Introduction
Idiopathic macular holes (IMH) are a major
vitreoretinal pathology that may cause metamorphopsia and poor
central vision (1,2). IMH mainly occurs in individuals aged
60–70 years, with ~66.7% of those affected being female (3–6).
In the past, MHs were considered to be incurable.
Vitrectomy in combination with intraocular gas tamponade was the
first remedy for MHs with a prominent success rate (7,8). Various
adjunctive therapeutic strategies have also been introduced for MH
treatment. Internal limiting membrane (ILM) peeling, an alternative
treatment method, may increase the probability of primary
anatomical closure of MHs (9–11).
Previous studies have confirmed the safety and reliability of the
25-gauge vitrectomy system with sutureless self-sealing
sclerotomies in the treatment of various vitreoretinal diseases
(12,13). The 23-gauge transconjunctival system
was then proposed, which combined the merits of 25- and 20-gauge
instrumentation (14). In general,
small-gauge transconjunctival vitrectomy has gained wide
recognition due to its intra- and post-operative superiority, as it
involves less pain, a shorter surgical time and a lower rate of
post-operative inflammation (13).
Closed pars plana vitrectomy remains the primary
treatment option for a large number of patients with IMH (15). Despite the great progress made in
transconjunctival vitrectomy and the high post-operative anatomic
success (7,16–18),
visual recovery is far from satisfactory among patients with MHs
(19,20). Cataract progression is the most
common complication following MH surgery, often deeming a second
surgery necessary within 2 years. As elderly patients commonly
present with MHs, numerous eyes affected may have concomitant IMH
and cataracts. ILM peeling may be a beneficial adjunctive procedure
for MH surgery and has been reported to increase MH closure rates
(15). However, it is a complex
procedure due to the lens opacity of the eyes of patients with IMH
accompanied by cataracts (21).
The aim of the present study was to assess the
curative effect of combined phacoemulsification, 23-gauge pars
plana vitrectomy with Brilliant blue G (BBG)-assisted ILM peeling
and gas tamponade in patients with IMH and age-associated
cataracts.
Patients and methods
Patients
A total of 21 consecutive patients with IMH
accompanied by age-associated cataracts who presented at the
Department of Ophthalmology, Hebei General Hospital (Shijiazhuang,
China) from January to November 2016 were enrolled in the present
study. All patients were subjected to 23-gauge microincision
vitrectomy, air tamponade and phacoemulsification. The study was
approved by the institutional review board of Hebei General
Hospital (Shijiazhuang, China). Written informed consent was
obtained from each patient prior to surgery and each patient
consented for their information to be used for the purpose of
research.
Prior to the surgery, all patients received
ophthalmic examinations, which included best-corrected visual
acuity (BCVA) assessment, slit-lamp biomicroscopic examination,
intraocular pressure measurement and indirect ophthalmoscopic
examinations. The diagnosis of IMH was made based on fundus
examination and optical coherence tomography (OCT). The IMH grading
system used was the Gass classification (22).
The inclusion criteria included stage 2–4 IMH
accompanied by age-associated cataracts. The exclusion criteria
were high myopia (>8D), history of retinal diseases, diabetes
and traumatic MH, history of vitreoretinal surgery, MH stage 1,
macular cysts or secondary MHs.
Surgical technique
All surgeries were performed by a single experienced
vitreoretinal surgeon using a Stellaris PC system (Bausch &
Lomb Inc., Bridgewater, NJ, USA). Following superficial anesthesia
with 3 ml proxymetacaine (Alcon, Fort Worth, TX, USA) and
retrobulbar anesthesia with a (1:1) mixture of 2% lidocaine and
0.75% bupivacaine (3 ml), phacoemulsification surgery was performed
via a 3.2-mm sclera tunnel incision. An ophthalmic viscosurgical
device was used to expand and stabilize the capsular bag, in order
to facilitate the implantation of a foldable water-based acrylic
intraocular lens into the capsular bag. A three-port 23-gauge
vitrectomy was then performed. During the vitrectomy, a posterior
vitreous detachment was performed and the remnant posterior
vitreous cortex was removed. Peripheral vitreous detachment was
then performed. Following visualization using BBG dye [ILM
Blue®; Dutch Ophthalmic Research Center (International)
B.V., Zuidland, the Netherlands], the ILM was peeled off around the
MH using a pair of ILM forceps. Fluid-air exchange and intraocular
tamponade with sterilized air was applied at the end of the
procedure. Following surgery, all patients were instructed to
maintain a face-down position for 2–3 days at the hospital.
