Introduction
Depression is a prevalent and recurrent
psychological illness that results in a significant burden for
individuals and the society (1).
Previous studies indicated that electroconvulsive therapy (ECT) is
an efficient and rapid treatment for major depression, especially
for patients with drug-resistant and refractory depression
(2,3). Modern ECT requires administration of
anesthetic agents and muscle relaxants to avoid the side effects of
treatment, including headache, muscle ache and bone fracture
(4). At present, propofol is the
most frequently used general anesthetic during ECT (5). Previous studies indicated that propofol
could alleviate the cognitive impairment induced by
electroconvulsive seizure (ECS) (6,7).
However, certain studies suggested that propofol may interfere with
the efficacy of ECT due to its anticonvulsant properties (8,9).
Therefore, an improved anesthetic regimen for ECT is required.
In the last 40 years ketamine has been administered
as an anesthetic to several million people (10,11).
Ketamine is considered an anesthetic and a rapid-acting
antidepressant (12,13). A clinical study revealed that a
single ketamine infusion could rapidly reduce the levels of
anhedonia in treatment-resistant major depression (14). Notably, the anesthetic dose of
ketamine was positively associated with significant adverse
psychological effects, including delusion and hallucination
(15,16). These disadvantages may limit the
clinical use of ketamine, especially for patients with psychiatric
disorders. Based on these concerns, a previous study revealed that
compared with propofol anesthesia, a low, subanesthetic dose of
ketamine combined with propofol could enhance the antidepressant
efficacy and further improve the cognitive performance following
ECS, an animal analog of human ECT, in stressed stressed rats
(17). As the stimulus intensity is
germane to both therapeutic and adverse effects of ECT (18), the appropriate stimulus intensity of
this modified ECS (MECS) with the introduction of compound
anesthetics, requires further study. Furthermore, the biological
processes underlying the protective effect of MECS on cognitive
function remain to be elucidated.
Previous studies revealed that the glutamatergic
transmitter system, including N-methyl-D-aspartic acid (NMDA) and
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)
glutamate receptors, was crucial in the pathological physiology of
depression and cognitive function (19,20). It
is commonly accepted that ketamine exerts its biological effects by
inhibiting the NMDA receptor (14).
However, another study suggested that ketamine may exert rapid
antidepressant-like effects by enhancing the AMPA receptor relative
to NMDA receptor throughput in critical neuronal circuits (21). Furthermore, previous studies
indicated that the antidepressant effects induced by ketamine or
MECS were associated with glutamate receptor 1 (GluR1) (17,21). The
AMPA receptor is made up of heterotetrameric complexes comprising
at least two of four subunits (GluR1-4) (22). Differences in subunit composition
confer specific physiological properties to AMPA receptor function
and the phosphorylation of subunits is necessary for maintaining
the function of this receptor (23).
GluR1 was reported to be involved in the cognitive function
(24,25). GluR1 subunit is recruited into the
hippocampal synapses to alter the cognitive behavior with the
phosphorylation at Ser831 [GluR1 (Ser831)] by
calcium/calmodulin-dependent protein kinase II (CaMKII), and at
Ser845 [pGluR1 (Ser845)] by protein kinase A (PKA) (24). In addition to the expression of
CaMKII and PKA, the activities of these two protein kinases could
also affect the phosphorylation of GluR1 (25). Therefore, the current study
hypothesized that the improved cognitive performance induced by
MECS may be associated with the phosphorylation levels of GluR1 and
the expression and activities of these two protein kinases in the
hippocampus. CaMKIIα is the dominant isoform of CaMKII in the brain
(26). The hallmark feature of
CaMKII regulation is the generation of autonomous kinase activity
by Thr286 autophosphorylation (26).
In contrast to CaMKII, the activity of PKA can be determined by the
relative expression of the regulatory subunit to the catalytic
subunit (25). The effects of
different charges of MECS on PKA- and CaMKIIα-dependent GluR1
phosphorylation have not been previously reported.
In the present study, five different stimulus
intensities of MECS were used to evaluate their efficacies on
depression-like and cognitive behaviors in stressed rats, and to
investigate the potential roles of the expression of GluR1, phospho
(p)GluR1 (Ser831), pGluR1 (Ser845), PKA regulatory subunit (PKR2),
PKA catalytic subunit (PKAβ), CaMKIIα, pCaMKIIα (Thr286),
cAMP-response element-binding protein (CREB) and pCREB (Ser133) in
these processes.
Materials and methods
Animals
A total of 104 healthy adult male Sprague-Dawley
rats (weight, 200–250 g; age, 2–3 months) were obtained from the
Laboratory Animal Center of Chongqing Medical University
(Chongqing, China). Animals were housed under standard laboratory
conditions (temperature, 22–24°C; air humidity, 55–60%; 12-h
light/dark cycle) with food and water available ad libitum.
Rats were allowed to acclimatize for 1 week before further
experiments. All procedures were approved by the Ethics Committee
of Chongqing Medical University in accordance with the animal care
guidelines of the National Institutes of Health and Use of
Laboratory Animals (27).
Chronic unpredictable mild stress
procedure
The rat model of depression was established by
chronic unpredictable mild stress (CUMS). This experimental
protocol was adopted from previous studies with a minor
modification (28–30). Briefly, the rats were randomly
administered the following stressors for 28 days: Damp sawdust for
24 h of continuous lighting, swimming in cold water (4°C) for 5
min, water deprivation for 24 h, food deprivation for 24 h, shaking
for 20 min (once per sec), tail pinching for 1 min, cage tilting
(45°) for 24 h, or social crowding for 24 h. All stressor stimuli
were randomly scheduled and repeated within 4 weeks to prevent
habituation and increase unpredictability.
