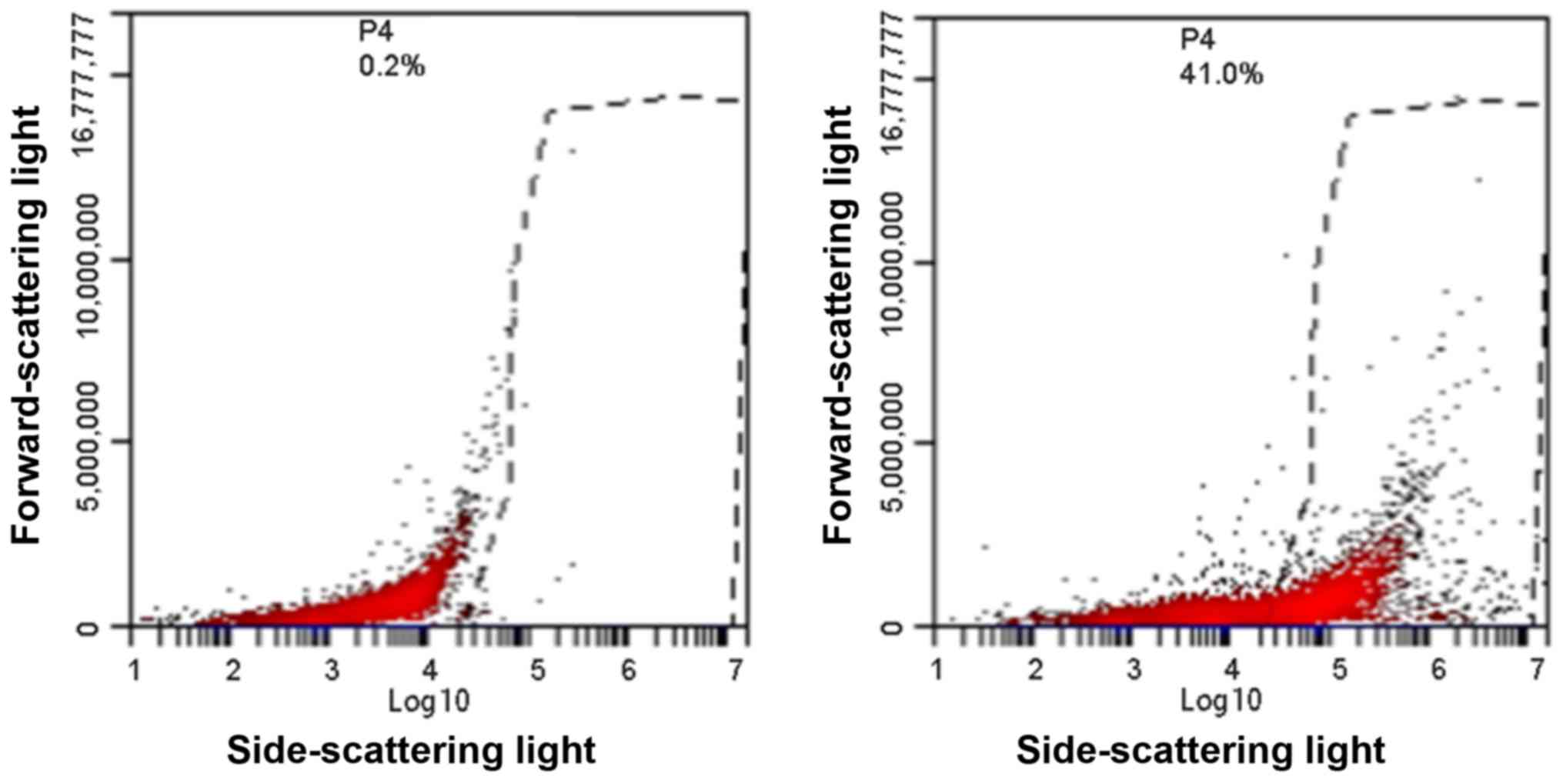
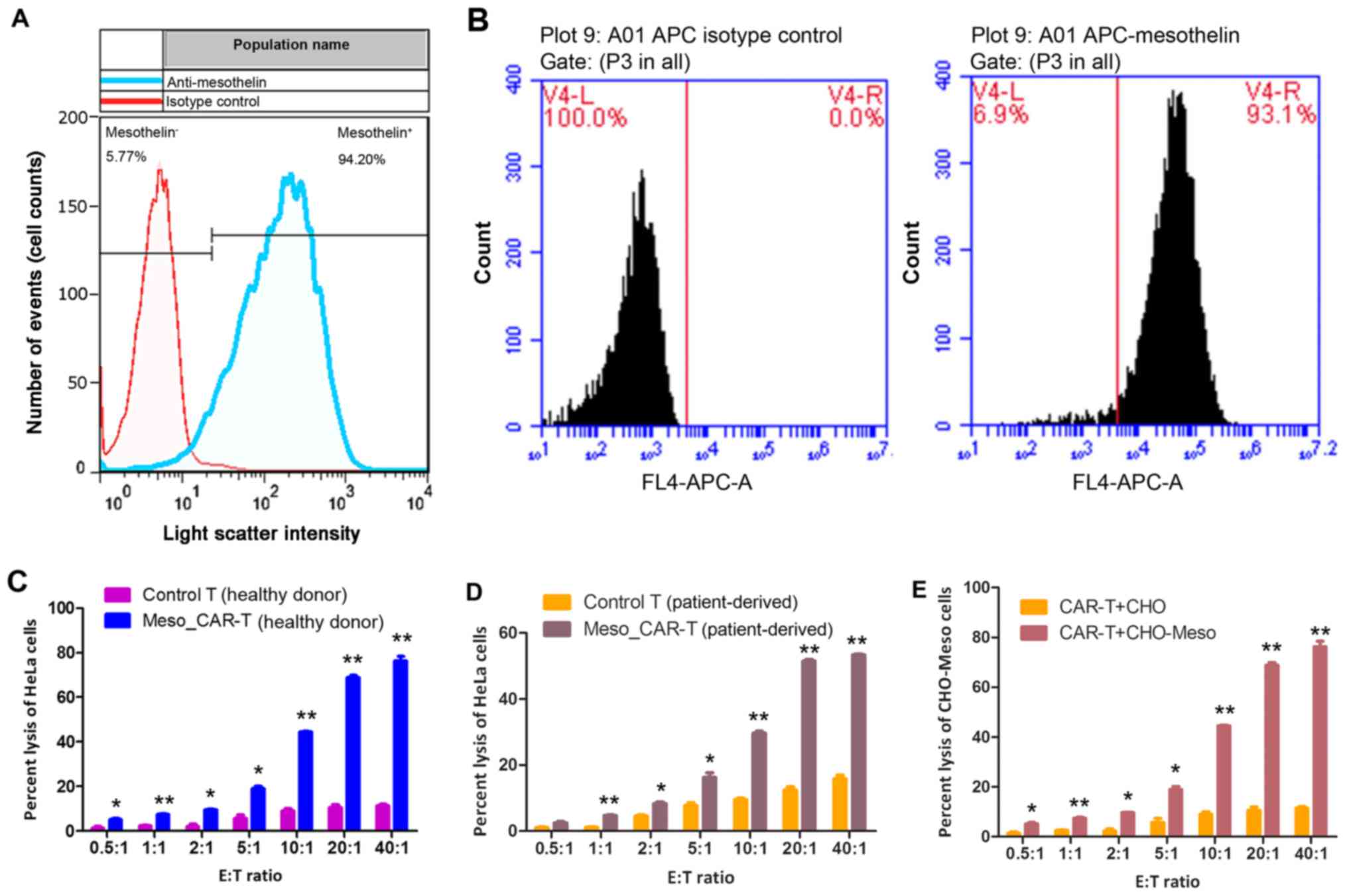
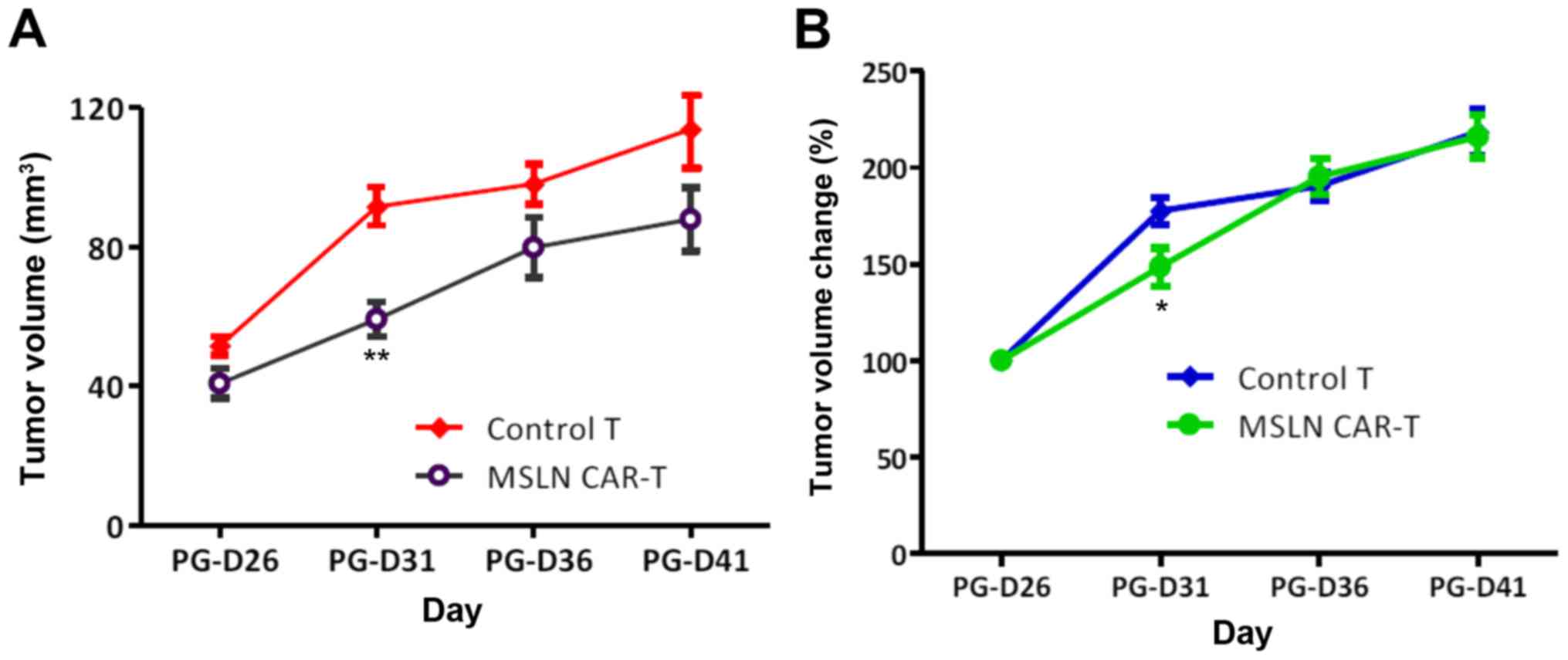
|
1
|
Kirkwood JM, Butterfield LH, Tarhini AA,
Zarour H, Kalinski P and Ferrone S: Immunotherapy of cancer in
2012. CA Cancer J Clin. 62:309–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A and Bolejack V: International Association for the Study
of Lung Cancer Staging and Prognostic Factors Committee, Advisory
Boards, and Participating Institutions, et al The IASLC lung
cancer staging project: Proposals for revision of the TNM stage
groupings in the forthcoming (eighth) edition of the TNM
Classification for lung cancer. J Thorac Oncol. 11:39–51. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ricciardi GR, Russo A, Franchina T,
Ferraro G, Zanghì M, Picone A, Scimone A and Adamo V: NSCLC and
HER2: Between lights and shadows. J Thorac Oncol. 9:1750–1762.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johnson DB, Peng C and Sosman JA:
Nivolumab in melanoma: Latest evidence and clinical potential. Ther
Adv Med Oncol. 7:97–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumarakulasinghe NB, van Zanwijk N and Soo
RA: Molecular targeted therapy in the treatment of advanced stage
non-small cell lung cancer (NSCLC). Respirology. 20:370–378. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weill H, Hughes JM and Churg AM: Changing
trends in US mesothelioma incidence. Occup Environ Med. 61:438–441.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Robinson BW and Lake RA: Advances in
malignant mesothelioma. N Engl J Med. 353:1591–1603. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schiller JH, Harrington D, Belani C,
Langer CP, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group, : Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S,
Manegold C, et al: Phase III study of pemetrexed in combination
with cisplatin versus cisplatin alone in patients with malignant
pleural mesothelioma. J Clin Oncol. 21:2636–2644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rosenberg SA, Yang JC and Restifo NP:
Cancer immunotherapy: Moving beyond current vaccines. Nat Med.
