Introduction
Heart failure (HF) is characterized by functional
and structural cardiac abnormalities, progressively leading to
clinical symptoms and signs (1). The
sympathetic nervous system, the rennin-angiotensin-aldosterone
system, the natiuretic peptide system and interactions between
endothelial, monocytes/macrophages and myocardial cells serve
primary roles in HF pathophysiology (2–6). These
can cause endothelial dysfunction, oxidative stress, inflammation,
thrombosis, adverse cardiac remodeling and haemodynamic disorders
(2–6). At present, treatment of HF includes
drug therapy, cardiac resynchronization therapy, left ventricular
assist devices and revascularization, lifestyle interventions
(7). Drug therapy that aims to
adequately decongest the volume overload state consists of
angiotensin converting enzyme inhibitors/angiotensin-II receptor
blockers, mineralocorticoid receptor antagonists, beta-blockers,
vasopressin antagonists, angiotensin receptor-neprilysin inhibitors
and diuretics (8,9). Common adverse effects of
pharmacotherapy include abnormal water homeostasis, worsening
kidney function, electrolyte disturbances and drug-drug
interactions (8,9). Resistance to diuretics and the
associated morbidities have led to the development of effective and
safe treatment strategies that maximize decongestion but minimize
the adverse impact on kidney function (8,9).
Statins are types of lipid regulating medicines.
They lower cholesterol by inhibiting HMG-CoA reductase (10). In addition, statins exhibit various
pleiotropic effects, including improvement in endothelial
dysfunction, increased nitricoxide bioavailability, antioxidant
properties, reduction of blood thrombogenicity, and inhibition of
proinflammatory responses (10–12).
Previous studies have reported that statins are able
to induce left ventricular remodeling and improve cardiac function
in rats with experimental myocardial infarction (13,14). A
number of clinical trials have demonstrated that treatment with
statins improves the prognosis of patients with heart failure (HF)
(15–17). Patients with HF have been reported to
benefit from statin treatment irrespective of cholesterol levels,
atherosclerosis or the root cause of HF (18). However, the exact mechanism for this
is yet to be elucidated. Small GTP-binding protein RhoA and its
downstream signal transduction protein, Rho kinase, participate in
several cell signaling functions, including cell proliferation,
smooth muscle and myocardial contraction, cytoskeleton
re-arrangement, cell migration and proliferation (19). Previous animal studies have indicated
that the RhoA/Rho kinase signaling pathway serves an important role
in left ventricular remodeling of HF (19–21). The
RhoA/Rho-kinase pathway is known to serve a number of important
roles in cellular functions, including contraction, motility
(22), proliferation and apoptosis;
excessive activation of this pathway induces oxidative stress and
increases the risk of cardiovascular diseases (23). In the present study, an
isopropylnoradrenaline (ISO)-induced chronic HF (CHF) rat model was
established to investigate the roles of the RhoA/Rho kinase
signaling pathway in left ventricular remodeling, as well as the
treatment effect of atorvastatin (Ato). The aim of the present
study was to explore the effects and possible mechanisms of Ato as
a preventative measure and treatment for HF to provide a
theoretical basis for its clinical use.
Materials and methods
Animals, grouping and treatment
A total of 95 6–8 week old male Wistar rats (220–250
g) were provided by the Experimental Animal Center of Harbin
Medical University (Harbin, China). Rats were housed at a
temperature of 23±2°C and a humidity of 50%. Animals were subjected
to a 12 h light/dark cycle and standard rat chow and tap water were
available ad libitum. Rats were randomly divided into 2
groups: The control group (C; n=15) and the HF group (n=80). The HF
group was administered with subcutaneous injections of 340 mg/kg
ISO (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) twice with an
interval of 24 h. At 2 months post-injection, a total of 34 rats in
the HF group had survived. HF rats were subsequently randomly
divided into two groups: The ISO + Ato group (n=17; orally
administered with 50 mg/kg Ato dissolved in saline once daily) and
the ISO group (n=17; orally administered with an equal volume of
saline once daily). Ato was supplied by Pfizer, Inc. (New York, NY,
USA). No additional treatments were administered to the C group. A
total of 14 rats in the ISO+Ato group, 11 rats in the ISO group and
15 rats in the C group survived following 4 weeks of continuous
treatment. The animal use protocol has been reviewed and approved
by the Institutional Animal Care and Use Committee of Heilongjiang
Provincial Hospital (Harbin, China).
Echocardiography
A Philips SONOS 7500 ultrasound scanner (Philips
Medical Systems, Inc., Bothell, WA, USA) was used for ECG. Each rat
was anesthetized by an intraperitoneal injection of 30 mg/kg sodium
pentobarbital (Shanghai Yiji Industrial Co., Ltd., Shanghai, China)
and the left ventricular end-diastolic diameter (LVEDD), left
ventricular end-systolic diameter (LVESD) and left ventricular
fractional shortening (FS) were measured. Results were calculated
automatically by the ultrasound system.
