Introduction
The aging of the Japanese population is currently
unprecedented (1). The percentage of
the population aged 65 years or older in 2017 was 27.3% according
to a report from the Japanese cabinet (2). The incidence of sarcoma increases
progressively with age (3), and the
number of sarcoma patients in Japan increases yearly according to
the Japanese Registry of Musculoskeletal Oncology (4).
At present, there are no established treatments for
elderly sarcoma patients, who are difficult to treat in the same
manner as their young counterparts because of potential
immunodeficiencies, underlying diseases, complications, and social
backgrounds. Moreover, studies addressing the management of elderly
sarcoma patients are few (4–10), with controversial results. Here we
present a retrospective study aimed at determining the best
treatment strategy for elderly sarcoma patients.
Materials and methods
Patients and treatments
The present retrospective study comprised 34
patients (21 men and 13 women) with malignant bone or soft tissue
tumors who underwent surgery in our department from September 2004
to March 2014 (Table I). The median
patient age was 72 years (range, 65–86 years); 11 patients were
65–69 years-old, 10 were 70–74 years-old, and 13 were ≥75
years-old. All patients provided written informed consent for this
retrospective study.
 | Table I.Characteristics of the study
population. |
Table I.
Characteristics of the study
population.
| Factor | Patients, n (%) |
|---|
| Age (years) |
|
|
65–69 | 11 (32) |
|
70–74 | 7 (20) |
| ≥75 | 16 (48) |
| Sex |
|
| Male | 21 (62) |
|
Female | 13 (38) |
| Tumor site |
|
| Arms | 3 (9) |
| Legs | 28 (82) |
|
Trunk | 3 (9) |
| Tumor size (cm) |
|
|
<5 | 3 (9) |
| 5–10 | 12 (35) |
|
>10 | 19 (56) |
| Histological
type |
|
| Soft
tissue |
|
|
Liposarcoma | 19 (56) |
|
Atypical
lipomatous tumor/well differentiated | 3 (9) |
|
Dedifferentiated | 4 (12) |
|
Myxoid | 2 (6) |
|
Pleomorphic | 10 (29) |
|
Leiomyosarcoma | 9 (26) |
|
Undifferentiated
pleomorphic sarcoma | 3 (9) |
|
Synovial
sarcoma | 1 (3) |
| Bone |
|
|
Chondrosarcoma | 2 (6) |
| Histological
grade |
|
|
Total |
|
|
Low | 11 (32) |
|
High | 23 (68) |
| Bone
tumor |
|
|
Grade 1 | 0 (0) |
|
Grade 2 | 1 (3) |
|
Grade 3 | 1 (3) |
| Soft
tissue (FNCLCC grading system) |
|
|
Grade 1 | 11 (32) |
|
Grade 2 | 3 (9) |
|
Grade 3 | 18 (53) |
| Complications |
|
|
Diabetes mellitus | 5 |
|
Hypertension | 15 |
|
Dementia | 2 |
|
Hepatitis | 1 |
| Metastasis at
initial visit |
|
|
(−) | 31 |
|
(+) | 3 |
| Surgical
margin |
|
| R0 | 25 (74) |
| R1 | 7 (20) |
| R2 | 2 (6) |
|
Adequate | 25 (74) |
|
Inadequate | 7 (20) |
|
Intralegional | 2 (6) |
| Surgical time
(min) |
|
|
<60 | 4 (12) |
|
60–120 | 19 (56) |
|
>120 | 11 (32) |
| Blood loss
(cc) |
|
|
<100 | 20 (59) |
|
100–200 | 6 (17) |
|
>200–300 | 5 (15) |
|
>300–400 | 3 (9) |
|
>400 | 3 (9) |
| Adjuvant
radiotherapy | 30 (88) |
|
(−) | 4 (12) |
|
(+) |
|
| Adjuvant
chemotherapy |
|
|
(−) | 31 (91) |
|
(+) | 3 (9) |
| Follow-up period
(years) |
|
|
<5 | 11 (32) |
| ≥5 | 23 (68) |
| Oncological
result |
|
|
CDF | 24 (71) |
|
AWD | 3 (9) |
|
DOD | 7 (20) |
| Recurrence |
|
|
(−) | 20 (59) |
|
(+) | 10 (41) |
| ECOG-PS |
|
| 0 | 9 (26) |
| 1 | 21 (62) |
| 2 | 4 (12) |
| 3 | 0 (0) |
| 4 | 0 (0) |
| ASA-PS |
|
| I | 9 (26) |
| II | 23 (74) |
|
III | 0 (0) |
| IV | 0 (0) |
| V | 0 (0) |
| VI | 0 (0) |
The tumor sites were the legs (28 cases), arms (3
cases), and trunk (3 cases) (Table
I). The histological tumor types were liposarcoma (19 cases),
leiomyosarcoma (9 cases), undifferentiated pleomorphic sarcoma (3
cases), chondrosarcoma (2 cases), and synovial sarcoma (1 case).
Among the 19 liposarcoma cases, 3 were well differentiated, 4 were
dedifferentiated, 2 were myxoid, and 10 were pleomorphic (11).