10:909–915. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weber JS: At the bedside: Adoptive cell
therapy for melanoma-clinical development. J Leukoc Biol.
95:875–882. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maude SL, Frey N, Shaw PA, Aplenc R,
Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et
al: Chimeric antigen receptor T cells for sustained remissions in
leukemia. N Engl J Med. 371:1507–1517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carpenito C, Milone MC, Hassan R, Simonet
JC, Lakhal M, Suhoski MM, Varela-Rohena A, Haines KM, Heitjan DF,
Albelda SM, et al: Control of large, established tumor xenografts
with genetically retargeted human T cells containing CD28 and CD137
domains. Proc Natl Acad Sci USA. 106:3360–3365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kreitman RJ, Hassan R, FitzGerald DJ and
Pastan I: Phase I Trial of continuous infusion anti-mesothelin
recombinant immunotoxin SS1P. Clin Cancer Res. 15:5274–5279. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Adusumilli PS, Cherkassky L,
Villena-Vargas J, Colovos C, Servais E, Plotkin J, Jones DR and
Sadelain M: Regional delivery of mesothelin-targeted CAR T cell
therapy generates potent and long-lasting CD4-dependent tumor
immunity. Sci Transl Med. 6:261ra1512014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Glienke W, Esser R, Priesner C, Suerth JD,
Schambach A, Wels WS, Grez M, Kloess S, Arseniev L and Koehl U:
Advantages and applications of CAR-expressing natural killer cells.
Front Pharmacol. 6:212015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pastan I and Hassan R: Discovery of
mesothelin and exploiting it as a target for immunotherapy. Cancer
Res. 74:2907–2912. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hassan R, Bera T and Pastan I: Mesothelin:
A new target for immunotherapy. Clin Cancer Res. 10:3937–3942.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng YM, Spirason N, Iannello P, Jelley L,
Lau H and Barr IG: A simplified Sanger sequencing method for full
genome sequencing of multiple subtypes of human influenza A
viruses. J Clin Virol. 68:43–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hollyman D, Stefanski J, Przybylowski M,
Bartido S, Borquez-Ojeda O, Taylor C, Yeh R, Capacio V, Olszewska
M, Hosey J, et al: Manufacturing validation of biologically
functional T cells targeted to CD19 antigen for autologous adoptive
cell therapy. J Immunother. 32:169–180. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akerstrom B and Bjorck L: Protein L: An
immunoglobulin light chain-binding bacterial protein.
Characterization of binding and physicochemical properties. J Biol
Chem. 264:19740–19746. 1989.PubMed/NCBI
|
|
23
|
Taghian DG and Nickoloff JA: Subcloning
strategies and protocols. Methods Mol Biol. 58:221–235.
1996.PubMed/NCBI
|
|
24
|
Topalian SL, Taube JM, Anders RA and
Pardoll DM: Mechanism-driven biomarkers to guide immune checkpoint
blockade in cancer therapy. Nat Rev Cancer. 16:275–287. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dudley ME, Wunderlich JR, Yang JC, Sherry
RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE,
Mavroukakis SA, et al: Adoptive cell transfer therapy following
non-myeloablative but lymphodepleting chemotherapy for the
treatment of patients with refractory metastatic melanoma. J Clin
Oncol. 23:2346–2357. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Currie GA: Eighty years of immunotherapy:
A review of immunological methods used for the treatment of human
cancer. Br J Cancer. 26:141–153. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rosenberg SA: IL-2: The first effective
immunotherapy for human cancer. J Immunol. 192:5451–5458. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rosenberg SA and Restifo NP: Adoptive cell
transfer as personalized immunotherapy for human cancer. Science.
348:62–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang L, Ren B, Li H, Yu J, Cao S, Hao X
and Ren X: Enhanced antitumor effects of DC-activated CIKs to
chemotherapy treatment in a single cohort of advanced
non-small-cell lung cancer patients. Cancer Immunol Immunother.
