Introduction
Melanoma is a type of malignant tumor derived from
melanocytes, which are abundant in the skin and are also present in
the mucosa, intestines and eye (1).
In 2015, an estimated 3.1 million cases of melanoma were diagnosed
globally, and 59,800 associated mortalities were registered
(2). Melanoma is the most malignant
tumor type in the skin with a high possibility of distant
metastasis (3). At present, skin
biopsy is widely used for clinical diagnosis. This is usually
followed by an extensive excision of the affected scar or tumor
(4). However, the 5-year survival
rates of patients with melanoma at stage I, II, III and IV are only
94, 44, 38 and 4.6%, respectively (5). Therefore, it is urgent to further
investigate the molecular mechanisms of melanoma in order to
develop novel strategies for its early diagnosis and treatment.
Long non-coding RNAs (lncRNAs) are transcripts of
>200 nucleotides in length that are not translated into protein
(6). An increasing number of studies
confirmed that certain lncRNAs participate in carcinogenesis and
metastasis of melanoma. For instance, long intergenic non-protein
coding RNA 673 has been reported to increase melanoma cell invasion
by upregulating the expression of matrix metalloproteinase 9, and
further affects melanoma survival (7). Furthermore, Wei et al (8) confirmed that urothelial cancer
associated 1 (UCA1) was negatively correlated with microRNA
(miR)-507, while it was positively correlated with forkhead box
(FOX)M1, and that the UCA1/miR-507/FOXM1 axis participates in the
invasion, proliferation and changes in the cell cycle associated
with the pathogenesis of melanoma. In vivo,
metastasis-associated lung adenocarcinoma transcript 1 was also
identified as a critical regulator in melanoma development by
participating in integrin subunit β1 signal activation (9).
lncRNA zinc finger E-box binding homeobox 1 (ZEB1)
antisense RNA 1 (ZEB1-AS1) originates from the promoters of ZEB1.
The molecular mechanisms of action of ZEB1-AS1 have been
investigated in various diseases, including osteosarcoma (10), glioma (11), prostate cancer (12) and non-small cell lung carcinoma
(13). In a previous study, ZEB1-AS1
was confirmed to promote metastasis of hepatocellular tumors and it
was indicated to serve as a biomarker to predict poor prognosis
(14). Furthermore, ZEB1-AS1 was
also confirmed to regulate the level of miR-200s and then
participate in cell proliferation and migration of osteosarcoma
(15).
While its role in the pathogenesis of cancer has
been previously studied, the molecular mechanisms of the roles of
ZEB1-AS1 in melanoma have remained largely elusive. Therefore, the
detailed mechanisms of action of ZEB1-AS1 and its target miRNAs
were herein investigated. The present study may provide novel
biomarkers or targets for the diagnosis and treatment of
melanoma.
Materials and methods
Sample collection
Melanoma and adjacent normal tissues were collected
from 46 melanoma patients (24 males, 22 females; age, 54±13 years)
at Xiangyang Central Hospital, Affiliated Hospital of Hubei
University of Arts and Science, (Xiangyang, China) between February
2013 to April 2016. All tissue samples were obtained and then
frozen and stored in liquid nitrogen at −80°C. According to the
median value of ZEB1-AS1, these samples were divided into a
ZEB1-AS1 high expression group and low expression group. The
present study was approved by the ethics committee of Xiangyang
Central Hospital, Affiliated Hospital of Hubei University of Arts
and Science, (Xiangyang, China) and written informed consent was
obtained by all patients.
Cell lines and culture
A total of 4 human melanoma cell lines, namely
SK-MEL-2, WM35, A375 and SK-MEL-5, were obtained from the Cell Bank
of The Chinese Academy of Sciences (Shanghai, China). Human primary
normal epidermal melanocytes (HEMa; cat. no. PCS-200-013™) were
obtained from the American Type Culture Collection (Manassas, VA,
USA). All of the cell lines were cultured in RPMI-1640 medium
supplemented with fetal bovine serum (10% v/v), penicillin (50
U/ml) and streptomycin (50 U/ml) (all from Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and incubated in a humidified
incubator (37°C and 5% CO2).
