Introduction
Hepatic malignant tumor is one of the most common
malignant tumors in the world. The morbidity and mortality rates of
males were, respectively, the 5th and the 2nd in the world, and for
females were the 9th and 6th in the world (1). Hepatic malignant tumors contain primary
hepatic carcinoma and secondary hepatic carcinoma, the common
clinical manifestations include liver pain, abdominal distension,
anorexia, fatigue, wasting, progressive liver enlargement or upper
abdominal mass, and some patients have symptoms containing low
fever, jaundice, diarrhea, upper gastrointestinal bleeding, and so
on (2). The etiology and exact
molecular mechanism of hepatic malignant tumors are not completely
clear. At present, it is considered that the pathogenesis of
hepatic cancer is a complex process of multi-factor and multi-step.
Epidemiological and experimental data showed that many factors,
consisting of hepatitis B virus (HBV) and hepatitis C virus (HCV)
infection, aflatoxin, drinking water pollution, alcohol, cirrhosis,
sex hormones, nitrosamines, trace elements and so on, are related
to the incidence of hepatic malignant tumors (3). Individualized comprehensive therapy
according to different stages of hepatic carcinoma is the key to
improving the curative effect. Therapeutic methods include surgery,
hepatic artery ligation, hepatic artery chemoembolization,
radiofrequency, cryotherapy, laser, microwave, chemotherapy and
radiotherapy. Biological therapy and traditional Chinese medicine
treatment are also useful in the treatment of hepatic malignant
tumors (4).
Hepatic liver malignant tumors are the most common
solid tumors, and surgical treatment has been regarded as the
preferred and the most efficient method (5). Surgical resection, which can
effectively prolong the survival time and improve the quality of
life of patients, is still the main treatment for hepatic cancer.
However, abdominal pain, jaundice, fatigue, weight loss and
unexplained fever symptoms are usually observed in patients after
treatment. Both increased AFP levels and imaging examination
results indicate the recurrence of hepatic cancer. In addition,
more than 30% of patients show distant metastasis within 1 year
after surgery. Studies have shown that there are 3 major causes of
postoperative recurrence of hepatic cancer, including the existence
of metastasis before surgery, preoperative assessment error leads
to incomplete surgery and reduced immune function (6).
A previous study confirmed that patients after
surgery of hepatic carcinoma may experience abnormal metabolism of
blood fat and glucose (7). However,
there remain few studies focusing on the levels of blood fat, blood
glucose and insulin as well as the correlations among them. Serum
leptin, a protein hormone secreted by adipocytes (8), is expressed in multiple organs and
systems, and can be used in metabolic disorders, such as losing
weight through regulating the metabolism of fat, carbohydrate and
protein, thereby suppressing the appetite, reducing the intake of
energetic substance (9) and
increasing the energy efficiency (10). A study (11) has confirmed that serum leptin is
significantly associated with the clinical efficacy and recurrence
of hepatic carcinoma patients. To better explore the variations in
metabolism of blood fat and glucose, and insulin functions of
hepatic carcinoma patients with recurrence after surgery, we
analyzed the alterations in these indicators and investigated the
correlations of serum leptin with the blood fat, fasting blood
glucose and insulin levels.
Patients and methods
Patients
We enrolled a total of 80 patients with primary
hepatocellular carcinoma who were admitted to The First Hospital of
Lanzhou University (Lanzhou, China) between October 2014 and June
2016 for open surgery, and these patients were diagnosed with
computed tomography in upper abdomen and biopsy with samples
collected from the surgery or before surgery. All participants
underwent surgical treatment with an estimated survival time over 1
year. Before enrollment, participants had signed the informed
consent and this study was approved by the Ethic Committee of The
First Hospital of Lanzhou University. Patients complicated with
severe liver or kidney dysfunction, hyperlipidemia before surgery,
cachexia, mellitus diabetes, decreased insulin function or
insulin-resistance and mental diseases, BMI >28 kg/m2
before surgery, and those refusing to be enrolled in this study
were excluded. After 1-year follow-up, patients were divided into
two groups according to the recurrence of hepatic carcinoma after
surgery, i.e., the recurrence group and non-recurrence group. The
basic information of patients are shown in Table I. Differences in sex, age, ratio of
patients complicated with HB, HB disease course, alcohol-intake
history and duration, staging of hepatic carcinoma showed no
statistical significance (P>0.05).
 | Table I.Basic information of patients in the
recurrence group and non recurrence group. |
Table I.
