Introduction
When treating certain types of vascular disease, it
is appropriate to perform parent vessel occlusion (PVO) of the
diseased area. PVO may be used as a pre-operative procedure prior
to tumor resection for patients suffering from neck or skull-base
tumors (1,2). In such cases, internal carotid artery
(ICA) endovascular occlusion is a necessary pre-operative maneuver.
However, as there is a significant risk of ischemic events
associated with ICA occlusion, patient selection is crucial.
Thromboembolic and hemodynamic ischemia are the major complications
associated with PVO (3). Therefore,
it is crucial to identify patients that are likely to develop
hemodynamic ischemia prior to PVO. A previous study demonstrated
that temporary balloon occlusion of the ICA can be used to identify
patients at risk for stroke during carotid artery sacrifice
(4). The temporary balloon occlusion
test (BOT) is useful in evaluating ischemic risk prior to permanent
ICA occlusion (5). During the BOT,
several methods may be used to assess the clinical tolerance of
PVO, which include clinical examination assessment, venous phase
delay assessment, stump pressure assessment, perfusion scanning
assessment and neurophysiological monitoring (3). The clinical examination assessment is
the most useful and broadly used method to determine whether a
patient is able to tolerate PVO (6).
There are several types of BOT assessment each with various
advantages and disadvantages. For example, the CT perfusion method
involves knowledge of image post-processing. This procedure also
involves the transfer of the patient to the CT suite with a balloon
catheter in the carotid artery, which may increase the risk of
carotid artery injury (3). Venous
phase delay assessment is a simple angiographic BOT criterion. To
investigate the utility of venous phase delay assessment in
evaluating the results from the BOT, the clinical examination
assessment and the venous phase delay assessment during BOT of the
ICA.
Materials and methods
Patients
A total of 38 patients without contraindications
underwent a BOT of the ICA between January 2012 and July 2016 at
The Second Xiangya Hospital of Central South University (Changsha,
China). Clinical examinations and venous phase assessments were
performed. Patients with a head and neck tumor or traumatic carotid
cavernous fistulae were included in the study. The present study
was approved by the Ethics Committee of the Second Xiangya Hospital
of Central South University (Changsha, China). All patients
provided informed consent.
BOT method
A 6-F femoral sheath was introduced into the femoral
artery using the single-wall puncture technique. Cervical and
cerebral angiograms were recorded with 4-F diagnostic catheters in
the anteroposterior and lateral projection, respectively. Each
patient was given 70–100 units per kg of heparin prior to the
angiogram. Subsequently, a 6-F guiding catheter was positioned in
the common carotid artery. A non-detachable 4×15 mm silicon balloon
catheter (cat. no. BCo415C; MicroVention Medical Technology, Co.,
Ltd., Hang Zhou, China) was positioned in the petrous ICA. After
the diseased vessel was completely filled, the balloon was inflated
for a total of 30 min. During the 30 min procedure, a neurological
examination was performed every 5 min (3). The patient was asked a series of
questions to assess their motor, sensory, speech and memory
capacity, as well as their analytical and/or calculation skills. If
any neurological deficits were detected during balloon occlusion,
the balloon catheter was immediately deflated. In this case, the
clinical examination was considered positive. If no neurological
deficit was detected by 30 min, the result was negative.
Anteroposterior angiography was performed on the
entire skull after the neurologic assessment. The venous phase
delay assessment was performed by comparing the time difference for
opacification of the first cortical vein between the territories of
the examined hemisphere and the occluded hemisphere.