Transfection
Specific small interfering (si)RNAs against ZEB1-AS1
(siZEB1-AS1#1, 5′-GGAGGUGACCUACAGUUGAAG-3′; and siZEB1-AS1#2,
5′-CGAGAGACCCUGUCUCAAAUA-3′), the scrambled negative control (siNC,
5′-AAUUCUCCGAACGUGUCACGU-3′), miR-1224-5p mimics
(5′-GUGAGGACUCGGGAGGUGG-3′) and miR-NC
(5′-ACAUCUGCGUAAGAUUCGAGUCUA-3′) were synthesized by Wuhan Genesil
Biotechnology Co., Ltd (Wuhan, China). For transfection,
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) was
used according to the manufacturer's protocol (16).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted and purified from cells,
melanoma tissues and adjacent normal tissues by using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
manufacturer's protocol and as described previously (17). The purity of the total RNA was
confirmed by measuring its absorbance ratio at 260 nm vs. 280 nm
using Nanodrop2000 spectrophotometry (Thermo Fisher Scientific,
Inc.). Total RNA (1 µg) was reverse transcribed into cDNA using the
PrimeScript™ RT reagent kit (Takara Biotechnology Co., Ltd.,
Dalian, China) with gDNA Eraser (Takara Biotechnology Co., Ltd.),
according to the manufacturer's protocol. qPCR was performed using
SYBR select master mix (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The thermocycling
conditions were as follows: Denaturation at 95°C for 10 min;
followed by 40 cycles of denaturation at 95°C for 15 sec and
elongation at 60°C for 1 min. The reaction was performed in an ABI
7500 system (Thermo Fisher Scientific, Inc.) and the gene
expression was calculated using the 2−ΔΔCq method
(18). Small nuclear RNA U6 was used
as the reference. The primer sequences were as follows: U6 forward,
5′-AACGAGACGACGACAGAC-3′ and reverse,
5′-GCAAATTCGTGAAGCGTTCCATA-3′; miR-1224-5p forward,
5′-AACGAGACGACGACAGAC-3′ and reverse, 5′-GTGAGGACTCGGGAGGTGG-3′;
ZEB1-AS1 forward, 5′-GAGAACGTGGTGGAATCAGA-3′ and reverse,
5′-TCCCATCCTCTTTCTTGTCC-3′.
Cell proliferation assay
A Cell Counting Kit-8 (CCK8) was purchased from
Dojindo Laboratories (Kumamoto, Japan) and used to determine the
cell proliferation. Based on the manufacturer's protocol, the
transfected cells were seeded into 96-well plates at a density of
2×103 cells/well and incubated at 37°C for set
time-points (24–96 h, every 24 h). Then cells were incubated with
CCK8 at 37°C for 1 h (19). The
absorbance was detected by a multi-detection microplate reader
(Tecan Group, Ltd, Maennedorf, Switzerland).
Cell invasion and migration assay
For each transfection group, cells were transfected
for 48 h. A serum-free cell suspension was then prepared, which was
seeded into the upper wells of Transwell plates (8 µm pore;
Corning, Incorporated, Corning, NY, USA) with a cell density of
2×104 cells/well. RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum was added to
the lower chamber. Subsequently, the plate was incubated at 37°C
for 24 h. The cells that had transgressed to the lower side of the
membrane were then fixed with 4% formaldehyde for 5 min at 25°C,
stained with 0.1% crystal violet for 10 min at 25°C, and images of
5 randomly selected fields of view were captured under an inverted
microscope. For the invasive ability assay, the
Matrigel® (1:10 dilution in 100 µl; Corning,
Incorporated) was evenly spread in the upper chambers of the
Transwell insert, followed by solidification in an incubator at
37°C for 1 h. The remaining steps were identical to those for the
migration assay.