62:65–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pan Y, Tao Q, Wang H, Xiong S, Zhang R,
Chen T, Tao L and Zhai Z: Dendritic cells decreased the concomitant
expanded tregs and tregs related IL-35 in cytokine-induced killer
cells and increased their cytotoxicity against leukemia cells. PLoS
One. 9:e935912014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miller JS, Soignier Y,
Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna
D, Le C, Defor TE, Burns LJ, et al: Successful adoptive transfer
and in vivo expansion of human haploidentical NK cells in patients
with cancer. Blood. 105:3051–3057. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fisher JP, Heuijerjans J, Yan M,
Gustafsson K and Anderson J: γδ T cells for cancer immunotherapy: A
systematic review of clinical trials. OncoImmunology. 3:e275722014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Smyth MJ, Godfrey DI and Trapani JA: A
fresh look at tumor immunosurveillance and immunotherapy. Nat
Immunol. 2:293–299. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ho WY, Blattman JN, Dossett ML, Yee C and
Greenberg PD: Adoptive immunotherapy: Engineering T cell responses
as biologic weapons for tumor mass destruction. Cancer Cell.
3:431–437. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sadelain M, Riviere I and Brentjens R:
Targeting tumours with genetically enhanced T lymphocytes. Nat Rev
Cancer. 3:35–45. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
van der Stegen SJ, Hamieh M and Sadelain
M: The pharmacology of second-generation chimeric antigen
receptors. Nat Rev Drug Discov. 14:499–509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Savoldo B, Ramos CA, Liu E, Mims MP,
Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, et al:
CD28 costimulation improves expansion and persistence of chimeric
antigen receptor-modified T cells in lymphoma patients. J Clin
Invest. 121:1822–1826. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Garçon F, Patton DT, Emery JL, Hirsch E,
Rottapel R, Sasaki T and Okkenhaug K: CD28 provides T-cell
costimulation and enhances PI3K activity at the immune synapse
independently of its capacity to interact with the p85/p110
heterodimer. Blood. 111:1464–1471. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fesnak AD, June CH and Levine BL:
Engineered T cells: The promise and challenges of cancer
immunotherapy. Nat Rev Cancer. 16:566–581. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hacein-Bey-Abina S, Garrigue A, Wang GP,
Soulier J, Lim A, Morillon E, Clappier E, Caccavelli L, Delabesse
E, Beldjord K, et al: Insertional oncogenesis in 4 patients after
retrovirus-mediated gene therapy of SCID-X1. J Clin Invest.
118:3132–3142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Durand S and Cimarelli A: The inside out
of lentiviral vectors. Viruses. 3:132–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Montini E, Cesana D, Schmidt M, Sanvito F,
Ponzoni M, Bartholomae C, Sergi Sergi L, Benedicenti F, Ambrosi A,
Di Serio C, et al: Hematopoietic stem cell gene transfer in a
tumor-prone mouse model uncovers low genotoxicity of lentiviral
vector integration. Nat Biotechnol. 24:687–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Srivastava S and Riddell SR: Engineering
CAR-T cells: Design concepts. Trends Immunol. 36:494–502. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cooper LJ, Al-Kadhimi Z, DiGiusto D, Kalos
M, Colcher D, Raubitschek A, Forman SJ and Jensen MC: Development
and application of CD19-specific T cells for adoptive immunotherapy
of B cell malignancies. Blood Cells Mol Dis. 33:83–89. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kochenderfer JN, Dudley ME, Kassim SH,
Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ,
Hughes MS, Sherry RM, et al: Chemotherapy-refractory diffuse large
B-cell lymphoma and indolent B-cell malignancies can be effectively
treated with autologous T cells expressing an anti-CD19 chimeric
antigen receptor. J Clin Oncol. 33:540–549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Schuster SJ, Svoboda J, Nasta SD, Porter
DL, Chong EA, Landsburg DJ, Mato AR, Lacey SF, Melenhorst JJ, Chew
A, et al: Sustained remissions following chimeric antigen receptor
modified T cells directed against CD19 (CTL019) in patients with
relapsed or refractory CD19+ lymphomas. Blood.