Results
Patient characteristics
The clinical characteristics of the 38 patients are
listed in Table I. Of the 38
patients enrolled, 18 (47%) were males and 20 (53%) were females,
average age 50 (16–72) years. There were 22 patients with carotid
aneurysms, 12 with extensive cervical tumors and 4 with traumatic
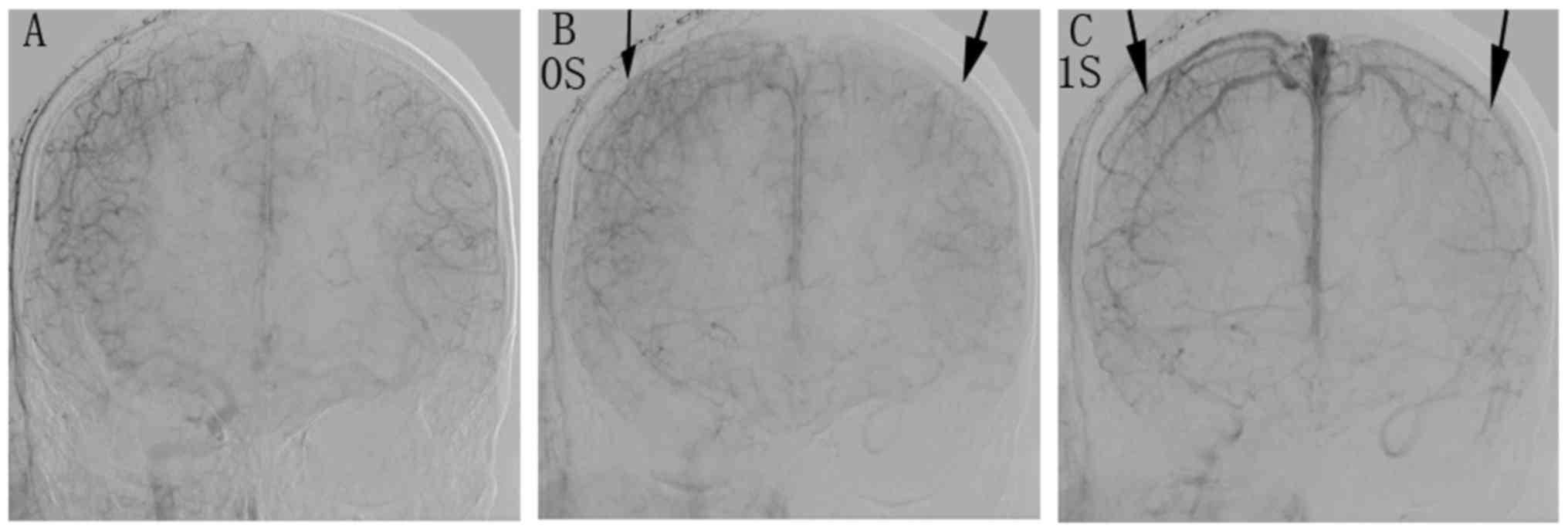
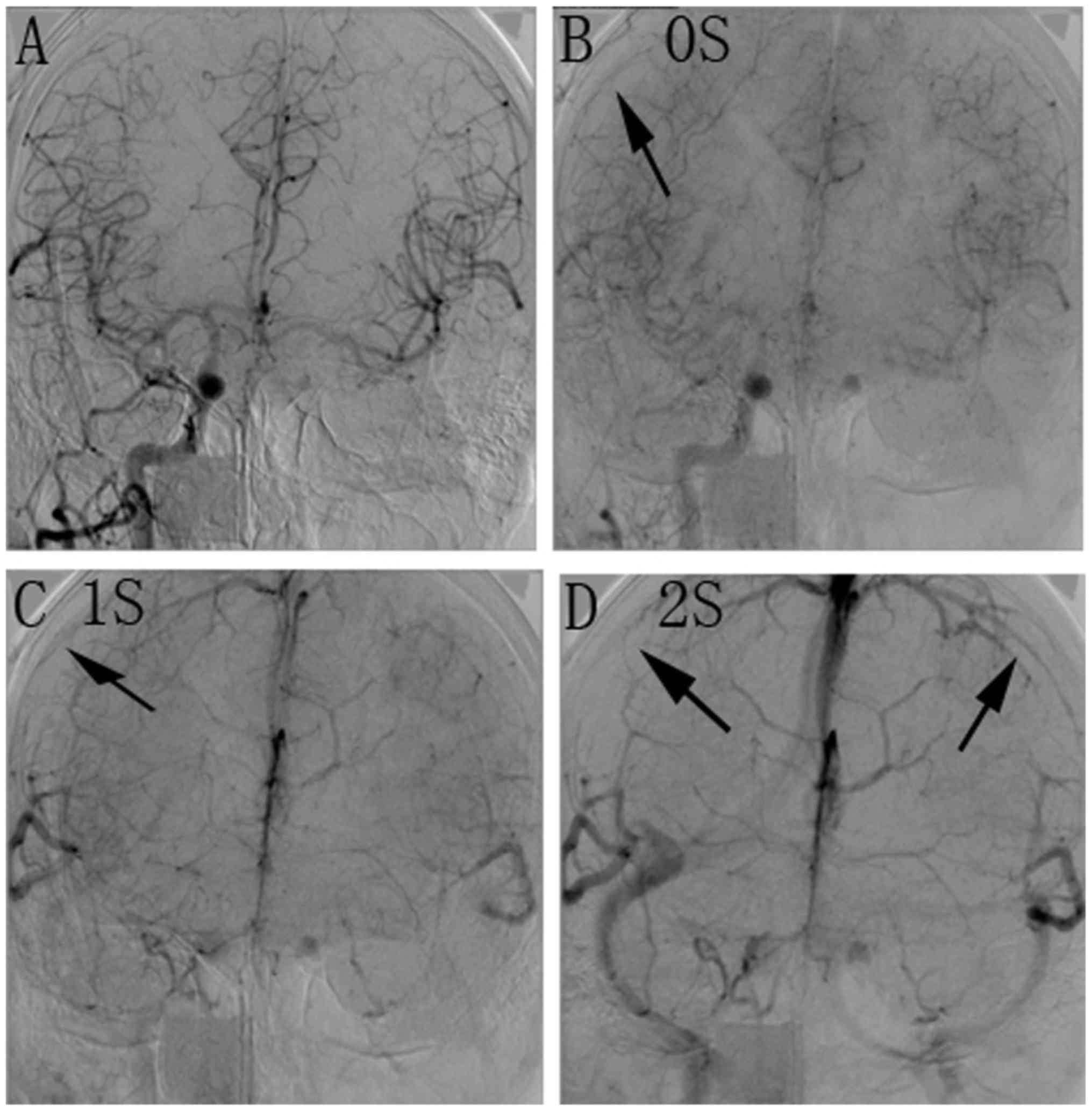
carotid cavernous fistulae. Fig. 1
displays a representative angiogram of a BOT of the right ICA in a
52-year-old male patient with a cervical tumor. A representative
angiogram of a BOT of the right ICA in a 47-year-old male patient
with a large cervical tumor is provided in Fig. 2.
 | Table I.Clinical characteristics of the 38
patients. |
Table I.
Clinical characteristics of the 38
patients.
| Sex | n | Carotid aneurysm | Extensive cervical
tumor | Traumatic carotid
cavernous fistulae |
|---|
| Female | 20 | 13 (65) | 5
(25) | 2 (10) |
| Male | 18 | 9
(50) | 7
(39) | 2 (11) |
| Total | 38 | 22 (58) | 12 (31) | 4 (11) |
Clinical and venous phase delay
examination during BOT
A comparison of the results of the clinical
examination and the venous phase assessment is provided in Table II. According to the neurological
examination during BOT, 30 patients (78.9%) were negative and 8
(21.1%) were positive. Of the 30 negative patients, venous phase
delay occurred within 2 sec in 28 patients (93.3%), and between 2
and 4 sec in two patients (6.7%). None of the patients with a
negative result had a delay of >4 sec. Of the eight patients
with positive results, the venous phase delay was within 2 sec for
one patient (12.5%), between 2 and 4 sec for two patients (25%) and