Luciferase reporter assay
By using a bioinformatics analysis (miRDB tool;
http://mirdb.org/miRDB/index.html),
miR-1224-5p was identified as a putative target of ZEB1-AS1. The
wild-type (WT) and mutant-type (Mut) miR-1224-5p binding sequences
of ZEB1-AS1 (synthesized by Sangon Biotech Co., Ltd., Shanghai,
China) were respectively inserted into the luciferase reporter
vector pmirGLO (Promega Corp., Madison, WI, USA). For the
luciferase reporter assay, miR-NC or miR-1224-5p mimics and
reporter plasmid were co-transfected into 2×104 A375
cells using Lipofectamine 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). After transfection for 48 h, the
luciferase activity of the sample was detected using the
Dual-Luciferase Reporter Assay System (E1910; Promega Corp.)
according to the manufacturer's protocols with normalization to the
luciferase activity of Renilla.
Statistical analysis
Values are expressed as the mean ± standard
deviation. SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA) was
used for statistical analysis. Student's t-test (for two-group
comparisons) and one-way analysis of variance followed by Tukey's
post-hoc test (for multiple-group comparisons) were performed. The
association between clinicopathological features and ZEB1-AS1
expression was analyzed using the Chi-squared test. The association
between ZEB1-AS1 levels and overall survival was estimated using
the Kaplan-Meier method, and differences between groups were
evaluated using the log-rank test. P<0.05 was considered to
indicate a statistically significant difference.
Results
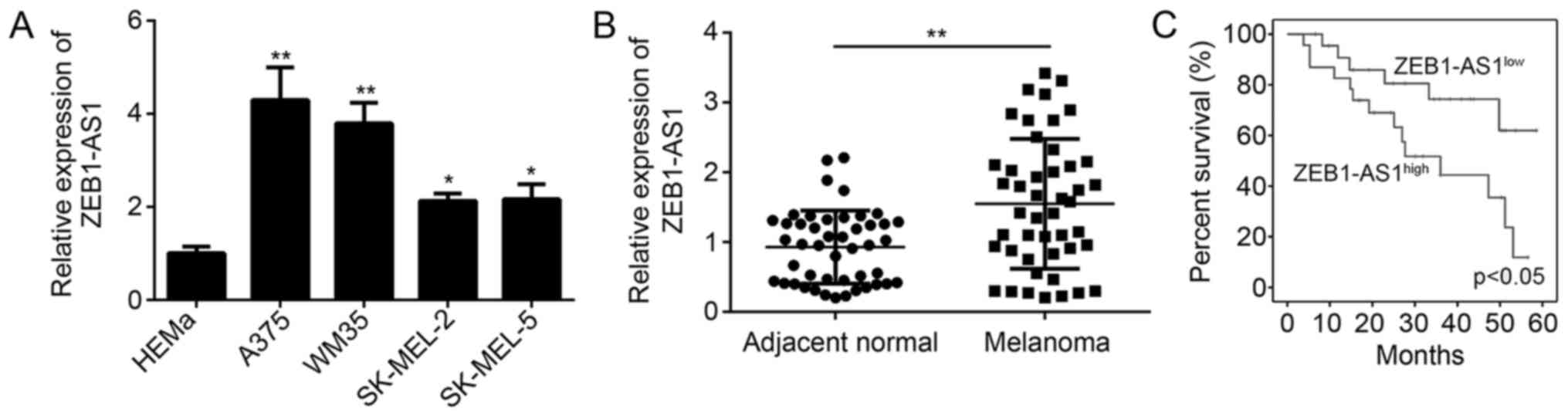
ZEB1-AS1 is increased in melanoma
In the present study, ZEB1-AS1 expression levels
were determined in melanoma cells and tissues by using RT-qPCR. The
results confirmed that ZEB1-AS1 was significantly upregulated in
melanoma cell lines, including A375, WM35, SK-MEL-2 and SK-MEL-5
cells, compared with that in normal HEMa cells (Fig. 1A). The relative expression levels of
ZEB1-AS1 were highest in the A375 cell line (Fig. 1A). Compared with the expression in
adjacent normal tissues, ZEB1-AS1 was significantly upregulated in
melanoma tissues (P<0.05; Fig.