The classification systems of the French Federation
of Comprehensive Cancer Centers (12) and Lee et al (13) were used for histological grading of
soft tissue and bone sarcomas, respectively. Twenty-three sarcomas
were categorized as high-grade and 11 as low-grade (Table I). Eleven soft tumor sarcomas were
grade 1; 3 were grade 2; and 18 were grade 3. One chondrosarcoma
was grade 2 and the other was grade 3. The complications were
diabetes mellitus (5 cases), hypertension (15 cases), dementia (2
cases), and hepatitis (1 case). Three patients had metastatic
lesions at their first visit.
Treatments included surgery with adequate margins
(25 cases), surgery with inadequate margins and radiotherapy (4
cases), surgery with inadequate margins and chemotherapy (3 cases),
and intralesional resection (2 cases). The postoperative
observation period ranged from 7 to 112 months (average, 49
months).
Surgery aimed to obtain adequate surgical margins in
all cases. Using previously described methods, surgical margins
were classified as adequate, inadequate, or intralesional (14) and R0, R1, or R2 (15). R1 and R2 margins are those in which
residual tumor is detectable microscopically and macroscopically,
respectively. R0 margins are tumor-free (15). The margins were inadequate in cases
where the tumors were intensive. These tumors were treated via
marginal resection because aggressive surgery may have impaired
performance status. Twenty-five (74%) cases were R0, 7 (20%) were
R1, and 2 (6%) were R2 (Table I).
The mean surgical time was 114.7 min (range, 50–465 min), with 23
(68%) surgeries requiring <120 min. The mean amount of blood
loss was 160.7 cc (range, 10–1501 cc); most patients (31, 91%) lost
<300 cc.
The indication for radiotherapy was a high risk of
recurrence after surgery with inadequate margins. Two patients with
dedifferentiated liposarcoma, 1 patient with leiomyosarcoma, and 1
patient with pleomorphic liposarcoma received postoperative
radiotherapy. A total dose of 60 Gy (2 Gy/day, 5 days/week) was
applied to the operative field.
Systemic chemotherapy was administered to patients
<70 years-old with preoperative metastasis or a locally
intensive tumor [Eastern Cooperative Oncology Group Performance
Status (ECOG-PS) score, 0–1]. Three patients with chondrosarcoma,
synovial sarcoma, and dedifferentiated liposarcoma, respectively,
received chemotherapy. In accordance with the Neoadjuvant
Chemotherapy for Osteosarcoma protocol in Japan (95 J, 80% dose)
(16), the preoperative regimen for
chondrosarcoma consisted of 8 g/m2 high-dose
methotrexate, 80 mg/m2 cisplatin, and 48
mg/m2 adriamycin; the postoperative regimen, which was
