Introduction
With the advancement in transportation options,
millions of people travel to areas of high-altitude for
recreational, sporting and military purposes. Thus, the occurrence
of accidental injuries at high-altitudes has risen rapidly by 12.7%
(1–3). Injuries from road traffic accidents
maintained high mortality rates also at high-altitudes (4) and traumatic brain injury (TBI) obtained
at high-altitudes are one of the most serious acute diseases
(5). The main feature of
high-altitude is hypobaric hypoxia, which often causes damage to
the body, including high-altitude cerebral edema or high-altitude
pulmonary edema, as a result, the central nervous system,
circulation and respiratory system function impairment occurs
(6). High-altitude cerebral oedema
(HACE) and dehydration are common in individuals who are not
acclimated to the altitude and can lead to mortality (7–9). TBI
following abrupt exposure to higher altitudes can be more
complicated than when observed at sea level. Mild-to-moderate
closed-head injury (mmCHI) accounts for 81.53% of all TBI cases
recorded in the higher regions of China (>3,000 m above sea
level) (10). The majority of
patients with mmCHI are conscious, exhibit concussions and mild
brain contusions (11). In mild
cases, certain patients are able to travel to nearby hospitals by
themselves or be transported by medical staff. However, mmCHI cases
at high-altitude are not well characterized. With inadequate
knowledge of the pathophysiological changes in high-altitude mmCHI,
clinicians often exclude mmCHI as a cause for TBI due to the high
Glasgow Coma Score observed, which evaluate the extent of
neurological damage (12). At
present, no relevant guidelines about high-altitude mmCHI have been
implemented (13,14).
In the present study, to simulate a rapid ascent to
high-altitudes causing acute hypobaric hypoxia (AHH), 6,000 m above
sea level was chosen as model high-altitude and hypobaric chambers
were automatically adjusted depending on the pre-set pressures
selected to simulate different high-altitude models in rats
(extreme high-altitude, 5,500-8,850 m; medium high-altitude,
3,500-5,500 m; high-altitude, 1,500-3,500 m) (15). Weight-drop models were used to
reproduce the physiopathology and the macro-and microscopic
alterations in TBI, which were observed via imaging techniques and
by examining neuronal damage markers to study new therapeutic
approaches. The aim of the present study was to investigate the
association between cerebral oedema, body weight (BW) and
neurological function following acute high-altitude mmCHI, using
magnetic resonance imaging (MRI) and glial fibrillary acidic
protein (GFAP) immunohistochemistry to analyse whether transporting
patients to different altitudes has an effect on injury outcome.
The effects of rapid transport to lower altitudes on brain injury,
pathophysiological association with altitude and the time window
for most effective transport were further evaluated. The results of
the current study may provide a reference for the clinical
treatment of mmCHI at high altitude.
Materials and methods
Animals
A total of 162 male Sprague-Dawley rats (weight,
200±20 g; age, 7–8 weeks) were supplied by the Experimental Center
for Medical Animals, Research Institute of Surgery, Daping Hospital
(Third Military Medical University, Chongqing, China). Animals were
allowed 2 days prior to experiments to acclimatize to the
facilities and for training according to the neurological severity
score (NSS) guidelines (16). All
rats were housed under a controlled temperature (18°C) with 12-h
light/dark cycles and the range of atmosphere (Atm) was 970–1,000
Pascal (Pa). Rats had access to food and water ad libitum.
Experiments were approved by the Ethics Committee of Third
Affiliated Hospital and Research Institute of Surgery, Third
Military Medical University (registration no. ChiCTR-RPC-15006770;
Chongqing, China).
Preparation of animal models and
grouping
The present study established models of AHH and
simulated conditions of different altitudes. Rats were placed into
a decompression chamber (Chongqing Key Laboratory of Vehicle
Crash/Bio-impact and Traffic Safety, Institute for Traffic
Medicine, Third Military Medical University, Chongqing, China) for
exposure to an extreme high-altitude of 6,000 m at a velocity of
6.7 m/sec for 15 min, with −52 to −57 kPa and the corresponding
oxygen content was 149 g/m3 for 24 h. The chamber humidity was set
to 40–50% and temperature was maintained at 18°C with a 12-h
light/dark cycle, in addition to free access to water and food. The
CHI model was established via brain injury using a weight-drop
device (17–19), modified for a pneumatic impact at a
force of 0.8 MPa (6.67 m/sec; 955.6±16.35 N). Rats were
anaesthetised with 2% isoflurane (1.5 l/min) using anaesthesia
equipment (ZS-M; Beijing Zhongshi Di Chuang Technology Development
Co., Ltd., Beijing, China) for ~2 min prior to injury. Animals that
perished following the trauma or due to skull fractures were
excluded from the present study. Cardiopulmonary resuscitation
(CPR) was performed immediately when rats were found to have apnea
following mmCHI. The oropharyngeal airway tube was placed
immediately and chest compressions were performed until rats
recovered spontaneous breathing. In the experimental study,
uninjured control rats were simulated to ascend rapidly from plain
(200 m above sea level) to plateau (6,000 m above sea level) and
were then maintained in a continuous hypobaric environment with
hypoxia at an altitude of 6,000 m for 48 h (n=6). mmCHI rats,
following exposure to 6,000 m altitude for 24 h, were divided into
groups according to altitude: Non-descending altitude group (ND;
maintained at 6,000 m); D-4,500 m group (descent to 4,500 m;
pressure: −42 to −47 kPa; oxygen content, 176 g/m3); and D-3,000 m
group (descent to 3,000 m; pressure, −30 to −35 kPa; oxygen
content, 206 g/m3). In order to observe changes in injuries over
time, each mmCHI group was divided into three subgroups: 6, 12 and
24 h following descend (n=18/subgroup).
Determination of BW, vital signs and
NSS
Weights of rats were determined prior to being
placed in the decompression chamber and at 24 h following exposure
to high-altitude. Spontaneous breathing, level of consciousness and
limb function following injury were observed and recorded.
According to the NSS standards (19), scoring was performed 30 min following
injury. The duration of coma and apnoea were further recorded.
Following the initial scoring, rats in each group were evaluated
and weighed again at 6, 12 and 24 h following descend.
Brain MRIscan
Rats in each group were randomly selected
(n=6/group) and brain MRI scans were recorded at 6, 12 and 24 h
following descend. Prior to MRI, rats were anaesthetised using the
aforementioned method. Body temperature was continuously maintained
at 37±0.5°C and blood oxygen saturation and heart rate were
monitored during the MRI scan. The 7.0T MRI (Bruker 7.0T/20 cm
BioSpec-Avance system; Bruker Corporation, Ettlingen, Germany) was
used to scan the brain tissues of rats through T2-weighted imaging
[T2WI; rapid acquisition relaxation-enhanced (RARE) echo time, 30
msec; repetition time, 4,000 msec; spatial resolution: 256×256
matrix; field of view, 30×30 mm; slice thickness, 0.5 mm;
interslice distance, 0 mm; RARE factor, 4], the field homogeneity
across the brain was optimized and coronal scout images were
obtained to orient the transverse slices throughout the brain
region of interest (ROI).
Determination of brain water content
(BWC)
Injured rats in each group (n=6/group) were
anesthetized with an intraperitoneal 1% sodium pentobarbital (v/v)
injection (45 mg/kg) and brain tissues were collected following
decapitation at 6, 12 and 24 h post-injury. The wet weight of each
brain was measured using a precision electronic balance
(BSA124S-CW; division value, d=0.1 mg; Sartorius AG, Göttingen,
Germany). Tissues were then placed in a thermostatic oven and
heated at 80°C for 48 h to obtain a constant weight. In accordance
with Elliot's formula (20), the
percentage of BWC was calculated as follows: Water content
(%)=[(wet weight-dry weight)/wet weight] ×100%.