Drugs and treatment
Propofol and ketamine were purchased from
AstraZeneca (cat no. H20080473; Cambridge, UK) and Jiangsu Hengrui
Medicine Co., Ltd. (cat no. H32022820; Lianyungang, China),
respectively. Based on previous results, a constant dosage of
anesthetics, ketamine (10 mg/kg) and propofol (80 mg/kg), was
administered intraperitoneally (i.p.) 10 min prior to ECS treatment
in each group (17). On the day of
the experiment, the rats were anesthetized to prepare them for MECS
treatments. Anesthesia time was defined as the time from the loss
of righting reflex until the regaining of it. The animals were
ventilated with a small animal ventilator (TKR-200C; Jiangxi Teli
Anaesthesia & Respiration Equipment Co., Ltd., Nanchang,
China). The mean arterial pressure, heart rate and body temperature
were continuously monitored using a multichannel
electrophysiological recording system (model, RM6240; Chengdu
Instrument Factory, Inc., Chengdu, China). The levels of
PaO2, PaCO2 and the acid-base status were
adjusted to ensure hemodynamic stability. The rats were excluded
from the experiment if they were hemodynamically unstable (mean
arterial pressure <10.7 kPa) or if their blood gas levels were
not within the normal range [PaO2 >13.6 kPa; pH
7.35–7.45; PaCO2 4.67–6.00 kPa; base excess of −2–2 mEq]
(31). In the present study, no rats
were excluded due to hemodynamic instability or gas levels.
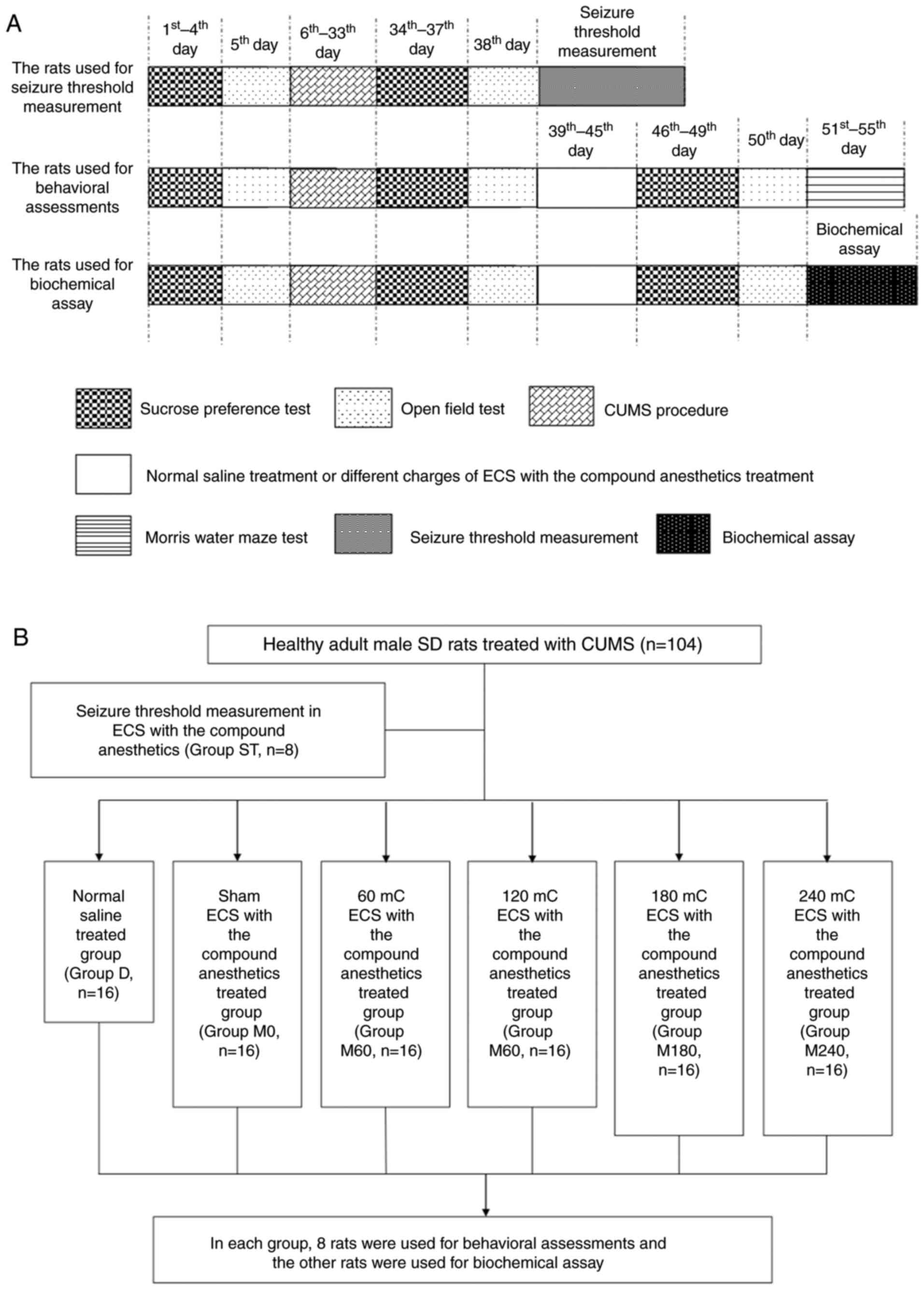
The experimental design is summarized in Fig. 1. Following the completion of the CUMS
procedure, one group of stressed rats was used for measuring
seizure threshold in ECS with the compound anesthetics (ST group,
n=8). The other stressed rats were allocated to six groups (n=96):
i) Group D received normal saline solution (8 ml/kg, i.p.) once a
day; ii) groups M0, M60, M120, M180 and M240 received ECS treatment
with stimulus intensities of 0, 60, 120, 180, and 240 mC,
respectively, following treatment with the same anesthetic protocol
[ketamine (10 mg/kg) and propofol (80 mg/kg), i.p.]. ECS was
delivered via ear clip electrodes using a Niviqure ECT system
(Niviqure Meditech, Pvt., Ltd., Bangalore, India) (32). The following stimulus parameters were
used: Bidirectional square wave pulses (amplitude, 0.8 A; width,
1.5 ms; 125 Hz) with a duration of 0.4, 0.8, 1.2 or 1.6 sec to
generate charges of 60, 120, 180 and 240 mC, respectively. These
treatments were administered once daily for 7 days. Oxygen was
given to the rats that underwent ECS and their saturation of blood
oxygen (SpO2) was monitored. Only rats with a value of
SpO2 ≥95% were included.