administered to 1 patient owing to a poor response, was the same
with the addition of 14 mg/m2 ifosfamide (16). The preoperative and postoperative
(the dedifferentiated liposarcoma only) regimen for the soft tissue
sarcomas consisted of 1–3 cycles of 60–75 mg/m2
doxorubicin, 4–5 mg/m2 ifosfamide, and lenograstim (100
µg) every 3 weeks (17). Toxic
effects were assessed using the National Cancer Institute common
toxicity criteria (version 1) (17).
Statistical analysis
The 5-year survival rate was determined using the
Kaplan-Meier method, and differences were assessed using the
log-rank test, and P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed with Stat Mate (Atms, Tokyo, Japan) software for Windows,
version 4.01. Recurrence rates, metastasis, ECOG-PS (18), and ASA-PS (19) were also examined. We compared the
outcomes in all cases to determine the best treatment strategy for
elderly sarcoma patients.
Results
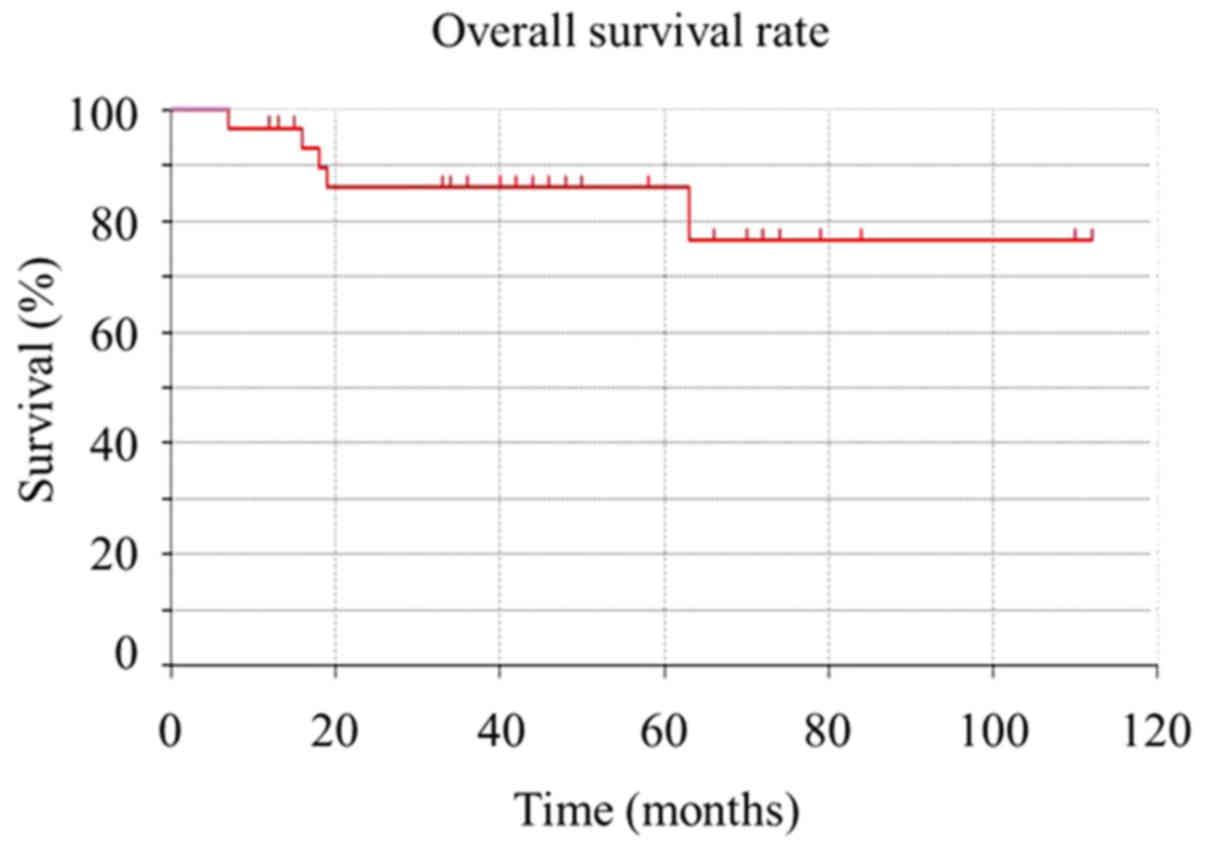
The 5-year overall survival rate for the patients in
this study was 86.02% (Fig. 1). It
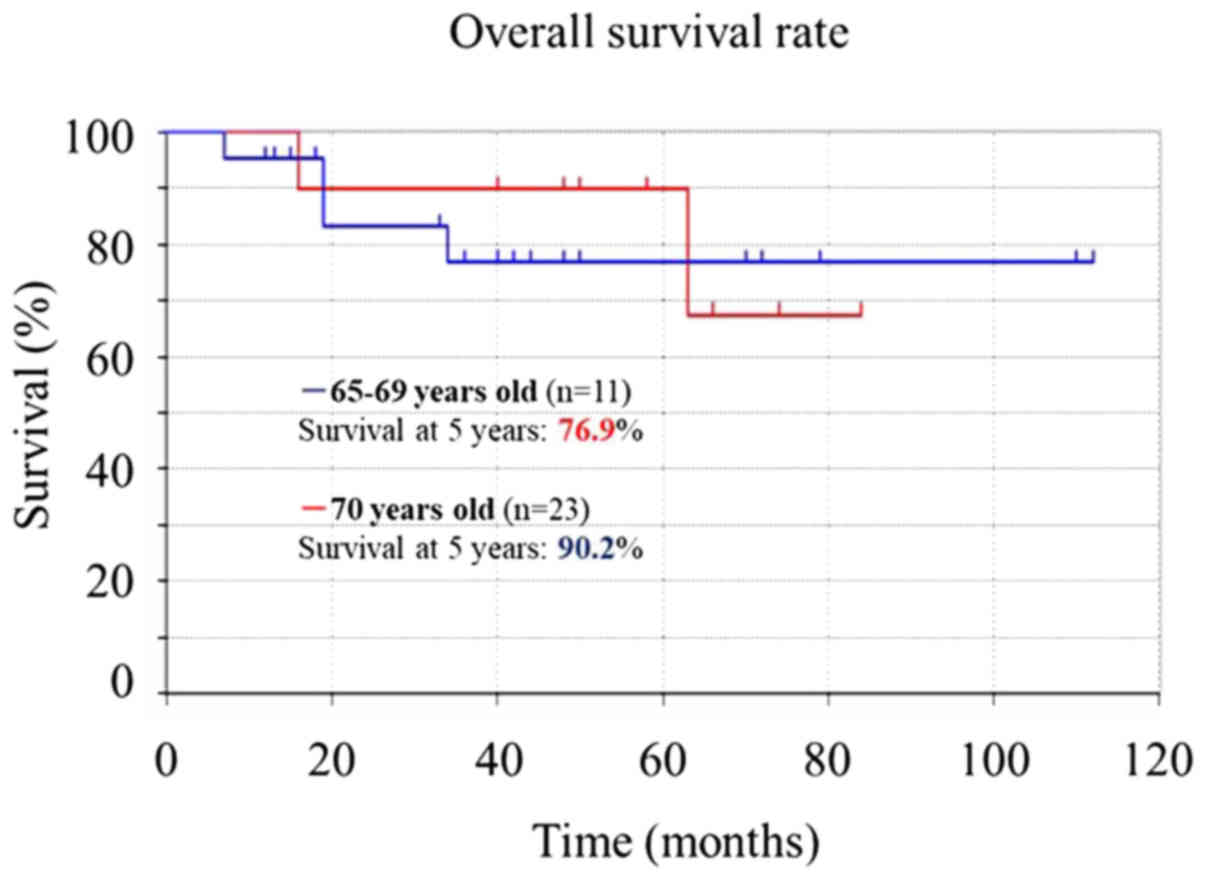
did not differ significantly in patients 65–69 (76.9%) vs. ≥70
(90.2%) years-old (P=0.65) (Fig. 2),
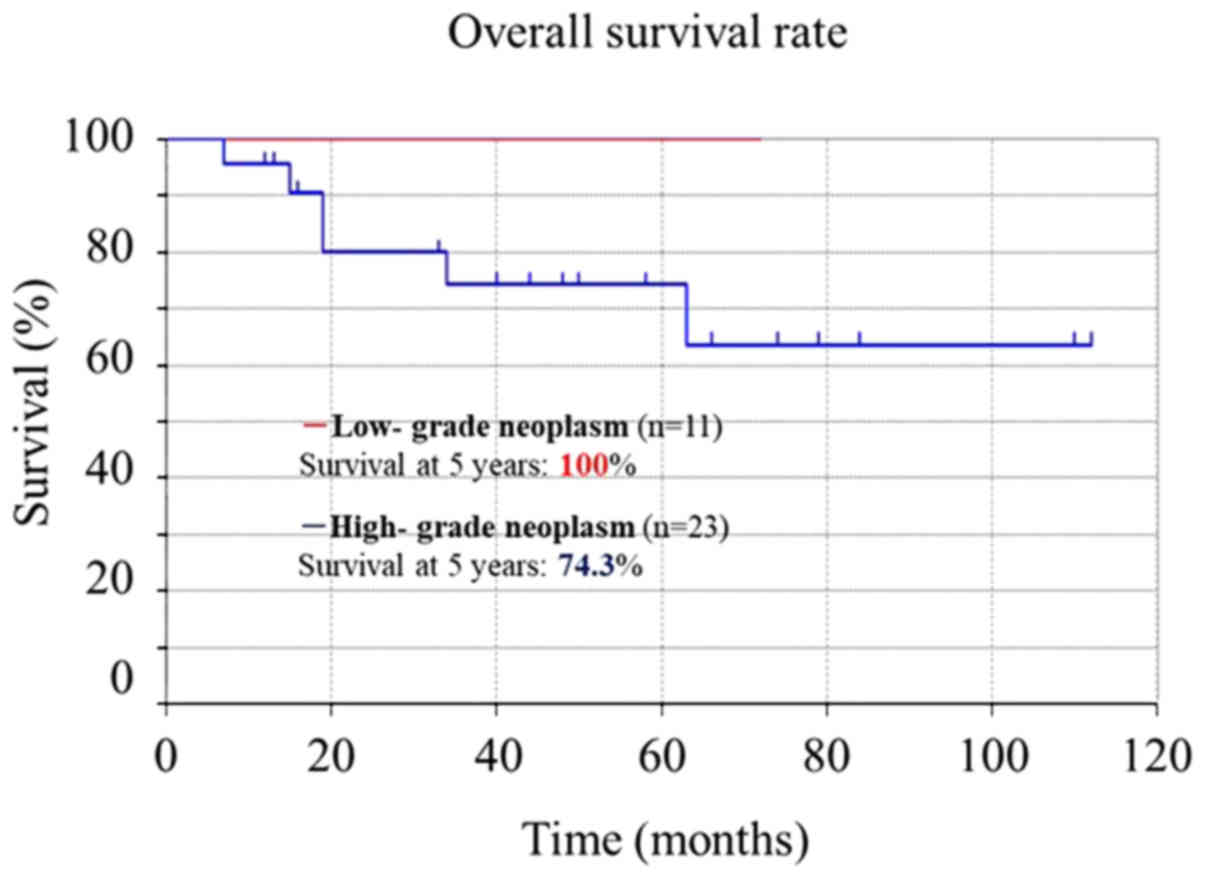
but was significantly higher in those with low-grade (100%) vs.
high-grade (74.3%) neoplasms (P<0.001) (Fig. 3).
