Introduction
It is well established that >90% of fatalities
associated with solid tumors are primarily due to tumor metastasis
(1). Lung cancer has been revealed
to be the leading cause of cancer-associated fatality, with 5-year
survival rates reportedly as low as 15% (2). Hence, the molecular mechanism of
metastasis in the tumorigenesis of lung cancer is of key research
significance. Recently, an increasing number of studies have
indicated that the altered expression of claudin (CLDN) tight
junction (TJ) proteins has an essential role in the tumorigenesis
and progression of various human cancer types by affecting the
structure and function of TJs and associated cellular signaling
pathways (3,4). TJs are located at the apex of cell
junctions, and function to regulate cell adhesion and maintain cell
polarity and permeability (5). CLDNs
are the key proteins that comprise TJs, and evidence has suggested
that the expression and localization of CLDNs is frequently altered
in tumor cells (6). For instance, it
was indicated that CLDN1 was overexpressed in colorectal cancer
(CRC) compared with in normal mucosa, and CLDN1 targeting with an
anti-CLDN1 monoclonal antibody resulted in decreased growth and
survival of CRC cells, suggesting that CLDN1 could be a novel
potential therapeutic target in CRC (7). In addition, high-level cytoplasmic
CLDN3 expression was revealed to be an independent predictor of
poor survival in triple-negative breast cancer (8). Furthermore, CLDN4 has been reported to
be overexpressed in advanced ovarian cancer (OC), and Kaplan-Meier
survival analysis and Log-Rank testing suggested that high
expression of CLDN4 may have prognostic value in OC (9). These observations indicate that
alterations in the expression of CLDNs may be associated with
tumorigenesis and cancer progression in various human
carcinomas.
The distinct expression patterns of CLDNs in various
cancer types present an opportunity for application in the
diagnosis and treatment of tumors, and in determining the
mechanisms of different therapeutic responses. It has been revealed
that the expression and function of CLDNs may be highly
tissue-specific and depend on the active molecular pathways in
epithelial cells (4). Previous
genetic and pharmacological assays confirmed that the expression of
CLDN3 was downregulated in colon cancer, and that loss of CLDN3
induced Wnt/β-catenin activation and thus promoted colon cancer
(10). However, the expression
pattern of claudin-12 (CLDN12) and its association with prognostic
value in human squamous cell carcinoma (SqCC) is not well
established. Therefore, the aim of the present study was to
determine the expression levels and prognostic value of CLDN12 in
SqCC tissues, as well as the impact and mechanism of CLDN12 with
regards to the tumorigenesis and progression of SqCC, in order to
identify novel targets to be utilized in the treatment of SqCC and
control of early metastasis.
Patients and methods
Patients
The SqCC sections were collected via surgery from 50
patients (27 males, 23 females; age range, 43–74 years; median age,
59 years) who were treated at The First Bethune Hospital, Jilin
University (Changchun, China) between June 2005 and July 2012, who
had pathologically confirmed diagnoses of SqCC. The cases were
selected based on the following criteria: Pathologically confirmed
diagnosis of SqCC; no previous malignant disease or second primary
tumor; and no history of radiotherapy and chemotherapy. All
patients with SqCC were graded and classified according to the
American Joint Committee on Cancer tumor node metastasis (TNM)
staging system. Additionally, 10 cases of lung adenocarcinoma
tissues (6 males, 4 females; age range, 49–69 years; median age, 67
years) were collected from patients who were treated at The First
Bethune Hospital, Jilin University between October 2007 and May
2013. A further 20 sections of histologically non-neoplastic lung
tissues were also obtained from patients with pneumonia (11 males,
9 females; age range, 45–67 years; median age, 52 years). The
medical records of the patients were reviewed to determine clinical
and pathological characteristics.
Immunohistochemistry
An immunohistochemistry assay was used to detect the
expression of CLDN12 and epithelial (E)-Cadherin in the tissue
samples from the 50 cases of SqCC, 10 cases of lung adenocarcinoma
and 20 cases of non-neoplastic mucosa. Patients who had smoked <
100 cigarettes in their lifetime were defined as non-smokers. No
neoadjuvant therapy was applied in any of the 50 patients with
SqCC. The pretreatment for the sections were performed as follows:
fixed in 10% formalin for 8 h at room temperature and embedded in
paraffin. Then the tissues were sectioned (1.5 mm thick) and
deparaffinized in xylene twice for 5 min each; hydrated with 100%
ethanol twice for 3 min each; hydrated with 95% ethanol for 1 min;
and Rinsed in distilled water. The experimental method was as
described previously (11) and
sections (1.5 mm thick) were incubated at 4°C overnight with the
rabbit anti-human CLDN12 (cat. no. ab107061; Abcam, Cambridge, UK)
and E-Cadherin (cat. no. 3195; Cell Signaling Technology, Inc.,
Danvers, MA, USA) at a dilution of 1:400. Sections were then
incubated with serum (provided by the UltraSensitive™ SP IHC kit;
cat. no. KIT-9710; Fuzhou Maixin Biotech co., Ltd., Fuzhou, China)
for 30 min at 20°C to block the non-specific binding of
immunoglobulin. Subsequently, slides were incubated with the goat
anti-rabbit amplification reagent (provided by the UltraSensitive™
SP IHC kit) for 30 min at room temperature. Samples were then
incubated with DAB for 5 min at room temperature. For negative
controls, tissue sections were incubated with rabbit polyclonal
antibodies against claudin-2 (cat. no. ab53032; 1:400; Abcam) at
4°C overnight. All sections were scored by two pathologists using a
light microscope (E100, Nikon Corporation, Tokyo, Japan;
magnification, ×400). The expression of CLDN12 and E-Cadherin that
were localized at the cell membrane were considered positive. The
staining and scoring of CLDN12 and E-Cadherin protein expression
levels were classified semi-quantitatively based on the total
combined scores of the percentage of positively stained tumor cells
together with the staining intensity as previously described
(12).
Follow-up
Patients were followed from the beginning of
diagnosis up to 60 months to evaluate the extent of metastasis and
to determine survival times. Survival time was calculated from the
beginning of diagnosis to the time of death or loss to follow-up.
By the end of May 2017, all patients had received follow-up either
on an outpatient basis or by telephone interview. The living status
of each patient was confirmed. Survival analysis was conducted
through use of Kaplan-Meier curves with the Log-Rank test.
Cell culture
The human embryonic kidney cells 293T cells, the
human bronchial epithelial cell line BEAS-2B and the SqCC cell
lines H226 and SK-MES-1 were purchased from the Shanghai Cell Bank
of the Chinese Academy of Sciences (Shanghai, China) and cultured
in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS) at 37°C in a humidified incubator
containing 5% CO2.
Plasmid construction and
transfection
A total of 3 mg of eukaryon expression vector
pNSE-IRES2-EGFP (+)-CLDN12 (GeneBank accession no. NM022890) was
synthesized and amplified by Nanjing KeyGen Biotech Co. Ltd.