126:1832015.
|
|
47
|
Garfall AL, Maus MV, Hwang WT, Lacey SF,
Mahnke YD, Melenhorst JJ, Zheng Z, Vogl DT, Cohen AD, Weiss BM, et
al: Chimeric antigen receptor T cells against CD19 for multiple
myeloma. N Engl J Med. 373:1040–1047. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hege KM, Bergsland EK, Fisher GA,
Nemunaitis JJ, Warren RS, McArthur JG, Lin AA, Schlom J, June CH
and Sherwin SA: Safety, tumor trafficking and immunogenicity of
chimeric antigen receptor (CAR)-T cells specific for TAG-72 in
colorectal cancer. J Immunother Cancer. 5:222017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kershaw MH, Westwood JA, Parker LL, Wang
G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S,
Rogers-Freezer L, et al: A phase I study on adoptive immunotherapy
using gene-modified T cells for ovarian cancer. Clin Cancer Res.
12:6106–6115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Park JR, Digiusto DL, Slovak M, Wright C,
Naranjo A, Wagner J, Meechoovet HB, Bautista C, Chang WC, Ostberg
JR and Jensen MC: Adoptive transfer of chimeric antigen receptor
re-directed cytolytic T lymphocyte clones in patients with
neuroblastoma. Mol Ther. 15:825–833. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Haso W, Lee DW, Shah NN, Stetler-Stevenson
M, Yuan CM, Pastan IH, Dimitrov DS, Morgan RA, FitzGerald DJ,
Barrett DM, et al: Anti-CD22-chimeric antigen receptors targeting
B-cell precursor acute lymphoblastic leukemia. Blood.
121:1165–1174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Arabi F, Torabi-Rahvar M, Shariati A,
Ahmadbeigi N and Naderi M: Antigenic targets of CAR T Cell Therapy.
A retrospective view on clinical trials. Exp Cell Res. 369:1–10.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mami-Chouaib F, Echchakir H, Dorothee G,
Vergnon I and Chouaib S: Antitumor cytotoxic T-lymphocyte response
in human lung carcinoma: Identification of a tumor-associated
antigen. Immunol Rev. 188:114–121. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rump A, Morikawa Y, Tanaka M, Minami S,
Umesaki N, Takeuchi M and Miyajima A: Binding of ovarian cancer
antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol
Chem. 279:9190–9198. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li M, Bharadwaj U, Zhang R, Zhang S, Mu H,
Fisher WE, Brunicardi FC, Chen C and Yao Q: Mesothelin is a
malignant factor and therapeutic vaccine target for pancreatic
cancer. Mol Cancer Ther. 7:286–296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ho M, Bera TK, Willingham MC, Onda M,
Hassan R, Fitzgerald DJ and Pastan I: Mesothelin expression in
human lung cancer. Clin Cancer Res. 13:1571–1575. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Huang CY, Cheng WF, Lee CN, Su YN, Chien
SC, Tzeng YL, Hsieh CY and Chen CA: Serum mesothelin in epithelial
ovarian carcinoma: A new screening marker and prognostic factor.
Anticancer Res. 26:4721–4728. 2006.PubMed/NCBI
|
|
58
|
Han SH, Joo M, Kim H and Chang S:
Mesothelin expression in gastric adenocarcinoma and its relation to
clinical outcomes. J Pathol Transl Med. 51:122–128. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Thomas A, Chen Y, Steinberg SM, Luo J,
Pack S, Raffeld M, Abdullaev Z, Alewine C, Rajan A, Giaccone G, et
al: High mesothelin expression in advanced lung adenocarcinoma is
associated with KRAS mutations and a poor prognosis. Oncotarget.
6:11694–11703. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bharadwaj U, Marin-Muller C, Li M, Chen C
and Yao Q: Mesothelin confers pancreatic cancer cell resistance to
TNF-α-induced apoptosis through Akt/PI3K/NF-κB activation and
IL-6/Mcl-1 overexpression. Mol Cancer. 10:1062011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chang MC, Chen CA, Chen PJ, Chiang YC,
Chen YL, Mao TL, Lin HW, Lin Chiang WH and Cheng WF: Mesothelin
enhances invasion of ovarian cancer by inducing MMP-7 through
MAPK/ERK and JNK pathways. Biochem J. 442:293–302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chang MC, Chen CA, Hsieh CY, Lee CN, Su
YN, Hu YH and Cheng WF: Mesothelin inhibits paclitaxel-induced
apoptosis through the PI3K pathway. Biochem J. 424:449–458. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cristaudo A, Foddis R, Vivaldi A,
Guglielmi G, Dipalma N, Filiberti R, Neri M, Ceppi M, Paganuzzi M,
Ivaldi GP, et al: Clinical significance of serum mesothelin in
patients with mesothelioma and lung cancer. Clin Cancer Res.