Post-operative treatment consisted of Tobramycin
Dexamethasone eye drops (Alcon), Pranoprofen eye drops (SENJU
Pharmaceutical Co., Ltd., Osaka, Japan) for anti-inflammatory
therapy and Tropicamide eye drops (Shenyang Xingqi Pharmaceutical
Co., Ltd., Shengyang, China) for mydriasis treatment.
Clinical evaluation
The recorded data included the pre-operative MH
diameter, the MH index (MHI), the LogMAR BCVA, as well as events of
post-operative MH closure and any potential intra- and
post-operative complications. The MH diameter, MHI and BCVA were
measured at 1 month post-surgery and at the last follow-up. MH
closure was considered an anatomical success when no neurosensory
defects were observed in the fovea on ophthalmoscopy with
confirmation by OCT (23).
Post-operative complications were observed by slit-lamp
biomicroscopy, indirect funduscopy and intraocular pressure
assessment.
Statistical analysis
Quantitative data are expressed as the mean ±
standard deviation and analyzed using repeated-measures analysis of
variance followed by the Bonferroni test. Qualitative data are
presented as numbers and/or percentages and were analyzed using the
χ2 or Fisher's exact test, as appropriate. Correlation
between the pre-operative MH diameter and the post-operative LogMAR
BCVA was analyzed using a Spearman rank correlation analysis.
Statistical analysis was performed using SPSS 19.0 software (IBM
Corp., Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics
A total of 21 patients, including 5 men (23.8%) and
16 women (76.2%) and 21 eyes, diagnosed with coexisting IMH and
age-associated cataracts were enrolled in the present study. The