Basic information of patients in the
recurrence group and non recurrence group.
| Variables | Non-recurrence group
(n=40) | Recurrence group
(n=40) |
|---|
| Sex |
| Male | 30 | 31 |
|
Female | 10 | 9 |
| Average years of
age | 69.5±2.0 | 69.6±2.0 |
| Patients complicated
with HB | 32 | 31 |
| HB disease course
(years) | 5–35 | 5–40 |
| Average HB disease
course (years) | 21.3±1.7 | 23.4±2.0 |
| Patients with
alcohol-intake history | 35 | 36 |
| Alcohol-intake
history (years) | 5–40 | 5–40 |
| Average
alcohol-intake history (years) | 23.3±2.1 | 23.4±2.0 |
| Staging of hepatic
carcinoma |
|
|
| Stage I | 25 | 26 |
| Stage II | 15 | 14 |
Methods
All enrolled participants underwent surgical
treatment followed by regular outpatient follow-up by abdominal
ultrasonic examination and CT examination to identify the
postoperative recurrence. We also compared the levels of serum
leptin before, one month and one year after surgery, changes in
blood fat, body mass index (BMI), waistline and hipline in one year
after surgery, alterations between the fasting blood glucose and
blood glucose at 2 h after meal, and the fasting insulin (FINS)
level and homeostasis model assessment insulin resistance (HOMA-IR)
index. Finally, we analyzed the correlations of serum leptin with
the total cholesterol, FINS and fasting blood glucose.
Evaluation methods
Serum leptin was detected by enzymelinked
immunosorbent assay (ELISA) (cat. no. E-EL-H0113c; Elabscience,
Wuhan, China). The procedures included:
Dilution-loading-incubation-dosing-washing-enzyme
addingincubation-washing-color development-termination and
determination (8). Normal reference
range was 0.69–11.46 µg/l. As for the detection of indicators
associated with blood fat, the normal reference range of total
cholesterol (TC) referred to the levels of triglyceride (TG),
low-density lipoprotein cholesterol (LDL-C) and high-density
lipoprotein cholesterol (HDL-C) detected by an automatic
biochemical analyzer (Abbott AEROSET; Diamond Diagnostics Inc.,
Holliston, MA, USA). Specimens were collected at the enrollment and
1 year after intervention from the fasting elbow venous blood in
the morning. BMI was calculated using the formula: BMI = body
weight (kg)/height × height (m); waistline was measured in supine
position and stable breath at 1 cm above the belly button, while
the hipline was measured in the fullest part of the hips. The
measurement of blood glucose, including fasting blood glucose and
blood glucose at 2 h after meal, was carried out with the HITACHI
7080 Automatic Biochemical Analyzer (Beckman Coulter, Inc., Brea,
CA, USA) (normal range of fasting blood glucose: 3.9~6.1 mmol/l;
normal range of blood glucose at 2 h after meal: <7.8 mmol/l).
The level of HOMA-IR was calculated with the formula:
HOMA-IR=[fasting blood glucose (mmol/l) × FINS (mU/l)]/22.5, in
which the normal range of FINS was 3.0–24.9 U/ml, and the normal
ratio of HOMA-IR was 1. In addition, the assay of FINS was carried
out with the Beckman Access DXI 800 spectrometer (Beckman Coulter,
Inc.).
Statistical analysis
Statistical Product and Service Solutions v21.0 (IBM
Corp., Armonk, NY, USA) was used for statistical processing.
Measurement data were presented as mean ± standard deviation (SD),
and Student's t-test was adopted for the comparison of mean between
the two groups. Enumeration data were presented as %, and
chi-square test was performed for the intergroup comparison of
rate. Scatter diagram was prepared with the correlation analysis.
