Introduction
Osteosarcoma (OS) is characterized by the direct
formation of immature bone or osteoid tissue by malignant cells
that occurs in children and adults (1). According to an estimate by the National
Cancer Institute in 2017, OS accounted for 0.2% (3,260) of all new
cancer cases and 0.3% (1,550) of cancer worldwide mortality
(2). Despite advance in diagnosis
and treatment of OS, including magnetic resonance imaging,
neoadjuvant chemotherapy and surgery, the overall survival rate
remains poor (<30%) due to the invasiveness and distant
metastasis largely affecting the lung (3,4).
Therefore, novel effective and reliable methods are required in the
treatment of OS.
Saikosaponin D (SSd) is one of the main bioactive
ingredients extracted and purified from Radix bupleuri L,
which is prescribed in traditional Chinese medicine for
inflammatory, infectious and vascular diseases or as a
hepatoprotective, antibacterial or antiviral (3,4).
Previous research reported that SSd exhibits significant antitumor
activities in cancer cells, including breast, lung, ovarian and
pancreatic cancer and certain types of OS (5–12). SSd
induces apoptosis in DU145 pancreatic cancer cells via intrinsic
apoptotic pathways and sensitizes cancer cells to cisplatin through
ROS-mediated apoptosis, which in combination with further
saikosaponins may be an effective therapeutic strategy.
Furthermore, SSd inhibited hepatocellular carcinoma (HCC)
development and downregulated syndecan-2, matrix metalloproteinase
(MMP)-2, MMP-13 and tissue inhibitor of metalloproteinase-2
expression in HCC liver tissue, suggesting SSd is a potential
candidate in antitumor treatment in OS.
In previous studies, the potential antitumor
mechanism of SSd influencing apoptosis and autophagic cell death
was demonstrated (7–9,13).
However, biological functions and associated molecular mechanisms
of SSd in OS have not yet been elucidated. In the present research,
tumor-suppressive functions and mechanisms of SSd in OS were
evaluated in order to test the potential of using SSd as an
antitumor drug in OS.
Materials and methods
Cell culture and chemicals
Human OS cells lines (143B and MG-63) were purchased
from the American type culture collection (Manassas, VA, USA). Cell
lines were cultured in RPMI-1640 containing 10% fetal bovine serum
(both Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at
37°C in a 5% CO2 humidified atmosphere, as previously
described (14). SSd (purity,
>95%; authenticated by Shanghai Academy of Life Sciences,
Shanghai, China) was mixed with RPMI-1640 medium and stored at room
temperature.
Nucleic acid extraction
143B and MG-63 cells (1×104 cells/well)
were cultured in 6-well plates in RPMI-1640 medium supplemented
with 10% FBS for 24 h at room temperature. The medium was replaced
with RPMI-1640 supplemented with 10% FBS containing 80 µmol/l SSd
and cells were cultured for 48 h at 37°C prior to harvesting. RNA
was extracted as reported previously (15). Total RNA was isolated from the cells
using TRIzol reagent (Thermo Fisher Scientific, Inc.). Isolated RNA
samples were quantified using spectrophotometry and stored at −80°C
until further use. RNA concentrations were detected by a NanoDrop
2000 spectrophotometer (Thermo Fisher Scientific, Inc.) and 2 µl
samples were separated using gel electrophoresis on a 5% agarose
gel to examine the purity of the RNA as reported previously
(15,16).
MTS assay
Cell viability was assessed by MTS assay (Beyotime
Institute of Biotechnology, Haimen, China). Cells (143B and MG-63)
were cultured in 96-well plates (2,000 cells/well) RMPI-1640
supplemented with 10% FBS for 24 h at room temperature (14,17).
Cells were incubated with RMPI-1640 plus 10% FBS and varying
concentrations of SSd (20, 40, 60 and 80 µmol/l) for 24, 48 and 72
h at room temperature. Following, 20 µl MTS solution was added to
each well and samples were incubated at room temperature for 3 h
prior to absorbance measurements at 450 nm using a microplate
reader. Each experiment was repeated three times.
EdU assay
EdU assays were performed using the Click-iT EdU
Imaging kit (Promega Corporation, Madison, WI, USA) and an apollo
fluorescent dye (Guangzhou RiboBio Co., Ltd., Guangzhou, China) in
accordance with the manufacturer's instructions. 143B and MG-63
cells (3×103 cells/well) were incubated in RMPI-1640
medium plus 10% FBS with 80 µmol/l SSd for 24 and 48 h at room
temperature. Cells were exposed to 100 µl EdU at 37°C for 3 h and
fixed at room temperature for 20 min with 4% paraformaldehyde. The
number of EdU-positive and total cells was counted using a
fluorescence microscope (magnification in blue light, ×20) with
four non-overlapping fields per coverslip. Each experiment was
repeated three times.