1B). In addition, Kaplan-Meier analysis confirmed that higher
levels of ZEB1-AS1 (The median value of ZEB1-AS1 was used as cutoff
to divide samples into two subgroups) expression predict poor
overall survival of patients with melanoma (Fig. 1C). These results suggested that
ZEB1-AS1 was upregulated in melanoma tissues and that the
expression level is significantly associated with overall survival.
The association between clinicopathological characteristics and
ZEB1-AS1 expression in malignant melanoma tissues is presented in
Table I. The high expression of
ZEB1-AS1 was positively associated with advanced TNM stage and
lymph node metastasis (Table I),
indicating that ZEB1-AS1 may promote the progression of
melanoma.
 | Table I.Association between
clinicopathological features and zinc finger E-box binding homeobox
1 antisense RNA 1 expression (low vs. high) in malignant melanoma
tissues. |
Table I.
Association between
clinicopathological features and zinc finger E-box binding homeobox
1 antisense RNA 1 expression (low vs. high) in malignant melanoma
tissues.
| Feature | Low (n=23) | High (n=23) | P-value |
|---|
| Age (years) |
|
| 0.189 |
| ≤60 | 14 | 19 |
|
|
>60 | 9 | 4 |
|
| Sex |
|
| 0.768 |
| Male | 13 | 11 |
|
|
Female | 10 | 12 |
|
| TNM stage |
|
| 0.037 |
| I+II | 16 | 8 |
|
|
III+IV | 7 | 15 |
|
| Lymph node
metastasis |
|
| 0.047 |
| No | 15 | 5 |
|
| Yes | 8 | 18 |
|
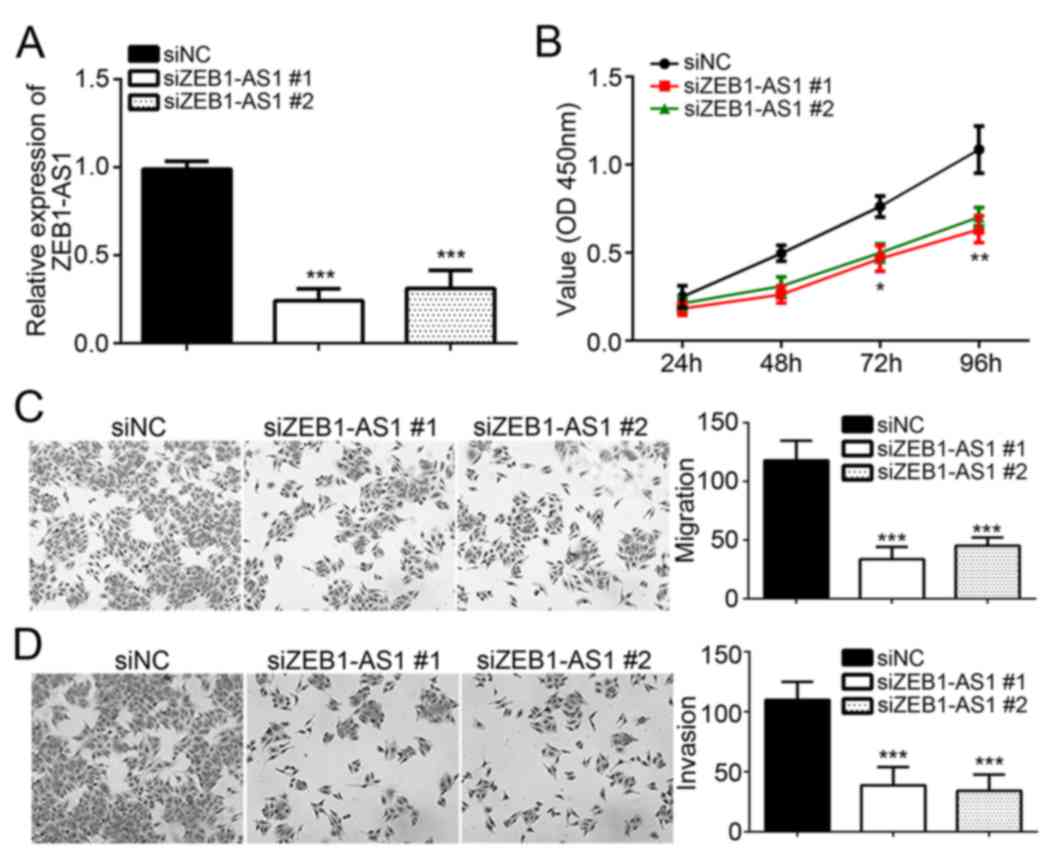
Knockdown of ZEB1-AS1 inhibits
melanoma cell proliferation, invasion and migration
To assess the roles of ZEB1-AS1 in melanoma,
specific siRNAs targeting ZEB1-AS1 (siZEB1-AS1#1 and siZEB1-AS1#2)
and siNC were individually transfected into the A375 cell line, and
the knockdown efficiency was confirmed by RT-qPCR (Fig. 2A). The CCK8 assay was then performed
to assess the effect of ZEB1-AS1 knockdown on melanoma cell
proliferation. Compared with that in the siNC group, knockdown of
ZEB1-AS1 significantly decreased the viability of A375 cells
(Fig. 2B), indicating that ZEB1-AS1
has a role in melanoma cell proliferation. Furthermore, Transwell
assays suggested that knockdown of ZEB1-AS1 significantly inhibited
the migration and invasion of A375 cells (P<0.05; Fig. 2C and D). These results demonstrate
that ZEB1-AS1 promotes the malignant behavior of melanoma
cells.
miR-1224-5p is directly regulated by