A previous study demonstrated that the seizure
threshold of rats with propofol anesthesia was ~40 mC (33). Furthermore, a clinical study reported
that ketamine may slightly increase the seizure duration induced by
ECT (13). Therefore, the current
study tested the initial ECS stimuli at 30 mC in each rat of the ST
group. If this stimulus did not induce a general tonic seizure, the
charge was increased by 1 mC for another ECS on the following day.
ECS stimulation was increased by 1 mC at a time until the rat
exhibited an obvious tonic seizure. The ECS charge at which a
general tonic seizure occurred was recorded as the seizure
threshold of the rat.
Sucrose preference test
Anhedonia, an indication of depressive disorder in
rats, was defined as a reduction in sucrose preference. Sucrose
preference was measured as described in a previous study (33). Following deprivation of water and
food for 23 h, the rats were given free access to two preweighed
bottles for 1 h, one filled with 1% sucrose solution and the other
filled with water. The position of the two bottles was rotated
every 30 min to prevent place preference. The two bottles were
subsequently weighed to measure the percentage of the consumed
sucrose solution. The sucrose preference percentage (SPP) was
calculated according to the following formula: SPP (%)=sucrose
solution consumption (g) × 100/[water consumption (g) + sucrose
solution consumption (g)] (7).
Open field test (OFT)
For the OFT, a spontaneous locomotor activity was
measured as described in a previous study (34). Experimental animals were placed at
the center of an open box (100×100×50 cm3). The floor
was divided into 25 equal squares with the walls painted black. The
horizontal locomotor activities (segments crossed with all four
paws) and vertical exploratory activities (standing on hind paws)
were scored. The number of horizontal activities (crossing) and
vertical activities (rearing) performed by each rat was observed
for 3 min.
Morris water maze (MWM)
To assess the spatial performance function, the rats
were tested in a MWM, which was a circular pool (diameter, 150 cm;
height, 50 cm) with a platform 1–2 cm below the surface of the warm
water (22±1°C) (35). The maze was
divided into four equal quadrants: SW, NW, SE and NE. Each rat was
placed into the water randomly from a quadrant and allowed a
maximum of 60 sec to find the submerged platform located at the
center of the NE quadrant. If the rats failed to complete the task
within 1 min, they were gently guided to the platform where they
remained for 15 sec. Each rat was given four trials/day for 5
consecutive days. The swimming speed, swimming distance and escape
latency (EL) to find the platform were recorded using a video
tracking system device with a data analysis program (Anhui Zhenghua
Bio-Tech Co., Ltd., Anhui, China). On day 6, the platform was
removed and the probe trial was performed for 60 sec. The swim time
spent in the platform quadrant was recorded as the space
exploration time.
Western blot analysis
Following the spatial memory retention test, eight
rats from each group were humanely sacrificed and the bilateral
hippocampus was rapidly removed on ice prior to storage at −80°C.
Tissues were homogenized using the RIPA lysis buffer (Beyotime
Institute of Biotechnology, Shanghai, China) and the supernatants
were collected after centrifugation at 12,000 × g for 15 min
at 4°C. After a bovine serum albumin microassay (Pierce BCA Protein
Assay Kit; cat. no. 23225; Pierce; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and spectrophotometry to assess the protein
levels, total protein was separated by 4–12% SDS-PAGE and
transferred to polyvinylidene fluoride membranes. The membranes
were blocked with 5% nonfat milk for 1 h at 37°C and sub sequently
incubated with appropriate primary antibodies overnight at 4°C:
GluR1 (cat. no. ab31232), pGluR1 (Ser831; cat. no. ab109464),
pGluR1 (Ser845; cat. no. ab76321), PKR2 (cat. no. ab38949), PKAβ
(cat. no. ab75993), CREB (cat. no. ab31387), pCREB (Ser133; cat.
no. ab32096; all Abcam, Cambridge, UK), CaMKIIα (cat. no. sc-9035),
pCaMKIIα (Thr286; cat. no. sc-12886-R; both Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and GAPDH (cat. no. AF1186;
Beyotime Institute of Biotechnology; all 1:1,000). After washing,
the membranes were incubated with biotinylated secondary antibodies
(cat. no. A0208; 1:2,000; Beyotime Institute of Biotechnology) for
1 h at 37°C. Blots were developed using a chemiluminescent
substrate (BeyoECL Plus kit; cat. no. P0018; Beyotime Institute of
Biotechnology) and analyzed semi-quantitatively using the Bio-Rad
Quantity One software (version 4.4.0; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). To determine the relative expression of these
proteins, they were normalised to GAPDH.