Flow cytometry analysis of cell cycle
and apoptosis
Cell cycle and apoptosis were determined by flow
cytometry as previously described (18). For cell cycle assays, cells were
plated in six-well plates at 1×105 cells/well and
cultured overnight at room temperature. RMPI-1640 medium plus 10%
FBS with 80 µmol/l SSd were added to the plates for 48 h and cells
were collected, digested and centrifuged (800 × g at room
temperature for 10 min). Following, cells were incubated with
ice-cold 70% ethanol at room temperature for 24 h and treated with
propidium iodide (PI) for 30 min at 4°C in the dark. Annexin
V-fluorescein isothiocyanate (FITC)/PI staining was performed for
apoptosis analysis following the manufacturer's guidelines (BD
Pharmingen; BD Biosciences, Franklin Lakes, NJ, USA) for 30 min at
4°C in the dark. Samples were analyzed using a flow cytometer. Data
were analyzed using CellQuest 16.0 software (BD Biosciences). Each
experiment was repeated three times. Apoptosis assays were
performed using the described procedure and staining was based on
FITC-Annexin V and PI using the FITC-Annexin V Apoptosis Detection
kit (BD Biosciences) according to manufacturer's instructions.
Experiments were performed in triplicate.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
RNA samples extracted from control and SSd-treated
cells were reverse transcribed using a Go-Taq DNA polymerase kit
(Promega Corporation) as reported previously (18,19). RT
was performed with the following protocol: 37°C for 15 min and 85°C
for 5 sec. β-actin functioned as the internal control. RT-qPCR
reactions were performed using the HT7500 system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and qPCR was performed
using the SYBR−Green PCR Master mix (Thermo Fisher
Scientific, Inc.). The PCR program began with an initial
denaturation at 95°C for 2 min, followed by 40 amplification
reaction cycles (95°C for 30 sec, 55°C for 30 sec and 72°C for 30
sec), with a final extension at 72°C for 3 min. Primer sequences
are reported in Table I. Each sample
was tested in triplicate and relative amounts of mRNA were
determined using the 2−ΔΔCt method (20).
 | Table I.Primers used in reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primers used in reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Sequence (5′-3′) | Fragment length
(bp) |
|---|
| p53-F |
TACACTGCCCAGGAGCCAGA | 103 |
| p53-R |
TGGCACCAGTGTCCGGATTA |
|
| p21-F |
TGGAGACTCTCAGGGTCGAAA | 65 |
| p21-R |
GGCGTTTGGAGTGGTAGAAATC |
|
| p27-F |
CGTGCGAGTGTCTAACGG | 206 |
| p27-R |
CGGATCAGTCTTTGGGTC |
|
| CyclinD1-F |
CTAGCAAGCTGCCGAACC | 90 |
| CyclinD1-R |
TCCGAGCACAGGATGACC |
|
| Bax-F |
TCCACCAAGAAGCTGACGAG | 275 |
| Bax-R |
GTCCAGCCCATGATGGTTCT |
|
| Caspase-3-F |
ATGGAGAACAATAAAACCCT | 50 |
| Caspase-3-R |
CTAGTGATAAAAGTAGAGTTC |
|
| β-actin-F |
TCCTGTGGCATCCACGAAACT | 315 |
| β-actin-R |
GAAGCATTTGCGGTGGACGAT |
|
Western blot analysis
Proteins from cells cultured in RMPI-1640 medium
plus 10% FBS with 80 µmol/l SSd were collected using RIPA buffer
containing fresh protease and phosphatase inhibitor cocktails (all
EMD Millipore, Billerica, MA, USA). To determine the concentration
of proteins, a BCA protein kit (Thermo Fisher Scientific, Inc.) was
used. Extracted proteins (50 µg/lane) were separated as previously
described (21). Proteins was
separated by SDS-PAGE on a 10% gel and transferred to
polyvinylidene difluoride membranes (EMD Millipore). Aliquoted
proteins were separated with 12% gels using SDS-PAGE and then
transferred to polyvinylidene fluoride membranes. After blocking
with 5% nonfat milk at room temperature for 1 h, the membranes were
incubated with primary antibodies at 4°C overnight. The following
antibodies were included: Tumor protein 53 (p53; cat. no.
ab131442); p21 (cat. no. ab188224); p27 (cat. no. ab190851);
cyclinD1 (cat. no. ab137875); B-cell lymphoma (Bcl)-2-like protein
4 (Bax; cat. no. ab182733); cleaved caspase-3 (cat. no. ab198447;
all 1:1,000); and β-actin (cat. no. ab8227; 1:2,000; all Abcam,
Cambridge, UK) as a control. Membranes were then incubated with
horseradish peroxidase-conjugated secondary goat anti-rabbit
antibodies (cat. no. sc-2004; 1:5,000; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) at room temperature for 1 h. The bound
antibodies were detected using enhanced chemiluminescence kit (EMD
Millipore) and protein blots were visualized with the Las4000
Imaging system (GE Healthcare Life Sciences, Little Chalfont,
UK).