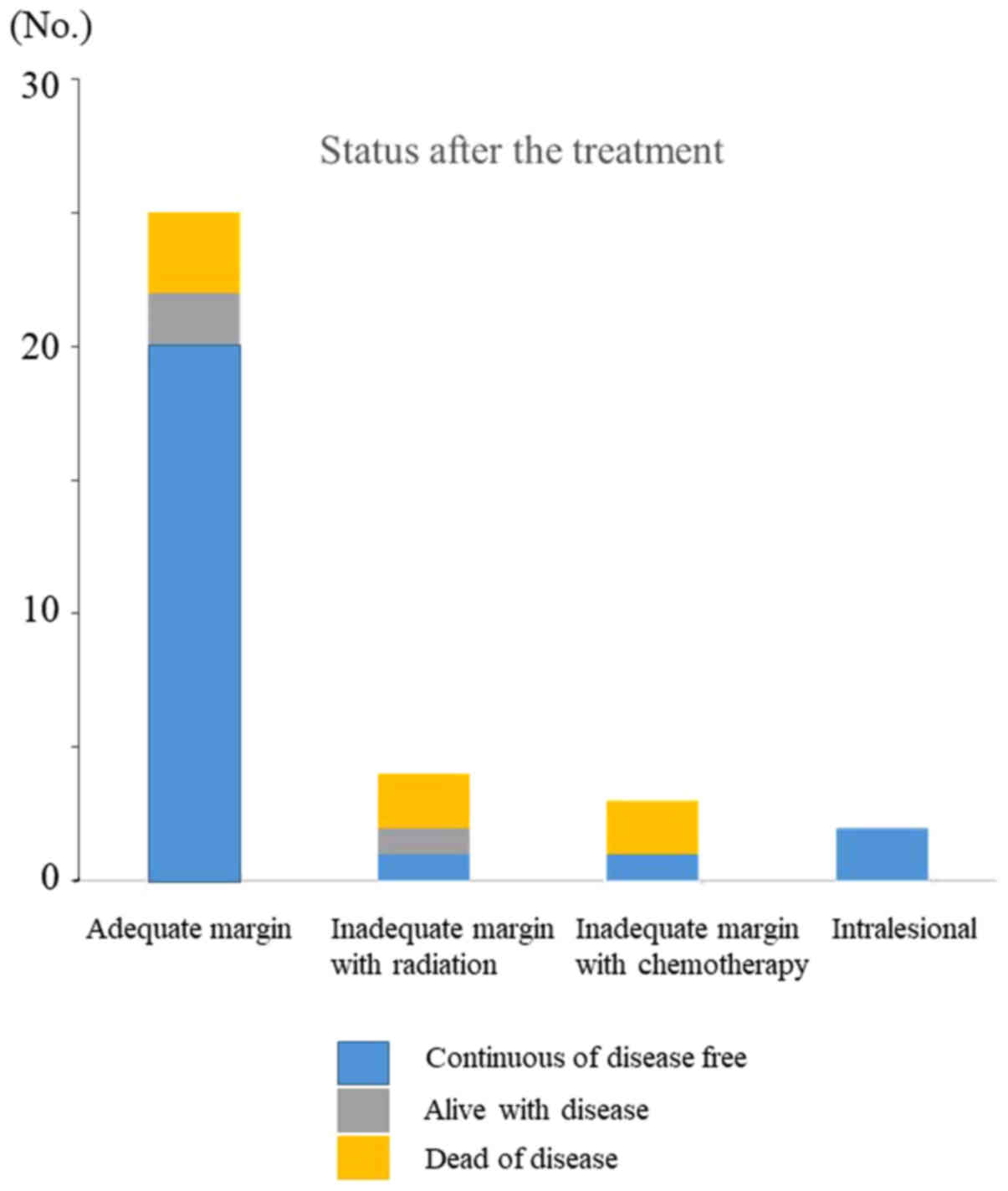
Tumor status was classified as continuously
disease-free (CDF) in 24 cases, alive with disease (AWD) in 3
cases, and dead of disease (DOD) in 7 cases (Table I). Tumor status according to
treatment was as follows: i) Surgery with adequate surgical
margins: CDF, 20 cases; AWD, 2 cases; and DOD, 3 cases; ii) surgery
with inadequate margins and radiotherapy: CDF, 1 case; AWD, 1 case
and DOD, 2 cases; iii) surgery with inadequate margins and
chemotherapy: CDF, 1 case and DOD, 2 cases; and iv) surgery with
intralesional margins: CDF, 2 cases (Fig. 4). After these treatments, tumors
recurred in 4/25 cases (16%), 3/4 cases (75%), 2/3 cases (67%), and
1/2 cases (50%), respectively. The recurrence rate was
significantly lower in cases with adequate margins (4/25, 16%) than
in cases with inadequate margins (6/9, 67%) (odds ratio, 0.24;
Fisher's exact test). Seven of the 10 recurrences were localized,
whereas 3 metastasized to the lung. The mean time before recurrence
was 10.3 months (range, 3–21 months). All metastatic lesions
present at the initial visit were also present after surgery, with
1 increasing in size.
Adverse events due to radiotherapy included
lymphedema, which occurred in 2 patients 6–12 months after the
therapy. Hematological and biological grade 0–2 adverse events were
observed during chemotherapy (Table
II).
 | Table II.Chemotherapy-associated
toxicities. |
Table II.
Chemotherapy-associated
toxicities.