Immunohistochemistry
The remaining rats in each group (n=8) were
anesthetized and transcardially perfused using 4% paraformaldehyde
(PFA) in normal saline. Their brains were removed and stored for at
least 24 h in 4% PFA at 4°C before dehydration and embedding in
paraffin. The hippocampi were cut into 10-µm thick sections in the
coronal plane and mounted on glass slides. The immunoreactivity of
pGluR1 (Ser831) and pCaMKIIα (Thr 286) was determined as described
in a previous study (36). Briefly,
sections of the rat brain were incubated overnight at 4°C with
anti-pGluR1 (Ser831) and anti-pCaMKIIα (Thr 286; both 1:100)
antibodies, and subsequently incubated for another 1 h at 37°C with
biotin-labeled secondary antibodies (1:50; Beyotime Institute of
Biotechnology, cat. no. A0208). The primary antibody was visualized
using brown 3,3′Diaminobenzidine substrate (Wuhan Boster Biological
Technology, Ltd., Wuhan, China). The CA1 region in the hippocampus
was observed using a light microscope (Olympus BX60; Olympus
Corporation, Tokyo, Japan) at a magnification of ×400 and analyzed
using the Image-Pro Plus software (version 6.0; Media Cybernetics,
MD, USA).
Statistical analysis
Statistical analysis was performed using SPSS
(version 17.0; SPSS Inc., IL, USA). All data are presented as the
mean ± standard deviation. Statistical significance was determined
using one-way analysis of variance. The Student-Newman-Keuls test
was used to compare differences among the groups. The repeatedly
measured data were analyzed using repeated-measures analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Seizure threshold
The seizure threshold of CUMS rats treated with
compound anesthesia was 41.25±5.50 mC.
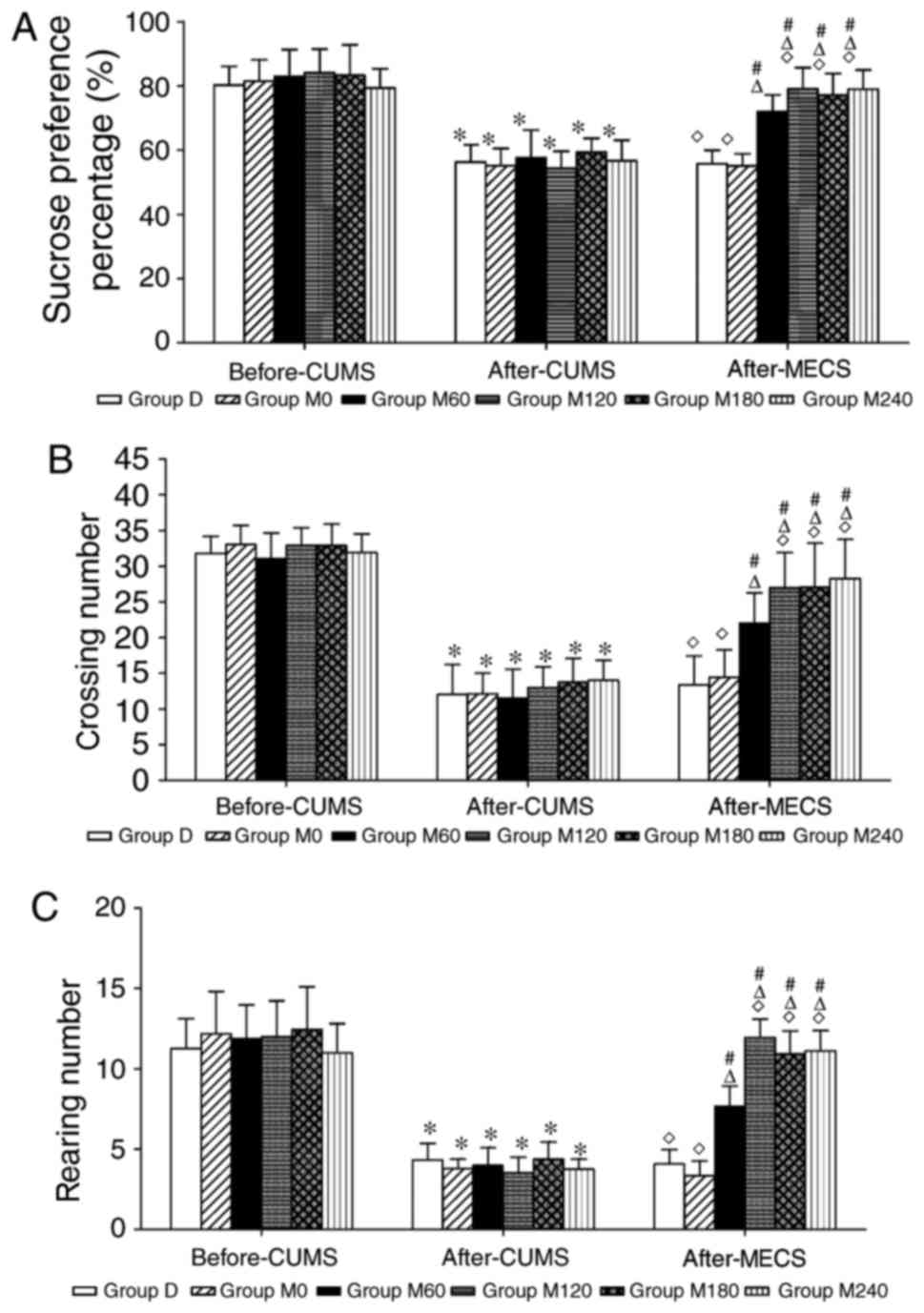
Sucrose preference test
The SPP in each CUMS group exhibited a significant
decrease compared with the respective group before CUMS (all
P<0.05), however no significant differences were found among the
groups (F=0.735, P=0.601; Fig. 2A).