mean age of the patients was 64.2±5.3 years (range, 51–72 years).
The demographic data of the patients are listed in Table I. The mean post-operative follow-up
duration was 8.28±3.52 months (range, 1–12 months) and the mean
value of the pre-operative MH diameter was 504.0±212.7 µm. Of the
21 cases, 8 patients (38.1%) presented with grade II and 12 (57.1%)
with grade III nuclear sclerosis.
 | Table I.Baseline characteristics. |
Table I.
Baseline characteristics.
| Item | Value |
|---|
| Sex
(male/female) | 5/16 |
| Age (years) | 64.2±5.3 |
| Eye
(left/right) | 13/8 |
| Hole diameter
(µm) | 504.0±212.7 |
| MHI | 1.10±1.13 |
| LogMAR BCVA |
|
|
Pre-operative | 1.22±0.42 |
| Last
follow-up | 0.75±0.47 |
|
Follow-up time (months) | 8.28±3.52 |
| Post-operative
complications (hemorrhage) | 2 (9.5) |
| Cataract grade
regarding lens nuclear sclerosis |
|
| II | 8 (38.1) |
|
III | 12 (57.1) |
| IV | 1 (4.8) |
Initial hole-closure rate
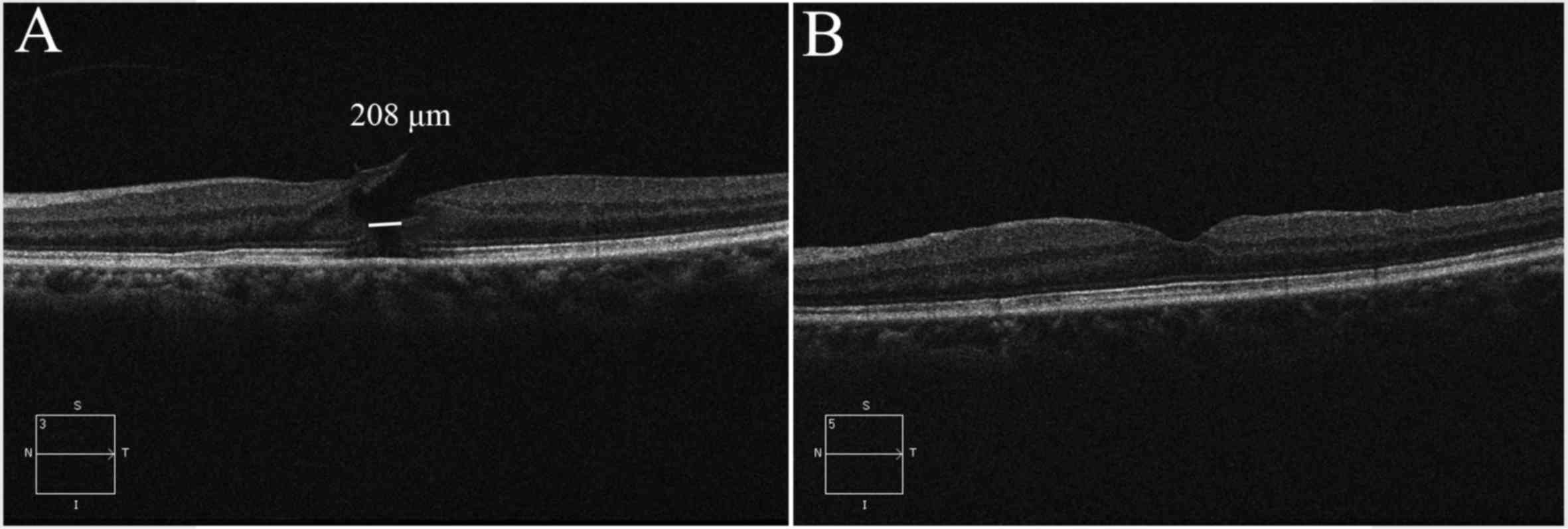
Anatomic MH closure was achieved in 19 eyes (90.4%)
with a single surgery (Fig. 1),
while 2 eyes did not achieve MH closure following initial surgery.
These latter 2 patients were asked to maintain a face-down position
for 2 weeks after the MHs were sealed using autologous serum and
silicone oil tamponades. Anatomical closure of the MHs and
improvement in VA was observed in these 2 cases at 1 month after
surgery. A higher MH closure rate was observed in patients with an
MH diameter of ≤600 µm, as compared with that in patients with an
MH diameter of >600 µm; however, the difference was not
significant (P=0.13). No significant association was observed