|
| Grade |
|---|
|
|
|
|---|
| Toxicity | 0 | 1 | 2 | 3 | 4 |
|---|
| Hematological,
n |
|
Hemoglobin | 2 | 1 | 0 | 0 | 0 |
| White
blood cells | 0 | 1 | 2 | 0 | 0 |
|
Neutrophils | 0 | 1 | 0 | 0 | 0 |
|
Platelets | 1 | 2 | 0 | 0 | 0 |
| Biochemical, n |
|
AST | 2 | 1 | 0 | 0 | 0 |
|
ALT | 2 | 1 | 0 | 0 | 0 |
|
Creatinine | 3 | 0 | 0 | 0 | 0 |
|
Bilirubin | 3 | 0 | 0 | 0 | 0 |
| Clinical, n |
|
Nausea | 2 | 1 | 0 | 0 | 0 |
|
Vomiting | 1 | 0 | 0 | 0 | 0 |
|
Diarrhea | 0 | 0 | 0 | 0 | 0 |
|
Alopecia | 2 | 1 | 0 | 0 | 0 |
|
Mucositis | 1 | 0 | 0 | 0 | 0 |
|
Infection | 0 | 0 | 0 | 0 | 0 |
|
Fever | 2 | 1 | 0 | 0 | 0 |
|
Neurological | 0 | 0 | 0 | 0 | 0 |
|
Cardiac | 0 | 0 | 0 | 0 | 0 |
The preoperative ECOG-PS scores were 0 in 9 cases, 1
in 21 cases, and 2 in 4 cases (Table
I). They were unchanged after treatment. The preoperative
ASA-PS scores were also I in 9 cases, and II in 23 cases (Table I).
Discussion
In general, almost all sarcoma patients are 40–60
years-old (3,12). However, the number of patients at the
upper end of this range is increasing (12). In the present study, the outcome for
sarcoma patients aged 65 years or older was almost always
favorable. Most patients in the study received surgery with
adequate margins (25/34), and such treatment may be necessary for a
good outcome.
In our study, the 5-year overall survival rate for
elderly patients with primary malignant bone or soft tissue tumors
was 86.02%, which is higher than previously reported rates
(35.0–83.0%, Table III) (4–10). This
may reflect our more frequent achievement of adequate margins
(73.5% of cases) (6,8–10) and
much lower ECOG-PS and ASA-PS scores (6,9,10).
 | Table III.Previous studies of elderly patients
with bone and soft tissue tumors. |
Table III.
Previous studies of elderly patients
with bone and soft tissue tumors.
| Author, year | Age (years) | No. of
patients | Surgical
margins | Prognostic
factors | 5-year survival
rate (%) | Follow-up, mean
(range) | (Refs.) |
|---|
| Osaka et al,
2003 | ≥65 | 25 | Wide (76%),
marginal | Age, grade | 79.6 | 91.6 months (4–180
months) | (5) |
|
|
|
| (16%),
intralesional (8%) |
|
|
|
|
| Boden, 2006 | ≥80 | 50 | Clear (76%) | Not identified | 46.0 | 22 months (12–3210
days) | (7) |
| Lahat et al,
2009 | ≥65 | 295 | Negative (63.1%),
positive (27.7%) | Age, tumor size,
grade | 63.0 | 65 months (1–162
months) | (6) |
| Torigoe et al,
2010 | ≥70 | 14 | Wide and
amputation | Bone tumor,
grade | 35.0 | 37 months (2–137
days) | (4) |
|
|
|
| (85.7%), marginal
(14.3%) |
|
|
|
|
| Kozawa et al,
2014 | ≥65 | 78 | Wide (71.8%),
marginal | Not identified | 72.0 | 38 months (1.4–160
days) | (8) |
|
|
|
| (11.5%),
intralesional (3.8%) |
|
|
|
|
| Yoneda et al,
2014 | ≥70 | 158 | Adequate wide
(66.5%), inadequate wide (33.5%) | Male sex,
grade | 83.0 | 38 months (12–186
months) | (9) |
| Iwai et al,
2015 | ≥65 | 90 | Wide (65.5%),
marginal | ASA-PS, grade | 77.5 | 44.8 months (1–163
months) | (10) |
|
|
|
| (18.9%),
intralesional (3.3%) |
|
|
|
|
| Present case | ≥65 | 34 | Adequate wide
(73.5%), inadequate wide (20.6%), intralesional (5.9%) | Not identified | 86.0 | 49 months (7–112
months) | – |
The tumor subtypes in our study are similar to those
in a previous study of all age groups (20), as are the tumor sites (mainly in the
limbs, with a minority in the head, neck, and trunk) (21). When age-matched cohorts are compared,
the percentage of high-grade tumors is higher in our study (68%)
than a previous study (32%) (22).
Although 68% of our cases had complications preoperatively, all
cases were amenable to surgical treatment, with no reductions in
both ECOG-PS and ASA-PS scores or worsening of complications
postoperatively.
Previously, it had been reported that 5-year
survival rates for elderly sarcoma patients ranged from 35.0–83.0%
(4–10), with various studies suggesting that
5-year survival rates were worse in older than in younger patients
(5,6). One study, for example, reported 5-year
survival rates of 46 and 63% for older and younger patients,
respectively (22). In the present
study, the 5-year survival rate for elderly sarcoma patients was
86.02%, which is better than previously reported rates for older
patients (70%) (23) and is like
that for younger patients (89.59%) (24). These findings suggest that age itself
may not affect survival of sarcoma patients.
As shown here and in previous studies, elderly
patients with high-grade sarcomas have a poorer prognosis than
those with low-grade sarcomas (4,5,10). Hence, the histological grade of the
sarcoma appears to affect the prognosis of elderly patients. Others
reported a local recurrence rate of 9% at the 20-month follow-up in
elderly sarcoma patients (9), as
well as a local recurrence rate of 22% at the 22-month follow-up in
sarcoma patients 80 years or older (7). Therefore, the recurrence rate may be
somewhat worse in older (22%) than younger sarcoma patients (9%)
(7,9).