Correlation analysis was performed using scatter chart Pearson's
correlation analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparison of the serum leptin levels
before, 1 month and 1 year after surgery between the two
groups
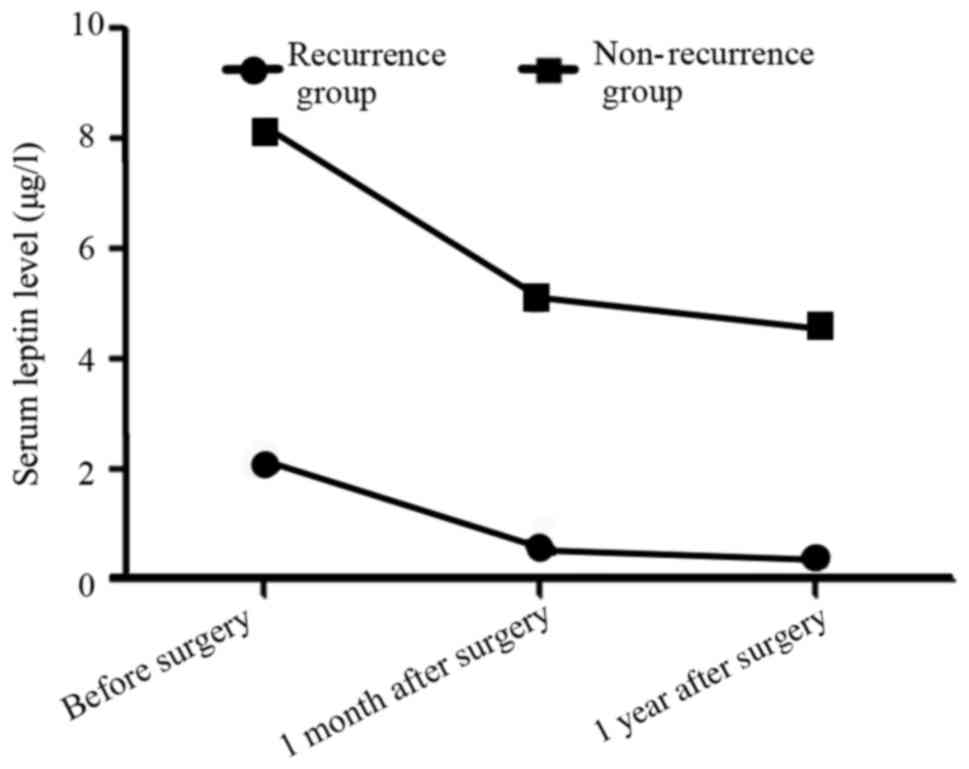
In the recurrence group, the serum leptin levels
before, 1 month and 1 year after surgery were (2.1±0.1), (0.6±0.1)
and (0.4±0.1) µg/l, significantly lower than those [(8.1±0.2),
(5.1±0.2) and (4.5±0.3) µg/l] in the non-recurrence group
(t=169.707, 127.279 and 82.000, P<0.05; Fig. 1).
Comparison of the blood fat at 1 year
after surgery between the two groups
There was no significant difference in the levels of
TC, TG, LDL-C and HDL-C between the two groups before operation
(P>0.05). One year after operation, LDL-C, TC and TG in both
groups was lower than that before operation (P<0.05) and HDL-C
was higher than that before operation (P<0.05). After 1-year
follow-up, we found that the levels of TC, TG and LDL-C (the
indicators of blood fat) in the recurrence group were significantly
higher than those in the non-recurrence group (P<0.05), while
the level of HDL-C was significantly lower than that in the
non-recurrence group (P<0.05; Table
II).
 | Table II.Comparison of the blood fat after
1-year follow-up between the two groups (mmol/l, mean ± SD). |
Table II.
Comparison of the blood fat after
1-year follow-up between the two groups (mmol/l, mean ± SD).
| Group | Time | TC | TG | LDL-C | HDL-C |
|---|
| Recurrence group | Before operation | 8.1±0.05 | 3.6±0.11 | 4.7±0.08 | 0.9±0.01 |
|
| 1 year after
operation | 6.2±0.03 | 2.4±0.02 | 3.5±0.03 | 1.1±0.01 |
| Non-recurrence
group | Before operation | 8.1±0.06 | 3.6±0.12 | 4.7±0.09 | 0.9±0.02 |
|
| 1 year after
operation | 5.4±0.01 | 1.8±0.01 | 2.5±0.02 | 1.4±0.01 |
| ta | – | 206.804 | 67.882 | 88.828 | 89.443 |
| P-value | – | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| tb | – | 280.733 | 94.541 | 150.919 | 141.421 |
| P-value | – | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| tc | – | 160.000 | 169.707 | 175.412 | 84.853 |
| P-value | – | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Comparison of the BMI, waistline and
hipline between the two groups
There was no significant difference in BMI, waist
circumference and hip circumference between the two groups before
operation (P>0.05). One year after operation, the BMI, waist
circumference and hip circumference of the two groups were lower
than those before operation (P<0.05). In the recurrence group,
the BMI was significantly higher than that in the non-recurrence
group (P<0.05), and similar results were also found in
comparison of waistline and hipline (P<0.05; Table III).
 | Table III.Comparison of the BMI, waistline and
hipline between the two groups (mean ± SD). |
Table III.
Comparison of the BMI, waistline and
hipline between the two groups (mean ± SD).