ZEB1-AS1
A search with a bioinformatics tool indicated that
miR-1224-5p is a target of ZEB1-AS1 and the corresponding binding
sites were determined (Fig. 3A). A
luciferase activity assay was then employed to verify this
interaction in vitro. A375 cells were co-transfected with
miR-1224-5p mimics and ZEB1-AS1-WT or ZEB1-AS1-Mut recombinant
luciferase reporter plasmids. The results indicated that
miR-1224-5p mimics had no significant effect on the luciferase
activity in the mutant ZEB1-AS1-Mut/miR-1224-5p plasmid group;
however, the luciferase activity in the ZEB1-AS1-WT/miR-1224-5p
reporter plasmid group was significantly reduced by ~70% (Fig. 3B). In another experiment,
transfection of miR-1224-5p mimics reduced the levels of ZEB1-AS1
(Fig. 3C), while knockdown of
ZEB1-AS1 increased the levels of miR-1224-5p (Fig. 3D). Furthermore, a negative
correlation between the levels of miR-1224-5p and ZEB1-AS1 in
melanoma tissues was identified (Fig.
3E). All of these results indicated that ZEB1-AS1 inhibits
miR-1224-5p in melanoma.
ZEB1-AS1 has a role in melanoma
development by regulating miR-1224-5p
To determine whether ZEB1-AS1 promotes melanoma
development via regulating miR-1224-5p, the effects of miR-1224-5p
mimics on melanoma cell proliferation, invasion and migration were
assessed using CCK8 and Transwell assays. The results confirmed
that miR-1224-5p levels were low in melanoma vs. normal adjacent
tissues (Fig. 4A). The successful
transfection of miR-1224-5p mimics was confirmed by RT-qPCR
(Fig. 4B). The results indicated
that miR-1224-5p mimics inhibited processes of melanoma development
and progression by suppressing cell proliferation, migration and
invasion (P<0.05; Fig. 4C-E).
Discussion
In the present study, the role of lncRNA ZEB1-AS1 in
the pathogenesis of melanoma and the underlying molecular
mechanisms were investigated. The results revealed that a negative
correlation between lncRNA ZEB1-AS1 levels and the overall survival
rate of melanoma patients. Furthermore, miR-1224-5p mimics reduced
the levels of ZEB1-AS1, while knockdown of ZEB1-AS1 significantly
increased the levels of miR-1224-5p, and a direct regulatory
interaction was identified between ZEB1-AS1 and miR-1224-5p. Of
note, transfection with miR-1224-5p mimics or with siZEB1-AS1
inhibited the proliferation, invasion and migration of melanoma
cells.