13:5076–5081. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Beatty GL, Haas AR, Maus MV, Torigian DA,
Soulen MC, Plesa G, Chew A, Zhao Y, Levine BL, Albelda SM, et al:
Mesothelin-specific chimeric antigen receptor mRNA-engineered T
cells induce anti-tumor activity in solid malignancies. Cancer
Immunol Res. 2:112–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Pauken KE and Wherry EJ: Overcoming T cell
exhaustion in infection and cancer. Trends Immunol. 36:265–276.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wherry EJ, Ha SJ, Kaech SM, Haining WN,
Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL and Ahmed
R: Molecular signature of CD8+ T cell exhaustion during
chronic viral infection. Immunity. 27:670–684. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Blackburn SD, Shin H, Haining WN, Zou T,
Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA and Wherry
EJ: Coregulation of CD8+ T cell exhaustion by multiple
inhibitory receptors during chronic viral infection. Nat Immunol.
10:29–37. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Paley MA, Kroy DC, Odorizzi PM, Johnnidis
JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner
SL and Wherry EJ: Progenitor and terminal subsets of
CD8+ T cells cooperate to contain chronic viral
infection. Science. 338:1220–1225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Johnston RJ, Comps-Agrar L, Hackney J, Yu
X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, et al:
The immunoreceptor TIGIT regulates antitumor and antiviral
CD8+ T cell effector function. Cancer Cell. 26:923–937.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Callahan MK and Wolchok JD: At the
Bedside: CTLA-4- and PD-1-blocking antibodies in cancer
immunotherapy. J Leukoc Biol. 94:41–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wherry EJ: T cell exhaustion. Nat Immunol.
12:492–499. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Brown CE, Badie B, Barish ME, Weng L,
Ostberg JR, Chang WC, Naranjo A, Starr R, Wagner J, Wright C, et
al: Bioactivity and safety of IL13R 2-redirected chimeric antigen
receptor CD8+ T cells in patients with recurrent
glioblastoma. Clin Cancer Res. 21:4062–4072. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Gomez GG: Adoptive T cell therapies for
glioblastoma. J Cancer Clin Trials. 1:1–5. 2015.
|
|
74
|
Ghosh A, Smith M, James SE, Davila ML,
Velardi E, Argyropoulos KV, Gunset G, Perna F, Kreines FM, Levy ER,
et al: Donor CD19 CAR T cells exert potent graft-versus-lymphoma
activity with diminished graft-versus-host activity. Nat Med.
23:242–249. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Qasim W, Amrolia P, Samarasinghe S,
Ghorashian S, Zhan H, Stafford S, et al: First clinical application
of talen engineered universal car19 T cells in B-ALL. Blood.
126:20462015.
|
|
76
|
Stepanenko AA and Dmitrenko VV: HEK293 in
cell biology and cancer research: Phenotype, karyotype,
tumorigenicity, and stress-induced genome-phenotype evolution.
Gene. 569:182–190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Jena B, Dotti G and Cooper LJ: Redirecting
T-cell specificity by introducing a tumor-specific chimeric antigen
receptor. Blood. 116:1035–1044. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Singh H, Manuri PR, Olivares S, Dara N,
Dawson MJ, Huls H, Hackett PB, Kohn DB, Shpall EJ, Champlin RE and
Cooper LJ: Redirecting specificity of T-cell populations for CD19
using the sleeping beauty system. Cancer Res. 68:2961–2971. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ma JS, Kim JY, Kazane SA, Choi SH, Yun HY,
Kim MS, Rodgers DT, Pugh HM, Singer O, Sun SB, et al: Versatile
strategy for controlling the specificity and activity of engineered
T cells. Proc Natl Acad Sci USA. 113:e450–e448. 2016. View Article : Google Scholar : PubMed/NCBI
|