between MH index and closure rate (P=0.48; Table II).
 | Table II.Correlation between the closure of
the MH and the MH diameter or the MH index at 1 month
post-operation. |
Table II.
Correlation between the closure of
the MH and the MH diameter or the MH index at 1 month
post-operation.
| Item | Post-operative
closure rate (%) | P-value |
|---|
| Pre-operative MH
diameter (µm) |
|
|
| <300
(n=6) | 100 |
|
| 300–600
(n=7) | 100 |
>0.999a |
| >600
(n=8) | 75 | 0.13b |
| Pre-operative MH
index |
|
|
| <0.5
(n=10) | 90 | 0.48 |
| ≥0.5
(n=11) | 100 |
|
BCVA
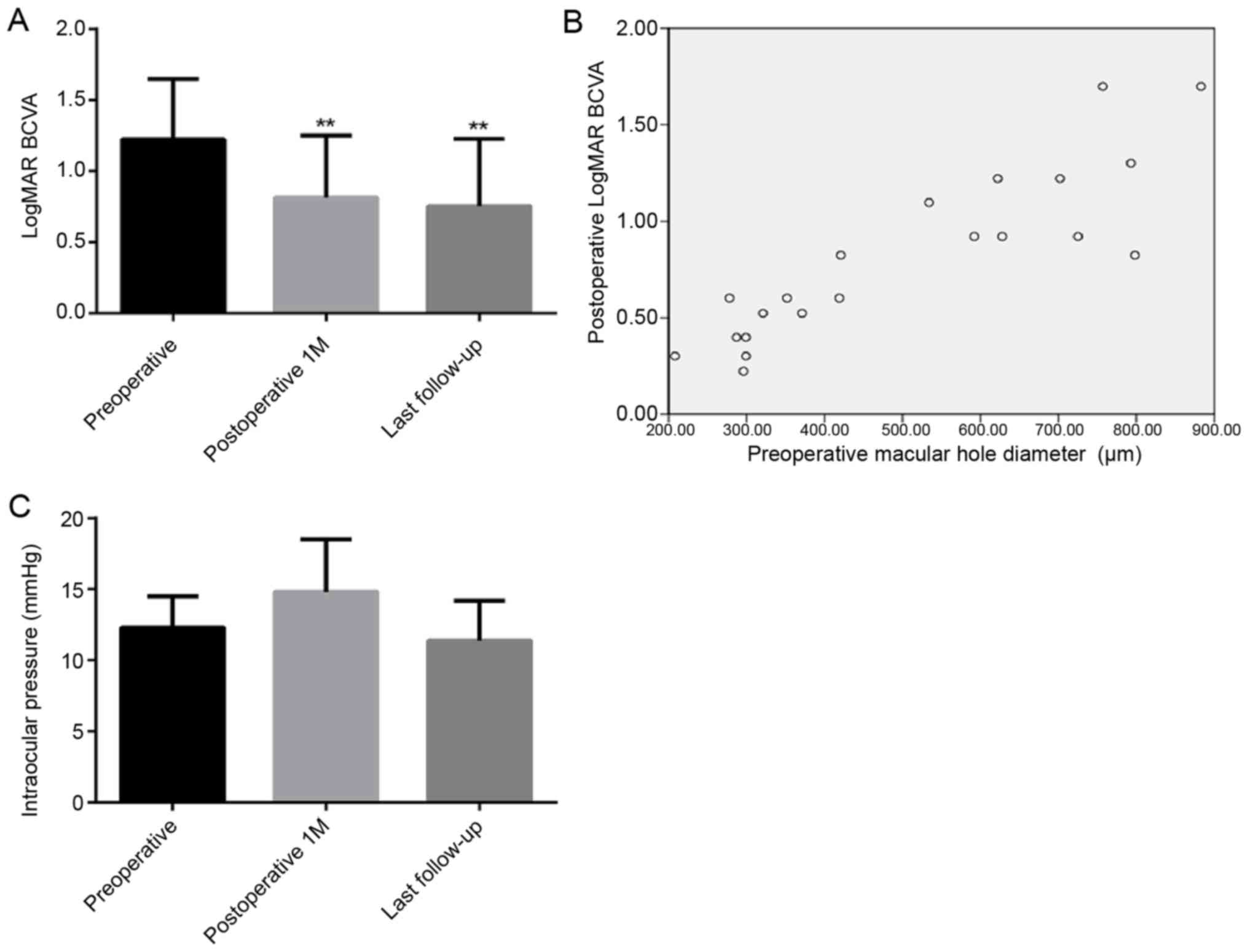
The mean pre-operative LogMAR BCVA was 1.22±0.42.
Following combined surgery, the mean LogMAR BCVA decreased to
0.82±0.43 and 0.75±0.47, at 1 month post-surgery and the last
follow-up, respectively. A significant decrease in the LogMAR BCVA
was observed at 1 month post-surgery (P=0.0036) and at the last
follow-up (P=0.0015), as compared with the baseline levels
(Fig. 2A). Spearman rank correlation
analysis revealed a positive correlation between the pre-operative
MH diameter and the post-operative LogMAR BCVA (r=0.869,
P<0.001; Fig. 2B).
Intraocular pressure
The intraocular pressure prior to surgery, at
post-operative month 1 and the last follow-up was 12.3±2.2,
14.8±3.7 and 11.4±2.8 mmHg, respectively. No statistically
significant difference in intraocular pressure from baseline was
observed at 1 month post-surgery and at the final follow-up (all
P>0.05; Fig. 2C).
Complications
Post-operative vitreous hemorrhage was observed in 2
eyes and they were successfully treated by vitreous lavage. No
choroidal detachment or endophthalmitis was recorded.