>4 sec for five patients (62.5%).
 | Table II.Results of the clinical examination
and venous phase assessment. |
Table II.
Results of the clinical examination
and venous phase assessment.
|
|
| Venous phase delay
(sec) |
|---|
|
|
|
|
|---|
| Clinical
examination | n | <2 | 2–4 | >4 |
|---|
| Negative | 30 | 28 (93) | 2 (7) | 0 (0) |
| Positive | 8 | 1 (12.5) | 2 (25) | 5 (62.5) |
| Total | 38 | 29 (76) | 4 (11) | 5 (13) |
Venous phase delay during BOT may be
utilized to determine suitability for PVO
Although the data were not sufficient from a
statistical perspective, the results allow for the prediction that
for most patients with a negative clinical examination the venous
phase delay was <2 sec. For the majority of patients with a
positive clinical examination, the delay was >2 sec. All
patients with a venous phase delay of >4 sec had a positive
clinical examination result.
Discussion
The treatment of complex vascular pathologies
depends on the evolution of endovascular technology (3). For the treatment of certain vascular
diseases, it is appropriate to perform PVO of the diseased area
(1,2). Certain conditions, including wide-neck
giant aneurysms, pseudoaneurysms, traumatic vascular injuries,
carotid blowout and arteriovenous fistulas, require PVO (1,2). PVO may
be used as a pre-operative treatment prior to tumor resection in
patients suffering from neck or skull-base tumors (7). In the early 1970s, Serbinenko first
reported on endovascular PVO with detachable balloons (8,9). It is
necessary to perform ICA endovascular occlusion as a pre-operative
maneuver for the treatment of certain head and neck diseases
(4).