ZEB1-AS1 may serve as a biomarker or target for
cancer diagnosis or treatment, respectively, and dysregulation of
ZEB1-AS1 may be a critical step in tumorigenesis and cancer
progression with a potential clinical application (20). For instance, ZEB1-AS1 has an
important role in the pathogenesis of bladder cancer by promoting
proliferation and inhibiting apoptosis (21). Of note, downregulation of ZEB1-AS1
caused an increase in B-cell lymphoma 2 (Bcl-2)-associated X
protein and a decrease in Bcl-2 expression to induce apoptosis
(11). ZEB1-AS1 was also confirmed
to be closely associated with metastasis and with a poor prognosis
for gastric cancer patients (22).
Consistent with these results, the present study indicated that the
survival rate of melanoma patients with lower ZEB1-AS1 was higher.
Collectively, it was suggested that ZEB1-AS1 has an important role
in the genesis and progression of melanoma.
In addition to the direct regulation of miR-1224-5p,
ZEB1-AS1 has been previously reported to regulate a number of
further miRs in various diseases. In the pathogenesis of
osteosarcoma, ZEB1-AS1 and miR-200s were indicated to have a
negatively regulatory association, and dysregulation of ZEB1-AS1 to
affect miR-200s contributed to the development of this disease
(23). Furthermore, Zhang et
al (24) reported that ZEB1-AS1
inhibits the levels of miR-335-5P to promote gastric cancer cell
invasion and proliferation in vitro. Wei et al
(25) reported that ZEB1-AS1
increased glioma cell migration, proliferation and invasion by
regulating the expression of miR-577. All of these studies offered
potential targets for disease treatment. Similarly, in the present
study, ZEB1-AS1 negatively regulated the expression of miR-1224-5p
to then affect the proliferation, invasion and migration of
melanoma. In malignant gliomas, miR-1224-5p was previously reported
to inhibit the expression of cyclic AMP responsive element binding
protein 1 and reduce its tumor-promoting activity, including
invasion, proliferation and apoptosis (26). Qian et al (27) also indicated that miR-1224-5p
inhibits the proliferation of glioma cells, and the expression
levels were associated with its clinical prognosis and grading of
this disease. Of note, overexpression of miR-1224-5pM was confirmed
to lead to suppression of keloid fibroblast proliferation, as well
as a decrease of invasion and migration, and promotion of apoptosis
in keloid fibroblasts via the transforming growth factor-β1/Smad3
signaling pathway (28). In the
present study, based on bioinformatics predictions and in
vitro experiments, we a negative regulatory interaction between
ZEB1-AS1 and miR-1224-5p was discovered. Furthermore, knockdown of
ZEB1-AS1 and transfection with miR-1224-5p mimics inhibited the
proliferation, invasion and migration of melanoma. However, to
better validate that ZEB1-AS1 regulates melanoma progression by
inhibiting miR-1225-4p, a rescue assay by simultaneous transfection
with siRNA targeting ZEB1-AS1 and miR-1224-55 mimics should be
performed in the future.
In conclusion, the present study indicated that
ZEB1-AS1 enhanced the proliferation, invasion and migration of
melanoma cells by directly inhibiting miR-1224-5p, and
overexpression of ZEB1-AS1 was also associated with a decrease in
the overall survival rate of melanoma patients. These results
indicated that ZEB1-AS1 and miR-1224-5p have a critical role in the
pathogenesis of melanoma and may serve as a predictive biomarker
and a potential target for melanoma treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
QW and DL initiated and designed the present study,
analyzed the data, interpreted the results and wrote the
manuscript. RZ performed several experiments. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Regarding the use of human samples, the protocol of
the present study was approved by the Institutional Ethics
Committee of Xiangyang Central Hospital, Affiliated Hospital of
Hubei University of Arts and Science (Xiangyang, China) and all
enrolled patients signed a written informed consent document.