Discussion
IMHs are full-thickness defects of the retinal
tissue involving the anatomic fovea. They may affect central visual
acuity and even cause metamorphopsia. As MH commonly occurs in aged
patients, numerous eyes affected may have concomitant MH and
cataract. Despite the great progress in transconjunctival
vitrectomy and high anatomic success after surgery, visual recovery
in MH patients is far from satisfactory. Therefore, a more
efficient surgical method is necessary. The success rate of MH
surgery has been increased to 90% since MH repair was first
described (24). However, few
studies have assessed combined phacoemulsifcation with 23-gauge
pars plana vitrectomy with BBG-assisted ILM peeling and gas
tamponade (13,25,26). The
present study combined cataract and MH surgery, including
phacoemulsifcation, 23-gauge pars plana vitrectomy, BBG-assisted
ILM peeling and gas tamponade. All patients received
phacoemulsification, pars plana vitrectomy, ILM peeling and
fluid-gas exchange, and a face-down position was adopted for
several days after the surgery. The MH closure rate of 90.4%
determined in the present study was consistent with the success
rates reported in previous studies (27–29).
Rizzo et al (27) revealed
that MH closure rate was 93% following the use of a sutureless
25-gauge vitrectomy (27).
Furthermore, the macular hole closure rate was ~90% with sulfur
hexafluoride gas or perfluoropropane gas (28). A comparatively higher MH closure rate
was observed in patients with a hole diameter of ≤600 µm, but no
significant difference was observed. These results were
inconsistent with those of previous studies reporting that a
smaller MH is associated with a better prognosis regarding
post-operative anatomical closure (30–32).
This contradiction may be due to the fact that the sample size of
the present study was too small to reach statistical significance.
Therefore, further studies with a larger sample size are required
to clarify this.
The effect of the pre-operative MHI on
post-operative anatomical closure was also determined. The present
data indicated that the MHI had no significant predictive value. By
contrast, Kusuhara et al (33) reported that an MHI of ≥0.5 is a
positive predictive factor for a favorable surgical outcome. This
contradiction may be due to the fact that the present study
included a small number of cases and that large inter-individual
differences were present. Furthermore, the results indicated a
positive correlation between the pre-operative MH diameter and the
post-operative LogMAR BCVA. This suggests that the pre-operative MH
size measured by OCT may provide a predictive factor for the LogMAR
BCVA after MH surgery. Therefore, apart from proposing another
effective combined surgical method, the present study may also
provide a potential predictor for the treatment outcome of MH. The
23-gauge vitrectomy system allows for a small incision,
self-sealing and sutureless transconjunctival pars plana
sclerotomies, which renders it superior to the traditional 20-gauge
vitrectomy system (34). In the
present study, 2 eyes presented with post-operative vitreous
hemorrhage, which was thought to be associated with sutureless
transconjunctival pars plana sclerotomies and low intraocular
pressure in the affected patients at the end of the air
tamponade.
MHs often occur in aged/elderly individuals, and
thus, certain patients may present with co-existing MH and
cataract. Due to blurred media, vitrectomy surgery becomes more
difficult. Combined cataract extraction and vitrectomy has been
regarded as an effective method for the treatment of various
vitreoretinal diseases (21,25,26,35–37).
Muselier et al (21) reported
that combined and consecutive surgeries produced equivalent
functional and anatomical results. Combined cataract and MH surgery
has been reported to be more advantageous than consecutive
surgeries, as it avoids a second surgical procedure and is more
convenient for the vitreoretinal surgeon (38). In addition, in this study, a faster
visual recovery was observed following the combined surgery; thus,
a second surgery was avoided and the overall surgical cost was
reduced. A previous study by Hikichi et al (13) has confirmed the effectiveness of
23-gauge vitrectomy with air tamponade and combined
phacoemulsification, but without the BBG-assisted ILM peeling, for
IMH. However, the cases included were of IMH with no cataracts. The
present study assessed the combined surgery in patients with
coexisting IMH and age-associated cataracts. Demetriades et
al (26) have reported that
combined phacoemulsification, intraocular lens implantation and
vitrectomy is a reasonable alternative in patients with coexisting
cataract and vitreoretinal pathology. However, the combined surgery
was not applied for the treatment of MH. Thus, the present study
provided a novel effective combined surgery for the treatment of
patients with coexisting MHs and cataracts.