(Nanjing, China). The final construct was confirmed by direct
sequence analysis. A total of 5 µg eukaryon expression vector
pNSE-IRES2-EGFP (+)-CLDN12 (1000 ng/µl) was transfected into cells
using the SuperFect Transfection Reagent (Takara Bio, Inc., Otsu,
Japan). Clones resistant to G418 (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) were expanded in culture at 37°C in a
humidified incubator containing 5% CO2 as a monoclonal
population for 3 weeks. A monoclonal strain of BEAS-2B transfected
with the p- IRES2-EGFP (+)-CLDN12 plasmid and a monoclonal strain
of BEAS-2B transfected with p-NSE-IRES2-EGFP plasmid (1000 ng/µl)
were obtained, which were termed as BEAS-CLDN12 and BEAS-Vector.
Cells transfected with the empty vector pNSE-IRES2-EGFP1-C1 (+)
(Nanjing KeyGen Biotech Co. Ltd., Nanjing, China) were used as a
vector control. Cells were collected and seeded for subsequent
experimentation at 48 h post-transfection.
Western blotting
Rabbit anti-human E-Cadherin (cat. no. 3195), rabbit
anti-human phospho(p)-signal transducer and activator of
transcription 1 (Stat1; cat. no. 7649), rabbit anti-human Stat1
antibody (cat. no. 14994), rabbit anti-human tyrosine kinase 2
(Tyk2; cat. no. 13531) and rabbit anti-human Vimentin (cat. no.
5741) antibodies were purchased from Cell Signaling Technology,
Inc. Rabbit polyclonal antibodies against CLDN12 (cat. no.
ab107061) and mouse anti-human β-actin (cat. no. ab8227) were
purchased from Abcam. Total protein was extracted from cells of the
respective groups using a protein extraction reagent (Beijing
Biolab Technology Co., Ltd., Beijing, China) following culture at
37°C in a humidified incubator containing 5% CO2 for 48
h. The protein concentration of cell lysates was determined using a
BCA protein assay kit (Pierce; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Following this, 30 µg of total protein per lane
was separated via 10% SDS-PAGE and transferred onto nitrocellulose
membranes (EMD Millipore, Billerica, MA, USA). Membranes were then
blocked with 4% skimmed milk powder for 2 h at room temperature and
incubated with the aforementioned primary antibodies (all 1:1,000)
against E-Cadherin, p-Stat1, Stat1, Tyk2, Vimentin, CLDN12 and
β-actin overnight at 4°C. Samples were washed and incubated with
horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G
antibodies (1:1,000; ab6721, Abcam) for 2 h at room temperature.
Immunoreactive bands were detected using enhanced chemiluminescent
western blotting reagents (GE Healthcare, Chicago, IL, USA) and
analyzed using Image Lab 6.0.1 software (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Immunofluorescence method
Cells were washed three times with PBS, fixed with
4% paraformaldehyde for 10 min at room temperature, permeabilized
with 0.1% Triton X-100 (cat. no. 9002-93-1; Sigma-Aldrich; Merck
KGaA) and blocked with 2% bovine serum albumin (Changchun Bote
Biological Technology Co., Ltd., Changchun, China) in PBS for 1 h
at room temperature. Staining for immunofluorescence was performed
with the following primary antibodies: Rabbit anti-human CLDN12,
rabbit anti-human E-Cadherin and rabbit anti-human Vimentin, which
were diluted in blocking solution (1:1,000) and incubated for 30
min at room temperature. Subsequently, cells were incubated with
Alexa Fluor 647-conjugated anti-rabbit IgG antibodies (cat. no.
ab150093; Santa Cruz Biotechnology, Inc.; 1:1,000). Images were
captured using a fluorescence microscope (Nikon Eclipse TE2000-S,
Nikon Corporation; magnification, ×400).
Cell Counting kit-8 (CCK-8) assay
A cell proliferation curve was determined using a
colorimetric water-soluble tetrazolium salt assay (CCK-8; Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) according to the
manufacturer's protocol. Cells were seeded into 96-well plates at a
density of 2×105 cells/well in triplicate and cultured
overnight at 37°C. Cell proliferation was recorded every 12 h for 4
days. The absorption of cell solution was measured at a wavelength
of 450 nm.
Wound-healing assay
Cells were cultured as a monolayer to 70% confluence
on gridded plastic dishes and wounded by scratching with a 100-µl
pipette tip. Following this, cells were washed three times with
PBS. The wounds were photographed with a light microscope (E100;
Nikon Corporation; magnification, ×200) at the same field of view
at 0, 12 and 24 h, respectively.
Transwell invasion assay
Cells were cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with 10% FBS to a 90%
confluence, then cultured at in FBS-free medium at 37°C in a
humidified incubator containing 5% CO2 for 24 h. The
medium containing chemotactic factors from the cell culture was
collected. For the cell invasion assay, 3×104 cells
suspended in 100 µl FBS-free medium were added into the upper
chamber of transwell chambers (8-mm pore size; BD Biosciences,
Franklin Lakes, NJ, USA) coated with Matrigel (cat. no. 356234; BD
Biosciences) and incubated at 37°C for 30 min. A total of 600 µl
the medium with chemotactic factors containing 10% FBS was added
into the lower chambers. Cells were incubated at 37°C in a
humidified 5% CO2 atmosphere for 6 h. Any cells that
remained on the upper chamber were removed carefully with a cotton
swab. Migrated or invaded cells that had traversed the membrane
were fixed in 95% methanol for 10 min at room temperature and
stained with 0.5% crystal violet for 10 min at room temperature.
The cells were subsequently photographed and counted in five
randomly selected visual fields under an inverted microscope
(Olympus Corporation; magnification, ×200).
RNA interference (RNAi)
Frozen glycerol bacterial stocks containing
pGCSIL-scramble and pGCSIL-Tyk2-RNAi were purchased from Nanjing
KeyGen Biotech Co., Ltd. pGCSIL-scramble was used as the control
insert sequence. Tyk2-RNAi (Target ID, 29473) was the targeting
sequence. 293T cells (0.2×107 per well) were seeded and
maintained for 24 h to achieve 70–80% confluence in 6-well dishes
(Corning Costar Co., Cambridge, MA, USA). The plasmids, including
pGCSIL-Tyk2-RNAi or pGCSIL-scramble, 5 µg of the packaging vector
pHelper 1.0 and 5 µg of a vesicular stomatitis virus glycoprotein
expression plasmid vector, were added to Opti-MEM (Corning Costar
Co.), to a final volume of 1.0 ml. Following this, 50 µl of
Lipofectamine (Invitrogen; Thermo Fisher Scientific, Inc.) was
added to 950 µl FBS-free DMEM (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). These two solutions were mixed and
combined with the cells. Lentiviral particles were harvested 48 h
following transfection, and the viral titer was determined using
counting green fluorescent protein-expressing cells under a
fluorescence microscope (Nikon Diaphot 300, Nikon Instruments,
Inc., Tokyo, Japan) with filters at 96 h post-transfection. The
BEAS-2B-CLDN12 cells which were transfected with the
pGCSIL-scramble and pGCSIL-Tyk2-RNAi plasmids were termed as
BEAS-Scramble or BEAS-siRNA groups.