Following the administration of MECS at different charges, the SPP
in groups M60, M120, M180 and M240 significantly increased compared
with the respective groups before MECS treatments (all P<0.05).
Compared with group M60, the SPP increased in group M120
(P<0.05). Furthermore, no significant differences were
identified in SPP between groups D and M0 (P=0.823).
Open field test
Following the CUMS treatment, rats exhibited
decreased locomotor activity (P<0.05) and rearing numbers
(P<0.05) in the OFT compared with the respective groups before
the CUMS administration (Fig. 2B and
C). No significant differences in OFT scores were found among
the CUMS groups (number of crossings, F=0.698 and P=0.628; rearing,
F=1.038 and P=0.408). Following the MECS treatment at different
charges, the number of crossings and rearing in groups M60, M120,
M180 and M240 significantly increased compared with the respective
groups before the MECS treatment (all P<0.05). The number of
crossings and rearing increased in the M120 group compared with the
M60 group (number of crossings, P<0.05; rearing, P<0.05).
Furthermore, no significant differences were observed in the number
of crossings and rearing between groups D and M0 (number of
crossings, P=0.672; rearing, P=0.235).
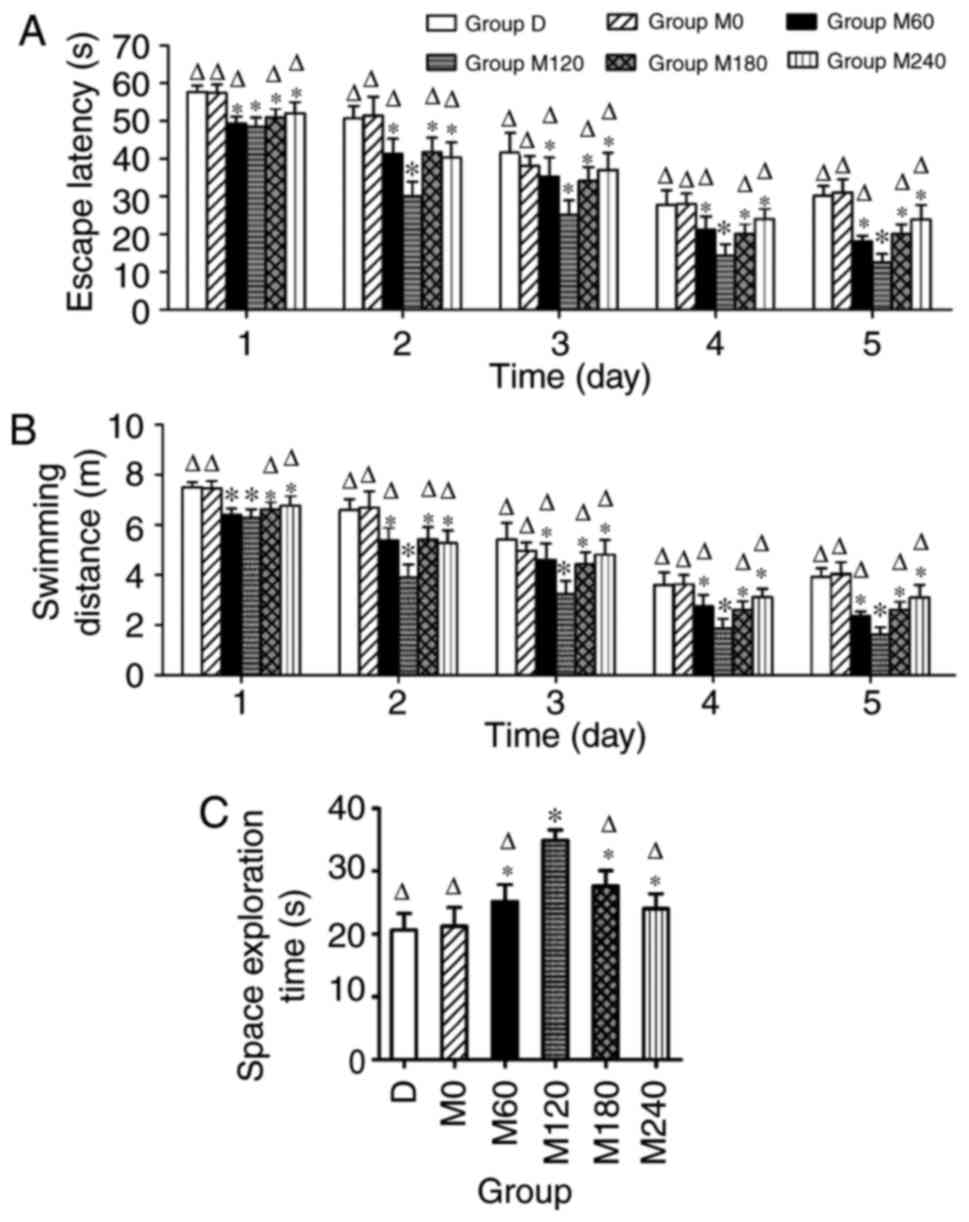
Morris water maze
During the MWM test, the swimming speed of CUMS rats
exhibited no significant differences among the groups (data not
shown). The mean latencies to find the submerged platform decreased
progressively during the five consecutive training days for all
animals. Following the MECS treatment, the time to find the
platform was shorter in the M120 group compared with the other
groups (P<0.05). Furthermore, group M0 exhibited no significant
decrease in EL compared with group D each day (P>0.05; Fig. 3A). Similar results were found for the
swimming distance (Fig. 3B). When
the platform was removed for spatial memory testing, group M120
exhibited a significantly longer dwelling time in the former
platform quadrant. No differences were identified between groups D
and M0 (Fig. 3C).