Determination of hemodynamics
Hemodynamic detection was performed immediately
following. Following anesthesia of the rats with an intraperitoneal
injection of 30 mg/kg sodium pentobarbital, the neck skin was cut
open and the right common carotid artery was fully exposed
approximately 1–1.5 cm long. Rats did not exhibit symptoms of
peritonitis following the administration of anesthesia. The distal
artery was ligated using a small arterial clip and then the
proximal artery was ligated. The outside of the two arteries were
tied using clips with surgical lines. The ligated arterial segment
was then punctured and fixed with one heparin saline-rinsed
micropressure sensing catheter. The artery clamp was opened and a
catheter was inserted into the left ventricle. A micropressure
sensor (YH type 4B physiological pressure sensor; Beijing Aerospace
Medical Engineering Research Institute, Beijing, China) was used to
determine left ventricular pressure changes at 400Hz. Spectrum
software was used to analyze the left ventricular end diastolic
pressure (LVEDP), the left ventricular end systolic pressure
(LVESP), the maximal left ventricular pressure increase rate
(dp/dtmax) and the maximal left ventricular pressure decrease rate
(dp/dtmin).
Preservation of specimens
Following Echocardiography, 2 ml of blood was
extracted from the right ventricle, centrifuged at 559 × g for 15
min at 4°C and stored at −70°C. After extracting blood, rats were
placed under general anesthesia as aforementioned. Each rat was
euthanized (75–150 mg/kg KCl intravenously into the femoral vein),
Then, the heart was taken out and washed with ice-cold saline. The
left and right atria, large blood vessels and attached connective
tissue were removed, following which the left ventricle was dried
using filter paper and weighed to calculate the left ventricular
mass index (left ventricular weight/body weight). The free wall
myocardial tissue was separated from the left ventricle. One
partial left ventricular tissue was used for morphological
detection. The remainder of the sample was used for molecular
biological tests.
Measurement of total blood
cholesterol
Plasma total cholesterol was determined
enzymatically using commercially available reagent kit according to
the manufacturer's protocol (Siemens Healthineers, Erlangen,
Germany; ADVIA Chemistry CHOL_2; cat. no. 10376501) (24).
Cardiac morphological examination
Left ventricular tissue sections were fixed in 4%
Bouins fluid at room temperature for 24 h, dehydrated, chloroform
transparentized and then immersed in paraffin. The
paraffin-embedded ventricular samples were cut to 5 µm sections for
Masson staining at room temperature for 17–22 min, followed by the
semi-quantitative analysis of collagens using a computer image
analysis system (Three high CMIAS99C multifunctional real color
pathological analysis system; Beijing University of Aeronautics and
Astronautics, Beijing, China). Collagen volume fraction = [area of
collagen under the visual field/(area of collagen + area of
myocardial tissue)].
Reverse transcription polymerase chain
reaction (RT-PCR)
Total RNA was extracted from the myocardial tissue
by the two-step guanidinium isothiocyanate-phenol-chloroform method
(25) and RT-PCR was performed
according to the kit instructions (A3500 reverse transcription kit;
Promega Corporation, Madison, WI, USA). The reaction mixture
comprised primers, mRNA samples, Taq enzyme (Takara Bio, Inc.,
Otsu, Japan) and deionized water to give a final volume of 20 µl.
RT was performed for 40 min at 42°C. The PCR conditions were as
follows: Pre-denaturation at 94°C for 5 min, denaturation at 94°C
for 30 sec, annealing at 60°C for 45 sec and extension at 72°C for
10 min for 35 cycles, followed by an extension step at 72°C for 5
min. Each PCR reaction was repeated at least 3 times. The primers
were synthetized by Shanghai Bioasia Co., Ltd. (Shanghai, China) as
follows: RhoA kinase (244 bp) forward, 5′-ACCAGTTCCCAGAGGTGTATGT-3′
and reverse, 5′-TTGGGACAGAAGTGCTTGACTTC-3′; Rho kinase (512 bp)
forward, 5′-GAGCAACTATGATGTGCCTGAAAAAT-3′ and reverse,
5′-GATGTCGTTTGATTTCTTCTAC-3′; endothelial nitric oxide synthase
(eNOS; 340 bp) forward, 5′-TACCGGCTGCCACCTGATCCTTAA-3′ and reverse,
5′-AACATGTGTCCTTGCTCGAGGCA-3′; β-actin (276 bp) forward,
5′-TCATGCCATCCTGCGTCTG-3′ and reverse, 5′-GCATCGGAACCGCTCATT-3′. A
total of 5 µl of the PCR products were then subjected to 1.5%
agarose gel electrophoresis and analyzed by a gel imaging system
(UV-visible light Gel detection and Imaging System; Kodak,
Rochester, NY, USA) to calculate the ratios of the RhoA/Rho kinase
and eNOS mRNA to β-actin.