Statistical analysis
All experiments were repeated three times and all
data were presented as the mean ± standard deviation of at least
three experimental results. Comparisons between two groups were
made using a Student's t-test. One-way analysis of variance
followed by a Dunnett's multiple comparisons test were used for
multiple group comparisons. Kaplan-Meier analysis was used for
survival analysis in patients with SqCC. Additionally, the
χ2 test was applied to assess the association of
clinical case indicators. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of CLDN12 in human SqCC
tissues is upregulated and negatively associated with
E-Cadherin
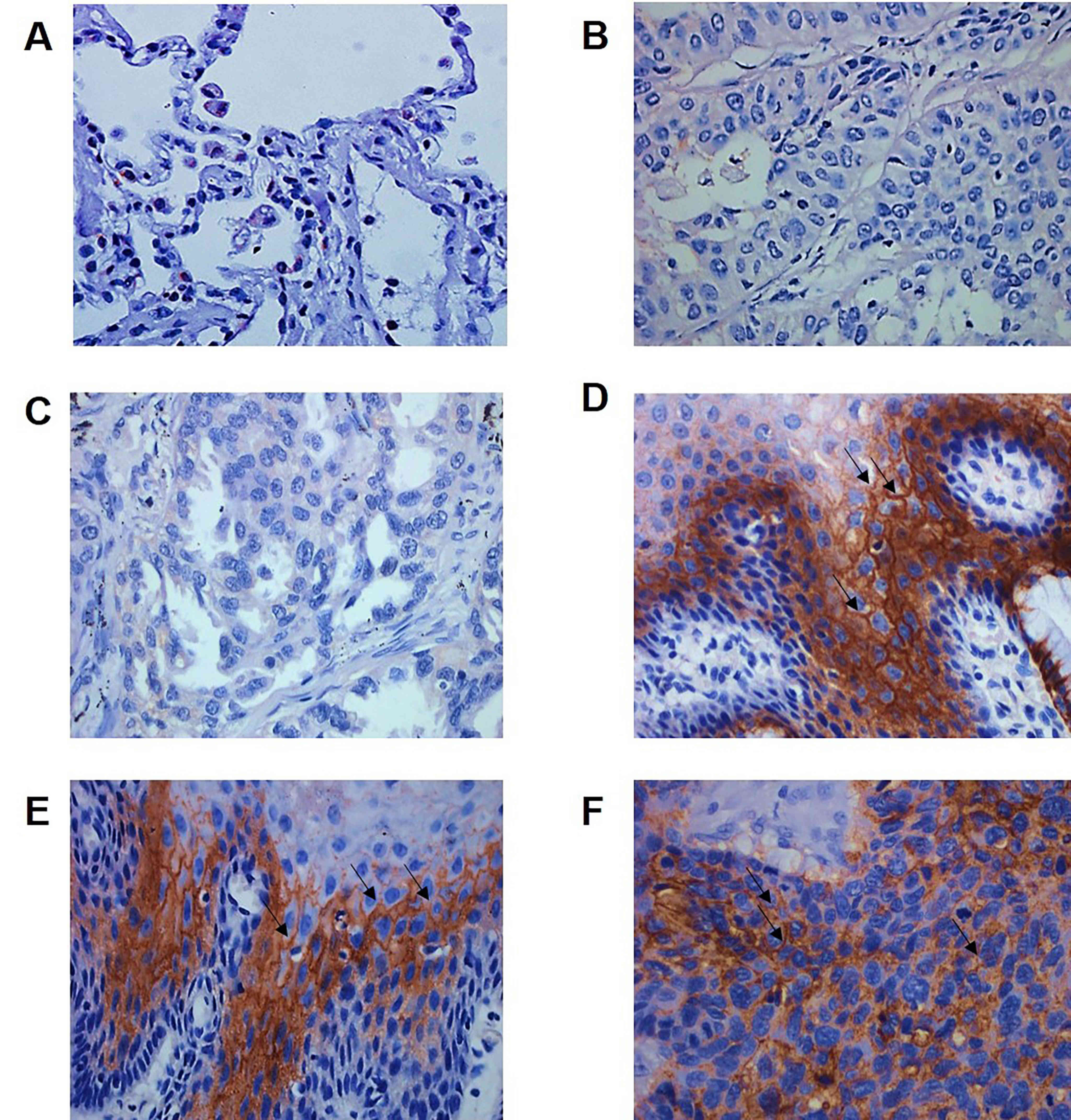
CLDN12 expression was assessed in the 50 specimens
of SqCC tissue, 10 specimens of lung adenocarcinoma tissue and 20
specimens of non-neoplastic mucosal tissue (Fig. 1). As indicated in Table I, high-level expression of CLDN12 was
detected in 64.0% (32/50) of the SqCC tissues and in 25.0% (5/20)
of the normal mucosal tissues (P=0.0002). The associations of
CLDN12 with clinical pathological indicators were analyzed, and it
was revealed that the expression of CLDN12 was not associated with
age (P=0.794), smoking status (P=0.372), degree of differentiation
(P=0.812) or clinical staging (P=0.736), but was associated with
lymph node metastasis (P=0.0001) and the expression of E-Cadherin
(P=0.0001; Table I). These data
revealed that the expression of CLDN12 was increased in SqCC
tissues and was associated with lymph node metastasis and
E-Cadherin expression.
 | Table I.Expression of CLDN12 and
clinicopathological characteristics of the patients with lung
squamous cell carcinoma. |
Table I.
Expression of CLDN12 and
clinicopathological characteristics of the patients with lung
squamous cell carcinoma.