| Group | Time | BMI
(kg/m2) | Waistline (cm) | Hipline (cm) |
|---|
| Recurrence
group | Before
operation | 27.9±1.1 | 95.2±2.4 | 98.1±2.0 |
|
| 1 year after
operation | 26.5±1.2 | 93.5±2.1 | 96.1±1.6 |
| Non-recurrence
group | Before
operation | 28.0±1.1 | 95.2±2.5 | 98.0±2.0 |
|
| 1 year after
operation | 23.3±1.0 | 89.8±1.6 | 93.0±1.5 |
| ta | – | 5.439 | 3.371 | 4.939 |
| P-value | – | <0.0001 | 0.001 | <0.0001 |
| tb | – | 19.995 | 11.506 | 12.649 |
| P-value | – | <0.0001 | <0.0001 | <0.0001 |
| tc | – | 12.956 | 8.864 | 8.940 |
| P-value | – | <0.0001 | <0.0001 | <0.0001 |
Comparison of the fasting blood
glucose and blood glucose at 2 h after meal
There was no significant difference in fasting blood
glucose and blood glucose at 2 h after meal between the two groups
before operation (P>0.05). At one year after operation, the
fasting blood glucose and blood glucose at 2 h after meal in the
two groups were lower than those before operation (P<0.05). In
the recurrence group, the fasting blood glucose was significantly
higher than that in the non-recurrence group (P<0.05), and the
blood glucose at 2 h after meal was also higher than that in the
non-recurrence group (P<0.05; Table
IV).
 | Table IV.Comparison of the levels of fasting
blood glucose and blood glucose at 2 h after meal between two
groups (mmol/l, mean ± SD). |
Table IV.
Comparison of the levels of fasting
blood glucose and blood glucose at 2 h after meal between two
groups (mmol/l, mean ± SD).
| Group | Time | Fasting blood
glucose | Blood glucose at 2
h after meal |
|---|
| Recurrence
group | Before
operation | 16.2±1.7 | 12.0±1.4 |
|
| 1 year after
operation | 8.9±1.1 | 14.2±1.3 |
| Non-recurrence
group | Before
operation | 16.2±1.6 | 12.1±1.4 |
|
| 1 year after
operation | 7.8±1.1 | 5.6±0.9 |
| ta | – | 24.700 | 27.361 |