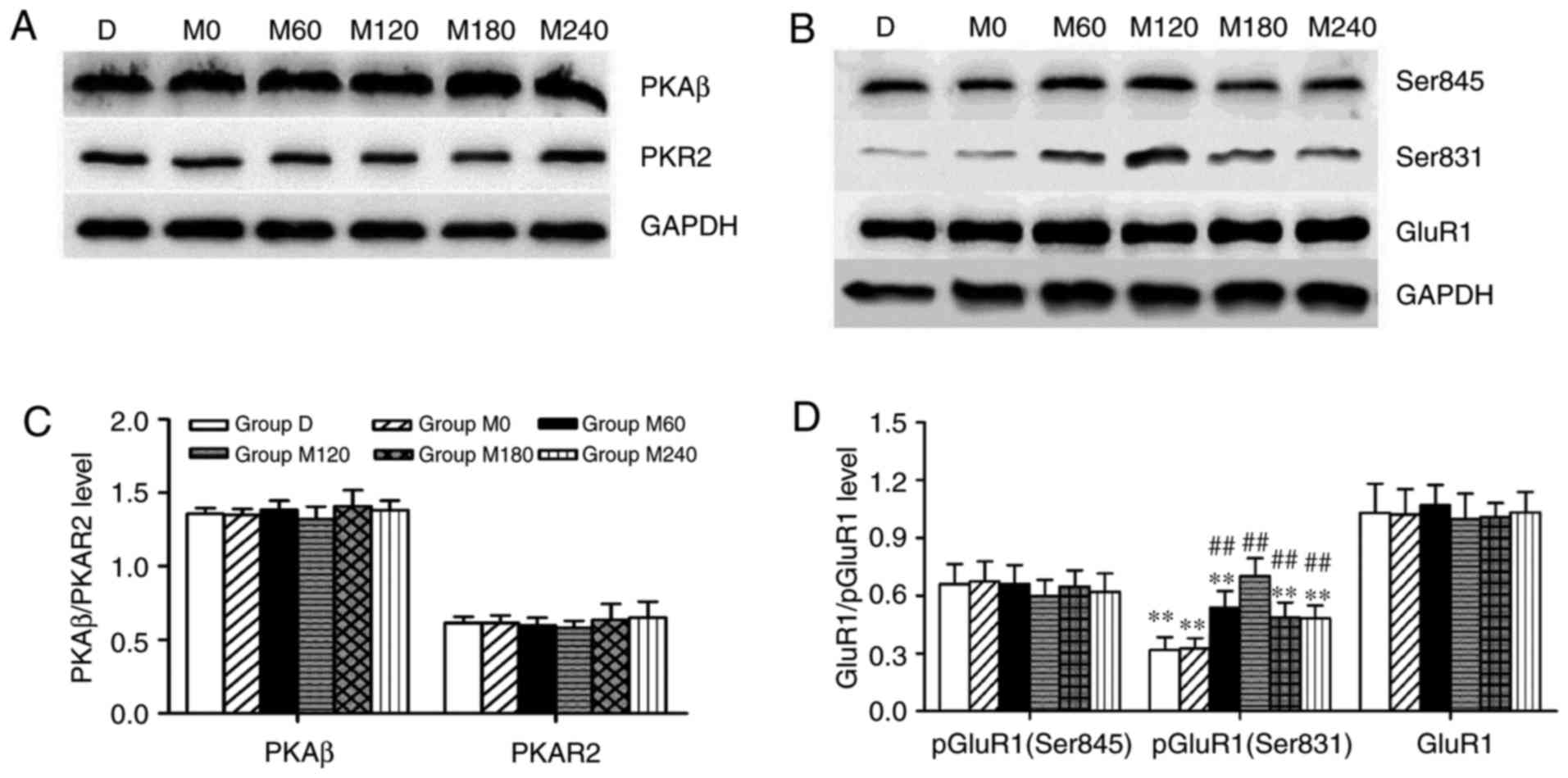
Expression levels of PKA subunits and
GluR1, and phosphorylation of GluR1 (Ser 845) in the
hippocampus
In the present study, PKAR2, PKAβ (Fig. 4A), GluR1 and pGluR1 (Ser845; Fig. 4B) were assessed via western blotting.
No significant differences were identified in the expression of
PKAR2 (F=0.448 and P=0.810) and PKAβ (F=0.803 and P=0.562) subunits
of PKA (Fig. 4C). Furthermore,
analysis of variance did not reveal any statistically significant
effects of MECS on the expression of GluR1 (F=1.059 and P=0.415)
and pGluR1 (Ser 845; F=0.365 and P=0.866) in the hippocampus
(Fig. 4D).