| Item | n | CLDN12 (+) | CLDN12 (−) | P-value |
|---|
| Tumor tissue | 50 | 32 | 18 | <0.001 |
| Normal mucosa | 20 | 5 | 15 |
|
| Age (years) |
|
|
| 0.794 |
| ≤60 | 22 | 14 | 8 |
|
|
>60 | 28 | 18 | 10 |
|
| Smoker |
|
|
| 0.372 |
|
Yes | 30 | 17 | 13 |
|
| No | 20 | 15 | 5 |
|
| E-Cadherin |
|
|
| 0.001 |
| + | 29 | 13 | 16 |
|
| − | 21 | 19 | 2 |
|
| Histological
grade |
|
|
| 0.812 |
| Well
and moderately differentiated | 24 | 15 | 9 |
|
| Poorly
differentiated | 26 | 17 | 9 |
|
| Lymph node
metastasis |
|
|
| 0.001 |
| + | 27 | 21 | 6 |
|
| − | 23 | 11 | 12 |
|
| Grade |
|
|
| 0.736 |
|
I–II | 35 | 22 | 13 |
|
|
III–IV | 15 | 10 | 5 |
|
Association with survival and clinical
outcomes
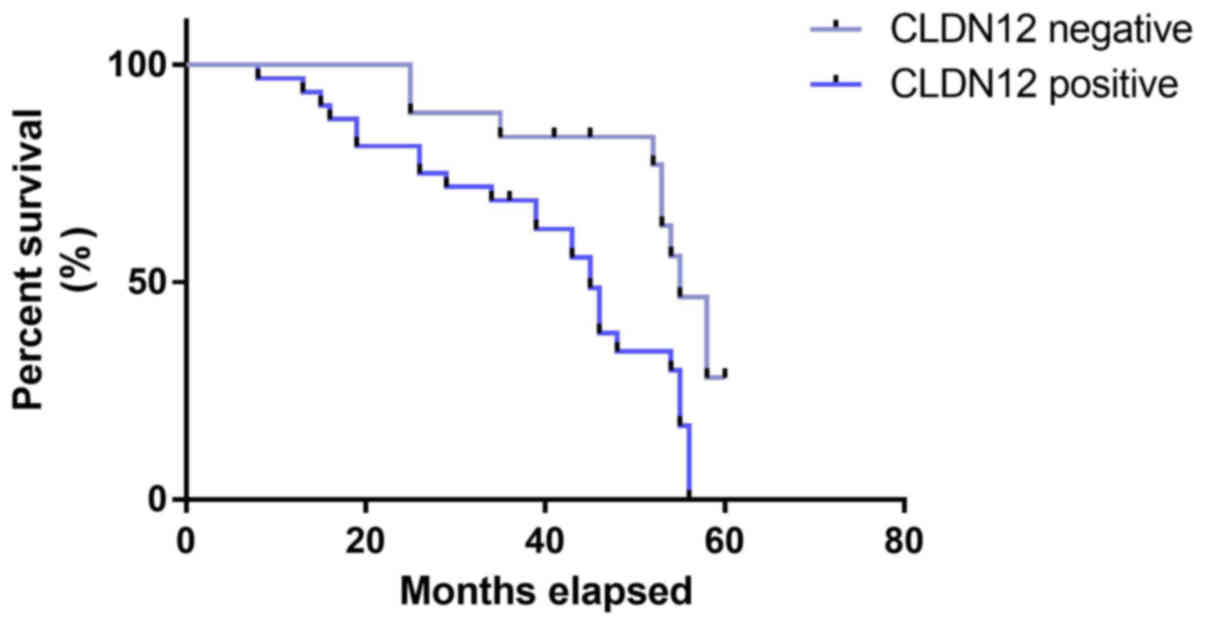
The length of follow-up ranged from 8 to 60 months.
Kaplan-Meier survival curves and the Log-Rank test were used to
determine whether there was an association between CLDN12 and
survival time. As indicated in Fig.
2, patients with tumors that were positive for CLDN12 protein
(median survival, 44.85 months) had a significantly shorter
survival time than those whose tumors were negative for CLDN12
protein (median survival, 55.23 months; χ2 =7.176,
P=0.0074).
Protein expression of CLDN12 in SqCC
cells and tissues
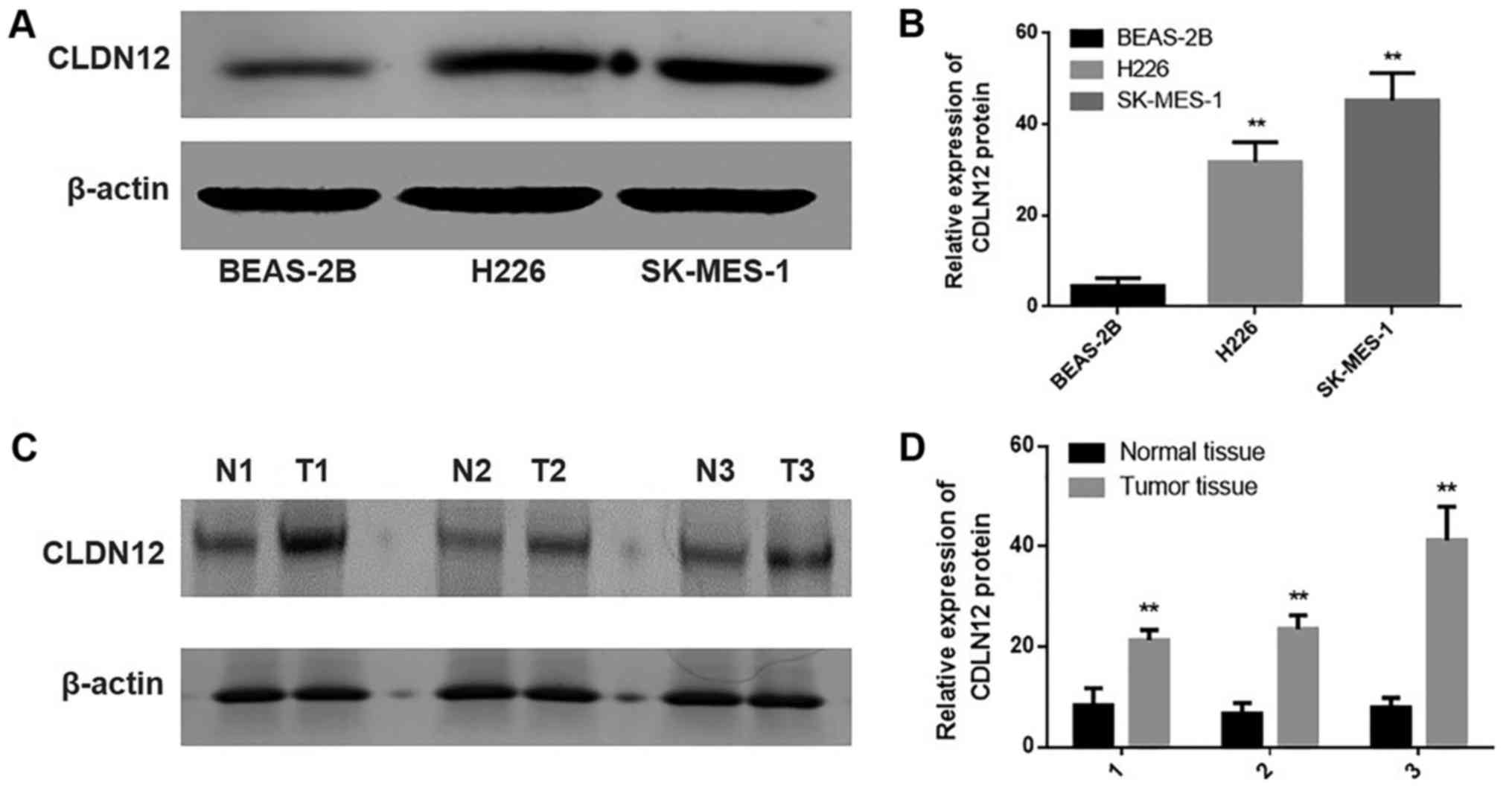
Analysis of the semiquantitative immunoblotting
results was performed to estimate and compare the statistical
differences in the expression of CLDN12 among the human bronchial
epithelial cell line BEAS-2B and SqCC cell lines H226 and SK-MES-1.
As depicted in Fig. 3, the protein
expression levels of CLDN12 were significantly increased in the
SqCC cell lines H226 and SK-MES-1 compared with the human bronchial
epithelial cell line BEAS-2B (P<0.01).
Semiquantitative immunoblotting analysis was also
performed to determine the statistical differences in CLDN12
expression among 3 cases of SqCC tissues and 3 cases of
non-neoplastic mucosal tissues, which were drawn from the 50 cases
of SqCC tissues and 20 cases of non-neoplastic mucosal tissues at
random. As indicated in Fig. 3, the
protein expression levels of CLDN12 were significantly upregulated
in the SqCC tissues compared with the non-neoplastic mucosal
tissues (P<0.01).