Western blot analysis
RhoA and eNOS proteins were extracted from the cell
membrane and cytoplasm as previously described (20,21,26,27). A
total of 100 mg of left ventricular myocardial tissue was
homogenized in 1 ml of lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) and centrifuged at 3,000 × g at 4°C
for 10 min to isolate the RhoA protein (in the supernatant) and the
eNOS protein (in the precipitate; 3,000 × g for 5 min at 4°C),
which were then centrifuged individually. The supernatant
containing the RhoA protein was centrifuged at 3,000 × g for 30 min
at 4°C to separate cell membrane and cytoplasmic proteins. Standard
protein (0.5 mg/ml) with a volume of 0, 1, 2, 4, 8, 12, 16 and 20
µl was added to the standard pore of a 96-well plate. A total of
20, 19, 18, 16, 12, 8, 4 and 0 µl distilled H2O was then
added to each pore with the standard protein. The sample
preparation solution was then added to each well to make 1 µl. A
total of 200 µl BCA working fluid was added to each well and
incubated at 56°C for 30 min. Light density was measured at 562 nm
using an enzyme scale ELIASA. Protein concentration was calculated
based on the standard curve.
The protein solution was adjusted so as to achieve a
consistent protein concentration. Proteins (50 µg) were
subsequently separated by SDS-PAGE using 15% (RhoA) and 5% (eNOS)
isolation gel and 5% spacer gel. Proteins were subsequently
transferred onto a nitrocellulose membrane using the semi-dry
electroblotting method (28), the
membrane was washed with TBS three times for 5 min, cultured in the
blocking solution (98% bovine serum albumin; Sigma-Aldrich; Merck
KGaA) for 30 min and incubated at 37°C for 1 h with the following
primary multiclonal antibodies: Rabbit RhoA (1:500; cat. no.
sant-sc-179; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), eNOS
(1:500; cat. no. FNABP0035; Wuhan Boster Biological Technology,
Ltd., Wuhan, China) and β-actin (1:1,000; cat. no. TA-09; OriGene
Technologies, Inc., Beijing, China) The membrane was subsequently
washed with TBS four times for 5 min and incubated with horseradish
peroxidase-labeled secondary rabbit anti-goat immunoglobulin G
antibodies (1:5,000; cat. no. HS-RG-HRP-100; Hebei Hanlin
Biological Technology, Co., Ltd., China) for 30 min at 37°C. The
membrane was washed with TBST four times for 5 min, TBS twice for 5
min and stained using a Beyo ECL kit (cat. no. P0018; Beyotime
Institute of Biotechnology) for 3–5 min at 37°C, following which
the optical densities were analyzed using an optical density
scanner (Universal Hood II 7218R02319; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Statistical analysis
All data were analyzed using SPSS version 12.0
(SPSS, Inc., Chicago, IL, USA) and expressed as the mean ± standard
deviation. One-way analysis of variance was used for multi-group
comparisons, and a post-hoc q-test was used for further comparison.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Echocardiography
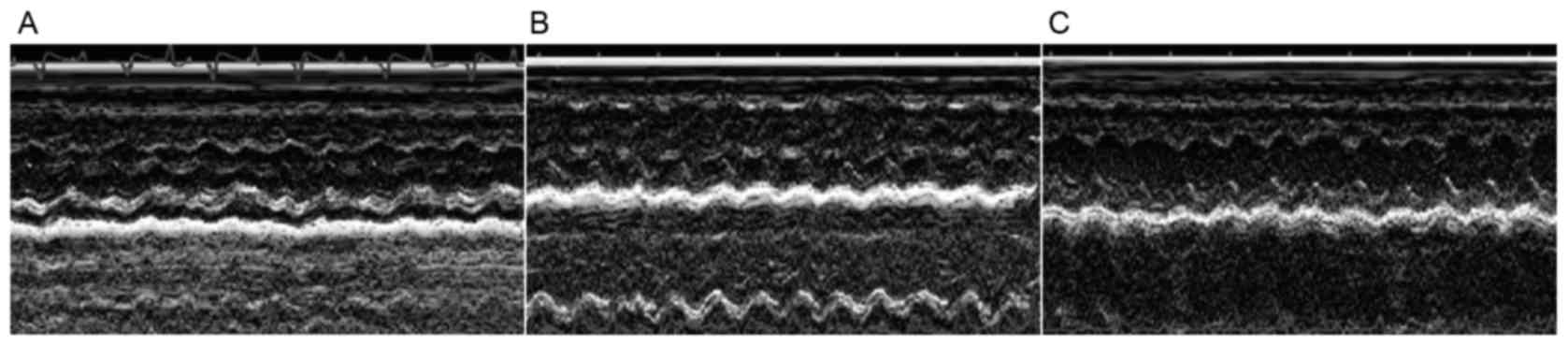
Compared with C group, the LVESD and LVEDD in the
ISO group were significantly increased, whereas the FS was
significantly deceased (P<0.01) (Fig.
1; Table I). The LVESD and LVEDD
were significantly decreased in the ISO+Ato group compared with the
ISO group, while the FS was significantly increased (Fig. 1; Table
I).
 | Table I.Comparison of ultrasound results
among different groups. |
Table I.