Morphological analysis
Injured rats of each group were randomly selected
(n=6/group). Brain tissues of each group were collected at 24 h
following mmCHI and fixed in 4% paraformaldehyde in 0.1 M PBS (pH
7.4) at 4°C overnight. Samples were sectioned (10 µm) using a
cryostat (Leica CM 1950; Leica Microsystems GmbH, Wetzlar, Germany)
and mounted on adhesion microscope slides. Pathological features of
the transverse sections of brain tissues from the width of the
lesion site were assessed by immunohistochemistry. Brain tissues
slides were blocked for 1 h using 10% bovine serum albumin
(Fraction V; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at room
temperature and 0.3% Triton X-100 and then incubated overnight at
4°C with glial fibrillary acidic protein antibodies (GFAP; cat. no.
YM3059; dilution, 0.5 µl/ml; ImmunoWay Biotechnology Company,
Plano, TX, USA). Following washing with PBS, tissues were incubated
for 1–2 h at room temperature with Alexa Fluor® 555 goat
anti-rabbit IgG (cat. no. A27017; dilution, 2 µl/ml; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Following several PBS washes,
nuclei were stained for 10 min at room temperature with DAPI
Fluoromount-G (SouthernBiotech, Birmingham, AL, USA) and staining
was detected via fluorescent microscopy (DM3000; Leica Microsystems
GmbH). The number of positive cells was manually counted at 20×
magnification and adjusted using image analysis software (Image-Pro
plus 5.0; Media Cybernetics, Inc., Rockville, MD, USA) by two
blinded, trained investigators. The ratio of positive cells was
calculated as following: (Number of positive cells/total number of
cells) ×100%.
Statistical analysis
Factorial design with SAS9.2 (SAS Institute Inc.,
Cary, NC, USA) was used for statistical analysis. The normal
distribution of data (BWC and GFAP-positive cell levels) was
measured and described using the mean ± standard deviation. The
double-factor experimental design was compared between groups using
two-way analysis of variance (ANOVA) with a Student-Newman-Keuls
(SNK) post hoc test. Abnormal data distribution (NSS and BW) was
measured and described using the median and quartile range (QR).
The median value of NSS reduced used the double-factor experimental
results were compared between groups using the Scheirer-Ray-Hare
test and the Wilcoxon matched pairs signed-rank test. In addition,
prior to using the SNK method, pair-wise comparisons of the primary
data were sorted to generate new variables. The location of lesions
in MRI images were determined using ImageJ (1.47v; National
Institutes of Health, Bethesda, MD, USA) by tracing the area of
quantification using the plug-in analysis tool. ROIs were segmented
by manual selection or setting thresholds, then the area (cm2) was
calculated automatically according to the scale. Values of ROIs are
presented as the mean ± standard deviation. Two-way ANOVA and post
hoc Tukey's test were conducted to determine statistical
significance using SPSS17.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Vital signs and NSS
Following mmCHI, the vital sign analysis revealed
that 57/126 rats exhibited either apnoea or seizure or both and
that, of these 57 rats, 22 (38.6%) exhibited apnoea alone, 21
(36.8%) exhibited seizure alone and 14 (24.6%) exhibited both
aponea and seizure. Rats underwent cardiopulmonary resuscitation
(CPR) for 1–3 min until breathing resumed; one rat that perished
due to failed CPR was excluded from the present study and was
replaced with another rat that underwent the same group-specific
procedures. The CHI procedure is the same as that of a previous
study (21) and the observed
mortality rate was 0.6% (1/162), which was consistent with data
that has been previously reported (0–10%) (16,22).
Mean coma duration was 13.13±10.4 min (n=162). NSS was determined
(6±2.5) and limb dysfunction was not observed at 30 min following
injury, which is in line with the reported effects of mmCHI
(23). NSS analysis revealed that
scores of injured rats in each group gradually improved over 24 h
regardless of altitude changes. When comparing the time point
effect using the Scheirer-Ray-Hare test between the groups, there
was a consistent change in NSS score at the corresponding time
points; however, the effect on the groups and the associations
between the time points and the different groups was not
statistically different. The results of different time point
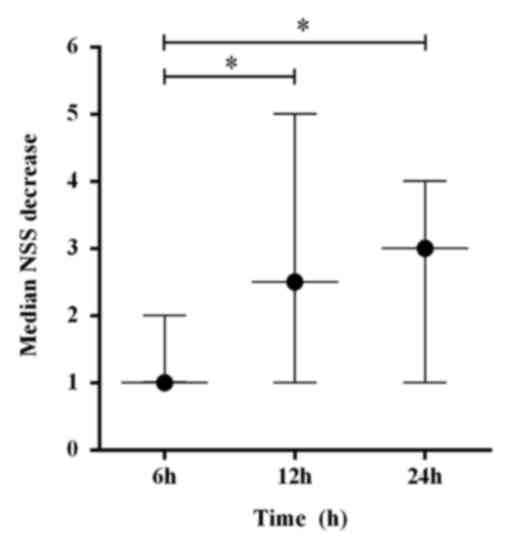
experiments on NSS revealed significant median reductions in NSS of
2.5 (QR, 1–5) at 12 h and 3 (QR, 1–4) at 24 h when compared with 1
(QR, 1–2) at 6 h (H=10.29; P<0.05; Fig. 1).
BWchanges
Following exposure to AHH for 24 h and prior to
injury, the median BW of rats decreased from 215.0 g (QR,
205.3–221.2 g) to 190.0 g (QR, 182.8–200.6 g). Results of Wilcoxon
matched pairs signed-rank test demonstrated that the mean BW of
rats significantly decreased by 23.9 g (QR, 12.3–26.4 g) at 24 h,
accounting for 9.90% (QR, 2.00–15.30%) of the initial BW
(P<0.05; Fig. 2A). The strongest
change in BW was observed in the ND group following mmCHI. The BW
loss for each group at each time point when compared with the
initial weight was as follows: ND [6 h, 15.42% (QR, 10.00–20.00%);
12 h, 14.83% (QR, 13.50–16.60%); 24 h, 20.20% (QR, 17.60–21.20%)];
D-4,500 m [6 h, 15.58% (QR, 14.00–18.10%); 12 h, 15.56% (QR,
9.60–17.80%); 24 h, 17.87% (QR, 20.90–13.50%)]; and D-3,000 m [6 h,
10.08% (QR, 6.90–12.80%); 12 h, 14.08% (QR, 10.50–16.70%); 24 h,
14.90% (QR, 12.10–18.80%)]. BW changes following mmCHI with
abnormal distribution were analysed using the Scheirer-Ray-Hare
test at 6, 12, and 24 h following mmCHI; the difference between the
final and initial BW for each group at different time points was
calculated. BW comparisons of each group following injury revealed
that the median BW loss in the D-3,000 m group [8.6 g (QR, 6.8–12.8
g)] was decreased compared with the ND group [11.15 g (QR,
9.15–14.35 g)] and the D-4,500 m group [10.3 g (QR, 8.4–13.8 g)].
It was demonstrated that weight loss significantly changed
following mmCHI at different high-altitudes (H=6.96; P<0.05;
Fig. 2B). Over time there
significant changes in weight loss were observed for the injured
rats of the ND, D-4,500 and D-3,000 m groups, with 6.95 g (QR,
6.3–8.4 g) between 0–6 h, 10.0 g (QR, 8.7–10.4 g) between 0–12 h,
and 14.4 g (QR, 12.8–16.5 g) between 0–24 h (H=36.43; P<0.001;
Fig. 2C). In the control group,
comprised of rats that were not injured following acute
high-altitude exposure, changes in BW between 0–24 h were 9.55 g
(QR, 7.3–12.2 g), which was significantly decreased compared with
the injured animals over the same period (P<0.05; Fig. 2C). No association between the various
injury groups at different time point were determined with regards
to the median BW (H=6.58; P=0.721).