Statistical analysis
SPSS 17.0 (SPSS, Inc. Chicago, IL, USA) was used for
statistical analyses. All data are expressed as means ± standard
deviation. Differences between two samples were assessed by
two-tailed Student's t-test and one-way analysis of variance
followed by the Bonferroni post hoc test to compare between
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
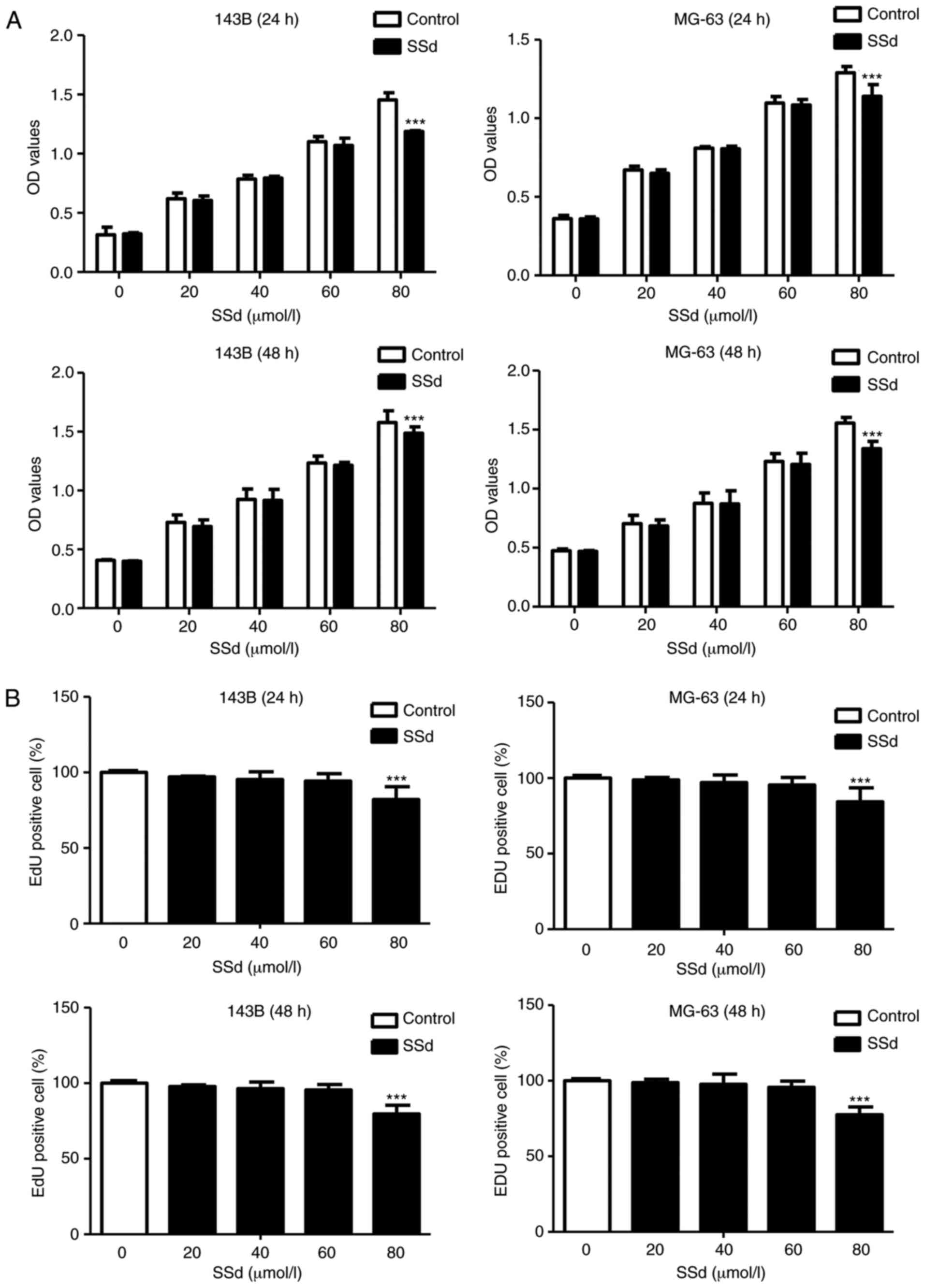
SSd inhibits cell proliferation
143B and MG-63 cell viability and proliferation were
tested by MTS and EdU assays. MTS assays assessed effects of
varying concentration of SSd (20, 40, 60 and 80 µmol/l) on cell
viability and results demonstrated that SSd had no significant
effects at 20–60 µmol/l when compared with the control group
(P>0.05; Fig. 1A). At 80 µmol/l
SSd significantly inhibited 143B and MG-63 cell viability following
24 and 48 h treatment compared with the control (P<0.001;
Fig. 1A). EdU incorporation assays
further suggested that SSd significantly inhibited cell
proliferation at 80 µmol/l compared with the control (P<0.001;
Fig. 1B). In 143B and MG-63 lower
doses of SSd (20, 40 and 60 µmol/l) had no significant influence on
proliferation following 24 or 48 h treatment compared with the
control (P>0.05; Fig. 1B).
Subsequent experiments were performed using 80 µmol/l SSd as cell
treatment.
