cancer. 95:331–338.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang YC, Shyong WY, Chang MS, Chen YJ, Lin
CH, Huang ZD, Wang, Hsu MT and Chen ML: Frequent gain of copy
number on the long arm of chromosome 3 in human cervical
adenocarcinoma. Cancer Genet Cytogenet. 131:48–53. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Kalkum M, Chait BT and Roeder RG:
The N-CoR-HDAC3 nuclear receptor corepressor complex inhibits the
JNK pathway through the integral subunit GPS2. Mol Cell. 9:611–623.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Perissi V, Aggarwal A, Glass CK, Rose DW
and Rosenfeld MG: A corepressor/coactivator exchange complex
required for transcriptional activation by nuclear receptors and
other regulated transcription factors. Cell. 116:511–526. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi HK, Choi KC, Yoo JY, Song M, Ko SJ,
Kim CH, Ahn JH, Chun KH, Yook JI and Yoon HG: Reversible
SUMOylation of TBL1-TBLR1 regulates β-catenin-mediated Wnt
signaling. Mol Cell. 43:203–216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hoberg JE, Yeung F and Mayo MW: SMRT
derepression by the IkappaB kinase alpha: A prerequisite to
NF-kappaB transcription and survival. Mol Cell. 16:245–255. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J and Wang CY: TBL1-TBLR1 and
beta-catenin recruit each other to Wnt target-gene promoter for
transcription activation and oncogenesis. Nat Cell Biol.
10:160–169. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Ou J, Guo Y, Dai T, Li X, Liu J,
Xia M, Liu L and He M: TBLR1 is a novel prognostic marker and
promotes epithelial-mesenchymal transition in cervical cancer. Br J
Cancer. 111:112–124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Liang W, Liu J, Lin C, Wu S, Song L
and Yuan Z: Transducin (β)-like 1 X-linked receptor 1 promotes
proliferation and tumorigenicity in human breast cancer via
activation of beta-catenin signaling. Breast Cancer Res.
16:4652014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen SP, Yang Q, Wang CJ, Zhang LJ, Fang
Y, Lei FY, Wu S, Song LB, Guo X and Guo L: Transducin β-like 1
X-linked receptor 1 suppresses cisplatin sensitivity in
nasopharyngeal carcinoma via activation of NF-κB pathway. Mol
Cancer. 13:1952014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuang X, Zhu J, Peng Z, Wang J and Chen Z:
Transducin (Beta)-like 1 X-linked receptor 1 correlates with
clinical prognosis and epithelial-mesenchymal transition in
hepatocellular carcinoma. Dig Dis Sci. 61:489–500. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y, Sun W, Zhang K, Zheng H, Ma Y, Lin
D, Zhang X, Feng L, Lei W, Zhang Z, et al: Identification of genes
differentially expressed in human primary lung squamous cell
carcinoma. Lung Cancer. 56:307–317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan J, Gumireddy K, Li A and Huang Q:
Regulation of mesenchymal phenotype by MicroRNAs in cancer. Curr
Cancer Drug Targets. 13:930–934. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giannelli G, Bergamini C, Fransvea E,
Sgarra C and Antonaci S: Laminin-5 with transforming growth
factor-beta1 induces epithelial to mesenchymal transition in
hepatocellular carcinoma. Gastroenterology. 129:1375–1383. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li N, Xu H, Fan K, Liu X, Qi J, Zhao C,
Yin P, Wang L, Li Z and Zha X: Altered beta1,6-GlcNAc branched
N-glycans impair TGF-β-mediated epithelial-to-mesenchymal
transition through Smad signalling pathway in human lung cancer. J
Cell Mol Med. 18:1975–1991. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang G, Liang Y, Zheng T, Song R, Wang J,
Shi H, Sun B, Xie C, Li Y, Han J, et al: FCN2 inhibits
epithelial-mesenchymal transition-induced metastasis of
hepatocellular carcinoma via TGF-β/Smad signaling. Cancer Lett.
378:80–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ji Q, Liu X, Han Z, Zhou L, Sui H, Yan L,
Jiang H, Ren J, Cai J and Li Q: Resveratrol suppresses
epithelial-to-mesenchymal transition in colorectal cancer through
TGF-β1/Smads signaling pathway mediated Snail/E-cadherin
expression. BMC Cancer. 15:972015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li C, Wan L, Liu Z, Xu G, Wang S, Su Z,
Zhang Y, Zhang C, Liu X, Lei Z and Zhang HT: Long non-coding RNA
XIST promotes TGF-β-induced epithelial-mesenchymal transition by
regulating miR-367/141-ZEB2 axis in non-small-cell lung cancer.
Cancer Lett. 418:185–195. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Daniels G, Li Y, Gellert LL, Zhou A,
Melamed J, Wu X, Zhang X, Zhang D, Meruelo D, Logan SK, et al:
TBLR1 as an androgen receptor (AR) coactivator selectively
activates AR target genes to inhibit prostate cancer growth. Endocr
Relat Cancer. 21:127–142. 2013. View Article : Google Scholar
|
|
28
|
Liu F, He Y, Cao Q, Liu N and Zhang W:
TBL1XR1 is highly expressed in gastric cancer and predicts poor
prognosis. Dis Markers. 2016:24365182016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Bio. 15:178–196. 2014. View
Article : Google Scholar
|
|
31
|
Zhai B, Yan HX, Liu SQ, Chen L, Wu MC and
Wang HY: Reduced expression of E-cadherin/catenin complex in
hepatocellular carcinomas. World J Gastroentero. 14:5665–5673.
2008. View Article : Google Scholar
|
|
32
|
Zhai X, Zhu H, Wang W, Zhang S, Zhang Y
and Mao G: Abnormal expression of EMT-related proteins, S100A4,
vimentin and E-cadherin, is correlated with clinicopathological
features and prognosis in HCC. Med Oncol. 31:9702014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee TK, Poon RT, Yuen AP, Ling MT, Kwok
WK, Wang XH, Wong YC, Guan XY, Man K, Chau KL and Fan ST: Twist
overexpression correlates with hepatocellular carcinoma metastasis
through induction of epithelial-mesenchymal transition. Clin Cancer
Res. 12:5369–5376. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guarino M: Epithelial-mesenchymal
transition and tumour invasion. Int J Biochem Cell Biol.
39:2153–2160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu H, Wang M, Wang H, Liu Z, Guan X, Yang
R, Huang R, Tang Q, Zou C, Wang G, et al: MEGF6 promotes the
epithelial-to-mesenchymal transition via the TGFβ/SMAD signaling
pathway in colorectal cancer metastasis. Cell Physiol Biochem.
46:1895–1906. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jung B, Staudacher JJ and Beauchamp D:
Transforming growth factor β superfamily signaling in development
of colorectal cancer. Gastroenterology. 152:36–52. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsang KJ, Tsang D, Brown TN and Crowe DL:
A novel dominant negative Smad2 mutation in a TGFbeta resistant
human carcinoma cell line. Anticancer Res. 22:13–19.
2002.PubMed/NCBI
|