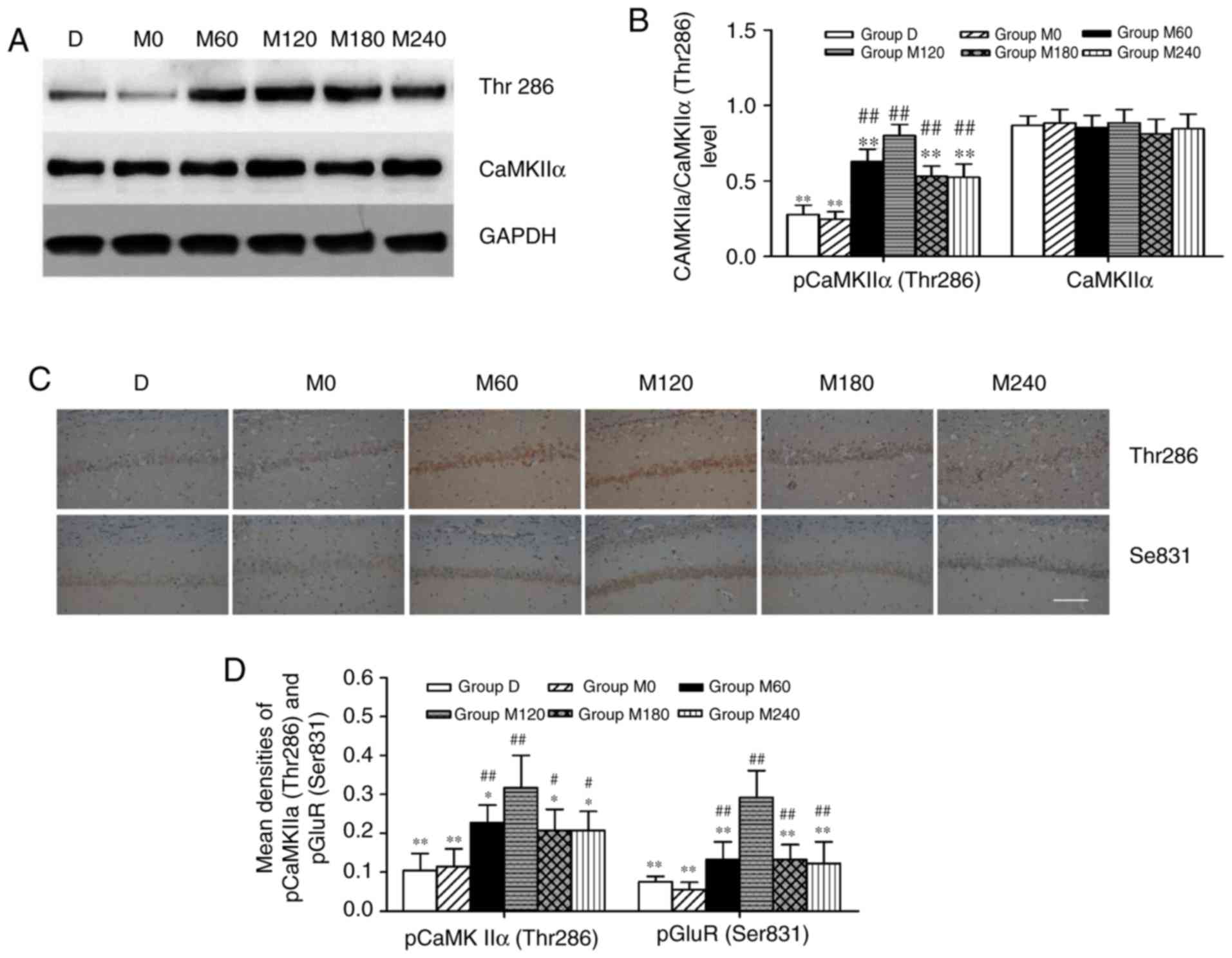
CaMKIIα activity and GluR1 (Ser831)
phosphorylation in the hippocampus
In the present study, CaMKIIα, pCaMKIIα (Thr286;
Fig. 5A) and pGluR1 (Ser831;
Fig. 5B) were assessed by western
blotting. As presented in Fig. 5B,
anesthesia combined with ECS treatments did not induce significant
effects on the hippocampal expression of CaMKIIα (F=0.462 and
P=0.799), however the treatments altered the pCaMKIIα (Thr 286)
levels (F=34.597 and P<0.01). Additionally, compared with the
other five groups, pCaMKIIα (Thr 286) expression significantly
increased in the M120 group (P<0.01), while the expression of
this protein was lower in groups D and M0 compared with all other
groups (all P<0.01). The expression of pCaMKIIα (Thr286) and
pGluR1 (Ser831) were also examined via immunohistochemistry
(Fig. 5C). The expression of pGluR1
(Ser831) was the highest in group M120 compared with all other
groups (all P<0.01; Fig. 4D). The
results of immunohistochemistry were similar to those of western
blotting, and indicated that the expression levels of pCaMKIIα (Thr
286) and pGluR1 (Ser831) in the CA1 region of the hippocampus
increased in group M120 compared with other groups (P<0.05 and
P<0.01; Fig. 5D).