Achieving adequate surgical margins is an important
component in sarcoma treatment for elderly patients, as shown both
here and by others (5,6,9). The
surgical resection protocol is very similar for older and younger
sarcoma patients, whereas the use of adjuvant radiotherapy may
differ. As a general policy, our unit recommends radiotherapy for
all patients with large high-grade tumors or intermediate- or
high-grade tumors of any size where the wide margin is not clear.
However, when dealing with very elderly patients, additional
considerations are warranted. A previous report suggests that
outpatient radiotherapy is too burdensome for elderly sarcoma
patients (7). In the present study,
we administered radiotherapy to 4 elderly sarcoma patients as an
in-hospital treatment. Although their physical status did not
decline, the efficacy of this treatment could not be confirmed.
According to the European Society for Medical
Oncology Guidelines (25), adjuvant
chemotherapy may delay or reduce the incidence of distant and local
recurrence in high-risk sarcoma patients. In elderly patients with
high-grade malignant bone tumors, the use of chemotherapy is
controversial. Adriamycin and ifosfamide are the main chemotherapy
drugs for sarcoma (26). Since
systemic chemotherapy may impair cardiac and renal function in
elderly sarcoma patients (27,28), it
should be limited to those patients without health problems. None
of the 3 patients in our study who received chemotherapy appeared
to benefit from the therapy. Chemotherapy did not worsen physical
function in the others. Regardless of whether it is low-dose (80%)
or high-dose, chemotherapy should be carefully administered to
patients selected on the basis of age and ECOG-PS.
Patient status is mainly evaluated by determining
ECOG-PS and ASA-PS scores (18,19).
ECOG-PS is based on the patient's daily activities (18), whereas ASA-PS is based on the
patient's medical condition (mainly the presence or absence of
systemic diseases) (19). Both
systems were useful for avoiding excessive treatment of the elderly
sarcoma patients in our study. ASA-PS is a potential prognostic
factor for elderly sarcoma patients (10). Whether ECOG-PS is also predictive is
not known and should be addressed in the future. We were unable to
do so in the present study, since we did not review the patients
with poor ECOG-PS scores (≥3). Studies focusing on elderly sarcoma
patients with poor ECOG-PS or ASA-PS (≥3) scores are needed.
Our study has some limitations. First, there was
potential selection bias since not all elderly patients are good
candidates for surgery. Second, the number of patients in our study
was low. The low number did not, however, preclude statistical
analysis of the data. Third, our study included only 2 cases of
malignant bone tumors. Fourth, we included well-differentiated
liposarcomas, which are currently classified as intermediate tumors
by the World Health Organization (29). However, at the time their treatment
(2004–2012), they were classified as malignant tumors.
Our results suggest that surgery with adequate
margins improves the prognosis of sarcoma patients 65 years-old.
However, further study is required to clarify the indications for
chemotherapy and radiotherapy in the elderly.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KH, SN, YH, NO, HT, SI and MA performed the study,
and collated, analyzed and interpreted the data. KH, SN, and MA
wrote the manuscript.
Ethics approval and consent to
participate
All patients provided written informed consent for
this retrospective study.
Patient consent for publication
All patients provided written informed consent for
the publication of this retrospective study.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ASA-PS
|
Anesthesiologists-Physical Status
|
|
ECOG-PS
|
Eastern Cooperative Oncology Group
Performance Status
|
References
|
1
|
Montgomery W, Ueda K, Jorgensen M, Stathis
S, Cheng Y and Nakamura T: Epidemiology, associated burden, and
current clinical practice for the diagnosis and management of
Alzheimer's disease in Japan. Clinicoecon Outcomes Res. 10:13–28.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cabinet office, Government of Japan: Wite
paper on aged society. http://www8.cao.go.jp/kourei/whitepaper/w-2017/zenbun/29pdf_index.htmlSeptember
4–2018
|
|
3
|
Balducci L and Erchler WB: Cancer and
aging: A nexus at several levels. Nat Rev Cancer. 5:655–662. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Torigoe T, Terakado A, Suehara Y, Kurosawa
H, Yazawa Y and Takagi T: Bone versus soft-tissue sarcomas in the
elderly. J Orthop Surg. 18:58–62. 2010. View Article : Google Scholar
|
|
5
|
Osaka S, Sugita H, Osaka E, Yoshida Y and
Ryu J: Surgical management of malignant soft tissue tumours in
patients aged 65 years or older. J Orthop Surg. 11:28–33. 2003.