Comparison of ultrasound results
among different groups.
| Group | n | LVEDD (mm) | LVESD (mm) | FS |
|---|
| C | 15 | 4.2±0.5 | 2.3±0.4 | 0.46±0.03 |
| ISO | 11 |
6.3±0.5a |
4.8±0.3a |
0.23±0.04a |
| ISO+Ato | 14 |
5.8±0.3a,b |
3.8±0.2a,b |
0.34±0.02a,b |
Hemodynamic indices
Compared with the C group, the LVESP and dp/dtmax in
the ISO group were significantly decreased (P<0.01), whereas the
LVEDP and dp/dtmin were significantly increased (P<0.01)
(Table II). In the ISO+Ato group,
the LVESP and dp/dtmax were significantly increased (P<0.05),
whereas the LVEDP and dp/dtmin was significantly decreased when
compared with the ISO group (P<0.05; Table II).
 | Table II.Hemodynamic results. |
Table II.
Hemodynamic results.
| Group | n | LVESP (mmHg) | LVEDP (mmHg) | dp/dtmax
(mmHg/sec) | dp/dtmin
(mmHg/sec) |
|---|
| C | 15 | 112.6±3.4 | 2.6±0.4 | 11294±796 | −7351±684 |
| ISO | 11 |
96.8±5.1a |
7.2±0.6a |
6879±512a |
−5426±526a |
| ISO+Ato | 14 |
106.3±4.3a,b |
4.3±0.7a,b |
8456±873a,b |
−5986±398a,b |
Left ventricular weight, body weight
and left ventricular mass index
Compared with the C group, left ventricular weight
and left ventricular mass indices in the ISO and ISO+Ato groups
were significantly increased (P<0.01). Compared with the ISO
group, left ventricular weight and left ventricular mass index was
decreased in the ISO+Ato group (P<0.05), whereas there was no
significant difference in the body weight among the three groups
(Table III).
 | Table III.Left ventricular weight, body weight
and left ventricular mass index. |
Table III.
Left ventricular weight, body weight
and left ventricular mass index.
| Group | n | LVW (mg) | BW (g) | LVW/BW (mg/g) |
|---|
| C | 15 | 767±13 | 324±12 | 2.37±0.38 |
| ISO | 11 | 918±17a | 326±17 |
2.80±0.43a |
| ISO+Ato | 14 | 871±20a,b | 323±22 |
2.68±0.48a,b |
Serum lipid level
No significant difference in the total cholesterol
was observed between the ISO and C groups (P>0.05; 1.55±0.084
and 1.54±0.068 mmol/l). Following 4 weeks of Ato treatment, total
cholesterol in the ISO+Ato group was no significantly decreased
compared with the ISO group (P>0.05; Table IV).
 | Table IV.Effects of atorvastatin on serum
TC. |
Table IV.
Effects of atorvastatin on serum
TC.
| Group | n | TC (mmo/l) |
|---|
| C | 15 | 1.54±0.068 |
| ISO | 11 |
1.55±0.084a |
| ISO+Ato | 14 |
1.52±0.066b |
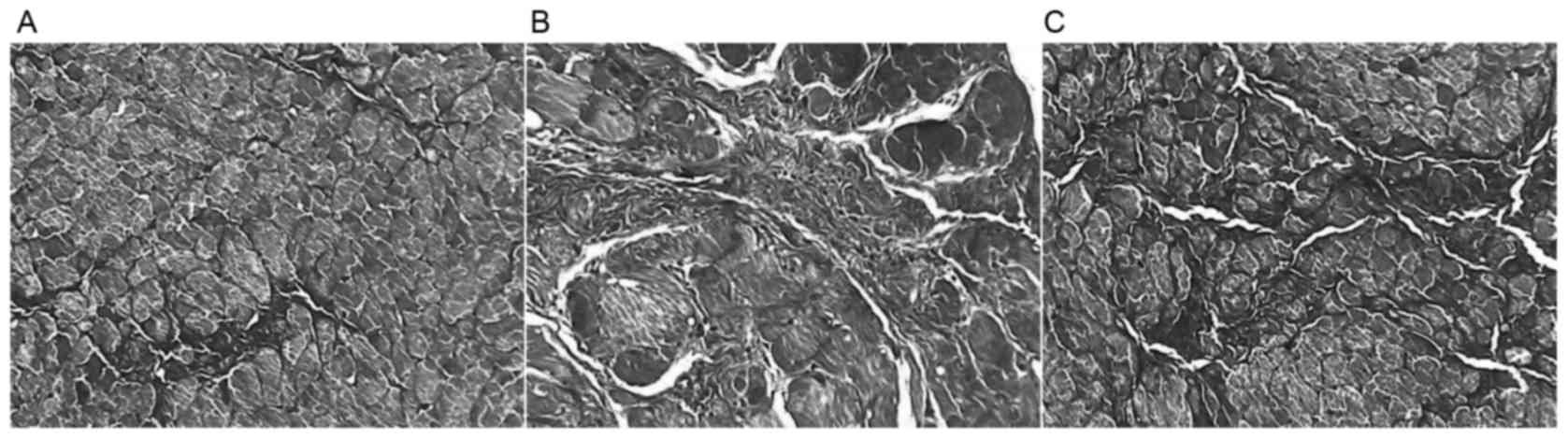
Collagen volume fraction
The collagen volume fraction in group ISO and
ISO+Ato were significantly higher compared with the C group
(6.48±0.61, 4.07±0.17 vs. 1.67±0.19%; P<0.01; Figs. 2 and 3); however, the myocardial collagen volume
fraction in the ISO+Ato group was significantly lower compared with
the ISO group (4.07±0.17 vs. 6.48±0.61%; P<0.05; Figs. 2 and 3).