Patient consent for publication
All patients within the present study provide
consent for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Levin DB, Wilson K, Valadares de Amorim G,
Webber J, Kenny P and Kusser W: Detection of p53 mutations in
benign and dysplastic nevi. Cancer Res. 55:4278–4282.
1995.PubMed/NCBI
|
|
2
|
Wallack MK, Sivanandham M, Balch CM, Urist
MM, Bland KI, Murray D, Robinson WA, Flaherty LE, Richards JM,
Bartolucci AA, et al: A phase III randomized, doúble-blind,
multiinstitutional trial of vaccinia melanoma oncolysate-active
specific immunotherapy for patients with stage II melanoma. Cancer.
75:34–42. 2015. View Article : Google Scholar
|
|
3
|
Busam KJ, Berwick M, Blessing K, Fandrey
K, Kang S, Karaoli T, Fine J, Cochran AJ, White WL, Rivers J, et
al: Tumor vascularity is not a prognostic factor for malignant
melanoma of the skin. Am J Pathol. 147:1049–1056. 1995.PubMed/NCBI
|
|
4
|
Surhone LM, Tennoe MT and Henssonow SF:
Treatment and prognosis of melanoma. Betascript Publishing.
2010.
|
|
5
|
Balch CM, Murad TM, Soong SJ, Ingalls AL,
Halpern NB and Maddox WA: A multifactorial analysis of melanoma:
Prognostic histopathological features comparing Clark's and
Breslow's staging methods. Ann Surg. 188:732–742. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yarmishyn AA and Kurochkin IV: Long
noncoding RNAs: A potential novel class of cancer biomarkers. Front
Genet. 6:1452015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmidt K, Joyce CE, Buquicchio F, Brown
A, Ritz J, Distel RJ, Yoon CH and Novina CD: The lncRNA SLNCR1
mediates melanoma invasion through a conserved SRA1-like Region.
Cell Rep. 15:2025–2037. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei Y, Sun Q, Zhao L, Wu J, Chen X, Wang
Y, Zang W and Zhao G: LncRNA UCA1-miR-507-FOXM1 axis is involved in
cell proliferation, invasion and G0/G1 cell cycle arrest in
melanoma. Med Oncol. 33:882016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yong S, Cheng H, Wang G, Yu G, Zhang D,
Wang Y, Fan W and Yang W: Deregulation of miR-183 promotes melanoma
development via lncRNA MALAT1 regulation and ITGB1 signal
activation. Oncotarget. 8:3509–3518. 2017.PubMed/NCBI
|
|
10
|
Liu C and Lin J: Long noncoding RNA
ZEB1-AS1 acts as an oncogene in osteosarcoma by epigenetically
activating ZEB1. Am J Transl Res. 8:4095–4105. 2016.PubMed/NCBI
|
|
11
|
Lv QL, Hu L, Chen SH, Sun B, Fu ML, Qin
CZ, Qu Q, Wang GH, He CJ and Zhou HH: A long noncoding RNA ZEB1-AS1
promotes tumorigenesis and predicts poor prognosis in Glioma. Int J
Mol Sci. 17(pii): E14312016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Su W, Xu M, Chen X, Chen N, Gong J, Nie L,
Li L, Li X, Zhang M and Zhou Q: Long noncoding RNA ZEB1-AS1
epigenetically regulates the expressions of ZEB1 and downstream
molecules in prostate cancer. Mol Cancer. 16:1422017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li L, Wang QF, Zou ML, He XA and Lv JJ:
Overexpressed lncRNA ZEB1-AS1 promotes cell invasion and
angiogenesis through Wnt/β-catenin signaling in non-small cell lung
cancer. Int J Clin Exp Patho. 10:3990–3997. 2017.
|
|
14
|
Li T, Xie J, Shen C, Cheng D, Shi Y, Wu Z,
Deng X, Chen H, Shen B, Peng C, et al: Upregulation of long
noncoding RNA ZEB1-AS1 promotes tumor metastasis and predicts poor
prognosis in hepatocellular carcinoma. Oncogene. 35:1575–1584.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu B, Ye B, Yang L, Zhu X, Huang G, Zhu
P, Du Y, Wu J, Qin X, Chen R, et al: Long noncoding RNA lncKdm2b is
required for ILC3 maintenance by initiation of Zfp292 expression.