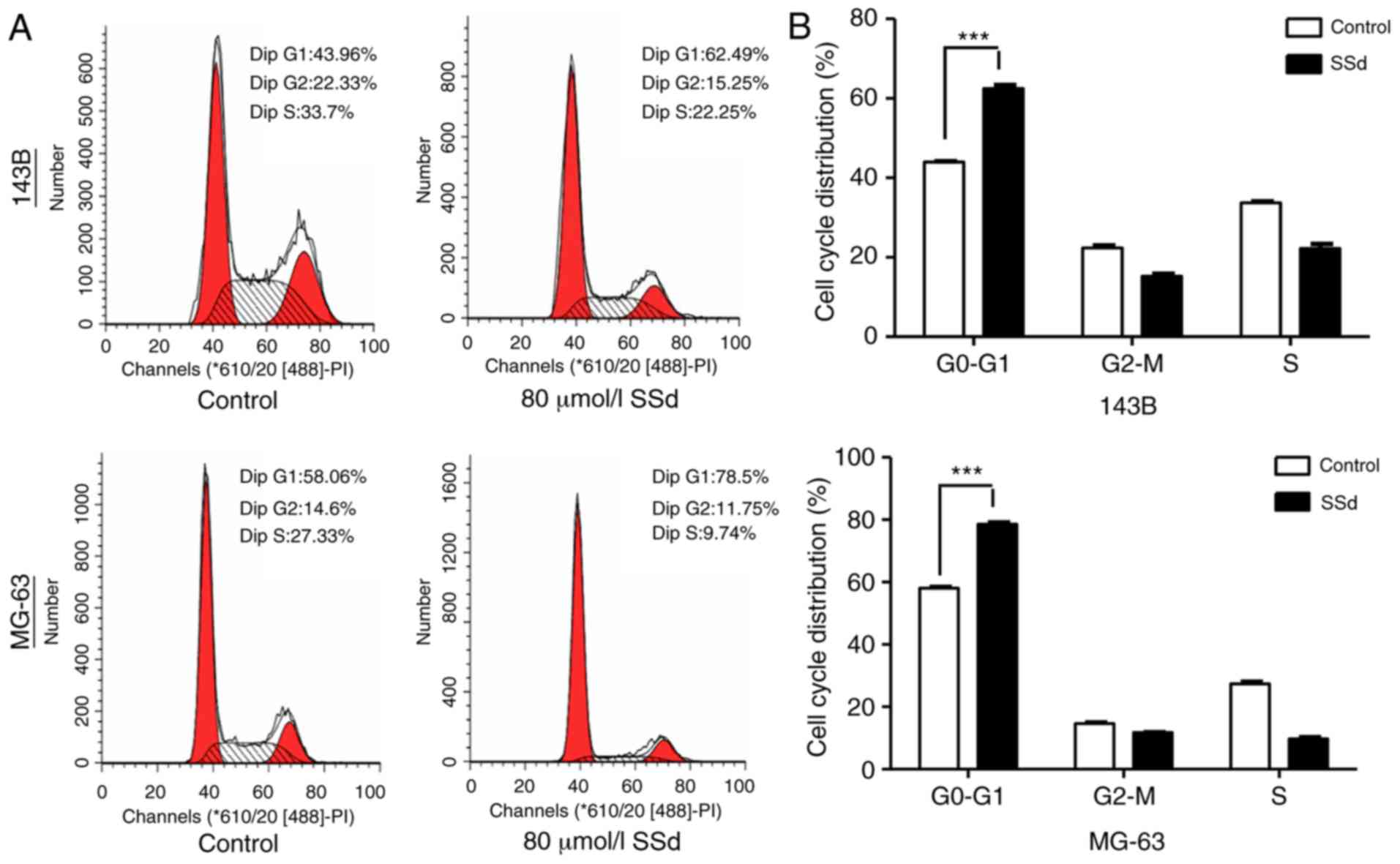
SSd causes cell cycle arrest in G0-G1
phase
Flow cytometric analyses of the cell cycle were used
to evaluate the underlying mechanisms of proliferation suppression
by SSd. It was observed that 80 µmol/l SSd significantly increased
the percentage of 143B and MG-63 cells in
G0-G1 phase by 18 and 23%, respectively
(P<0.001; Fig. 2).
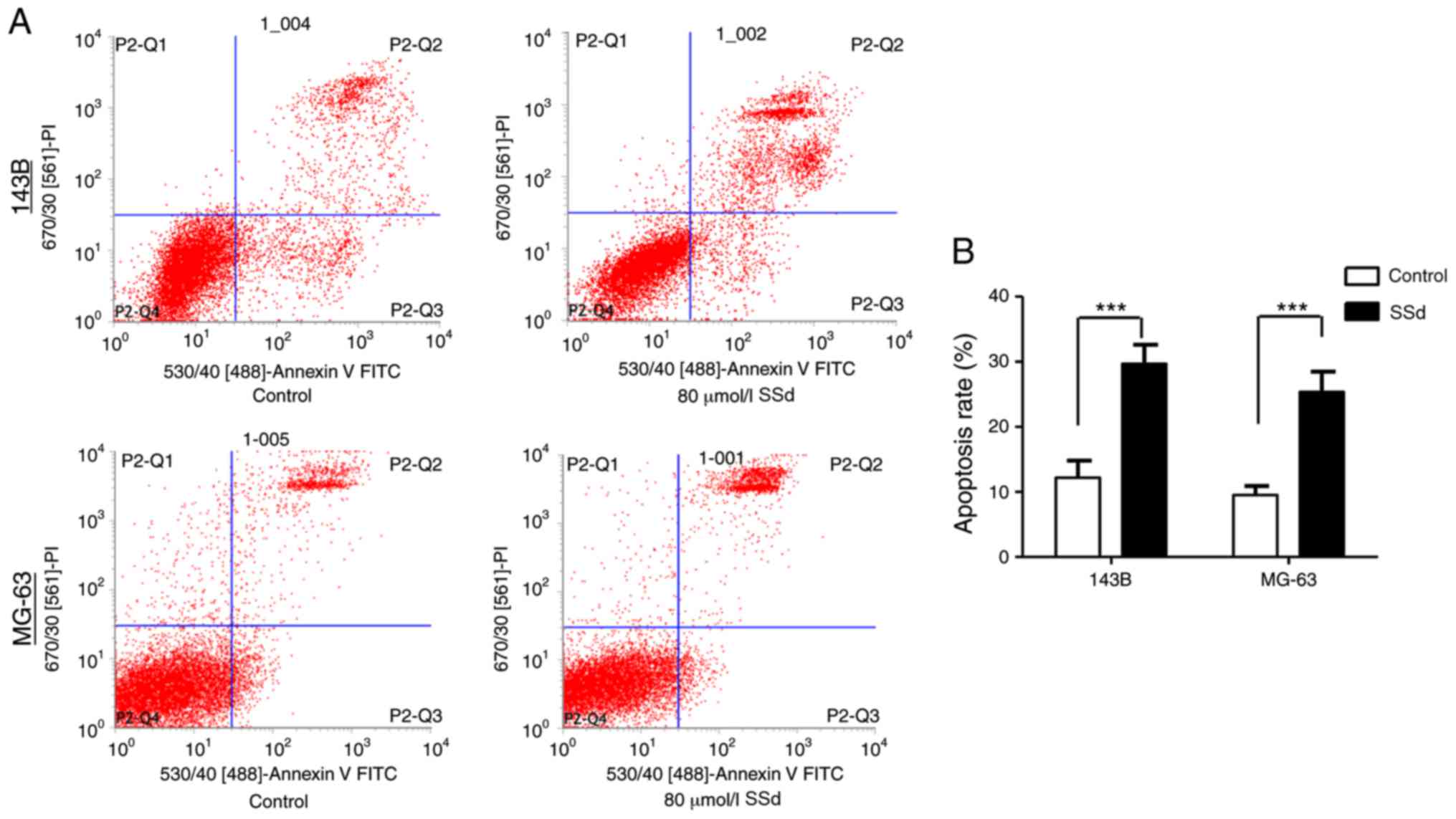
SSd induces apoptosis
A significant increase in apoptosis was observed
compared with the control group following the treatment of 143B and
MG-63 with 80 µmol/l SSd for 48 h (P<0.001; Fig. 3), indicating that SSd may serve a
vital role in the apoptosis of OS cells.
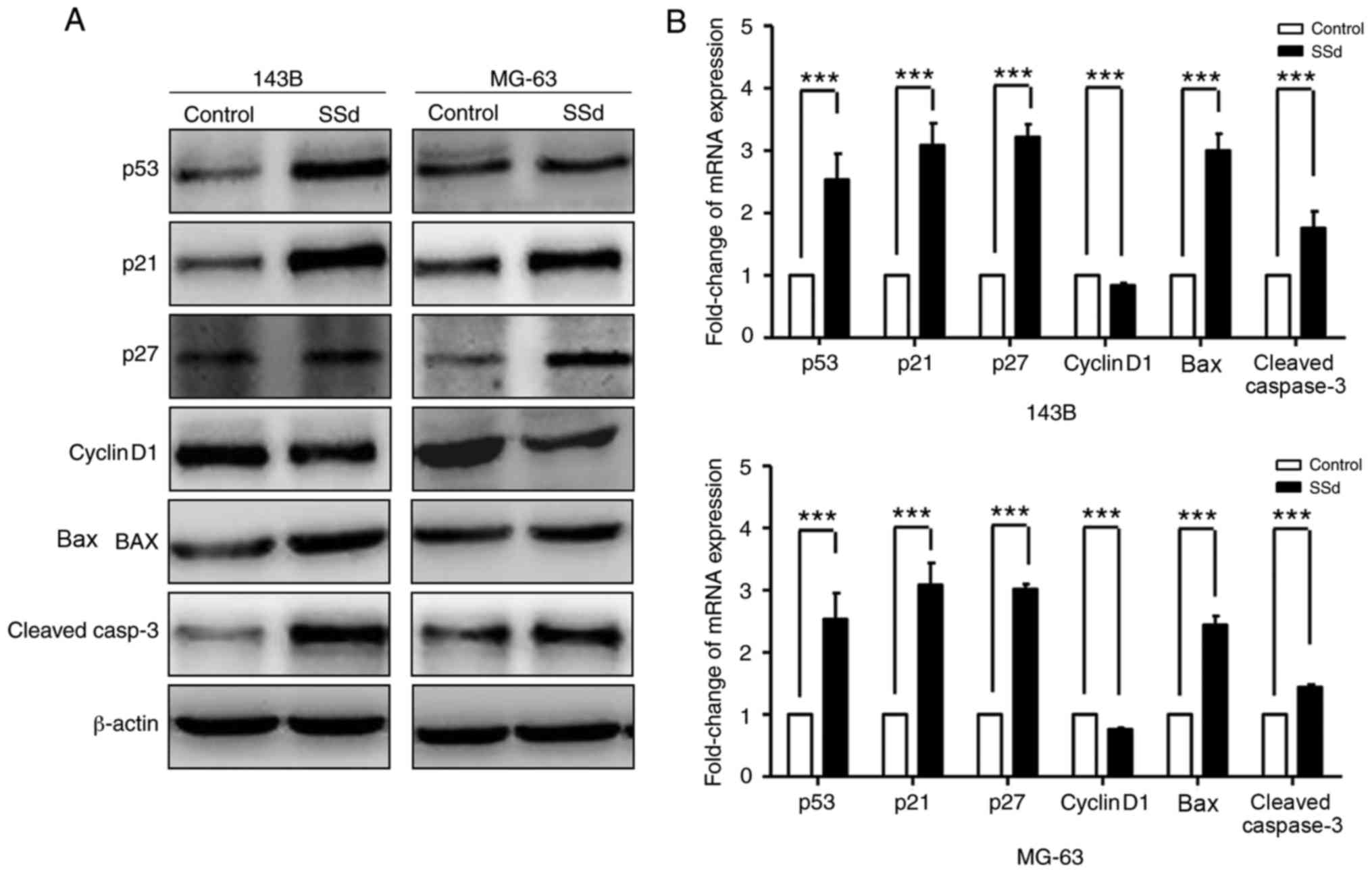
Effects of SSd on cell cycle and
apoptosis are associated with p53 accumulation
While cell cycle arrest and apoptosis are known to
be associated with the activation of the p53 signaling pathway, SSd
has been demonstrated to lead to p53 accumulation in ovarian cancer
cell lines, Hey and SKOV3 (9). A
potential association of SSd and p53 was evaluated. p53 expression
was assessed in 143B and MG-63 treated with 80 µmol/l SSd. Western
blot analysis revealed that SSd promoted p53 accumulation in OS
cells. Furthermore, it was demonstrated that SSd treatment was
associated with the upregulation of p53 downstream targets,
including p21, p27, Bax, cleaved caspase-3 and the downregulation
of cyclinD1 (Fig. 4A). RT-qPCR was
performed to evaluate the mRNA expression of p53 signaling pathway