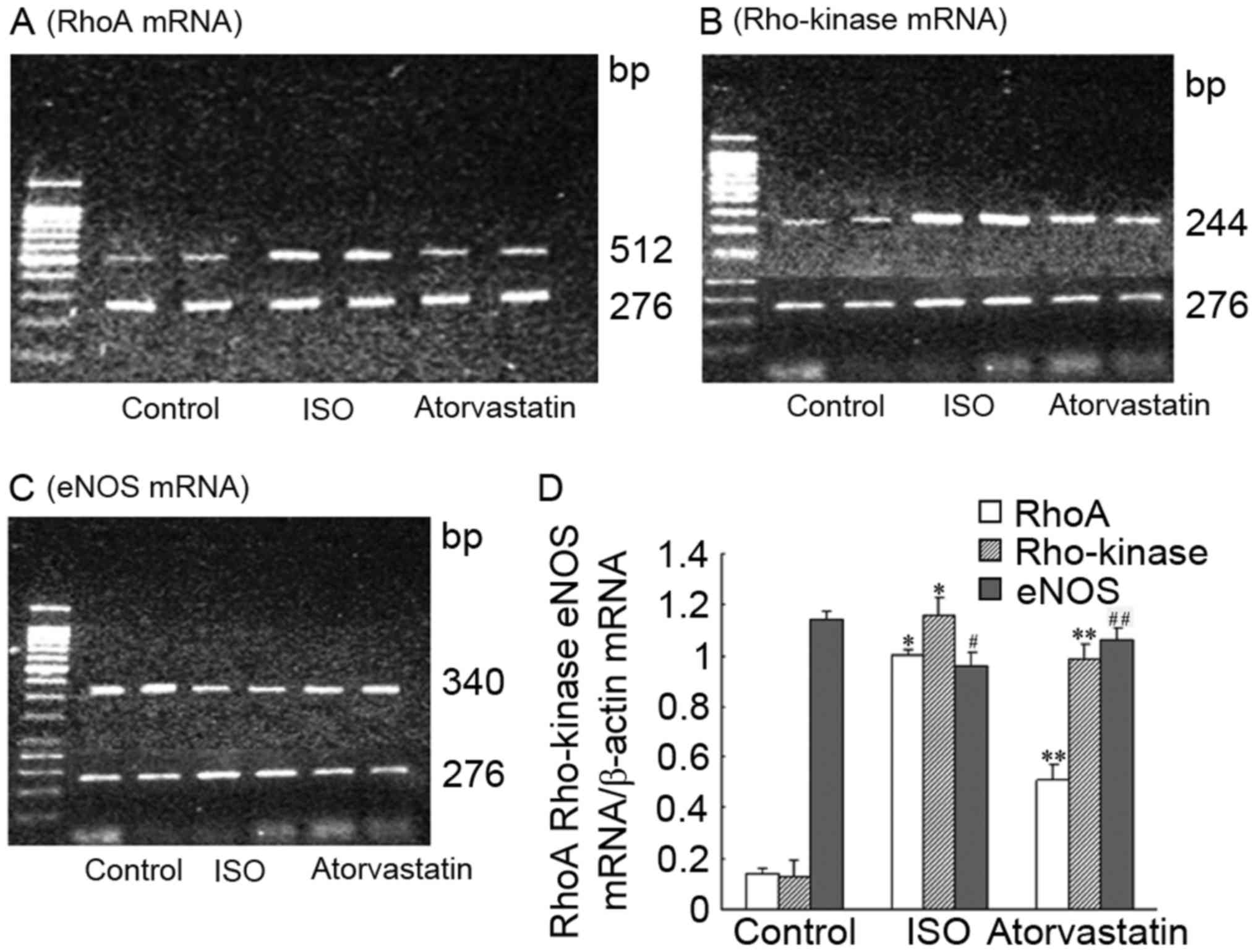
mRNA expression of RhoA/Rho kinase and
eNOS
The results are expressed as the grey value ratios
of RhoA/Rho kinase and eNOS mRNA to β-actin mRNA. The levels of
RhoA/Rho kinase and eNOS mRNA are presented in Fig. 4. Compared with the C group, the
expression of RhoA/Rho kinase mRNA was significantly increased in
the ISO group (P<0.01), whereas the expression of eNOS mRNA was
significantly decreased (P<0.05). Ato is able to significantly
decrease the expression of RhoA/Rho kinase mRNA (P<0.01) and
increase the expression of eNOS mRNA (P<0.05).
Expressions of RhoA and eNOS
protein
Under normal conditions, the RhoA protein is mainly
expressed in the cytoplasm; however, in rats with CHF, expression
is significantly increased (P<0.01) on the myocardial cell
membrane (group ISO). Ato markedly reduces the expression of RhoA
protein on the myocardial cell membrane (Fig. 5A and B). Compared with the C group,
eNOS expression was downregulated in the ISO group and this effect
was markedly reversed by Ato (Fig.
5C).