| P-value | – | <0.0001 | <0.0001 |
| tb | – | 11.012 | 5.911 |
| P-value | – | <0.0001 | <0.0001 |
| tc |
| 14.685 | 23.769 |
| P-value |
| <0.0001 | <0.0001 |
Comparison of the levels of FINS and
HOMA-IR index between the two groups
There was no significant difference in the levels of
FINS and HOMA-IR between the two groups before operation
(P>0.05), and the levels of FINS and HOMA-IR 1 year after
operation were lower than those before operation (P<0.05). In
the recurrence group, the level of FINS was significantly lower
than that in the non-recurrence group, while the HOMA-IR index
level was significantly higher than that in the non-recurrence
group (P<0.05; Table V).
 | Table V.Comparison of the levels of FINS and
HOMA-IR index between the two groups (mean ± SD). |
Table V.
Comparison of the levels of FINS and
HOMA-IR index between the two groups (mean ± SD).
| Group | Time | FINS (mU/l) | HOMA-IR |
|---|
| Recurrence
group | Before
operation | 9.3±0.2 | 1.7±0.03 |
|
| 1 year after
operation | 4.5±0.1 | 1.5±0.02 |
| Non-recurrence
group | Before
operation | 9.3±0.2 | 1.7±0.03 |
|
| 1 year after
operation | 8.4±0.3 | 0.9±0.01 |
| ta | – | 135.765 | 35.082 |
| P-value | – | <0.0001 | <0.0001 |
| tb | – | 15.787 | 160.000 |
| P-value | – | <0.0001 | <0.0001 |
| tc | – | 78.000 | 169.706 |
| P-value | – | <0.0001 | <0.0001 |
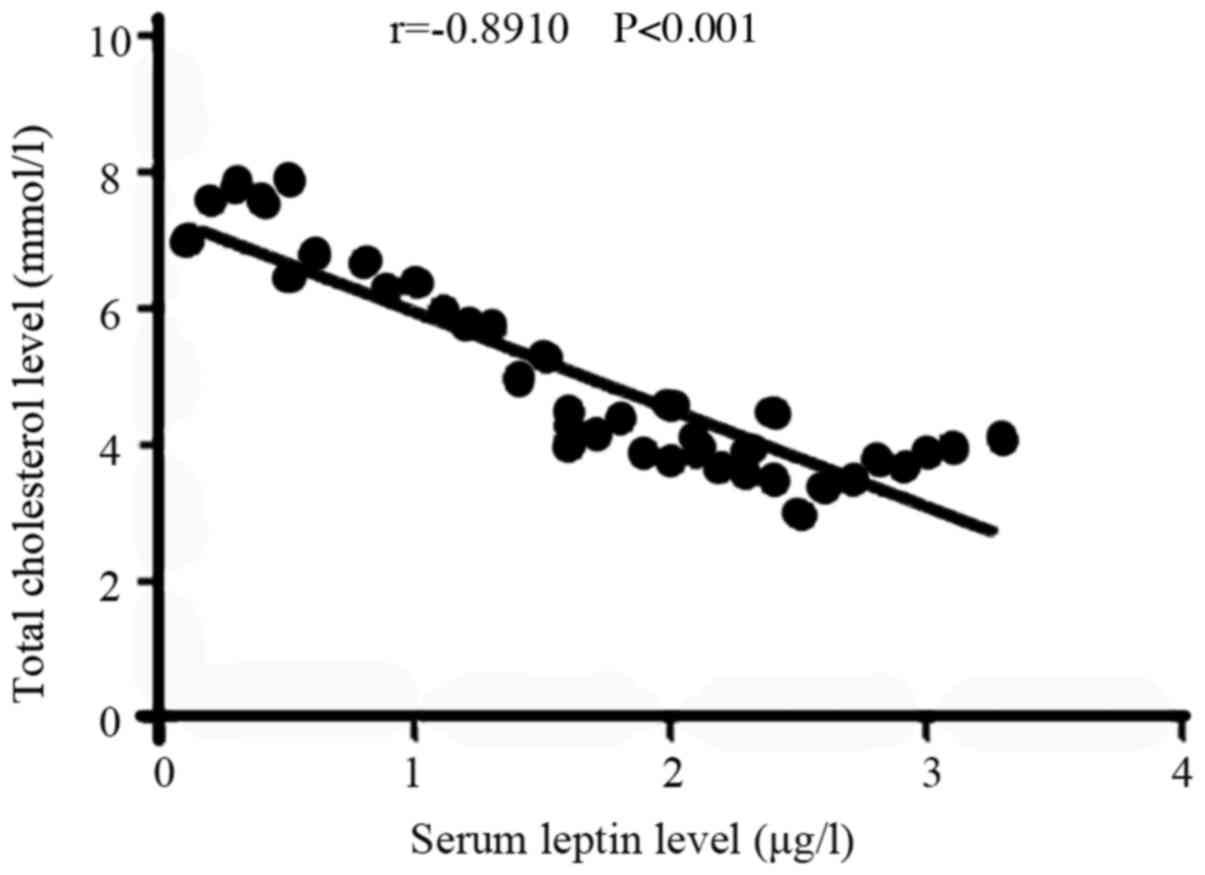
Correlation between the serum leptin
and TC levels in the recurrence group
In the recurrence group, the level of serum leptin
was negatively correlated with that of TC (r=−0.8910, P<0.001;
Fig. 2).
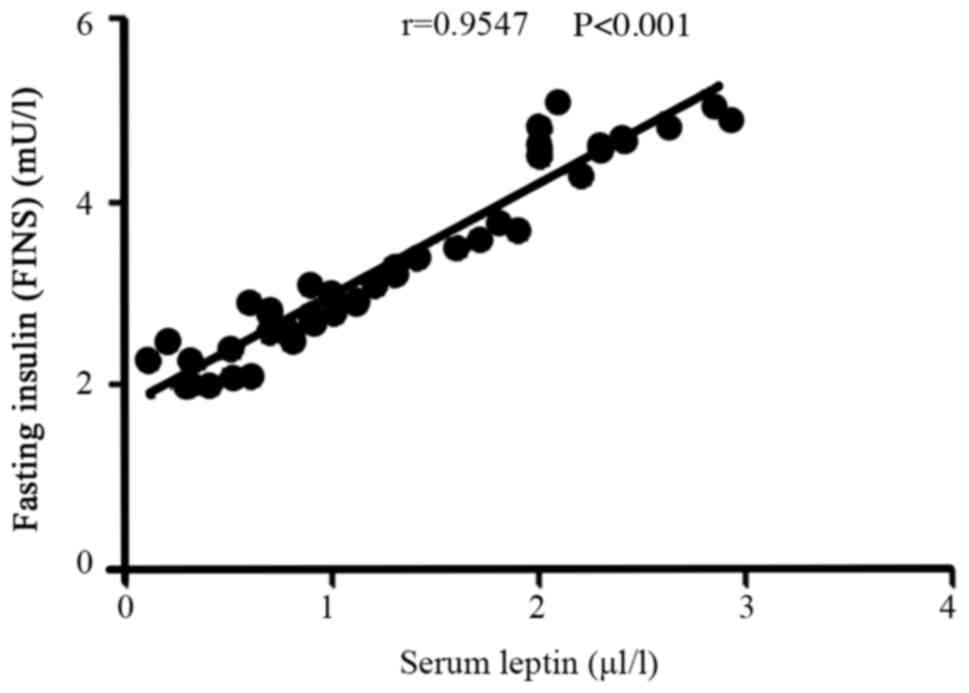
Correlation between the serum leptin
level and FINS level in the recurrence group
In the recurrence group, the level of serum leptin
was positively correlated with the FINS level (r=0.9547,
P<0.001; Fig. 3).