View Article : Google Scholar
|
|
6
|
Lahat G, Dhuka AR, Lahat S, Lazar AJ,
Lewis VO, Lin PP, Feig B, Cormier JN, Hunt KK, Pisters PW, et al:
Complete soft tissue sarcoma resection is a viable treatment option
for select elderly patients. Ann Surg Oncol. 16:2579–2586. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boden RA, Clark MA, Neuhaus SJ, A'hern JR,
Thomas JM and Hayes AJ: Surgical management of soft tissue sarcoma
in patients over 80 years. Eur J Surg Oncol. 32:1154–1158. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kozawa E, Sugiura H, Tsukushi S, Urakawa
H, Arai E, Futamura N, Nakashima H, Yamada Y, Ishiguro N and
Nishida Y: Multiple primary malignancies in elderly patients with
high-grade soft tissue sarcoma. Int J Clin Oncol. 19:384–390. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoneda Y, Kunisada T, Naka N, Nishida Y,
Kawai A, Morii T, Takeda K, Hasei J, Yamakawa Y and Ozaki T:
Japanese Musculoskeletal Oncology Group: Favorable outcome after
complete resection in elderly soft tissue sarcoma patients:
Japanese musculoskeletal oncology group study. Eur J Surg Oncol.
40:49–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iwai T, Hoshi M, Takada J, Oebisu N, Aono
M, Takami M, Ieguchi M and Nakamura H: Prognostic factors for
elderly patients with primary malignant bone and soft tissue
tumors. Oncol Lett. 10:1799–1804. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fletcher CD; Unni KK and Mertens F: World
Health Organization Classification of Tumors. Pathology and
Genetics of Tumors of Soft Tissue and Bone. Rydholm A, Singer S,
Sundaram M and Coinche JM: IARC Press; Lyon: pp. 12–18. 2002,
PubMed/NCBI
|
|
12
|
Weiss SW and Goldblum JR: Enzinger and
Weiss's Soft Tissue Tumors. Folpe AL: 4th. Mosby, St; Louis, MO:
pp. 1–10. 2001
|
|
13
|
Lee FY, Mankin HJ, Fondren G, Gebhardt MC,
Springfield DS, Rosenberg AE and Jennings LC: Chondrosarcoma of
bone: An assessment of outcome. J Bone Joint Surg Am. 81:326–338.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawaguchi N, Ahmed AR, Matsumoto S, Manabe
J and Matsushita Y: The concept of curative margin in surgery for
bone and soft tissue sarcoma. Clin Orthop Relat Res. 419:165–172.
2004. View Article : Google Scholar
|
|
15
|
Gundle KR, Kafchinski L, Gupta S, Griffin
AM, Dickson BC, Chung PW, Catton CN, O'Sullivan B, Wunder JS and
Ferguson PC: Analysis of margin classification systems for
assessing the risk of local recurrence after soft tissue sarcoma
resection. J Clin Oncol. 36:704–709. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iwamoto Y, Tanaka K, Isu K, Kawai A,
Tatezaki S, Ishii T, Kushida K, Beppu Y, Usui M, Tateishi A, et al:
Multiinstitutional phase II study of neoadjuvant chemotherapy for
osteosarcoma (NECO study) in Japan: NECO-93J and NECO-95J. J Orthop
Sci. 14:397–404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Woll PJ, Reichardt P, Le Cesne A, Bonvalot
S, Azzarelli A, Hoekstra HJ, Leahy M, Van Coevorden F, Verweij J,
Hogendoorn PC, et al: Adjuvant chemotherapy with doxorubicin,
ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC
62931): A multicentre randomised controlled trial. Lancet Oncol.
13:1045–1054. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hosking MP, Warner MA, Lobdell CM, Offord
KP and Melton LJ III: Outcomes of surgery in patients 90 years of
age and older. JAMA. 261:1909–1915. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pitcher ME, Fish S and Thomas JM:
Management of soft tissue sarcoma. Br J Surg. 81:1136–1139. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Clark MA, Fisher C, Judson I and Thomas
JM: Soft-tissue sarcomas in adults. N Engl J Med. 353:701–711.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ramanathan RC, A'Hern R, Fisher C and
Thomas JM: Prognostic index for extremity soft tissue sarcoma with
isolated local recurrence. Ann Surg Oncol. 8:278–289. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harrison DJ, Geller DS, Gill JD, Lewis VO
and Gorlicl R: Current and future therapeutic approaches for
osteosarcoma. Expert Rev Anticancer Ther. 18:39–50. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hager S, Makowiec F, Henne K, Hopt UT and
Wittel UA: Significant benefits in survival by the use of surgery
combined with radiotherapy for retroperitoneal soft tissue sarcoma.
Radiat Oncol. 12:292017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
ESMO/European Sarcoma Network Working
Group: Soft tissue, visceral sarcomas: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 23
Suppl 7:vii92–vii99. 2012.PubMed/NCBI
|
|
26
|
Linch M, Miah AB, Thway K, Judson IR and
Benson C: Systemic treatment of soft-tissue sarcoma-gold standard
and novel therapies. Nat Rev Clin Oncol. 11:187–202. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Farry JK, Flombaum CD and Latcha S: Long
term renal toxicity of ifosfamide in adult patients-5 year data.
Eur J Cancer. 48:1326–1331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matushansky I, Dela Cruz F, Insel BJ,
Hershman DL and Neugut AI: Chemotherapy use in elderly patients
with soft tissue sarcoma: A population-based study. Cancer Invest.
31:83–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fletcher CD, Bridge JA, Hogendoom PC and
Mertens F: WHO Classification of Tumors of Soft Tissue and Bone.
Mertens F (eds). 4th edition. IARC Publications, France,. 5:33–38.
2013.
|