Combining surgeries has numerous advantages
(21,38–41).
First, patients do not require a second surgical procedure.
Furthermore, it is beneficial for extending vitrectomy, which
contributes to the removal of the anterior vitreous. Increasing the
gas volume may provide a longer tamponade, which may prove
beneficial for the closure of the MH. Nuclear sclerotic cataract
progression has been associated with the use of vitrectomy for the
treatment of MHs (42). Therefore,
combined cataract extraction and vitrectomy may help avoid the
adverse effects of cataract progression despite improvement in
visual acuity. Muselier et al (21) reported a marked difference in visual
acuity improvement at 0.5 years after surgery between patients who
underwent vitrectomy alone and those who underwent vitrectomy
combined with cataract extraction for the treatment of MHs. The
study concluded that cataract progression following vitreous
surgery may have been due to the slower recovery of patients who
received a separate vitrectomy (21).
The present study had certain limitations. First, it
lacks a concurrent control group. Furthermore, all procedures were
performed by a single surgeon, thereby failing to compare the
differences in clinical outcomes between different surgical
procedures or surgeons. Finally, the size of the study population
was small due to the strict inclusion and exclusion criteria.
However, the present results may be representative for a patient
population similar to that of the present study, namely those with
coexisting IMH and age-associated cataract.
In conclusion, combined phacoemulsification with
23-gauge pars plana vitrectomy with BBG-assisted ILM peeling and
gas tamponade yielded satisfactory anatomic and functional results
for patients with IMH and age-associated cataracts. The
pre-operative MH size measured by OCT may be a predictive factor
for the LogMAR BCVA after MH surgery.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QM drafted the manuscript. ZJ designed the study. QM
and FF analyzed and interpreted the data. ZZ collected and
assembled the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
Hebei General Hospital. All patients provided written informed
consent.
Patient sconsent for publication
All patients have provided informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang S, Xu L and Jonas JB: Prevalence of
full-thickness macular holes in urban and rural adult Chinese: The
Beijing Eye Study. Am J Ophthalmol. 141:589–591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jackson TL, Donachie PH, Sparrow JM and
Johnston RL: United Kingdom National Ophthalmology Database study
of vitreoretinal surgery: Report 2, macular hole. Ophthalmology.
120:629–634. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Casuso LA, Scott IU, Flynn HW Jr, Gass JD,
Smiddy WE, Lewis ML and Schiffman J: Long-term follow-up of
unoperated macular holes. Ophthalmology. 108:1150–1155. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim JW, Freeman WR, el-Haig W, Maguire AM,
Arevalo JF and Azen SP: Baseline characteristics, natural history,
and risk factors to progression in eyes with stage 2 macular holes.
Results from a prospective randomized clinical trial. Vitrectomy
for Macular Hole Study Group. Ophthalmology. 102:1818–1828;
discussion 1828–1819. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jackson TL, Donachie PH, Sparrow JM and
Johnston RL: United Kingdom National Ophthalmology Database study
of vitreoretinal surgery: Report 1; case mix, complications, and
cataract. Eye (Lond). 27:644–651. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Risk factors for idiopathic macular holes.
The Eye Disease Case-Control Study Group. Am J Ophthalmol.
118:754–761. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kelly NE and Wendel RT: Vitreous surgery
for idiopathic macular holes. Results of a pilot study. Arch
Ophthalmol. 109:654–659. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wendel RT, Patel AC, Kelly NE, Salzano TC,
Wells JW and Novack GD: Vitreous surgery for macular holes.
Ophthalmology. 100:1671–1676. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tognetto D, Grandin R, Sanguinetti G,
Minutola D, Di Nicola M, Di Mascio R and Ravalico G: Macular Hole
Surgery Study G: Internal limiting membrane removal during macular
hole surgery: Results of a multicenter retrospective study.
Ophthalmology. 113:1401–1410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sheidow TG, Blinder KJ, Holekamp N, Joseph
D, Shah G, Grand MG, Thomas MA, Bakal J and Sharma S: Outcome
results in macular hole surgery: An evaluation of internal limiting
membrane peeling with and without indocyanine green. Ophthalmology.