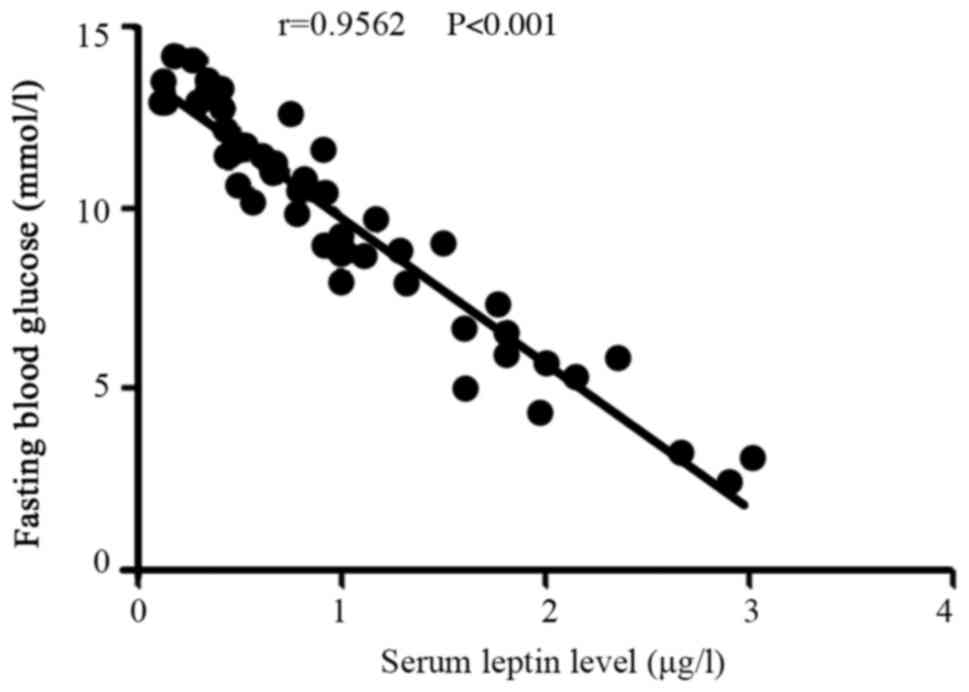
Correlation between the serum leptin
level and the fasting blood glucose in the recurrence group
In the recurrence group, the level of serum leptin
was negatively correlated with the fasting blood glucose level
(r=−0.9562, P<0.001; Fig. 4).
Discussion
Liver is a major organ for energy metabolism. Once
the hepatic functions are damaged, abnormalities will emerge in the
metabolism of blood fat and glucose (12). However, elder patients are more
susceptible to hyperlipidemia, hyperglycemia, hypertension and
chronic obstructive pulmonary disease. Particularly for the elder
hepatic carcinoma patients, their liver functions are significantly
damaged with an obvious decline in compensation function, plus the
adverse effect of surgical treatment, anesthesia and postoperative
chemotherapy, severely compromising the normal functions of the
liver (13). Previous studies
confirmed that in over 90% of hepatic carcinoma patients, the level
of serum leptin is decreased (14)
with the abnormality in metabolism of blood fat and glucose
(15). However, for hepatic
carcinoma patients who experienced recurrence after surgery, there
are few studies reporting the variations in levels of serum leptin,
blood glucose and blood fat. Thus, more studies are required to
attest whether the changes in these indicators are significant.
In this study, all participants were divided into
the recurrence group and non-recurrence group based on the
postoperative recurrence. We found that in the recurrence group,
the levels of serum leptin before, one month and one year after
surgery were significantly lower than those in the non-recurrence
group (P<0.05), suggesting that in the hepatic carcinoma
patients with postoperative recurrence, the serum leptin level was
significantly decreased, thereby remarkably attenuating its
regulatory effect on the energy metabolism. In addition,
comparisons of the indicators of blood fat, BMI, waistline and
hipline one year after surgery in the two groups showed that the
levels of TC, TG and LDL-C in the recurrence group were
significantly higher than those in the non-recurrence group, while
the level of HDL-C was significantly lower than that in the
non-recurrence group; besides, the BMI, waistline and hipline in
the recurrence group was significantly larger than those in the
non-recurrence group; these results suggested that hepatic
carcinoma patients with postoperative recurrence are more
susceptible to the abnormality of blood fat, thereby affecting the
weight and fat distribution. Furthermore, we compared the fasting
blood glucose, blood glucose at 2 h after meal, FINS and HOMA-IR
index, and found that compared with the non-recurrence group, the
levels of fasting blood glucose, blood glucose at 2 h after meal
and HOMA-IR index were significantly higher, and the FINS level was
decreased; these results revealed that in the hepatic carcinoma
patients with postoperative recurrence, the abnormal regulation of
blood glucose will be further exacerbated, making patients more
susceptible to the simultaneous increases in fasting blood glucose
and blood glucose after meal, and frequently complicated with the
abnormality in functions of insulin and emergence of
insulin-resistance. The analysis of correlation of serum leptin
with TC, FINS and fasting blood glucose showed that in the
recurrence group, the level of serum leptin was negatively
correlated with TC and fasting blood glucose levels, and positively
correlated with the FINS level.