targets. In 143B and MG-63, SSd treatment (80 µmol/l) significantly
decreased mRNA levels of cyclinD1 and significantly increased mRNA
levels of p21, p27, Bax and cleaved caspase-3 (P<0.001; Fig. 4B). Thus, SSd increased the expression
of p53 in protein and mRNA levels in 143B and MG-63.
Discussion
OS is the leading cause of death among primary bone
malignancy, which originates from bone mesenchymal cells (22). It occurs mainly in adolescents and
children (~90% of patients with OS are <20 years of age)
(22,23). The OS survival rate in the US was
~20%, and the five-year OS survival rates for children and
adolescents in Europe were similar (22,23).
Long-term survival rates of patients who underwent surgical
resection of OS were ~20% worldwide (23). In recent years, cisplatin-based
combination chemotherapy has improved the prognosis of patients
with OS and the 5-year overall survival rate for non-metastasis
accounts for 60–70% (24).
Resistance to chemotherapy describes an important factor
influencing the treatment outcomes in OS (23,25).
Over the past decades, traditional Chinese
medicines, based on herbs and botanicals, have been applied in the
treatment of osteoarthrosis, diabetes disease and malaria, and
clinical evaluation deemed the formulations as extremely safe,
efficient and to exhibit lower toxicity (25). For example, artemisinin was used in
the treatment of malaria (24) and
Chinese medicines, such as rosmarinic, were used as diabetic
retinopathy therapies (26). SSd
regulates T lymphocyte function, prevents the progression