Discussion
It has been reported that high doses of ISO result
in time and dose-dependent cardiac dysfunction; furthermore, 2-week
application induces an increase in left ventricular filling
pressure, left ventricular hypertrophy and left ventricular
dilatation, thus leading to the decrease of cardiac functions
(29). The results of the present
study indicate that the FS, LVESP and dp/dtmax in the ISO group
were significantly decreased compared with the C group; however,
the LVEDP and dp/dtmin were increased. This suggests that cardiac
functions were decreased in the ISO group; however, the LVEDD,
LVESD, left ventricular weight and left ventricular mass index were
significantly increased, as well as the myocardial collagen volume
fraction, which suggest that rats in the ISO group underwent
cardiac remodeling.
Ato is a lipid-regulating statin that functions by
inhibiting the synthesis of 3-hydroxy-3-methyl glutaryl coenzyme A
reductase (30). However, its
non-lipid-regulating roles have not yet been fully elucidated. The
results of the present study demonstrated that FS, LVESP and
dp/dtmax are significantly increased in rats with CHF following 4
weeks of Ato treatment, whereas the dp/dtmin, LVEDP, LVEDD, LVESD
and left ventricular mass index are significantly decreased. The
myocardial collagen volume fraction was also decreased; however,
there was no significant reduction in blood cholesterol, indicating
that Ato reduces left ventricular remodeling and improves cardiac
function independently of cholesterol.
It was also observed that RhoA mRNA and protein in
the ISO group were significantly upregulated compared with the C
group and the expression of Rho kinase was significantly increased.
This indicates that RhoA activation induces Rho kinase activation,
thus leading to left ventricular remodeling and cardiac
dysfunction. The mRNA and protein expression of RhoA in rats with
CHF was decreased, whereas the mRNA expression of Rho kinase was
downregulated, suggesting that Ato blocks the RhoA/Rho kinase
pathway to improve ventricular remodeling and cardiac
functions.
RhoA belongs to the small GTP-binding protein Rho
family and is normally present in the cytoplasm in its inactive
form, which undergoes isoprenylation due to the action of
geranylgeranyl pyrophosphate (GGPP), is transferred onto the cell
membrane and becomes the active form to serve its biological
functions (19). It serves an
important role in regulating the contraction of vascular smooth
muscle cells and other cellular functions, including cell
proliferation and migration (19).
It has previously been reported that the RhoA/Rho kinase pathway is
associated with hypertensive left ventricular hypertrophy and HF
(21,26,27,31).
Hattori et al (32)
demonstrated that the RhoA kinase pathway is associated with left
ventricular remodeling in rats with experimental myocardial
infarction. Dong et al (33)
used a pressure overload-induced HF rat model to observe the roles
of RhoA/Rho kinase; their results revealed that the RhoA/Rho kinase
pathway participates in the occurrence and development of CHF.
These findings indicate that RhoA and Rho kinases may be associated
with the pathophysiology of cardiac dysfunction and cardiovascular
remodeling, which is in agreement with the findings on the present
study.
The mechanism of RhoA/Rho kinase-induced left
ventricular remodeling in HF has not yet been determined. Kobayashi
et al (21) applied
RhoA-specific inhibitor Y-27632 to treat rats with CHF and
salt-sensitive hypertension. The results indicated that Y-27632
inhibited RhoA, following which the expression of eNOS mRNA and
protein increased, indicating that RhoA/Rho kinase induces left
ventricular remodeling by inhibiting eNOS in the myocardium
(34). Previous studies (35–37) have
demonstrated that eNOS has beneficial effects on ventricular
remodeling and improving cardiac functions. In the present study,
the mRNA and protein expression of eNOS was significantly
downregulated in the ISO group and upregulated following Ato
treatment, indicating that Ato improves left ventricular remodeling
and cardiac functions in rats with CHF by inhibiting the RhoA/Rho
kinase pathway to upregulate eNOS expression.
Statins are able to block
3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, which
reduces the cholesterol synthesis (30) and blocks the production of
isoprenylated products in the mevalonate pathway (38). As RhoA protein can therefore not be
prenylated, a large number of inactive RhoA protein accumulate in
the cytoplasm, extending the half-life of eNOS mRNA (38).
In the present study, the roles of Ato in improving
left ventricular remodeling and cardiac functions in rats with CHF
were not associated with a reduction of blood cholesterol. A
previous study also reported that competitive inhibitors of HMG-CoA
reductase had no effect on blood cholesterol in rats, whereas they
had been demonstrated to significantly reduce blood cholesterol in
other species, including monkeys and humans (39). The reason for this anomaly in rats
has not been fully elucidated and may be associated with the
activity increase of HMG-CoA reductase in rats' livers (35).
In conclusion, the findings of the present study
indicate that Ato improves left ventricular remodeling and cardiac
functions in rats with CHF by inhibiting RhoA/Rho kinase
overexpression in the myocardial tissue, thereby further
upregulating eNOS. Large-scale clinical trials are required to
confirm these results and provide a clinical basis for the use of
Ato as a treatment for CHF.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LA contributed to the conception of the study, and
was a major contributor in writing the manuscript. SA wrote and
revised the manuscript and gave final approval of the version to be
published. ZJ performed the experiments and wrote the manuscript.