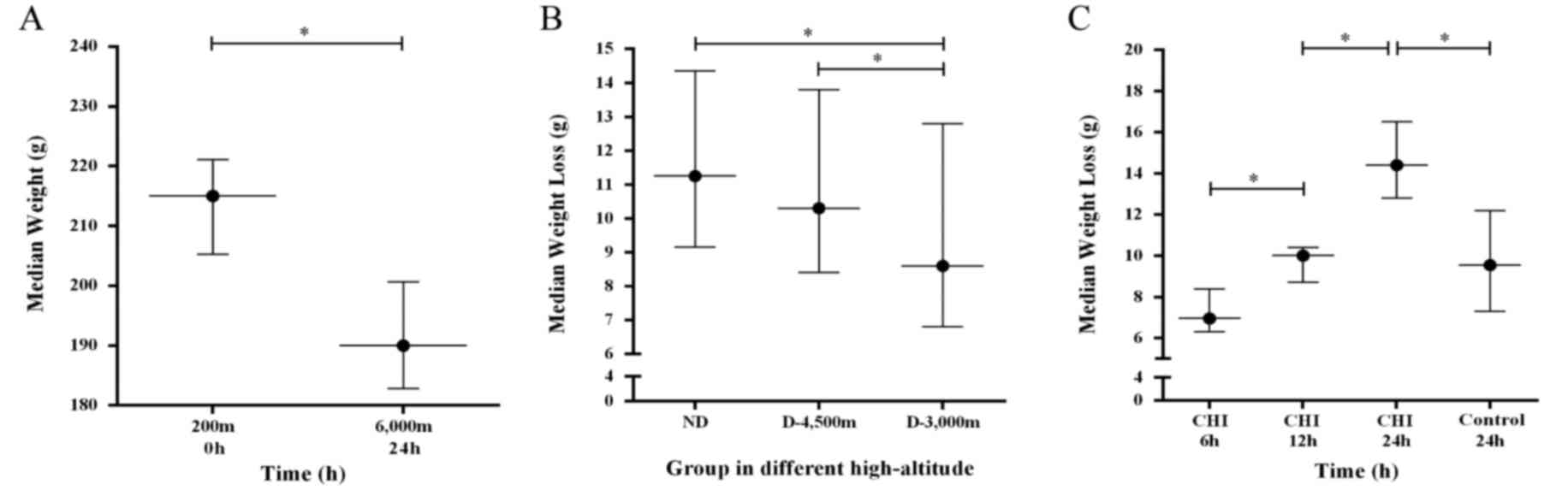 | Figure 2.BW decreases over the time for rats
in the high-altitude groups. (A) Wilcoxon matched pairs signed-rank
test was applied to compare the BW of rats; The median weight from
200 m ascended to 6,000 m and exposure for 24 h was 23.9
(26.4–12.3) g, the difference was statistically significant,
*P<0.05. (B) Median weight loss of rats with mmCHI in the
various experimental groups; the difference to the initial BW is
reported. *P<0.05. (C) Weight loss over time with the results
for the groups combined. The control describes the median weight
loss of rats not injured following acute high-altitude exposure at
48 h; all other animals were exposed to acute high-altitude for 24
h prior to injury and subsequent descend. *P<0.05. Plain, 200 m
above sea level; Plateau, 6,000 m above sea level exposure for 24
h; BW, body weight; mmCHI, mild-to-moderate closed head injury; ND,
non-descending altitude; D-4,500 m, descent to 4,500 m following
injury; D-3,000 m, descent to 3,000 m following injury. |
BWC
BWC continuously increased in the ND group following
mmCHI and it continuously decreased in the D-4,500 m and the
D-3,000 m groups. A two-way ANOVA revealed no significant
differences in BWC among the different groups or the time points
(Fig. 3A). The present study
calculated the ratio of BWC to BW (‰). Two-way ANOVA demonstrated
that following mmCHI, BWC/BW increased in the ND group over time (6
h, 4.304±0.206‰; 12 h, 4.482±0.193‰; and 24 h, 4.884±0.217‰). In
the D-4,500 and D-3,000 m groups at 6 h following injury, the
BWC/BW was 4.389±0.215 and 3.987±0.275‰, respectively, and peaked
at 12 h following injury with 4.548±0.264 and 4.451±0.224‰,
respectively. In the following measurement at 24 h following mmCHI,
the BWC/BW decreased in the D-4,500 and D-3,000 m groups
(4.183±0.228 vs. 4.414±0.395‰). All changes exhibited by the
various groups were statistically significant (F=7.22; P<0.05).
When comparing the different time points following mmCHI at
high-altitudes, results revealed that at 6 h the BWC/BW for D-3,000
m was the lowest and the difference was statistically significant
compared with the ND and the D-4,500 m groups (P<0.05). At 12 h
following mmCHI, no significant differences were observed between
the groups. At 24 h following injury, the highest ratio was
observed in the ND group, which was significantly increased
compared with the D-3,000 and D-4,500 m groups (P<0.05).
Additionally, an association between the different time-points and
various groups was determined (F=8.68; P<0.05; Fig. 3B).
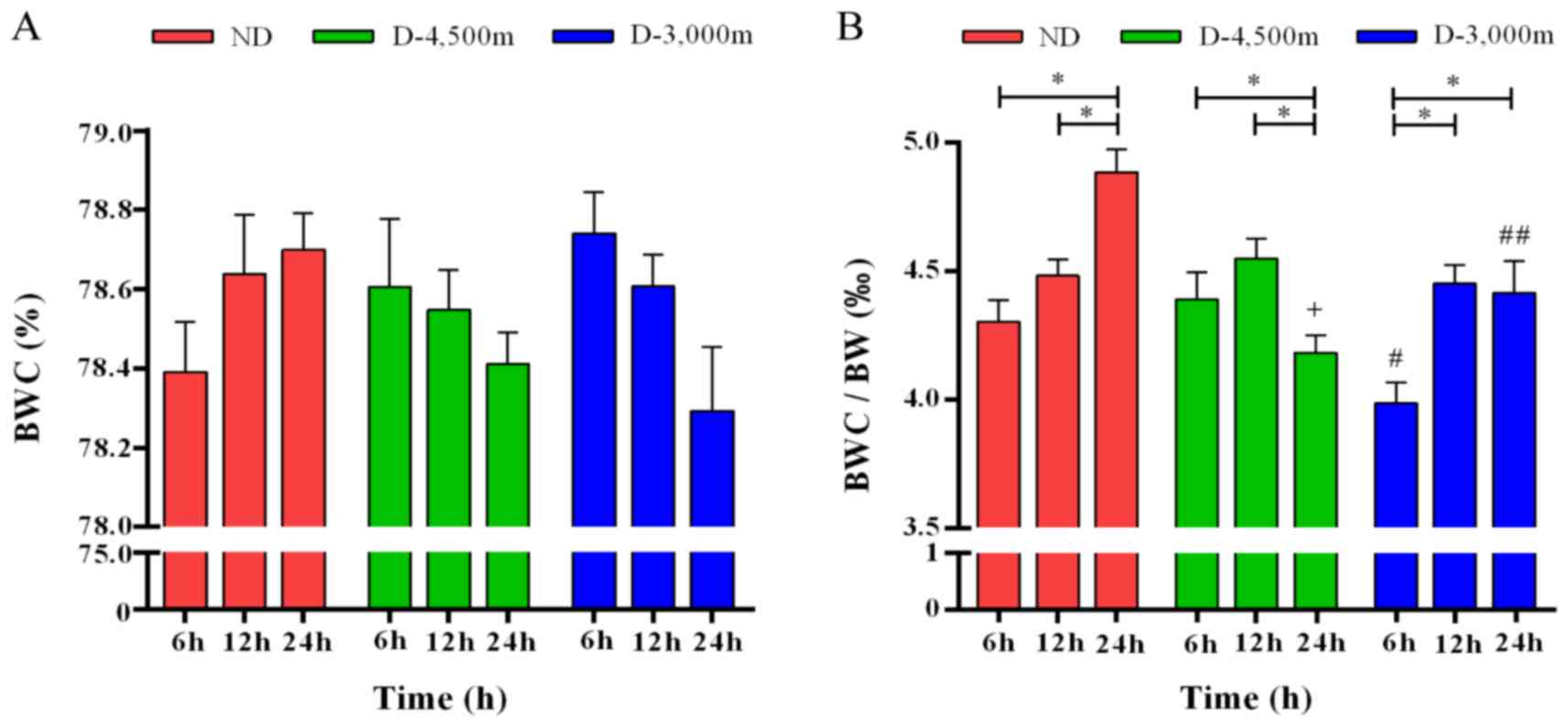 | Figure 3.BWC is associated with BW changes
overtime in rats with mild-to-moderate closed head injury exposed
to high-altitudes. (A) BWC in various groups over time did not
change significantly. No association between time and altitude was
observed. (B) The ratio of BWC/BW (‰) significantly changed over
time in the various groups, *P<0.05. Differences between the
altitude groups were significant at 6 and 24 h following injury:
+P<0.05 vs. ND 24 h; #P<0.05 vs. ND 6 h; ##P<0.05 vs. ND
24 h (n=6/group). P<0.05 was considered to indicate a
statistically significant difference. BWC, brain water content; BW,
body weight; ND, non-descending altitude; D-4,500 m, descent to
4,500 m following injury; D-3,000 m, descent to 3,000 m following
injury. |
MRI analysis
To evaluate the diagnostic value of MRI on
determining the level of brain damage in rats with mmCHI following
AHH, brain tissues of rats were examined at 6, 12 and 24 h
following injury at varying altitudes using T2WI. The MRI scan in
T2WI revealed that the signals of cortex and white matter were
uniform and there was no cerebral oedema, contusion or haemorrhage
in the cortex of the impact region. Furthermore, it did not
identify subarachnoid haemorrhages (SAH) or intraventricular
haemorrhage (IVH) (data not shown). The region of the corpus
callosum (CC) produced high signals, attributed to an increase in
the BWC in these tissues. The lateral ventricles (LV) were markedly
dilated at 6 h following injury in each group. Furthermore, the
area of the ROIs were analyzed (Fig. 4A
and B).