The impact of CLDN12 on human
bronchial epithelial cell EMT
Previous findings revealed that the expression of
CLDN12 was low or absent in the human bronchial epithelial cell
line BEAS-2B. To detect the impact of CLDN12 on the metastasis of
BEAS-2B cells, a p-EGFP-C1/CLDN12 plasmid was transfected into the
cells. Following G418 screening, a monoclonal strain of BEAS-2B
transfected with p-EGFP-C1/CLDN12 plasmid and a monoclonal strain
of BEAS-2B transfected with p-EGFP-C1/vector plasmid was obtained,
which were termed as BEAS-CLDN12 and BEAS-vector.
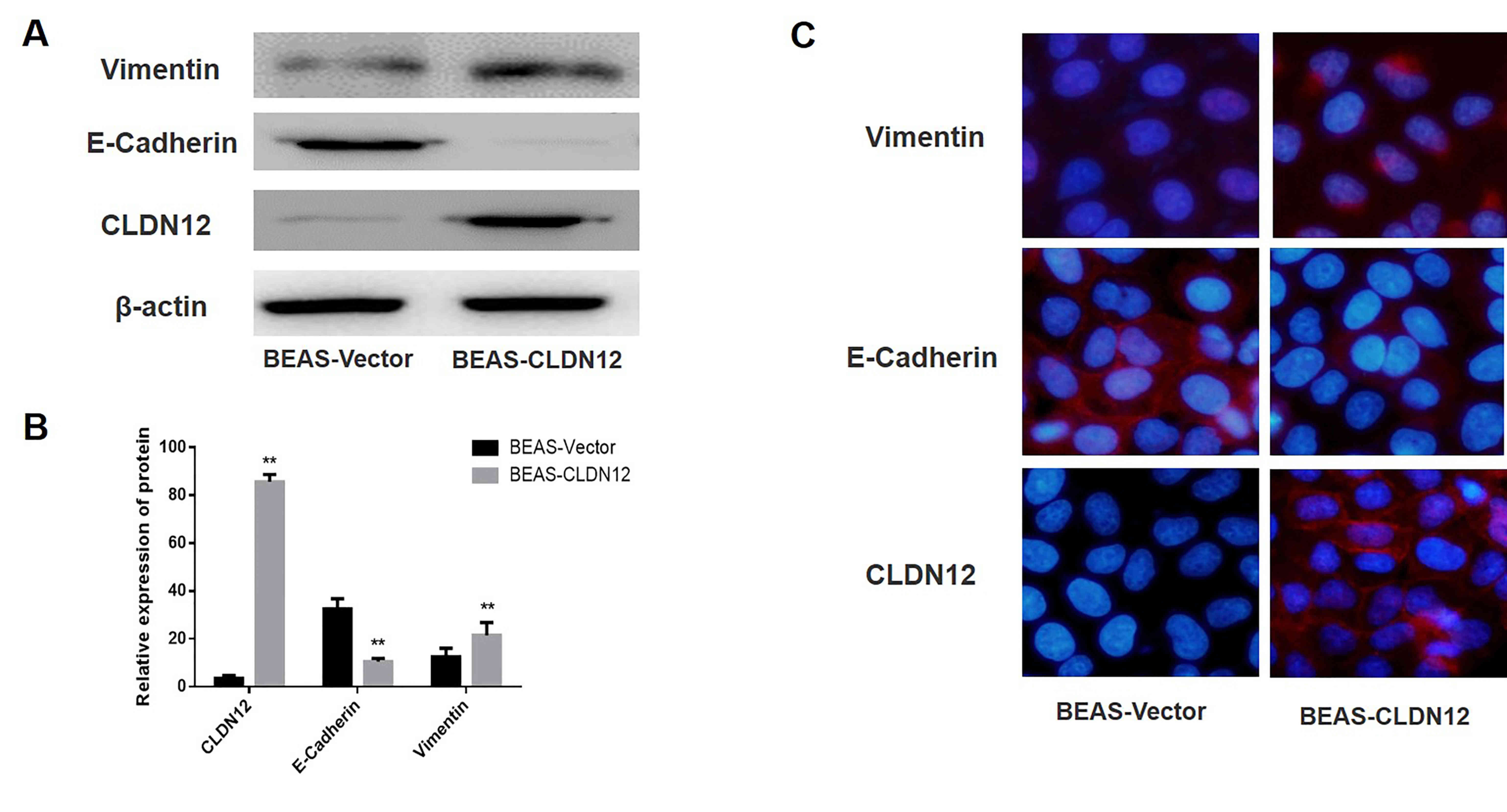
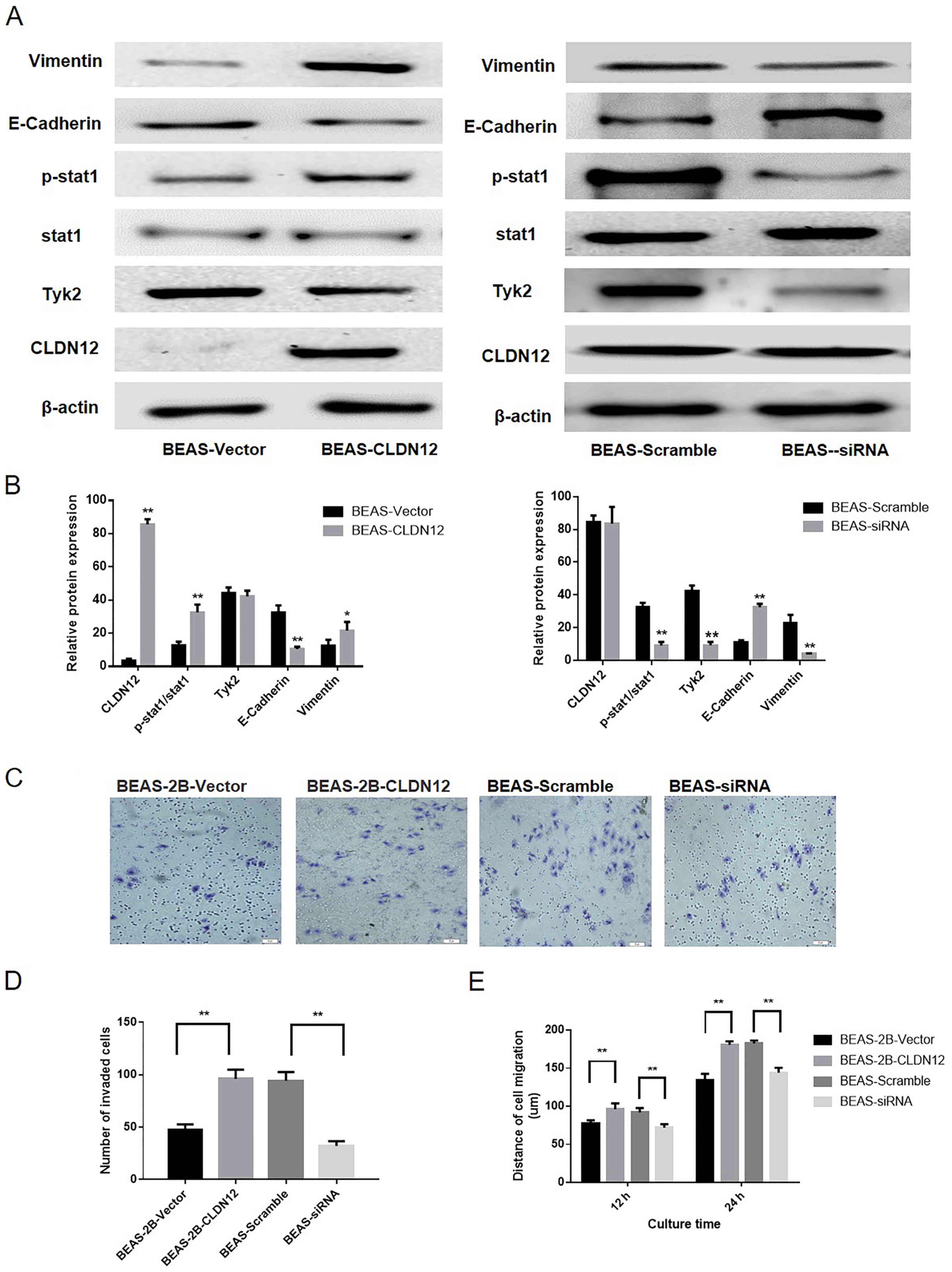
Western blotting was used to detect the expression
of CLDN12, E-Cadherin and Vimentin in the BEAS-2B cells. The
results revealed that CLDN12 and Vimentin protein expression levels
in the BEAS-CLDN12 cells were significantly increased compared with
those in the empty vector group, whereas the protein expression
levels of E-Cadherin in the clonal BEAS-2B cells was significantly
decreased compared with the empty vector group (P<0.01; Fig. 4A and B).