In hepatic carcinoma patients, the serum leptin
level is significantly correlated with the metabolism of blood fat
and glucose. Adipose tissue accumulation
(central-visceral-waistline and peripheral-hipline) was negatively
correlated with serum leptin level, and the leptin receptor
sensitivity was significantly reduced in pathological state,
resulting in imbalance of the normal fat-islet axis feedback
mechanism and the accumulation of fat, especially visceral fat
accumulation (16,17). Decrease in serum leptin (18) will result in the decline in appetite
of patients with weight loss. But sufficient nutrition is usually
provided for hepatic carcinoma patients with postoperative
recurrence, and massive intake of nutrients cannot timely
participate in the metabolism (19,20),
which will further lead to the abnormality in blood fat metabolism,
the increase in BMI and accumulation of fat in abdomen and hips
(21), thereby inducing an increase
in blood glucose; besides, the long-term hyperglycemia can further
stimulate the hepatic carcinoma cells, which can induce the
accumulation of nutrients (22),
thicken the basilemma in capillary, and decrease the membrane
permeability (23), thus
facilitating the cell proliferation (24–26), and
further giving rise to the postoperative recurrence.
In conclusion, leptin levels decreased in patients
with postoperative recurrence, and accumulation of visceral adipose
tissue and abnormal blood glucose metabolism also occurred. Serum
leptin level is negatively correlated with total cholesterol level
and fasting blood glucose and positively correlated with FINS
levels in patients with postoperative recurrence. However, more
studies are needed to investigate whether serum leptin is the
relevant factor for hepatic cancer recurrence, and to explore the
prognostic value of serum leptin in hepatic cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by Natural Science
Foundation of Gansu province (grant no. 1308RJZA219).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL drafted the manuscript. SL and LZ collected and
interpreted the data. YZ and JW performed the ELISA experiments. SL
and XY conceived and designed the study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Hospital of Lanzhou University (Lanzhou,
China). Written informed consent was obtained from the patients or
their guardians prior to their inclusion in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee J, Liu K, Stiles B and Ou JJ:
Mitophagy and hepatic cancer stem cells. Autophagy. 14:715–716.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuruvilla SP, Tiruchinapally G, Kaushal N
and El Sayed MEH: Effect of N-acetylgalactosamine ligand valency on
targeting dendrimers to hepatic cancer cells. Int J Pharm.
545:27–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qu YB, Liao ZX, Liu C, Wang XZ and Zhang
J: EFLDO induces apoptosis in hepatic cancer cells by caspase
activation in vitro and suppresses tumor growth in vivo. Biomed
Pharmacother. 100:407–416. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiong F, Zhang F, Jin Y, Weng Q, Song J,
Zhou G, Shin D, Zheng C and Yang X: Orthotopic hepatic cancer:
Radiofrequency hyperthermia-enhanced intratumoral herpes simplex
virus-thymidine kinase gene therapy. Oncotarget. 9:14099–14108.
2017.PubMed/NCBI
|
|
5
|
Pelage JP, Fohlen A, Mitry E, Lagrange C,
Beauchet A and Rougier P: Chemoembolization of neuroendocrine liver
metastases using streptozocin and tris-acryl microspheres: Embozar
(EMBOsphere + ZAnosaR) study. Cardiovasc Intervent Radiol.
40:394–400. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hocine L, Merzouk H, Merzouk SA, Ghorzi H,
Youbi M and Narce M: The effects of alpha-cypermethrin exposure on
biochemical and redox parameters in pregnant rats and their
newborns. Pestic Biochem Physiol. 134:49–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lilienberg E, Dubbelboer IR, Sjögren E and
Lennernäs H: Lipiodol does not affect the tissue distribution of
intravenous doxorubicin infusion in pigs. J Pharm Pharmacol.
69:135–142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao Y, Banerjee S, Huang P, Wang X,
Gladson CL, Heston WD and Foster CB: Selenoprotein P neutralizes
lipopolysaccharide and participates in hepatic cell endoplasmic
reticulum stress response. FEBS Lett. 590:4519–4530. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chaurasia B, Kaddai VA, Lancaster GI,
Henstridge DC, Sriram S, Galam DL, Gopalan V, Prakash KN, Velan SS,
Bulchand S, et al: Adipocyte ceramides regulate subcutaneous
adipose browning, inflammation, and metabolism. Cell Metab.