Nat Immunol. 18:499–508. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dalby B, Cates S, Harris A, Ohki EC,
Tilkins ML, Price PJ and Ciccarone VC: Advanced transfection with
Lipofectamine 2000 reagent: primary neurons, siRNA, and
high-throughput applications. Methods. 33:95–103. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Dong Y, Wang X, Ma H, Sheng Z, Li
G, Lu G, Sugimura H and Zhou X: Expression of EphA1 in gastric
carcinomas is associated with metastasis and survival. Oncol Rep.
24:1577–1584. 2010.PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiao B, Zhu ED, Li N, Lu DS, Li W, Li BS,
Zhao YL, Mao XH, Guo G, Yu PW and Zou QM: Increased miR-146a in
gastric cancer directly targets SMAD4 and is involved in modulating
cell proliferation and apoptosis. Oncol Rep. 27:559–566.
2012.PubMed/NCBI
|
|
20
|
Li J, Li Z, Leng K, Xu Y, Ji D, Huang L,
Cui Y and Jiang X: ZEB1-AS1: A crucial cancer-related long
non-coding RNA. Cell Prolif. 51:2018.doi: 10.1111/cpr.12423.
View Article : Google Scholar
|
|
21
|
Lin J, Zhan Y, Liu Y, Chen Z, Liang J, Li
W, He A, Zhou L, Mei H, Wang F and Huang W: Increased expression of
ZEB1-AS1 correlates with higher histopathological grade and
promotes tumorigenesis in bladder cancer. Oncotarget.
8:24202–24212. 2017.PubMed/NCBI
|
|
22
|
Liu XJ, Li SL, Li JS, Lu H, Yin LL, Zheng
WF and Wang WC: Long non-coding RNA ZEB1-AS1 is associated with
poor prognosis in gastric cancer and promotes cancer cell
metastasis. Eur Rev Med Pharmacol Sci. 22:2624–2630.
2018.PubMed/NCBI
|
|
23
|
Liu C, Pan C, Cai Y and Wang H: Interplay
between long noncoding RNA ZEB1-AS1 and miR-200s regulates
osteosarcoma cell proliferation and migration. J Cell Biochem.
118:2250–2260. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang LL, Zhang LF, Guo XH, Zhang DZ, Yang
F and Fan YY: Downregulation of miR-335-5p by long noncoding RNA
ZEB1-AS1 in gastric cancer promotes tumor proliferation and
invasion. DNA Cell Biol. 37:46–52. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei N, Wei H and Zhang H: Long non-coding
RNA ZEB1-AS1 promotes glioma cell proliferation, migration and
invasion through regulating miR-577. Eur Rev Med Pharmacol Sci.
22:3085–3093. 2018.PubMed/NCBI
|
|
26
|
Jin Q, Rui L, Wang YY, Shi Y, Luan WK, Tao
T, Zhang JX, Xu YC and You YP: MiR-1224-5p acts as a tumor
suppressor by targeting CREB1 in malignant gliomas. Mol Cell
Biochem. 403:33–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qian J, Li R, Wang YY, Luan WK, Tao T,
Zhang JX, Xu YC and You YP: MiR-1224-5p acts as a tumor suppressor
by targeting CREB1 in malignant gliomas. Mol Cell Biochem.
403:33–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yao X, Cui X, Wu X, Xu P, Zhu W, Chen X
and Zhao T: Tumor suppressive role of miR-1224-5p in keloid
proliferation, apoptosis and invasion via the TGF-β1/Smad3
signaling pathway. Biochem Biophys Res Commun. 495:713–720. 2018.
View Article : Google Scholar : PubMed/NCBI
|