Immunofluorescence was used to detect the expression
and localization of CLDN12, E-Cadherin and Vimentin in BEAS-2B
cells. The results suggested that CLDN12 expression levels in the
CLDN12 overexpressed group were markedly increased compared with
the empty vector group, and that CLDN12 was primarily localized on
cell membranes. Conversely, the expression levels of E-Cadherin on
the membranes of the CLDN12 overexpressed group were decreased
compared with the empty vector group. Furthermore, the expression
levels of Vimentin in the cytoplasm of the CLDN12 overexpressed
group were increased compared with the empty vector group (Fig. 4C).
These results demonstrated that BEAS-2B clones
stably expressing CLDN12 were successfully established, and that
cells transitioned from an epithelial phenotype to interstitial
phenotype following the overexpression of CLDN12; i.e., the EMT
process was promoted in these BEAS-2B cells.
Impact of CLDN12 on the proliferation
rate and metastasis ability of human bronchial epithelial
cells
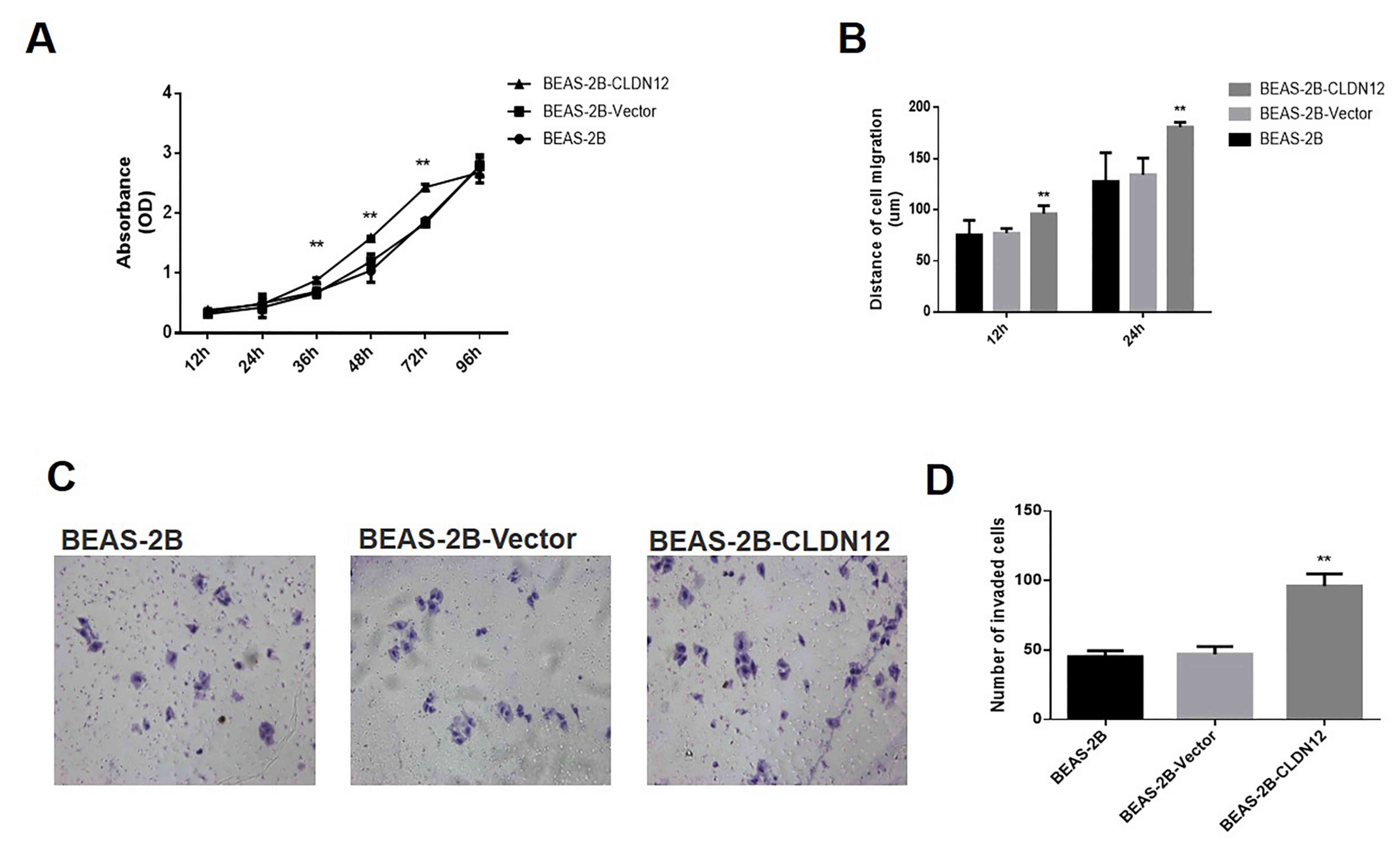
Growth curves of BEAS-2B cells were determined using
the CCK-8 method. As revealed in Fig.
5A, the proliferation rate of BEAS-CLDN12 cells was
significantly higher than that of the empty vector group at 36, 48
and 72 h (P<0.01).