Hemodynamic ischemia and thromboembolism are the
major complications associated with PVO. Thromboembolic
complications may be managed by anti-coagulation treatment
(5). However, it is difficult to
avoid immediate or delayed hemodynamic cerebral ischemia during
carotid artery occlusion, even during uncomplicated procedures.
Identification of patients that are likely to develop hemodynamic
ischemia prior to PVO is crucial for preventing this type of
complication (3).
A temporary BOT is useful for evaluating ischemic
risk prior to permanent ICA occlusion. Several techniques may be
used for the BOT. The primary purpose of the BOT is to assess the
ability of the intracranial collateral circulation to maintain
perfusion of the affected vascular territory during temporary
occlusion of the major arterial supply. An interventionist assesses
whether a patient is able to tolerate PVO by applying the BOT. A
clinical BOT of the ICA contributes to lower post-occlusion
morbidity (10,11). A study by Linskey et al
(5), which included 516 patients,
demonstrated that the use of an ICA BOT reduced the morbidity of
permanent ICA occlusion from 26 to 13%.
Several methods may be used to assess the clinical
tolerance to PVO. These may include neurological examination
assessment, venous phase delay assessment (6), stump pressure assessment (12), perfusion scanning assessment
[including single-photon emission computed tomography (SPECT)
(13), xenon CT perfusion (14), CT perfusion with an acetazolamide
challenge (15)], as well as
magnetic resonance (MR) perfusion (16) and neurophysiological monitoring (NPM)
(17). Other methods include
electroencephalography, somatosensory-evoked potentials and brain
stem-evoked potentials (3),
transcranial Doppler (TCD) ultrasonography (18) and induced hypotension (19). The aim of these techniques is to
enhance the predictive value of a successful BOT. In certain cases,
numerous assessment methods have been employed in conjunction with
clinical examinations to determine in which patients PVO is safe
and to thereby reduce the incidence of ischemic complications
(20).
The neurological examination assessment is the most
useful and widely applied method during BOT to determine whether a
patient is able to tolerate PVO (21). When the BOT procedure is performed
for clinical assessment, the patient is asked a series of questions
to assess their motor, sensory, speech and memory capacity, as well
as their analytical or calculation skills (3). Although the clinical assessment is the
basis for all BOT paradigms, certain disadvantages exist. For
instance, a clinical examination may increase the risk of
thromboembolic complications, which may require a prolonged balloon
inflation time. In addition, patients must be fully conscious and
the occlusion procedure must be performed under local anesthesia to
facilitate the clinical examination (3).
For most types of BOT assessment, certain challenges
prevail, as the systemic pressure is affected by several factors.
General anesthesia, patient mood and even environmental conditions
may affect stump pressure assessment (20). In addition, although previous studies
have identified a significant correlation between stump pressure
and cerebral perfusion (22), the
usefulness of stump pressure assessment remains controversial, as
other studies did not identify a correlation with cerebral
perfusion (23). If perfusion
scanning assessment is performed, the patient must be transferred
to a CT, MRI or SPECT scanning room. The balloon is placed in the
ICA during transportation, which may increase the risk of carotid
artery injury (13–15). The disadvantage of NPM is that the
result is not reliable due to the high false-positive and -negative
rates (17). Although TCD
ultrasonography has the advantage of being non-invasive, it does
not provide a consistently stable result (18). The correlation between cerebral blood
flow and mean middle cerebral artery velocity is not linear, as the
mean velocity may be affected by the hematocrit, viscosity and
vessel caliber (14). A previous
study indicated that the induced hypotension assessment was not
superior to the traditional BOT (19). Dare et al (19) suggested that the false-negative rate
of the induced hypotension assessment may be increased due to the
direct vasodilator effect of nitroprusside on the cerebral
circulation.
The venous phase delay assessment is based on the
assumption that an adequate intracranial collateral circulation
exists to confirm the symmetry of the two hemispheres when one ICA
is occluded during a BOT (6). Venous
phase delay is a simple angiographic BOT assessment performed under
general anesthesia removing the need for any further neurological
examination. This type of evaluation is rapid and straightforward
(6). A reduced balloon inflation
time in the ICA thus indicates a reduced risk of thrombus formation
associated with an occluded ICA (3).