110:1697–1701. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lois N, Burr J, Norrie J, Vale L, Cook J,
McDonald A, Boachie C, Ternent L and McPherson G: Full-thickness
Macular Hole and Internal LimitingMembrane Peeling Study (FILMS)
Group: Internal limiting membrane peeling versus no peeling for
idiopathic full-thickness macular hole: A pragmatic randomized
controlled trial. Invest Ophthalmol Vis Sci. 52:1586–1592. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rizzo S, Genovesi-Ebert F, Murri S,
Belting C, Vento A, Cresti F and Manca ML: 25-gauge, sutureless
vitrectomy and standard 20-gauge pars plana vitrectomy in
idiopathic epiretinal membrane surgery: A comparative pilot study.
Graefes Arch Clin Exp Ophthalmol. 244:472–479. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hikichi T, Kosaka S, Takami K, Ariga H,
Ohtsuka H, Higuchi M, Matsushita T and Matsushita R: 23- and
20-gauge vitrectomy with air tamponade with combined
phacoemulsification for idiopathic macular hole: A single-surgeon
study. Am J Ophthalmol. 152(114–121): e12011.
|
|
14
|
Goncu T, Gurelik G and Hasanreisoglu B:
Comparison of efficacy and safety between transconjunctival
23-gauge and conventional 20-gauge vitrectomy systems in macular
surgery. Korean J Ophthalmol. 26:339–346. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Steel DH and Lotery AJ: Idiopathic
vitreomacular traction and macular hole: A comprehensive review of
pathophysiology, diagnosis, and treatment. Eye (Lond). 27 Suppl
1:S1–S21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wittich W, Overbury O, Kapusta MA and
Faubert J: Visual function assessment and metamorphopsia after
macular hole surgery. Ophthalmic Physiol Opt. 25:534–542. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grigoropoulos VG, Theodossiadis GP and
Theodossiadis PG: Association of the preoperative photoreceptor
layer defect as assessed by optical coherence tomography with the
functional outcome after macular hole closure: A long follow-up
study. Ophthalmologica. 225:47–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Passemard M, Yakoubi Y, Muselier A, Hubert
I, Guillaubey A, Bron AM, Berrod JP and Creuzot-Garcher C:
Long-term outcome of idiopathic macular hole surgery. Am J
Ophthalmol. 149:120–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haritoglou C, Gass CA, Schaumberger M,
Ehrt O, Gandorfer A and Kampik A: Macular changes after peeling of
the internal limiting membrane in macular hole surgery. Am J
Ophthalmol. 132:363–368. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tranos PG, Ghazi-Nouri SM, Rubin GS, Adams
ZC and Charteris DG: Visual function and subjective perception of
visual ability after macular hole surgery. Am J Ophthalmol.
138:995–1002. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Muselier A, Dugas B, Burelle X, Passemard
M, Hubert I, Mathieu B, Berrod JP, Bron AM and Creuzot-Garcher C:
Macular hole surgery and cataract extraction: Combined vs
consecutive surgery. Am J Ophthalmol. 150:387–391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gass JD: Reappraisal of biomicroscopic
classification of stages of development of a macular hole. Am J
Ophthalmol. 119:752–759. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abdelkader MF and Moharram HM: Internal
limiting membrane closure of idiopathic macular hole. J Clin Exp
Ophthalmol. 7:6182016. View Article : Google Scholar
|
|
24
|
Wu D, Ho LY, Lai M and Capone A Jr:
Surgical outcomes of idiopathic macular hole repair with limited
postoperative positioning. Retina. 31:609–611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koenig SB, Mieler WF, Han DP and Abrams
GW: Combined phacoemulsification, pars plana vitrectomy, and
posterior chamber intraocular lens insertion. Arch Ophthalmol.