A wound-healing experiment was used to detect the
impact of CLDN12 on the migratory ability of human bronchial
epithelial cells. The results indicated that at 12 and 24 h, the
migration distances of BEAS-CLDN12 cells were significantly greater
compared with those of the empty vector group (P<0.01; Fig. 5B). Additionally, the Transwell
invasion assay was used to assess invasive ability in the human
bronchial epithelial cells. At 6 h after the cells were seeded,
those cells that invaded under the membrane of the chamber were
observed. The results demonstrated that the number of invasive
BEAS-CLDN12 cells was increased compared with the empty vector
group (Fig. 5C). Statistical
analysis revealed the difference was significant (P<0.01;
Fig. 5D). These results suggested
that CLDN12 significantly promoted the proliferation and metastasis
of BEAS-2B cells in vitro.
Impact of Tyk2/Stat1 signaling pathway
activation on the invasion and migration abilities of BEAS-2B
cells
Western blotting was utilized to investigate the
activation state of the Tyk2/Stat1 signaling pathway. Following the
overexpression of CLDN12, the phosphorylation levels of Stat1 were
significantly increased in BEAS-2B cells compared with the control
(P<0.01; Fig. 6A and B). To
determine the impact of the Tyk2/Stat1 signaling pathway on the
invasion and migration abilities of BEAS-2B cells, pGCSIL-scramble
and pGCSIL-Tyk2-RNAi plasmids were transfected into BEAS-2B-CLDN12
cells. Following the knockdown of Tyk2, the phosphorylation levels
of Stat1 were significantly downregulated in the BEAS-2B cell line
overexpressing CLDN12 (P<0.01; Fig.
6A and B). Furthermore, the expression levels of E-Cadherin in
Tyk2-RNAi cells were increased and the expression of levels of
Vimentin in Tyk2-RNAi cells were significantly decreased
(P<0.01; Fig. 6A and B).
A Transwell chamber assay and wound-healing assay
were also used to analyze the effect of Tyk2 on the invasive and
migratory abilities of the examined cells. The results demonstrated
that the number of invasive BEAS-2B cells in the CLDN12
overexpressed group was significantly decreased following Tyk2
silencing (P<0.01; Fig. 6C and
D). The migration distances of the Tyk2-RNAi cells were
significantly decreased compared with those of the scramble group
at 12 and 24 h (P<0.01; Fig.
6E).
Discussion
Certain reports have indicated CLDN to function as a
tumor suppressor gene, where loss of CLDN contributed to enhanced
tumorigenic properties of tumor cells (13,14). In
one study, expression of CLDN1 was identified to be reduced in
stage II and III rectal cancer and was established as a factor that
significantly correlated with recurrence and poor prognosis
(15). Furthermore, the expression
of CLDN6 has been revealed to be downregulated in cervical
carcinoma tissues, whereas the gain of CLDN6 expression suppressed
cell proliferation and the colony formation capacity of cervical
carcinoma cells in vitro, and tumor growth in vivo
(12). However, in contrast to these
results, increasing evidence suggests that CLDNs may serve as
pro-oncogenes in various types of human cancer. For instance, it
was highlighted that CLDN1 had a key role in inflammation-induced
growth and progression of colorectal carcinoma (16). Furthermore, Philip et al
(17) reported that CLDN7 expression
in colorectal cancer contributed to cell motility and invasion.
Therefore, specific CLDNs may have differential impacts on the
biological behavior of a given tumor (18–20). One
potential reason for the discrepancy in results may be that the
function of CLDNs is specific and relies on different interacting
molecules in various cells (21,22).
Recently, a number of studies have focused on the
role of CLDNs in the tumorigenesis of human lung carcinoma. For
instance, the expression of CLDN1 was identified as a positive
prognostic factor in cases of SqCC (23). Notably, CLDN2 has also been indicated
to be overexpressed in human lung adenocarcinoma tissues and a
novel target in lung adenocarcinoma (24). Additionally, CLDN3 was reported to
inhibit the metastatic phenotype of SqCC via suppression of the
Wnt/β-catenin signaling pathway (25). Other studies have revealed that
downregulation of CLDN7 has been reported to promote the survival
capacity of lung cancer cells under the hypoxic conditions of the
tumor microenvironment (26,27). CLDN12 is among the 27 members of the
CLDN protein family, and current understanding of the biological
function of CLDN12 is primarily limited to its role in epithelial
and epidermal permeability, barrier protection and cell
connections, with limited reports on the association between CLDN12
and tumors (28). The present data
suggested that CLDN12 expression was upregulated in SqCC, not in
lung adenocarcinoma, and was involved with the lymph node
metastasis of SqCC. Additionally, the association between CLDN12
and the expression level of E-Cadherin in SqCC was investigated.
The results indicated that the expression of E-Cadherin was
inversely associated with that of CLDN12. These data suggested that
CLDN12 may be negatively associated with the expression of
E-Cadherin during the tumorigenesis and progression of SqCC, and
therefore, that the combination of CLDN12 and E-Cadherin expression
may be useful as an independent predictor for the diagnosis of SqCC
as well as for the determination of distant metastasis and
prognosis. To verify this hypothesis, a human bronchial epithelial
cell line, BEAS-2B, that stably expressed CLDN12 was established.
It was indicated that overexpression of CLDN12 significantly
enhanced the metastasis and migratory abilities of this human
bronchial epithelial cell line.
To date, certain studies have demonstrated that CLDN
proteins can bind with various proteins associated with cellular
signal transduction, and thereby regulate a series of cell
behaviors, including the EMT process (29). For instance, Philip et al
(17) reported that CLDN7 expression
in colorectal cancer contributed to motility and invasion by
promoting a shift towards EMT through recruiting epithelial cell
adhesion molecule towards TACE/presenilin2. However, to date, there
have been few reports on the roles of CLDN12 in human tumors, and
the specific molecular mechanisms involved remain to be clarified.
In the present study, a preliminary investigation of the molecular
mechanism associated with the effect of CLDN12 on the metastasis
ability of bronchial epithelial cells was performed. The present
study identified that CLDN12 upregulation affected the Stat1
signaling pathway via Tyk2, and ultimately enhanced the EMT of the
human bronchial epithelial cell line BEAS-2B in vitro. To
date, few studies have indicated that Tyk2/Stat1 signaling impacts
the role of various CLDNs in human tumorigenesis. One of the most
interesting findings in the present research is that upregulated
CLDN12 contributed to an enhancement of cell invasion and migration
through the Tyk2/Stat1 signaling pathway in SqCC. Considering the
limited therapeutic options for patients with SqCC, the role of
CLDN12 as a therapeutic target merits further investigation.
In the present study, it was suggested that CLDN12
may be a protooncogene in SqCC, as the overexpression of CLDN12
significantly enhanced the metastasis ability of the human
bronchial epithelial cell line BEAS-2B. Furthermore, the induction
of Tyk2/Stat1 signaling appeared an important mechanism by which
CLDN12 promoted the metastatic phenotype of SqCC cells.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LS and LF performed the experiments and analyzed the
data. JC contributed to the conception and design of the study. LF
also revised the manuscript critically for important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. This present study is retrospective and patient consent
was obtained at the time of data collection. Ethics approval
(approval no. JLU00896 and JLU01125) was granted by the Ethics
Committee of Jilin University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TJ
|
Tight junction
|
|
CLDNs
|
claudins
|
|
EMT
|
epithelial-mesenchymal transition
|
|
SqCC
|
lung squamous cell carcinoma
|
|
CCK8
|
Cell Counting kit-8
|
|
OC
|
ovarian cancer
|
References
|
1
|
Tabaries S and Siegel PM: The role of
claudins in cancer metastasis. Oncogene. 36:1176–1190. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matsuda A and Katanoda K: Five-year
relative survival rate of lung cancer in the USA, Europe and Japan.