24:820–834. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hussain A, Yadav MK, Bose S, Wang JH, Lim
D, Song YK, Ko SG and Kim H: Daesiho-tang is an effective herbal
formulation in attenuation of obesity in mice through alteration of
gene expression and modulation of intestinal microbiota. PLoS One.
11:e01654832016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu L, Ren H, Gao G, Zhou L, Malik MA and
Li P: The progress and challenges in metabolic research in China.
IUBMB Life. 68:847–853. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsu YJ, Huang WC, Chiu CC, Liu YL, Chiu
WC, Chiu CH, Chiu YS and Huang CC: Capsaicin supplementation
reduces physical fatigue and improves exercise performance in mice.
Nutrients. 8:pii: E648. 2016. View Article : Google Scholar
|
|
13
|
Li J, Zhou M, Liu F, Xiong C, Wang W, Cao
Q, Wen X, Robertson JD, Ji X, Wang YA, et al: Hepatocellular
carcinoma: Intra-arterial delivery of doxorubicin-loaded hollow
gold nanospheres for photothermal ablation-chemoembolization
therapy in rats. Radiology. 281:427–435. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vardhanabhuti V, Lo AW, Lee EY and Law SY:
Dual-tracer PET/CT using 18F-FDG and 11C-acetate in gastric
adenocarcinoma with liver metastasis. Clin Nucl Med. 41:864–865.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Asperti M, Stuemler T, Poli M, Gryzik M,
Lifshitz L, Meyron-Holtz EG, Vlodavsky I and Arosio P: Heparanase
overexpression reduces hepcidin expression, affects iron
homeostasis and alters the response to inflammation. PLoS One.
11:e01641832016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carey VJ, Walters EE, Colditz GA, Solomon
CG, Willett WC, Rosner BA, Speizer FE and Manson JE: Body fat
distribution and risk of non-insulin-dependent diabetes mellitus in
women. The nurses' health study. Am J Epidemiol. 145:614–619. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kissebah AH, Vydelingum N, Murray R, Evans
DJ, Hartz AJ, Kalkhoff RK and Adams PW: Relation of body fat
distribution to metabolic complications of obesity. J Clin
Endocrinol Metab. 54:254–260. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lim A, Zhou J, Sinha RA, Singh BK, Ghosh
S, Lim KH, Chow PK, Woon EC and Yen PM: Hepatic FTO expression is
increased in NASH and its silencing attenuates palmitic
acid-induced lipotoxicity. Biochem Biophys Res Commun. 479:476–481.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gonzalez FJ, Jiang C and Patterson AD: An
intestinal microbiota-farnesoid X receptor axis modulates metabolic
disease. Gastroenterology. 151:845–859. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Q, Yang F, Li X, Zhang HY, Chu XG,
Zhang H, Wang LW and Gong ZJ: Trichostatin A protects against
intestinal injury in rats with acute liver failure. J Surg Res.
205:1–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Salvia R, D'Amore S, Graziano G,
Capobianco C, Sangineto M, Paparella D, de Bonfils P, Palasciano G
and Vacca M: Short-term benefits of an unrestricted-calorie
traditional Mediterranean diet, modified with a reduced consumption
of carbohydrates at evening, in overweight-obese patients. Int J
Food Sci Nutr. 68:234–248. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lv J, Lv CQ, Wang BL, Mei P and Xu L:
Membrane glycolipids content variety in gastrointestinal tumors and
transplantable hepatomas in mice. Med Sci Monit Basic Res.
22:87–90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang X, Huang J, Xiong H, Zhang K, Chen C,
Wei X, Xu X, Xie Q and Huang R: Anti-tumor effects of the
polysaccharide isolated from tarphochlamys affinis in H22
tumor-bearing mice. Cell Physiol Biochem. 39:1040–1050. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thakkar A, Chenreddy S, Thio A, Khamas W,
Wang J and Prabhu S: Preclinical systemic toxicity evaluation of
chitosan-solid lipid nanoparticle-encapsulated aspirin and curcumin
in combination with free sulforaphane in BALB/c mice. Int J
Nanomed. 11:3265–3276. 2016. View Article : Google Scholar
|
|
25
|
Makary MS, Kapke J, Yildiz V, Pan X and
Dowell JD: Conventional versus drug-eluting bead transarterial
chemoembolization for neuroendocrine tumor liver metastases. J Vasc
Interv Radiol. 27:1298–1304. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Honda T and Inagawa H: Gene expression in
lipopolysaccharide-treated human monocytes following interaction
with hepatic cancer cells. Anticancer Res. 36:3699–3704.
2016.PubMed/NCBI
|