110:1101–1104. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Demetriades AM, Gottsch JD, Thomsen R,
Azab A, Stark WJ, Campochiaro PA, de Juan E Jr and Haller JA:
Combined phacoemulsification, intraocular lens implantation, and
vitrectomy for eyes with coexisting cataract and vitreoretinal
pathology. Am J Ophthalmol. 135:291–296. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rizzo S, Belting C, Cresti F and
Genovesi-Ebert F: Sutureless 25-gauge vitrectomy for idiopathic
macular hole repair. Graefes Arch Clin Exp Ophthalmol.
245:1437–1440. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim SS, Smiddy WE, Feuer WJ and Shi W:
Outcomes of sulfur hexafluoride (SF6) versus perfluoropropane
(C3F8) gas tamponade for macular hole surgery. Retina.
28:1408–1415. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lahey JM, Francis RR, Fong DS, Kearney JJ
and Tanaka S: Combining phacoemulsification with vitrectomy for
treatment of macular holes. Br J Ophthalmol. 86:876–878. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Haritoglou C, Neubauer AS, Reiniger IW,
Priglinger SG, Gass CA and Kampik A: Long-term functional outcome
of macular hole surgery correlated to optical coherence tomography
measurements. Clin Exp Ophthalmol. 35:208–213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamamoto K and Hori S: Long-term outcome
of vitrectomy combined with internal limiting membrane peeling for
idiopathic macular holes. Nippon Ganka Gakkai Zasshi. 115:20–26.
2011.(In Japanese). PubMed/NCBI
|
|
32
|
Ullrich S, Haritoglou C, Gass C,
Schaumberger M, Ulbig MW and Kampik A: Macular hole size as a
prognostic factor in macular hole surgery. Br J Ophthalmol.
86:390–393. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kusuhara S, Teraoka Escaño MF, Fujii S,
Nakanishi Y, Tamura Y, Nagai A, Yamamoto H, Tsukahara Y and Negi A:
Prediction of postoperative visual outcome based on hole
configuration by optical coherence tomography in eyes with
idiopathic macular holes. Am J Ophthalmol. 138:709–716. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Misra A, Ho-Yen G and Burton RL: 23-gauge
sutureless vitrectomy and 20-gauge vitrectomy: A case series
comparison. Eye (Lond). 23:1187–1191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Androudi S, Ahmed M, Fiore T, Brazitikos P
and Foster CS: Combined pars plana vitrectomy and
phacoemulsification to restore visual acuity in patients with
chronic uveitis. J Cataract Refract Surg. 31:472–478. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chung TY, Chung H and Lee JH: Combined
surgery and sequential surgery comprising phacoemulsification, pars
plana vitrectomy, and intraocular lens implantation: Comparison of
clinical outcomes. J Cataract Refract Surg. 28:2001–2005. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dugas B, Ouled-Moussa R, Lafontaine PO,
Guillaubey A, Berrod JP, Hubert I, Bron AM and Creuzot-Garcher CP:
Idiopathic epiretinal macular membrane and cataract extraction:
Combined versus consecutive surgery. Am J Ophthalmol. 149:302–306.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Treumer F, Bunse A, Rudolf M and Roider J:
Pars plana vitrectomy, phacoemulsification and intraocular lens
implantation. Comparison of clinical complications in a combined
versus two-step surgical approach. Graefes Arch Clin Exp
Ophthalmol. 244:808–815. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Satchi K and Patel CK: Posterior chamber
compartments demonstrated by optical coherence tomography, in
silicone-filled eyes, following macular hole surgery. Clin Exp
Ophthalmol. 33:619–622. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Grusha YO, Masket S and Miller KM:
Phacoemulsification and lens implantation after pars plana
vitrectomy. Ophthalmology. 105:287–294. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Creuzot-Garcher C, Aubé H, Candé F, Dupont
G, Guillaubey A, Malvitte L, Arnavielle S and Bron A: Vitreoretinal
outpatient surgery: Clinical and financial considerations. J Fr
Ophtalmol. 31:871–876. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Thompson JT, Glaser BM, Sjaarda RN and
Murphy RP: Progression of nuclear sclerosis and long-term visual
results of vitrectomy with transforming growth factor beta-2 for
macular holes. Am J Ophthalmol. 119:48–54. 1995. View Article : Google Scholar : PubMed/NCBI
|