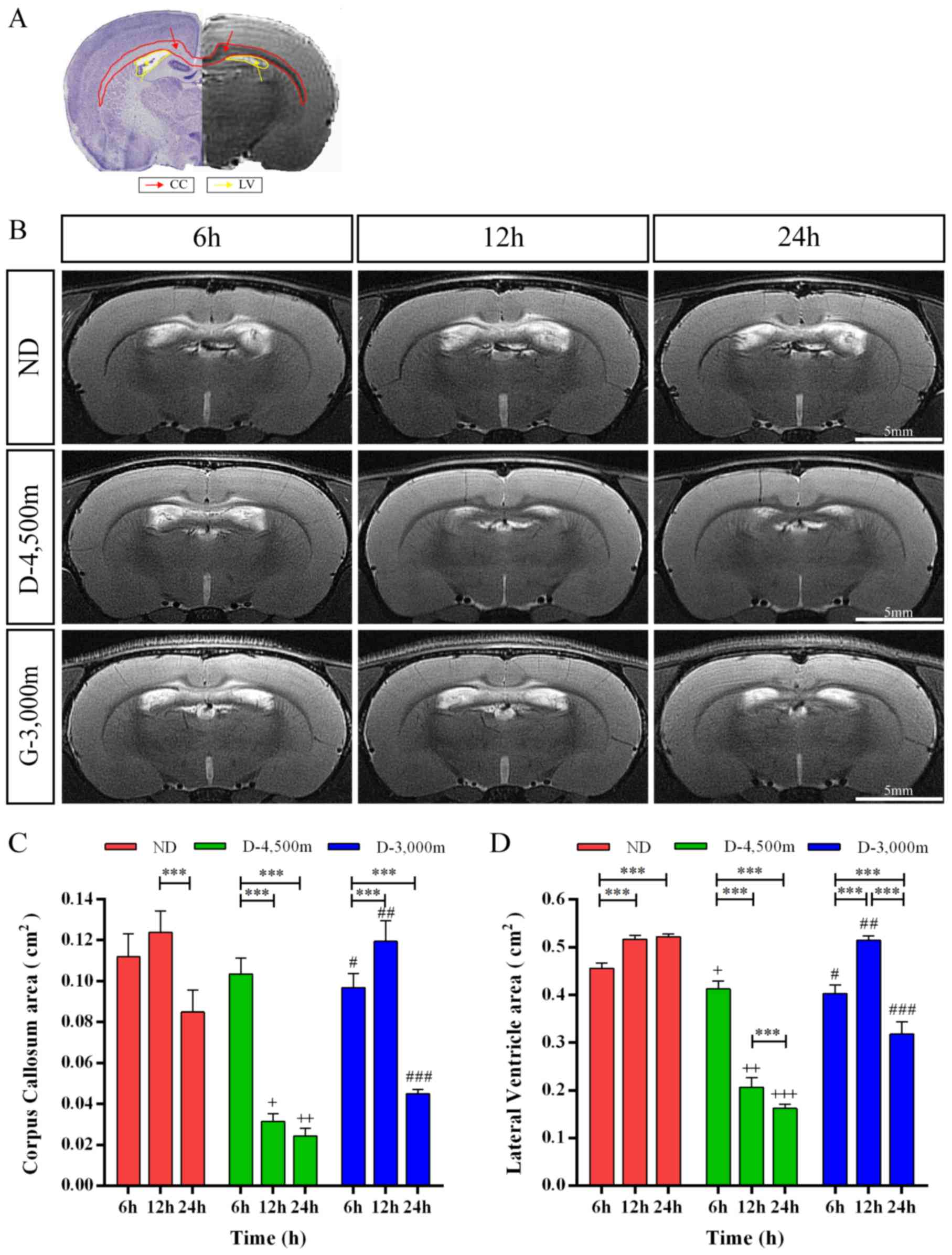 | Figure 4.ROIs vary between time points for
various altitude groups of rats with mmCHI. (A) Haematoxylin-eosin
staining and coronal T2 imaging revealed the location of ROIs in
the normal brain aiding the calculation of the area of the CC (red)
and the LV (yellow). (B) Time-dependent changes in the T2-weighted
anatomical MRI of various groups following AHH mmCHI at 6, 12 and
24 h; a hyper-intense signal covering the CC and LV dilation were
observed in the brains. Scale bar, 5 mm. CC and LV changes overtime
in rats with mild-to-moderate closed head injury exposed to
high-altitudes. (C) The effect of time in each group on CC
swelling, ***P<0.001; Differences among groups on CC swelling:
+P<0.05 vs. ND and D-3,000 m 12 h; ++P<0.05 vs. ND and
D-3,000 m 24 h; #P<0.05 vs. ND 6 h; ##P<0.05 vs. D-4,500 m 12
h; ###P<0.05 vs. ND and D-4,500 m 24 h. (D) The effect of time
effects in each group on LV dilation.***P<0.001; Differences
among different groups on CC swelling: +P<0.05 vs. ND 6 h;
++P<0.05 vs. ND and D-3,000 m 12 h; +++P<0.05 vs. ND and
D-3,000 m 24 h; #P<0.05 vs. ND 6 h; ##P<0.05 vs. D-4,500 m 12
h; ###P<0.05 vs. ND and D-4,500 m 24 h. ROIs, regions of
interest; AHH, acute hypobaric hypoxia; mmCHI, mild-to-moderate
closed head injury; MRI, magnetic resonance imaging; CC, corpus
callosum; LV, lateral ventricle; ND, non-descending altitude;
D-4,500 m, descent to 4,500 m following injury; D-3,000 m, descent
to 3,000 m following injury. |
Two-way ANOVA revealed that time (F=211.376;
P<0.001), altitude (F=206.061; P<0.001) and interactions
between time and altitude (F=69.561; P<0.001) had significant
effects on CC swelling. At 6 h post-mmCHI, CC swelling was
significantly higher in the ND group (0.11±0.01 cm2; P<0.05)
compared with the D-3,000 m group (0.10±0.01 cm2). However, there
were no significant differences between the D-4,500 m group
(0.10±0.01 cm2) and the ND or D-3,000 m group. At 12 h post-mmCHI,
CC swelling was significantly lower in the D-4,500 m group
(0.03±0.01 cm2; P<0.05) compared with the ND (0.12±0.01 cm2) or
D-3,000 m groups (0.12±0.01 cm2). At 24 h post-mmCHI, CC swelling
was significantly higher in the D-3,000 m group (0.05±0.01 cm2;
P<0.05) compared with the D-4,500 m group (0.02±0.01 cm2) and
results in the altitude groups were significantly decreased
compared with the ND group (0.08±0.01 cm2; P<0.05). Tukey's post
hoc tests were applied to identify significance for P≤0.05 and the
following was observed: CC swelling was significantly lower at 24 h
following mmCHI compared with 12 h in the ND group. In the D-4,500
m group CC swelling was significantly higher at 6 h compared with
12 or 24 h. For the D-3,000 m group, levels of CC swelling were
significantly lower at 6 h compared with 12 h and significantly
higher at 6 h compared with 24 h following mmCHI (Fig. 4C).