Even though venous phase delay has several advantages and has
recently attracted extensive attention, neurological examination
remains the most commonly used method to assess a patient's ability
to tolerate PVO (21). Neurological
examination is straightforward, practicable and does not require
any specialized equipment. Accordingly, in the present
retrospective study, the results of the venous phase delay
assessment were compared with those of a neurological examination
during a BOT of the ICA to investigate a preliminary comparison
between the two types of assessment.
In the present study, for most patients with a
negative neurological examination, the venous phase delay was <2
sec. Therefore, patients with a venous phase delay <2 sec were
identified as having the ability to tolerate PVO. This result was
consistent with that of a study by Abud et al (6), which demonstrated that patients with a
venous phase delay <3 sec were identified as having the ability
to tolerate carotid occlusion without developing neurologic
deficit. For most patients with a positive neurological
examination, the venous phase delay was >2 sec, and all patients
with a venous phase delay of >4 sec had a positive neurological
examination. Therefore, a venous phase delay of >2 sec, which is
associated with a positive neurological examination during BOT, may
be used to indicate patients which cannot tolerate PVO.
Furthermore, patients with a venous phase delay of >4 sec will
definitely not be able to tolerate PVO. Accordingly, if the venous
phase delay is 2–4 sec, the BOT result requires to be determined by
additional factors along with the venous phase delay.
In conclusion, according to the present
retrospective study and previous studies, venous phase delay
assessment is a reliable method for evaluating a BOT of the ICA. In
addition, a delay <2 sec should be considered to indicate that
PVO of the ICA is safe.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81601471
and 81571784); the Scientific and Technological Support Project in
the Field of Social Development of Hunan Province (grant no.
2015SF2020-4) and the Project of Development and Reform Commission
of Hunan Province (grant no. Xiangcai enterprise means [2015]
83).
Availability of data and materials
All datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EX and HZ designed the study. ZC and LH performed
the examinations and analyzed the data. ZC and LH prepared the
manuscript. EX and HZ read and approved the final manuscript.
Ethical approval and consent to
participate
The present study was approved by the Ethics
Committee of the Second Xiangya Hospital of Central South
University (Changsha, China). All patients provided informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Snelling BM, Sur S, Shah SS, Wolfson RI,
Ambekar S, Yavagal DR, Elhammady MS and Peterson EC: Venous phase
timing does not predict SPECT results during balloon test occlusion
of the internal carotid artery. World Neurosurg. 102:229–234. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bavinzski G, Killer M, Ferraz-Leite H,
Gruber A, Gross CE and Richling B: Endovascular therapy of
idiopathic cavernous aneurysms over 11 years. AJNR Am J
Neuroradiol. 19:559–565. 1998.PubMed/NCBI
|
|
3
|
Elias AE, Chaudhary N, Pandey AS and
Gemmete JJ: Intracranial endovascular balloon test occlusion:
Indications, methods, and predictive value. Neuroimaging Clin N Am.
23:695–702. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mathis JM, Barr JD, Jungreis CA, Yonas H,
Sekhar LN, Vincent D, Pentheny SL and Horton JA: Temporary balloon
test occlusion of the internal carotid artery: Experience in 500
cases. AJNR Am J Neuroradiol. 16:749–754. 1995.PubMed/NCBI
|
|
5
|
Linskey ME, Jungreis CA, Yonas H, Hirsch
WL Jr, Sekhar LN, Horton JA and Janosky JE: Stroke risk after
abrupt internal carotid artery sacrifice: Accuracy of preoperative
assessment with balloon test occlusion and stable xenon-enhanced
CT. AJNR Am J Neuroradiol. 15:829–843. 1994.PubMed/NCBI
|
|
6
|
Abud DG, Spelle L, Piotin M, Mounayer C,
Vanzin JR and Moret J: Venous phase timing during balloon test
occlusion as a criterion for permanent internal carotid artery
sacrifice. AJNR Am J Neuroradiol. 26:2602–2609. 2005.PubMed/NCBI
|
|
7
|
Hertel A, Görling S, Schwager K and
Hofmann E: Angiography and cerebral perfusion scintigraphy in
balloon test occlusion of carotid artery in head and neck tumors.
Rofo. 184:214–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Serbinenko F: Catheterization and
occlusion of major cerebral vessels and prospects for the
development of vascular neurosurgery. Vopr Neirokhir. 35:17–27.