HW contributed to the establishmen of animal models and specimen
collection. ZY performed the experiments. CX performed the specimen
collection. XT helped establish the animal models and performed the
experiments. JW contributed to the determination of hemodynamics.
XL performed the data analyses. QC analyzed and interpreted the
data, and SW analyzed the data acquired.
Ethics approval and consent to
participate
The animal use protocol has been reviewed and
approved by the Institutional Animal Care and Use Committee of
Heilongjiang Provincial Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Strandberg TE: Lipid-lowering drugs and
heart failure: Where do we go after the statin trials? Curr Opin
Cardiol. 25:385–393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Volpe M, Carnovali M and Mastromarino V:
The natriuretic peptides system in the pathophysiology of heart
failure: From molecular basis to treatment. Clin Sci (Lond).
130:57–77. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Glezeva N, Horgan S and Baugh JA: Monocyte
and macrophage subsets along the continuum to heart failure:
Misguided heroes or targetable villains? J Mol Cell Cardiol.
89:136–145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Münzel T, Gori T, Keaney JF Jr, Maack C
and Daiber A: Pathophysiological role of oxidative stress in
systolic and diastolic heart failure and its therapeutic
implications. Eur Heart J. 36:2555–2564. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shantsila E, Wrigley BJ, Blann AD, Gill PS
and Lip GY: A contemporary view on endothelial function in heart
failure. Eur J Heart Fail. 14:873–881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brower GL, Gardner JD, Forman MF, Murray
DB, Voloshenyuk T, Levick SP and Janicki JS: The relationship
between myocardial extracellular matrix remodeling and ventricular
function. Eur J Cardiothorac Surg. 30:604–160. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin W: Heart failure, can be very special
(N). Zhongguo yi xue lun tan bao Dec. 16:12–14. 2017.(In
Chinese).
|
|
8
|
Freda BJ, Slawsky M, Mallidi J and Braden
GL: Decongestive treatment of acute decompensated heart failure:
Cardiorenal implications of ultrafiltration and diuretics. Am J
Kidney Dis. 58:1005–1017. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yancy CW, Jessup M, Bozkurt B, Butler J,
Casey DE, Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz
MM, et al: 2016 ACC/AHA/HFSA Focused update on new pharmacological
therapy for heart failure: An update of the 2013 ACCF/AHA Guideline
for the management of heart failure. A report of the American
College of Cardiology/American Heart Association Task Force on
Clinical Practice Guidelines and the Heart Failure Society of
America. Circulation. 134:e282–e293. 2016.PubMed/NCBI
|
|
10
|
Babelova A, Sedding DG and Brandes RP:
Anti-atherosclerotic mechanisms of statin therapy. Curr Opin
Pharmacol. 13:260–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Birnbaum Y and Ye Y: Pleiotropic effect of
statins: The role of eicosanoid production. Curr Atheroscler Aep.
14:135–139. 2012. View Article : Google Scholar
|
|
12
|
Wang CY, Liu PY and Liao JK: Pleiotropic
effects of statin therapy: Molecular mechanisms and clinical
results. Trends Mol Med. 14:37–44. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bauersachs J, Galuppo P, Fraccarollo D,
Christ M and Ertl G: Improvement of left ventricular remodeling and
function by hydroxymethylglutaryl coenzyme a reductase inhibition
with cerivastatin in rats with heart failure after myocardial
infarction. Circulation. 104:982–985. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayashidani S, Tsutsui H, Shiomi T,
Suematsu N, Kinugawa S, Ide T, Wen J and Takeshita A: Fluvastatin,
a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor,
attenuates left ventricular remodeling and failure after
experimental myocardial infarction. Circulation. 105:868–873. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alehagen U, Benson L, Edner M, Dahlström U
and Lund LH: Association between use of statins and mortality in
patients with heart failure and ejection fraction of ≥50. Circ
Heart Fail. 8:862–870. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wojnicz R, Wilczek K, Nowalany-Kozielska
E, Szyguła-Jurkiewicz B, Nowak J, Poloński L, Dyrbuś K, Badziński
A, Mercik G, Zembala M, et al: Usefulness of atorvastatin in
patients with heart failure due to inflammatory dilated
cardiomyopathy and elevated cholesterol levels. Am J Cardiol.
97:899–904. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sola S, Mir MQ, Lerakis S, Tandon N and
Khan BV: Atorvastatin improves left ventricular systolic function
and serum markers of inflammation in nonischemic heart failure. J
Am Coll Cardiol. 47:332–337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Horwich TB, MacLllan R and Fonarow GC:
Statin therapy is associated with improved survival in ischemic and
non-ischemic heart failure. J Am Coll Cardiol. 43:642–648. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ren J and Fang CX: Small guanine
nucleotide-binding protein Rho and myocardial function. Acta
Pharmacologica Sinica. 26:279–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kobayashi N, Horinaka S, Mita S, Nakano S,
Honda T, Yoshida K, Kobayashi T and Matsuoka H: Critical role of
Rho-kinase pathway for cardiac performance and remodeling in
failing rat hearts. Cardiovasc Res. 55:757–767. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kobayashi N, Nakano S, Mita S, Kobayashi
T, Honda T, Tsubokou Y and Matsuoka H: Involvement of Rho-kinase
pathway for angiotensin II-induced plasminogen activator
inhibitor-1 gene expression and cardiovascular remodeling in
hypertensive rats. J Pharmacol Exp Ther. 301:459–466. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Serra N, Rosales R, Masana L and Vallvé
JC: Simvastatin increases Fibulin-2 expression in human coronary
artery smooth muscle cells via RhoA/Rho-Kinase signaling pathway
inhibition. PLoS One. 10:e01338752015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shimokawa H, Sunamura S and Satoh K:
RhoA/Rho-Kinase in the cardiovascular system. Circ Res.