In addition, two-way ANOVA revealed that time
(F=123.760; P<0.001), altitude (F=753.681; P<0.001) and
interactions between time and altitude (F=188.876; P<0.001) had
significant effects on LV dilation. At 6 h following mmCHI, LV
dilation was significantly higher in the ND group (0.46±0.03 cm2;
P<0.05) compared with the D-4,500 m (0.41±0.02 cm2; P<0.05)
or D-3,000 m groups (0.40±0.01 cm2; P<0.05). At 12 h post-mmCHI,
LV dilation was lower in the D-4,500 m group (0.21±0.02 cm2;
P<0.05) compared with the ND (0.52±0.02 cm2) or D-3,000 m groups
(0.51±0.01 cm2). At 24 h post-mmCHI, LV dilation was significantly
higher in the D-3,000 m group (0.32±0.03 cm2; P<0.05) compared
with the D-4,500 m group (0.16±0.01 cm2), and significantly lower
in the D-3,000 m group compared with the ND group (0.52±0.01 cm2;
P<0.05). Tukey's post hoc tests were applied to identify
significance for P≤0.05 and the following was observed: In the ND
group, LV dilation was significantly lower at 6 h compared with 12
or 24 h following mmCHI. In the D-4,500 m group, LV dilation was
significantly lower at 12 h compared with 6 h and significantly
higher at 12 h compared with 24 h following mmCHI. LV dilation was
significantly lower at 6 h compared with 12 h and significantly
higher at 6 h compared with 24 h following mmCHI in the D-3,000 m
group (Fig. 4D).
GFAP immunohistochemistry
To determine the effect of high-altitudes on
reactive astrogliosis following AHH mmCHI in vivo, the
present study conducted immunofluorescence staining and discovered
that GFAP-positive astrocytes were observed in injured brains at 24
h post-mmCHI. Quantitatively, the ratio of GFAP-positive astrocytes
in the D-3,000 m group (45.32±4.17%; P<0.05) was significantly
higher compared with the D-4,500 m group (36.26±3.55%) and
significantly decreased compared with the ND group (56.88±5.62%;
Fig. 5A and B).
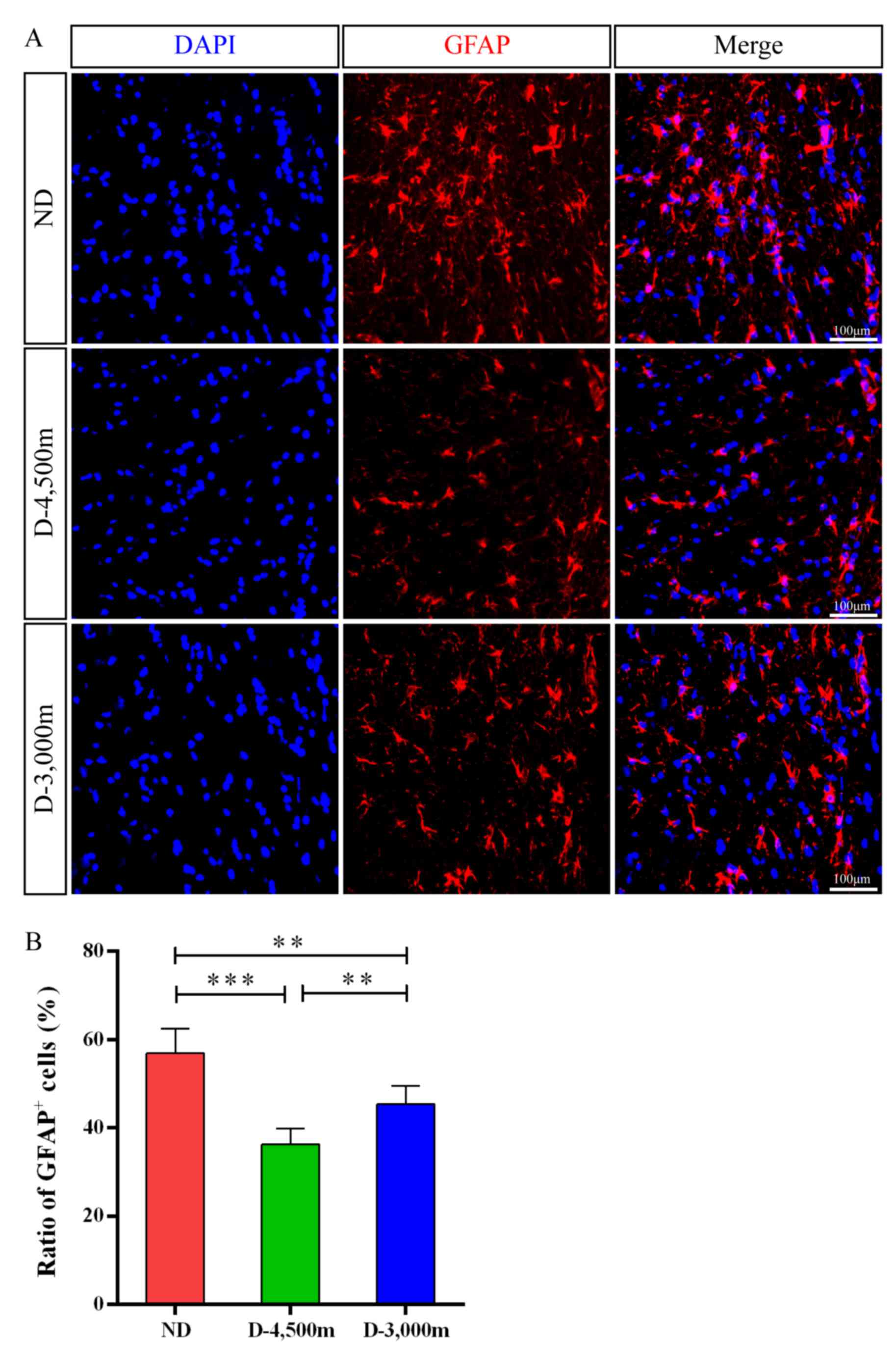 | Figure 5.GFAP+ cell number changes in the
brains of rats with mmCHI at 24 h following injury. (A)
Representative images for GFAP+ cells (red) revealed the
distribution of astrocytes in the injured brain among the ND,
D-4,500 and D-3,000 m groups at 24 h following TBI. Nuclei were
counterstained with DAPI (blue); scalebar, 100 µm. (B) GFAP+ cells
in the injured brain for the ND, D-4,500 and D-3,000 m groups at 24
h post-TBI (n=6/group). ***P<0.001 vs. D-4,500 m group;
**P<0.01 vs. D-3,000 m group. GFAP, glial fibrillary acidic
protein; mmCHI, mild-to-moderate closed head injury; TBI, traumatic
brain injury; ND, non-descending altitude; D-4,500 m, descent to
4,500 m following injury; D-3,000 m, descent to 3,000 m following
injury. |
Discussion
Abrupt exposure to higher altitudes (>3,500 m
above sea level) can cause acute mountain sickness and can lead to
complications, including HACE, high-altitude pulmonary oedema,
reduced body heat metabolism in plateau, increased evaporation, and
significant dehydration, which in turn lead to increased difficulty
in treatment of TBI (24,25). To the best of our knowledge, no
previous reports have described the neurological, pathophysiology
and imaging characteristics of the acute phase of mmCHI at varying
high-altitudes.