Jpn J Clin Oncol. 43:1287–1288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Osanai M, Takasawa A, Murata M and Sawada
N: Claudins in cancer: Bench to bedside. Pflugers Arch. 469:55–67.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kwon MJ: Emerging roles of claudins in
human cancer. Int J Mol Sci. 14:18148–18180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Balda MS and Matter K: Tight junctions at
a glance. J Cell Sci. 121:3677–3682. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Escudero-Esparza A, Jiang WG and Martin
TA: The Claudin family and its role in cancer and metastasis. Front
Biosci (Landmark Ed). 16:1069–1083. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ouban A: Claudin-1 role in colon cancer:
An update and a review. Histol Histopathol. 33:1013–1019.
2018.PubMed/NCBI
|
|
8
|
Szasz MA: Claudins as prognostic factors
of breast cancer. Magy Onkol. 56:209–212. 2012.(In Hungarian).
PubMed/NCBI
|
|
9
|
de la Fuente Martin L, Malander S, Hartman
L, Jönsson JM, Ebbesson A, Nilbert M, Måsbäck A and Hedenfalk I:
Claudin-4 expression is associated with survival in ovarian cancer
but not with chemotherapy response. Int J Gynecol Pathol.
37:101–109. 2018.PubMed/NCBI
|
|
10
|
Ahmad R, Kumar B, Chen Z, Chen X, Müller
D, Lele SM, Washington MK, Batra SK, Dhawan P and Singh AB: Loss of
claudin-3 expression induces IL6/gp130/Stat3 signaling to promote
colon cancer malignancy by hyperactivating Wnt/β-catenin signaling.
Oncogene. 36:6592–6604. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang X, Ruan Y, Li Y, Lin D, Liu Z and
Quan C: Expression of apoptosis signal-regulating kinase 1 is
associated with tight junction protein claudin-6 in cervical
carcinoma. Int J Clin Exp Pathol. 8:5535–5541. 2015.PubMed/NCBI
|
|
12
|
Zhang X, Ruan Y, Li Y, Lin D and Quan C:
Tight junction protein claudin-6 inhibits growth and induces the
apoptosis of cervical carcinoma cells in vitro and in vivo. Med
Oncol. 32:1482015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ouban A and Ahmed A: Claudins in human
cancer, A review. Histol Histopathol. 25:83–90. 2010.PubMed/NCBI
|
|
14
|
Zavala-Zendejas VE, Torres-Martinez AC,
Salas-Morales B, Fortoul TI, Montano LF and Rendon-Huerta EP:
Claudin-6, 7, or 9 overexpression in the human gastric
adenocarcinoma cell line AGS increases its invasiveness, migration,
and proliferation rate. Cancer Invest. 29:1–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoshida T, Kinugasa T, Akagi Y, Kawahara
A, Romeo K, Shiratsuchi I, Ryu Y, Gotanda Y and Shirouzu K:
Decreased expression of claudin-1 in rectal cancer: A factor for
recurrence and poor prognosis. Anticancer Res. 31:2517–2525.
2011.PubMed/NCBI
|
|
16
|
Cherradi S, Ayrolles-Torro A, Vezzo-Vie N,
Gueguinou N, Denis V, Combes E, Boissière F, Busson M,
Canterel-Thouennon L, Mollevi C, et al: Antibody targeting of
claudin-1 as a potential colorectal cancer therapy. J Exp Clin
Cancer Res. 36:892017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Philip R, Heiler S, Mu W, Buchler MW,
Zoller M and Thuma F: Claudin-7 promotes the epithelial-mesenchymal
transition in human colorectal cancer. Oncotarget. 6:2046–2063.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
D'Souza T, Agarwal R and Morin PJ:
Phosphorylation of claudin-3 at threonine 192 by cAMP-dependent
protein kinase regulates tight junction barrier function in ovarian
cancer cells. J Biol Chem. 280:26233–26240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
D'Souza T, Indig FE and Morin PJ:
Phosphorylation of claudin-4 by PKCepsilon regulates tight junction
barrier function in ovarian cancer cells. Exp Cell Res.
313:3364–3375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li X, Li Y, Qiu H and Wang Y:
Downregulation of claudin-7 potentiates cellular proliferation and
invasion in endometrial cancer. Oncol Lett. 6:101–105. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu Z: Functions of claudin-7 in human lung
cancer (unpublished PhD thesis). 2012.
|
|
22
|
Micke P, Mattsson JS, Edlund K, Lohr M,
Jirström K, Berglund A, Botling J, Rahnenfuehrer J, Marincevic M,
Pontén F, et al: Aberrantly activated claudin 6 and 18.2 as
potential therapy targets in non-small-cell lung cancer. Int J
Cancer. 135:2206–2214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moldvay J, Fabian K, Jackel M, Németh Z,
Bogos K, Furák J, Tiszlavicz L, Fillinger J, Döme B and Schaff Z:
Claudin-1 protein expression is a good prognostic factor in
non-small cell lung cancer, but only in squamous cell carcinoma
cases. Pathol Oncol Res. 23:151–156. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Du W, Xu X, Niu Q, Zhang X, Wei Y, Wang Z,
Zhang W, Yan J, Ru Y, Fu Z, et al: Spi-B-mediated silencing of
claudin-2 promotes early dissemination of lung cancer cells from
primary tumors. Cancer Res. 77:4809–4822. 2017.PubMed/NCBI
|
|
25
|
Che J, Yue D, Zhang B, Zhang H, Huo Y, Gao
L, Zhen H, Yang Y and Cao B: Claudin-3 inhibits lung squamous cell
carcinoma cell epithelial-mesenchymal transition and invasion via
suppression of the Wnt/β-catenin signaling pathway. Int J Med Sci.
15:339–351. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu Z, Liu Y, Xu J, Yin H, Yuan H, Gu J,
Chen YH, Shi L, Chen D and Xie B: Immunohistochemical
quantification of expression of a tight junction protein,
claudin-7, in human lung cancer samples using digital image
analysis method. Comput Methods Programs Biomed. 155:179–187. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu Z, Kim DH, Fan J, Lu Q, Verbanac K,
Ding L, Renegar R and Chen YH: A non-tight junction function of
claudin-7-Interaction with integrin signaling in suppressing lung
cancer cell proliferation and detachment. Mol Cancer. 14:1202015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koval M: Claudin heterogeneity and control
of lung tight junctions. Annu Rev Physiol. 75:551–567. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kominsky SL: Claudins: Emerging targets
for cancer therapy. Expert Rev Mol Med. 8:1–11. 2006. View Article : Google Scholar : PubMed/NCBI
|