118:352–366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li JZ: Method of determination of blood
lipid recommended by Chinese Society of Laboratory Medicine: Serum
total cholesterol determination by enzymatic method. Zhonghua jian
yan yi xue za zhi. 18:185–187. 1995.(In Chinese).
|
|
25
|
Giovambattista A, Gaillard RC and Spinedi
E: Ghrelin gene-related peptides modulate rat white adiposity.
Vitam Horm. 77:171–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Landmesser U, Engberding N, Bahlmann FH,
Schaefer A, Wiencke A, Heineke A, Spiekermann S, Hilfiker-Kleiner
D, Templin C, Kotlarz D, et al: Statin-induced improvement of
endothelial progenitor cell mobilization, myocardial
neovascularization, left ventricular function, and survival after
experimental myocardial infarction requires endothelial nitric
oxide synthase. Circulation. 110:1933–1939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sauzeau V, Le Jeune H, Cario-Toumaniantz
C, Smolenski A, Lohmann SM, Bertoglio J, Chardin P, Pacaud P and
Loirand G: Cyclic GMP-dependent protein kinase signaling pathway
inhibits RhoA-induced Ca2+ sensitization of contraction in vascular
smooth muscle. J Biol Chem. 275:21722–21729. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fleming JE and Paull TT: Semi-dry
electroblotting of DNA. Biotechniques. 6(926): 928–929. 1988.
|
|
29
|
Chappel GI, Rona G, Balazs T and Gaudry R:
Comparison of cardiotoxic actions of certain sympathomimetic
amines. Can J Biochem Physiol. 37:35–42. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ray S, Jindal AK, Sengupta S and Sinha S:
Statins: Can we advocate them for primary prevention of heart
disease? Med J Armed Forces India. 70:270–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Satoh S, Ueda Y, Koyanagi M, Kadokami T,
Sugano M, Yoshikawa Y and Makino N: Chronic inhibition of Rho
kinase blunts the process of left ventricular hypertrophy leading
to cardiac contractile dysfunction in hypertension-induced heart
failure. J Mol Cell Cardiol. 35:59–70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hattori T, Shimokawa H, Higashi M, Hiroki
J, Mukai Y, Tsutsui H, Kaibuchi K and Takeshita A: Long-term
inhibition of Rho-kinase suppresses left ventricular remodeling
after myocardial infarction in mice. Circulation. 109:2234–2239.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dong M, Liao JK, Yan B, Li R, Zhang M and
Yu CM: A combination of increased Rho kinase activity and
N-terminal pro-B-type natriuretic peptide predicts worse
cardiovascular outcome in patients with acute coronary syndrome.
Int J Cardiol. 167:2813–2819. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mutlu E, İlhan S, Onat E, Kara M and Şahna
E: The effects of novokinin, an AT2 agonist, on blood pressure,
vascular responses, and levels of ADMA, NADPH oxidase, and Rho
kinase in hypertension induced by NOS inhibition and salt. Turk J
Med Sci. 46:1249–1257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mossion PB and Balligand JL: Modulation of
cardiac contraction, relaxation and rate by the endothelial
nitricoxide synthase (eNOS): Lessons from genetically modified
mice. J Physiol. 546:63–75. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Koyanagi M, Egashira K, Kitamoto S, Ni W,
Shimokawa H, Takeya M, Yoshimura T and Takeshita A: Role of
monocyte chemoattractant protein-1 in cardiovascular remodeling
induced by chronic blockade of nitric oxide synthesis. Circulation.
102:2243–2248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Talukder MA, Fujiki T, Morikawa K,
Motoishi M, Matsuo Y, Hatanaka M, Tsutsui M, Takeshita A and
Shimokawa H: Endothelial nitric oxide synthase-independent effects
of an ACE inhibition on coronary flow response to bradykinin in
aged mice. J Cardiovasc Pharmacol. 44:557–563. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Laufs U, Endres M, Stagliano N,
Amin-Hanjani S, Chui DS, Yang SX, Simoncini T, Yamada M, Rabkin E,
Allen PG, et al: Neuroprotection mediated by changes in the
endothelial actin cytoskeleton. J Clin Invest. 106:15–24. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Endo A, Tsujita Y, Kuroda M and Tanzawa K:
Effects of ML-236B on cholesterol metabolism in mice and rats: Lack
of hypocholesterolemic activity in normal animals. Biochim Biophys
Acta. 575:266–276. 1979. View Article : Google Scholar : PubMed/NCBI
|