In the present study, the neurological function of
rats was evaluated using the NSS, which was developed to define the
clinical condition of rats following trauma. In contrast to
previous research (26), the present
results revealed that there was a higher proportion of respiratory
depression and seizure following mmCHI at high-altitudes, which
maybe caused by partial pressure changes in brain tissues due to
significantly decreasing oxygen levels (27). The mortality rate following injury
was 0.6%, and absence of breathing recovery was determined as the
cause of death. No significant difference was identified between
the different altitude groups in the NSS evaluation. However, the
overall trend of injury changes exhibited varying patterns at
different time points. Neurological function recovered slowly
during an acute period, which maybe due to increased disturbance in
consciousness and respiratory depression following mmCHI at
high-altitudes. Rats resumed breathing and regained conscious and
the neurological function further recovered, when promptly provided
with CPR and maintenance of the airway. This suggested that the NSS
may have limitations at high-altitude. Hypoxia is not conducive to
the recovery of neurological function, but cerebral ischaemia and
hypoxia are the final common pathways of secondary brain damage
(28); therefore, early restoration
of respiration and continuous supplemental oxygen were important in
the treatment of mmCHI at high-altitudes. Neurological recovery at
24 h and altitude were not directly associated with NSS.
Previous studies have demonstrated that in early
hypoxia, humans and animals adapt to a hypoxic environment via
reduced water intake, a modest decrease or increase in urine
production, and a gradual increase in perspiration to increase
blood oxygen content as soon as possible (29,30). In
these integrated factors, decreased water intake is an essential
measure for animals to adapt to a hypoxic environment during early
stages and it further is the main reason for weight loss. The
present study confirmed that decreases in BW were significantly
lower following acute high-altitude exposure for 24 h compared with
rats at sea level. Subsequently, rats that underwent mmCHI lost an
increased amount of weight compared with healthy individuals at
high-altitude. Following high-altitude injury, rats that rapidly
descended to lower altitudes lost the least amount of weight. In
addition, previous studies have demonstrated that rats were less
active and had a reduced desire for food and water following AHH
injury (31,32). This suggested that changes in BW
following brain injury were closely associated with the hypoxic
environment and may be due to excessive stress reactions during
head injury, including hyperventilation, acute dieresis, metabolism
decreases and a decline in food and water intake (33).
Certain studies have demonstrated that altitude
hypoxia is responsible for acute mountain sickness (34,35). It
can generate HACE, which may be more serious and can be fatal
following TBI (36). In the present
study, the absolute BWC of rats following mmCHI at varying
high-altitudes was not significantly different among the groups.
However, the BWC/BW ratio was significantly different at the
various altitudes and times. The analysis demonstrated that within
24 h following mmCHI at high-altitudes, the BWC/BW ratio in the ND
group was still increasing, while the ratio at 6 h in the D-3,000 m
group was the lowest, which maybe due to low levels of weight loss
observed among the rats that rapidly descended to 3,000 m following
injury. This indicated that BWC may be associated with BW changes
and altitude. Although there was a reduction in the degree of
weight loss and improved neural function recovery following injury
at decreased altitude, the BWC was not reduced. Compared with the
absolute BWC, the relative BWC, referring to the BWC/BW ratio,
maybe more informative of the actual cerebral oedema. Previous
studies have demonstrated that brain oedema occurs for the first
time following 24 h of closed head injury in low-altitude areas and
peaks on days 5–8 (37–39). The ratio of the rats that descended
to altitudes of D-4,500 m and D-3,000 m reached a peak at 12 h
following injury prior to ratios starting to decline. At 24 h, the
BWC/BW ratio of the D-4,500 m group was significantly lower
compared with the D-3,000 m group. This suggested that there may be
a correlation between BWC/BW, times and altitude levels following
mmCHI at high-altitudes. The brain oedema following TBI at
high-altitudes occurred earlier and was more severe compared with
that observed in areas of lower altitude.
Although brain MRI has been used in various studies
of simulated high-altitude (40,41), the
present study first investigated the dynamic change of brain MRI
during 24 h at varying high-altitudes following AHH mmCHI. The MRI
scan in T2WI revealed that the model of head injury was due to
mmCHI. In patients with mmCHI, MRI findings are often accessible
and include diffuse axonal injury (DAI) lesions and subarachnoid
haemorrhages (SAH) (42). The
pathology of DAI is characterized histologically by microscopic
axonal damage observed in the parasagittal white matter and
grey-white matter junctions of the cerebral cortex and in the CC
and brainstem (43). Ventricular
dilation is a frequent phenomenon in patients with TBI and can
present in follow-up examinations and develop into post-traumatic
hydrocephalus (PTH) (44). Various
studies have indicated that SAH and intraventricular haemorrhage
(IVH) may be associated with hydrocephalus development following
TBI, which can result in acute ventricular dilation at 24 h
following TBI (45,46). As a result of the tearing of
subependymal veins that line ventricular cavities, IVH has been
associated with severe injuries (47). The mechanisms of ventricular dilation
are still not well understood. In the current study, the location
of CC exhibiteds ignificantly higher signalling and the LVs were
enlarged markedly in injured rats at 6 h following AHH. This result
indicated that there was an axonal injury in mmCHI following AHH.
In addition, mmCHI following AHH resulted in the earlier occurrence
of acute hydrocephalus, but MRI scanning did not identify SAH or
IVH in our study. In the control group, no such observations were
made; indicating that CC swelling and acute ventricular dilatation
of mmCHI may be closely associated with AHH. PTH may cause
increased intracranial pressure, which has been recognized as an
indicator of poorer outcomes following TBI (48). The dynamic brain MRI manifestations
were more gradually improved by hypobaric-hypoxia conditions
(slowly descending altitude) compared with rapid reoxygenation
(rapidly descending altitude) and continuous hypoxia (maintained at
extreme high-altitude) in mmCHI following AHH at 12 and 24 h.
Exposure to a hypobaric environment following TBI
increases the neuroinflammatory response to injury and the severity
of secondary brain injury (49).
Various studies have indicated that increased local tissue GFAP
immunoreactivity is a sensitive indicator of neuronal injury and
the increase in GFAP immunoreactivity is a sensitive marker of
reactive astrocytosis (50,51). Additionally, GFAP levels increase
when cerebral tissues are damaged due to trauma (52). GFAP is an early diagnostic indicator
of TBI and a sensitive indicator of mortality following TBI
(53). GFAP is further the major
protein of glial intermediate filaments in astrocytes and is often
used as a hallmark of astrocyte reactivity (54). An increase in GFAP expression is a
feature of various pathological conditions of the central nervous
system (55). Although the NSS in
the current study was similar following mmCHI at 24 h in the
various groups, levels of GFAP-positive cells varied. Levels of
GFAP-positive cells following continuous hypoxia were the highest
in the ND group, however, in the other two groups, gradual
reoxygenation produced lower levels in the D-4,500 m group compared
with rapid reoxygenation in the D-3,000 m group. Furthermore,
levels of GFAP-positive cells reflected on the degree of neuronal
damage under mmCHI hypobaric-hypoxia. Due to the different oxygen
contents at different altitudes, continuous hypobaric-hypoxia
following TBI significantly aggravated secondary brain injury and
rapid improvement of hypobaric and hypoxic conditions following AHH
TBI was not conducive to neurological recovery.
In conclusion, increasing attention should focus on
the initial 24 h of mmCHI following AHH exposure. Subjects may
benefit from being transported at the earliest possible time and
avoiding large-span descent altitude was beneficial to reduce
neurological impairment. Brain-specific biomarkers and MRI results
may further the understanding altitude mmCHI and can be
translatable to clinical practice. Further studies are required in
order to understand the precise mechanisms and long-term effects of
brain damage caused by mmCHI in rats following AHH. Levels of
inflammation and mechanisms of secondary brain injury following
exposure to AHH need to be investigated further.