proteinuria, modulates macrophage function and enhances nonspecific
resistance against aeruginosa infection (7,27,28). The
compound further possesses anti-inflammatory effects and causes
cell death in human HCC cell lines (29). Other studies reported that SSd
sensitizes tumor cells to cisplatin via ROS-mediated apoptosis and
the combination of SSd with cisplatin may describe an effective
therapeutic strategy (29). Wang
et al (30) suggested that
SSd potentiates effects of radiation in SMMC-7721 hepatocytes;
thus, it may be a promising radiosensitizer further affecting the
cell cycle (30). However, functions
and underlying mechanisms of SSd in OS remain to be
investigated.
The current study focused on antitumor activities of
SSd. The results suggested that 143B and MG-63 incubated with 80
µmol/l SSd exhibited significantly reduced cell viability when
compared with the control group. In addition, DNA synthesis in 143B
and MG-63 cells was significantly suppressed at the G1
phase after incubation with high doses of SSd. It has been reported
that SSd is associated with various anticancer functions,
influencing cell proliferation, apoptosis and migration and
invasion of various cancer types (7,27). The
current study focused on antitumor activities od SSd in OS and it
was demonstrated that SSd induced G0/G1 phase
arrest and caused cells apoptosis, consistent with previous reports
(30,31).
p53 serves a critical role in regulation of cell
cycle checkpoints, DNA damage and the prevention of normal cell
developing malignant phenotypes (32,33).