1971.(In Russian). PubMed/NCBI
|
|
9
|
Serbinenko FA: Balloon catheterization and
occlusion of major cerebral vessels. J Neurosurg. 41:125–145. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Detre JA, Samuels OB, Alsop DC,
Gonzalez-At JB, Kasner SE and Raps EC: Noninvasive magnetic
resonance imaging evaluation of cerebral blood flow with
acetazolamide challenge in patients with cerebrovascular stenosis.
J Magn Reson Imaging. 10:870–875. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuwabara Y, Ichiya Y, Sasaki M, Akashi Y,
Yoshida T, Fukumura T and Masuda K: A comparison of the
cerebrovascular responses to CO2 and Diamox in patients with
unilateral occlusive cerebral arteries: A H2(15)O PET study. Kaku
Igaku. 32:569–577. 1995.(In Japanese). PubMed/NCBI
|
|
12
|
Morishima H, Kurata A, Miyasaka Y, Fujii K
and Kan S: Efficacy of the stump pressure ratio as a guide to the
safety of permanent occlusion of the internal carotid artery.
Neurol Res. 20:732–736. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tansavatdi K, Dublin AB, Donald PJ and
Dahlin B: Combined balloon test occlusion and SPECT analysis for
carotid sacrifice: Angiographic predictors for success or failure?
J Neurol Surg B Skull Base. 76:249–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kofke WA, Brauer P, Policare R, Penthany
S, Barker D and Horton J: Middle cerebral artery blood flow
velocity and stable xenon-enhanced computed tomographic blood flow
during balloon test occlusion of the internal carotid artery.
Stroke. 26:1603–1606. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jain R, Hoeffner EG, Deveikis JP, Harrigan
MR, Thompson BG and Mukherji SK: Carotid perfusion CT with balloon
occlusion and acetazolamide challenge test: Feasibility. Radiology.
231:906–913. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma J, Mehrkens JH, Holtmannspoetter M,
Linke R, Schmid-Elsaesser R, Steiger HJ, Brueckmann H and Bruening
R: Perfusion MRI before and after acetazolamide administration for
assessment of cerebrovascular reserve capacity in patients with
symptomatic internal carotid artery (ICA) occlusion: Comparison
with 99mTc-ECD SPECT. Neuroradiology. 49:317–326. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu AY, Lopez JR, Do HM, Steinberg GK,
Cockroft K and Marks MP: Neurophysiological monitoring in the
endovascular therapy of aneurysms. AJNR Am J Neuroradiol.
24:1520–1527. 2003.PubMed/NCBI
|
|
18
|
Eckert B, Thie A, Carvajal M, Groden C and
Zeumer H: Predicting hemodynamic ischemia by transcranial Doppler
monitoring during therapeutic balloon occlusion of the internal
carotid artery. AJNR Am J Neuroradiol. 19:577–582. 1998.PubMed/NCBI
|
|
19
|
Dare AO, Chaloupka JC, Putman CM, Fayad PB
and Awad IA: Failure of the hypotensive provocative test during
temporary balloon test occlusion of the internal carotid artery to
predict delayed hemodynamic ischemia after therapeutic carotid
occlusion. Surg Neurol. 50:147–156. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang AY, Chen CC, Lai HY and Lee ST:
Balloon test occlusion of the internal carotid artery with stump
pressure ratio and venous phase delay technique. J Stroke
Cerebrovasc Dis. 22:e533–e540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Asai K, Imamura H, Mineharu Y, Tani S,
Adachi H, Narumi O, Sato S, Sakai C and Sakai N: X-ray angiography
perfusion analysis for the balloon occlusion test of the internal
carotid artery. J Stroke Cerebrovasc Dis. 24:1506–1512. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tomura N, Omachi K, Takahashi S, Sakuma I,
Otani T, Watarai J, Ishikawa K, Kinouchi H and Mizoi K: Comparison
of technetium Tc 99m hexamethylpropyleneamine oxime single-photon
emission tomograph with stump pressure during the balloon occlusion
test of the internal carotid artery. AJNR Am J Neuroradiol.
26:1937–1942. 2005.PubMed/NCBI
|
|
23
|
Barker DW, Jungreis CA, Horton JA,
Pentheny S and Lemley T: Balloon test occlusion of the internal
carotid artery: Change in stump pressure over 15 minutes and its
correlation with xenon CT cerebral blood flow. AJNR Am J
Neuroradiol. 14:587–590. 1993.PubMed/NCBI
|