Acknowledgements
The authors would like to thank Professor MinHui Xu
(Department of Neurosurgery, Daping Hospital, Third Military
Medical University, Chongqing), Professor Hui Zhao (Chongqing Key
Laboratory of Vehicle Crash/Bio-impact and Traffic Safety,
Institute for Traffic Medicine, Third Military Medical University,
Chongqing) and Dr Mou Gao (Affiliated Bayi Brain Hospital P.L.A
Army General Hospital, Beijing) for their helpful discussions and
excellent technical support.
Funding
The present study was supported by grants from the
National Key Research and Development Program (grant no.
2016YFC0800702), Natural Science Foundation of China (grant no.
31470913), The National Science Fund for Distinguished Young
Scholars (grant no. 81300965) & Open Fund Project of Shanghai
Key Laboratory of Forensic Medicine (grant no. KF1501).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
HW and XZ designed the current study, performed the
experiments, analyzed the data and wrote the paper. HX and ZL
performed the experiments, administered food and anaesthesia to
animals, and contributed experimental equipment. MR and PW designed
the current study and performed the experiments. MG, YL and YZ
anlyzed and searched for literature. HZ and MX designed the current
study, provided experimental equipment, analysed the data and
presented results. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Experiments were approved by the Ethics Committee of
Third Affiliated Hospital and Research Institute of Surgery, Third
Military Medical University (registration no. ChiCTR-RPC-15006770;
Chongqing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dicianno BE, Aguila ED, Cooper RA,
Pasquina PF, Clark MJ, Collins DM, Fitzgerald SG and Wichman TA:
Acute mountain sickness in disability and adaptive sports:
Preliminary data. J Rehabil Res Dev. 45:479–487. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhong JM, Wan QI, Yang YL and Juan DU:
Epidemiological investigation of 11718 hospitalized patients with
trauma in plateau. J Mil Surg Southwest China. 14:2012.(In
Chinese).
|
|
3
|
Chengliang H, Jinlong H, Chengyi L,
Haichuan X and sheng Z: Characteristics and treatment of
carniocerebral traffic injuries in plateau: Lasa. Chin J
Neuromedicine. 443–450. 2003.(In Chinese).
|
|
4
|
Zhao H, Yin Z, Xiang H, Liao Z and Wang Z:
Preliminary study on alterations of altitude road traffic in China
from 2006 to 2013. PLoS One. 12:e1710902017.
|
|
5
|
Vickers ML, Coorey CP, Milinovich GJ,
Eriksson L, Assoum M and Reade MC: Bibliometric analysis of
military trauma publications: 2000-2016. J R Army Med Corps.
164:142–149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Woods DR, O'Hara JP, Boos CJ, Hodkinson
PD, Tsakirides C, Hill NE, Jose D, Hawkins A, Phillipson K,
Hazlerigg A, et al: Markers of physiological stress during exercise
under conditions of normoxia, normobaric hypoxia, hypobaric
hypoxia, and genuine high altitude. Eur J Appl Physiol.
117:893–900. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deb SK, Brown DR, Gough LA, Mclellan CP,
Swinton PA, Andy Sparks S and Mcnaughton LR: Quantifying the
effects of acute hypoxic exposure on exercise performance and
capacity: A systematic review and meta-regression. Eur J Sport Sci.
18:243–256. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Simancas-Racines D, Arevalo-Rodriguez I,
Osorio D, Franco JV, Xu Y and Hidalgo R: Interventions for treating
acute high altitude illness. Cochrane Database Syst Rev.
6:CD0095672018.PubMed/NCBI
|
|
9
|
Natah SS, Srinivasan S, Pittman Q, Zhao Z
and Dunn JF: Effects of acute hypoxia and hyperthermia on the
permeability of the blood-brain barrier in adult rats. J Appl
Physiol (1985). 107:1348–1356. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei L, Feng G, Lu G, Dong H, Li Z, Ye D,
et al: Analysis of epidemiological characteristics of 628 patients
with traumatic brain injury in Linzhi China. Med J Natl Defending
Forces Southwest China. 427–429. 2014.doi:
10.3969/j.issn.1004-0188.2014.04.032.
|
|
11
|
Hou J, Nelson R, Wilkie Z, Mustafa G,
Tsuda S, Thompson FJ and Bose P: Mild and mild-to-moderate
traumatic brain injury-induced significant progressive and enduring
multiple comorbidities. J Neurotrauma. 34:2456–2466. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chieregato A, Martino C, Pransani V, Nori
G, Russo E, Noto A and Simini B: Classification of a traumatic
brain injury: The Glasgow Coma scale is not enough. Acta
Anaesthesiol Scand. 54:696–702. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Armed Forces Health Surveillance Center
(AFHSC), . Incident diagnoses of common symptoms (‘sequelae’)
following traumatic brain injury, active component, U.S. Armed
Forces, 2000-2012. MSMR. 20:9–13. 2013.
|
|
14
|
Dongsheng P, Zewen L, Kejun Q and Guoqiang
S: Analysis of the cause of death of mild-to-moderate brain injury
in plateau area. Zhongguo Shiyong Yiyao. 101–102. 2013.(In
Chinese).
|
|
15
|
Luks AM: Physiology in Medicine: A
physiologic approach to prevention and treatment of acute
high-altitude illnesses. J Appl Physiol (1985). 118:509–519. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sauerbeck AD, Fanizzi C, Kim JH, Gangolli
M, Bayly PV, Wellington CL, Brody DL and Kummer TT: modCHIMERA: A
novel murine closed-head model of moderate traumatic brain injury.
Sci Rep. 8:76772018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Flierl MA, Stahel PF, Beauchamp KM, Morgan
SJ, Smith WR and Shohami E: Mouse closed head injury model induced
by a weight-drop device. Nat protoc. 4:1328–1337. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tu TW, Lescher JD, Williams RA, Jikaria N,
Turtzo LC and Frank JA: Abnormal injury response in spontaneous
mild ventriculomegaly wistar rat brains: A pathological correlation
study of diffusion tensor and magnetization transfer imaging in
mild traumatic brain injury. J Neurotrauma. 34:248–256. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khalin I, Jamari NL, Razak NB, Hasain ZB,
Nor MA, Zainudin MH, Omar AB and Alyautdin R: A mouse model of
weight-drop closed head injury: Emphasis on cognitive and
neurological deficiency. Neural Regen Res. 11:630–635. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Genet GF, Bentzer P, Ostrowski SR and
Johansson PI: Resuscitation with pooled and pathogen-reduced plasma
attenuates the increase in brain water content following traumatic
brain injury and hemorrhagic shock in rats. J Neurotrauma.
34:1054–1062. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang H, Zhu X, Liao Z, Xiang H, Ren M, Xu
M and Zhao H: Novel-graded traumatic brain injury model in rats
induced by closed head impacts. Neuropathology. 38:484–492. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan EB, Johnstone VP, Alwis DS,
Morganti-Kossmann MC and Rajan R: Characterising effects of impact
velocity on brain and behaviour in a model of diffuse traumatic
axonal injury. Neuroscience. 248:17–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hellewell SC, Ziebell JM, Lifshitz J and
Morganti-Kossmann MC: Impact acceleration model of diffuse
traumatic brain injury. Methods Mol Biol. 1462:253–266. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Burtscher M, Gatterer H, Burtscher J and
Mairbaurl H: Extreme terrestrial environments: Life in thermal
stress and hypoxia. A narrative review. Front Physiol. 9:5722018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gatterer H, Wille M, Faulhaber M, Lukaski
H, Melmer A, Ebenbichler C and Burtscher M: Association between
body water status and acute mountain sickness. PLoS One.