Additionally, the tumor suppressor protein p53 is a vital component
in the apoptotic pathway, and Bcl-2 and Bax are important
transcriptional targets of p53. Bax, an anti-apoptotic protein, is
essential for apoptosis and p53 can initiate cell death through
activating target genes via the upregulation of Bax expression
(31). Bcl-2 family proteins serve
central roles in mitochondria-mediated apoptosis and are divided
into three groups: BH3-only proteins, BAX/bcl-2 homologous
antagonist/killer (BAK) proteins and pro-survival proteins
(34). Activated BAX/BAK forms
homo-oligomers that permeabilize mitochondria and allow the release
of pro-apoptotic factors, such as cytochrome c, which promote the
activation of caspases (35,36). In the present study, SSd
significantly increased transcription of p53 and its downstream
targets, including p21, p27 and Bax, and decreased cyclinD1
expression. CyclinD1 has been demonstrated to activate the G1-S
transition of the cell cycle (35,36).
Gumz et al (37) reported
that cyclinD1 was up-regulated in clear cell RCC and that an
antagonist of the Wnt signaling pathway, sFRP1, inhibited cyclinD1
expression. The results of the current study demonstrated that SSD
had a similar effect on cyclinD1.
In conclusion, the current study describes the role
of SSd in inhibiting cell growth and inducing apoptosis through
activation of the p53 signaling pathway in OS. SSd may serve as a
potential tumor inhibitor in OS. However, large-scale in
vivo experiments may be needed to validate its mechanism.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ and JL conducted the nucleic acid extraction, MTS
and EdU assays and western blot analyses, and wrote the manuscript.
Z-BS, CS and Z-HY collected the data and performed the cell
culture, flow cytometry and the reverse transcription-quantitative
polymerase chain reaction analysis. XG designed the study. The
final version of the manuscript has been read and approved by all
authors, and each author believes that the manuscript represents
honest work.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Widhe B and Widhe T: Initial symptoms and
clinical features in osteosarcoma and Ewing sarcoma. J Bone Joint
Surg Am. 82:667–674. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin JS, Chen R, Yan W and Chen DD:
Enhancing soft-tissue reattachment with artificial mesh in joint
endoprosthetic reconstruction for bone tumors. Zhonghua Zhong Liu
Za Zhi. 39:540–544. 2017.(In Chinese). PubMed/NCBI
|
|
3
|
Lu CN, Yuan ZG, Zhang XL, Yan R, Zhao YQ,
Liao M and Chen JX: Saikosaponin a and its epimer saikosaponin d
exhibit anti-inflammatory activity by suppressing activation of
NF-κB signaling pathway. Int Immunopharmacol. 14:121–126. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wong VK, Zhang MM, Zhou H, Lam KY, Chan
PL, Law CK, Yue PY and Liu L: Saikosaponin-d enhances the
anticancer potency of TNF-α via overcoming its undesirable response
of activating NF-Kappa B signalling in cancer cells. Evid Based
Complement Alternat Med. 2013:7452952013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tundis R, Bonesi M, Deguin B, Loizzo MR
and Menichini F, Conforti F, Tillequin F and Menichini F: Cytotoxic
activity and inhibitory effect on nitric oxide production of
triterpene saponins from the roots of Physospermum verticillatum
(Waldst & Kit) (Apiaceae). Bioorg Med Chem. 17:4542–4547. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hsu YL, Kuo PL and Lin CC: The
proliferative inhibition and apoptotic mechanism of Saikosaponin D
in human non-small cell lung cancer A549 cells. Life Sci.
75:1231–1242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hsu YL, Kuo PL, Chiang LC and Lin CC:
Involvement of p53, nuclear factor kappaB and Fas/Fas ligand in
induction of apoptosis and cell cycle arrest by saikosaponin d in
human hepatoma cell lines. Cancer Lett. 213:213–221. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Motoo Y and Sawabu N: Antitumor effects of
saikosaponins, baicalin and baicalein on human hepatoma cell lines.
Cancer Lett. 86:91–95. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsuyoshi H, Wong VKW, Han Y, Orisaka M,
Yoshida Y and Tsang BK: Saikosaponin-d, a calcium mobilizing agent,
sensitizes chemoresistant ovarian cancer cells to cisplatin-induced
apoptosis by facilitating mitochondrial fission and G2/M arrest.
Oncotarget. 8:99825–99840. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhuang Y, Gan Y and Tang T: Saikosaponin-d
promotes apoptosis of osteosarcoma cells. International Journal of
Orthopaedics. 38:115–120. 2017.
|
|
11
|
Zhang P, Wang X and Guo X: Saikosaponin-D
inhibits migration and invasion of osteosarcoma cell line MG-63 by
reversing EMT. J Modern Oncol. 25:2561–2564. 2017.
|
|
12
|
Yang C, Fan W, Ou X and Yuan B: Inhibitory
effect of Saikosaponin D on proliferation of osteosarcoma 143B
cells. Chin Pharm. 1:9–13. 2018.
|
|
13
|
Wong VK, Li T, Law BY, Ma ED, Yip NC,
Michelangeli F, Law CK, Zhang MM, Lam KY, Chan PL and Liu L:
Saikosaponin-d, a novel SERCA inhibitor, induces autophagic cell
death in apoptosis-defective cells. Cell Death Dis. 4:e7202013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fan Y, Zhan Q, Xu H, Li L, Li C, Xiao Q,
Xiang S, Hui T, Xiang T and Ren G: Epigenetic identification of
ZNF545 as a functional tumor suppressor in multiple myeloma via
activation of p53 signaling pathway. Biochem Biophys Res Commun.
474:660–666. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiong J, Liu Y, Jiang L, Zeng Y and Tang
W: High expression of long non-coding RNA lncRNA-ATB is correlated
with metastases and promotes cell migration and invasion in renal
cell carcinoma. Jpn J Clin Oncol. 46:378–384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu X, Zhang P, Zhu H, Li S, Chen X and Shi
L: Long noncoding RNA FEZF1-AS1 indicates a poor prognosis of
gastric cancer and promotes tumorigenesis via activation of Wnt
signaling pathway. Biomed Pharmacother. 96:1103–1108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao L, Zhang H, Bao J, Liu J and Ji Z:
Saikosaponin-d protects renal tubular epithelial cell against high
glucose induced injury through modulation of SIRT3. Int J Clin Exp
Med. 8:6472–6481. 2015.PubMed/NCBI
|
|
18
|
Cao W, Peng T and Zhou Y: Long noncoding
RNA activated by transforming growth factor-β promotes cancer
development and is a prognostic marker in cervical cancer. J Cancer
Res Ther. 13:801–806. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gou X, Zhao X and Wang Z: Long noncoding
RNA PVT1 promotes hepatocellular carcinoma progression through
regulating miR-214. Cancer Biomark. 20:511–519. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu Y, Hu J, Zhang C and Liu Y:
MicroRNA-320 targets mitogen-activated protein kinase 1 to inhibit
cell proliferation and invasion in epithelial ovarian cancer. Mol
Med Rep. 16:8530–8536. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He SX, Luo JY, Zhao G, Xu JL, Wang YL, Fu
H and Dong L: Effect of saikosaponins-d on cyclooxygenase-2
expression of human hepatocellular carcinoma cell line SMMC-7721.
Zhonghua Gan Zang Bing Za Zhi. 14:712–714. 2016.(In Chinese).
|
|
24
|
Tu Y: The discovery of artemisinin
(qinghaosu) and gifts from Chinese medicine. Nat Med. 17:1217–1220.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Girdhani S, Bhosle SM, Thulsidas SA, Kumar
A and Mishra KP: Potential of radiosensitizing agents in cancer
chemo-radiotherapy. J Cancer Res Ther. 1:129–131. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song W and Zhu YW: Chinese medicines in
diabetic retinopathy therapies. Chin J Integr Med. 28:2018.
|
|
27
|
Wang Q, Zheng XL, Yang L, Shi F, Gao LB,
Zhong YJ, Sun H, He F, Lin Y and Wang X: Reactive oxygen
species-mediated apoptosis contributes to chemosensitization effect
of saikosaponins on cisplatin-induced cytotoxicity in cancer cells.
J Exp Clin Cancer Res. 29:1592010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qian L, Murakami T, Kimura Y, Takahashi M
and Okita K: Saikosaponin A-induced cell death of a human hepatoma
cell line (HuH-7): The significance of the ‘sub-G1 peak’ in a DNA
histogram. Pathol Int. 45:207–214. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng JT and Tsai CL: Anti-inflammatory
effect of saikogenin A. Biochem Pharmacol. 35:2483–2487. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang BF, Dai ZJ, Wang XJ, Bai MH, Lin S,
Ma HB, Wang YL, Song LQ, Ma XL, Zan Y, et al: Saikosaponin-d
increases the radiosensitivity of smmc-7721 hepatocellular
carcinoma cells by adjusting the g0/g1 and g2/m checkpoints of the
cell cycle. BMC Complement Altern Med. 13:2632013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li M, Li Y, Sun L, Song JL and Lv C: High
mobility group box 1 promotes apoptosis of astrocytes after oxygen
glucose deprivation/reoxygenation by regulating the expression of
Bcl-2 and Bax. Beijing Da Xue Xue Bao Yi Xue Ban. 50:785–791.
2018.(In Chinese). PubMed/NCBI
|
|
32
|
Friedman MA and Carter SK: The therapy of
osteogenic sarcoma: Current status and thoughts for the future. J
Surg Oncol. 4:482–510. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kastan MB, Onyekwere O, Sidransky D,
Vogelstein B and Craig RW: Participation of p53 protein in the
cellular response to DNA damage. Cancer Res. 51:6304–6311.
1991.PubMed/NCBI
|
|
34
|
Edlich F: BCL-2 proteins and apoptosis:
Recent insights and unknowns. Biochem Biophys Res Commun.
500:26–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kanthan R, Radhi JM and Kanthan SC:
Gallbladder carcinomas: An immunoprognostic evaluation of P53,
Bcl-2, CEA and alpha-fetoprotein. Can J Gastroenterol. 14:181–184.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang YW, Zhang K, Zhao S, Lv Y, Zhu J, Liu
H, Feng J, Liang W, Ma R and Wang J: HPV status and its correlation
with BCL2, p21, p53, Rb, and survivin expression in breast cancer
in a Chinese population. Biomed Res Int. 2017:63153922017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gumz ML, Zou H, Kreinest PA, Childs AC,
Belmonte LS, LeGrand SN, Wu KJ, Luxon BA, Sinha M, Parker AS, et
al: Secreted frizzled-related protein 1 loss contributes to tumor
phenotype of clear cell renal cell carcinoma. Clin Cancer Res.
13:4740–4748. 2007. View Article : Google Scholar : PubMed/NCBI
|