8:e731852013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hou J, Nelson R, Wilkie Z, Mustafa G,
Tsuda S, Thompson FJ and Bose P: 27Mild and mild-to-moderate
traumatic brain injury-induced significant progressive and enduring
multiple comorbidities. J Neurotrauma. 34:2456–2466. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ostergaard L, Aamand R, Karabegovic S,
Tietze A, Blicher JU, Mikkelsen IK, Iversen NK, Secher N, Engedal
TS, Anzabi M, et al: The role of the microcirculation in delayed
cerebral ischemia and chronic degenerative changes after
subarachnoid hemorrhage. J Cereb Blood Flow Metab. 33:1825–1837.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan EB, Hellewell SC, Bellander BM,
Agyapomaa DA and Morganti-Kossmann MC: Post-traumatic hypoxia
exacerbates neurological deficit, neuroinflammation and cerebral
metabolism in rats with diffuse traumatic brain injury. J
Neuroinflammation. 8:1472011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang F, Zhou L, Wang D, Yang LL, Yuan GR
and Huang QY: Suppression of TRPV4 channels ameliorates
anti-dipsogenic effects under hypoxia in the subfornical organ of
rats. Sci Rep. 6:301682016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Siebenmann C, Robach P and Lundby C:
Regulation of blood volume in lowlanders exposed to high altitude.
J Appl Physiol (1985). 123:957–966. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Westerterp KR, Meijer EP, Rubbens M,
Robach P and Richalet JP: Operation Everest III: Energy and water
balance. Pflugers Arch. 439:483–488. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shtemberg AS, Uzbekov MG and Farber IuV:
Certain mechanisms of development of types of body tolerance to
acute hypobaric hypoxia. Izv Akad Nauk Ser Biol. 444–453. 2007.(In
Russian). PubMed/NCBI
|
|
33
|
Matu J, O'Hara J, Hill N, Clarke S, Boos
C, Newman C, Holdsworth D, Ispoglou T, Duckworth L, Woods D, et al:
Changes in appetite, energy intake, body composition, and
circulating ghrelin constituents during an incremental trekking
ascent to high altitude. Eur J Appl Physiol. 117:1917–1928. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luks AM, Swenson ER and Bärtsch P: Acute
high-altitude sickness. Eur Respir Rev. 26(pii): 1600962017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reinthaler M, Jung F and Empen K: Remote
ischemic preconditioning of the heart: Combining lower limb
ischemia and electronic stimulation of the gastrocnemius muscle.
Clin Hemorheol Microcirc. Oct 9–2018.(Epub ahead of print).
View Article : Google Scholar
|
|
36
|
Kedzierewicz R and Cabane D: Acute
mountain sickness and high altitude cerebral and pulmonary edema.
Rev Prat. 63:18–26. 2013.(In French). PubMed/NCBI
|
|
37
|
Tang G and Yang GY: Aquaporin-4: A
potential therapeutic target for cerebral edema. Int J Mol Sci.
17(pii): E14132016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marmarou A, Signoretti S, Fatouros PP,
Portella G, Aygok GA and Bullock MR: Predominance of cellular edema
in traumatic brain swelling in patients with severe head injuries.
J Neurosurg. 104:720–730. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bennett Colomer C, Solari Vergara F, Tapia
Perez F, Miranda Vasquez F, Horlacher Kunstmann A, Parra Fierro G
and Salazar Zenkovich C: Delayed intracranial hypertension and
cerebral edema in severe pediatric head injury: Risk factor
analysis. Pediatr Neurosurg. 48:205–209. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hunt JJ Jr, Theilmann RJ, Smith ZM,
Scadeng M and Dubowitz DJ: Cerebral diffusion and T(2): MRI
predictors of acute mountain sickness during sustained
high-altitude hypoxia. J Cereb Blood Flow Metab. 33:372–380. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marussi V, Pedroso JL, Piccolo AM,
Barsottini OG, Moraes FM, Oliveira ASB, Freitas LF and Amaral LLFD:
Teaching NeuroImages: Typical neuroimaging features in
high-altitude cerebral edema. Neurology. 89:e176–e177. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mata-Mbemba D, Mugikura S, Nakagawa A,
Murata T, Ishii K, Kushimoto S, Tominaga T, Takahashi S and Takase
K: Traumatic midline subarachnoid hemorrhage on initial computed
tomography as a marker of severe diffuse axonal injury. J
Neurosurg. 1–8. 2018.PubMed/NCBI
|
|
43
|
Fidan E, Foley LM, New LA, Alexander H,
Kochanek PM, Hitchens TK and Bayir H: Metabolic and structural
imaging at 7 tesla after repetitive mild traumatic brain injury in
immature rats. Asn Neuro. 10:17590914187705432018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhao J, Chen Z, Xi G, Keep RF and Hua Y:
Deferoxamine attenuates acute hydrocephalus after traumatic brain
injury in rats. Transl Stroke Res. 5:586–594. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bonow RH, Oron AP, Hanak BW, Browd SR,
Chesnut RM, Ellenbogen RG, Vavilala MS and Rivara FP:
Post-traumatic hydrocephalus in children: A retrospective study in
42 pediatric hospitals using the pediatric health information
system. Neurosurgery. 83:732–3739. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dorner RA, Burton VJ, Allen MC, Robinson S
and Soares BP: Preterm neuroimaging and neurodevelopmental outcome:
A focus on intraventricular hemorrhage, post-hemorrhagic
hydrocephalus, and associated brain injury. J Perinatol.
38:1431–1443. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mata-Mbemba D, Mugikura S, Nakagawa A,
Murata T, Kato Y, Tatewaki Y, Li L, Takase K, Ishii K, Kushimoto S,
et al: Intraventricular hemorrhage on initial computed tomography
as marker of diffuse axonal injury after traumatic brain injury. J
Neurotrauma. 32:359–365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Garcia M, Poza J, Santamarta D,
Romero-Oraá R and Hornero R: Continuous wavelet transform in the
study of the time-scale properties of intracranial pressure in
hydrocephalus. Philos Trans A Math Phys Eng Sci. 376(pii):
201702512018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Scultetus AH, Haque A, Chun SJ, Hazzard B,
Mahon RT, Harssema MJ, Auker CR, Moon-Massat P, Malone DL and
McCarron RM: Brain hypoxia is exacerbated in hypobaria during
aeromedical evacuation in swine with traumatic brain injury. J
Trauma Acute Care Surg. 81:101–107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ye L, Yang Y, Zhang X, Cai P, Li R, Chen
D, Wei X, Zhang X, Xu H, Xiao J, et al: The role of bFGF in the
excessive activation of astrocytes is related to the inhibition of
TLR4/NFκB signals. Int J Mol Sci. 17(pii): E372015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Maeda J, Higuchi M, Inaji M, Ji B, Haneda
E, Okauchi T, Zhang MR, Suzuki K and Suhara T: Phase-dependent
roles of reactive microglia and astrocytes in nervous system injury
as delineated by imaging of peripheral benzodiazepine receptor.
Brain Res. 1157:100–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gill J, Latour L, Diaz-Arrastia R,
Motamedi V, Turtzo C, Shahim P, Mondello S, DeVoto C, Veras E,
Hanlon D, et al: Glial fibrillary acidic protein elevations relate
to neuroimaging abnormalities after mild TBI. Neurology.
91:e1385–e1389. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xuan W, Huang L and Hamblin MR: Repeated
transcranial low-level laser therapy for traumatic brain injury in
mice: Biphasic dose response and long-term treatment outcome. J
biophotonics. 9:1263–1272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kernie SG, Erwin TM and Parada LF: Brain
remodeling due to neuronal and astrocytic proliferation after
controlled cortical injury in mice. J Neurosci Res. 66:317–326.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Damodaran TV and Abou-Donia MB:
Alterations in levels of mRNAs coding for glial fibrillary acidic
protein (GFAP) and vimentin genes in the central nervous system of
hens treated with diisopropyl phosphorofluoridate (DFP). Neurochem
Res. 25:809–816. 2000. View Article : Google Scholar : PubMed/NCBI
|