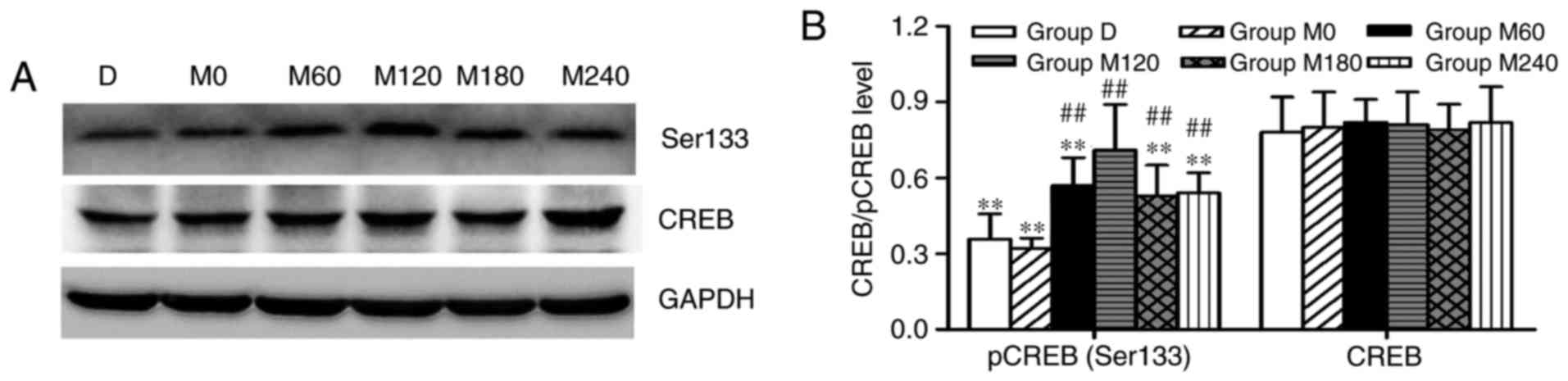
Expression levels of CREB and pCREB
(Ser 133) in the hippocampus
The western blot analysis was used to analyze and
quantify the protein expression of CREB and pCREB (Ser133) in the
hippocampus. No significant alterations in the expression levels of
CREB were detected (F=1.036 and P=0.427). However, significant
differences were identified in the expression levels of pCREB
(Ser133) among the six groups (F=13.156 and P<0.01). Group M120
exhibited a significantly increased level of pCREB (Ser133) in the
hippocampus (P<0.01) compared with the other five groups.
Additionally, the expression levels of pCREB were lower in groups D
and M0 compared with other groups (all P<0.01; Fig. 6A and B).
Discussion
The current study primarily demonstrated that
different charges of MECS affected the behavioral performance and
protein expression levels in stressed rats. Notably, the medium
stimulus intensity (120 mC) of MECS was optimal for improving the
antidepressant efficacy and cognitive performance in rats with
depression. Furthermore, the underlying cognitive protective
mechanisms of the stimulus intensity were associated with the
upregulation of pCaMKIIα (Thr286), pGluR1 (Ser831) and pCREB
(Ser133).
The CUMS procedure, which has been successfully used
in previous studies to establish animal models of depression
(17,29,37), was
first successfully established by Willner et al (38) in 1987. However, it should be noted
that CUMS procedures may differ slightly in certain aspects between
different laboratories, primarily due to convenience and logistics
(29,39). While a certain extent of
standardization of the procedure may be desirable, there are no
standard factors that distinguish the laboratories in which the
procedure operates reliably from those where it does not (39). Anhedonia is one of the primary
symptoms of depression, which can be inferred from a reduction in
the intake of sweet liquid food (1,40). As
expected, the SPP of rats decreased significantly following the
CUMS procedure in the present study, which was consistent with
results of a previous study (7). In
addition, the CUMS model could also be used to simulate human
bradykinesia, which is one of the core symptoms of depression
(41,42). OFT is widely used to evaluate the
locomotor and exploratory behavior in experimental animals, a
reduction in which is a sign of depression (34,37). The
stressed rats in the present study exhibited decreased crossing and
rearing numbers, indicating apathy and reduced exploration. These
results indicated that the depression-like behaviors were
successfully modeled using the CUMS procedure. Furthermore,
following administration of different charges of MECS, the SPP and
OFT performances differed between groups treated with different
charges. In addition, the SPP and OFT performances remained
unaltered following an increase in charges from medium to high (≥20
mC). These results indicated that the ceiling effect of the
antidepressant therapy using ECS with the compound anesthetics was
reached at the 120 mC charge. One possible explanation for the lack
of improvement in antidepressant efficacy following administration
of higher stimulus intensities (180 and 240 mC) in the present
study may be associated with the seizure duration, which was an
important determinant of the treatment efficacy. A previous study
reported that the ECT seizure duration did not exhibit a linear
association with the stimulus intensity, and increased intensities
may reduce seizure duration (43).
However, further studies are required to clarify the underlying
mechanism of this phenomenon, as the present study could not
monitor the brain electrical activity of the stressed rats due to
the lack of equipment. A previous study indicated that pretreatment
with propofol could further improve the antidepressant effect
induced by ketamine in the forced swimming test (44). However, no statistically significant
improvement was found in the depression-like behaviors in rats
administered the compound anesthetics only (group M0). The present
study hypothesized that this result may be associated with the
anesthetic regimen and animal models (exposed to acute or chronic
stressors). Wang et al (44)
used a subanesthetic adjuvant dose of propofol (5, 10, 15 and 20
mg/kg, i.p.) combined with ketamine (15 mg/kg, i.p.) to evaluate
their safety and efficacy in rats with acute depression-like
behavior. In the current study, a low dose of ketamine (10 mg/kg,
i.p.) combined with propofol (80 mg/kg, i.p.), which was equivalent
to the clinical dose in humans, was selected as the anesthetics
during ECS in chronically stressed rats. Furthermore, Wang et
al (44) found that the
subanesthetic adjuvant dose of propofol with ketamine could not
increase the crossing and rearing of the rats compared with the
control group. Therefore, combined with clinical-dose propofol, a
lower-dose ketamine may not be able to exert a direct
antidepressant effect in the chronic stress model of depression. To
the best of the authors' knowledge, the anesthetic regimen used in
the present study has not been previously reported. However,
according to previous studies and relevant preliminary experiments,
propofol (100 mg/kg, i.p.) or ketamine (50–100 mg/kg, i.p.) could
be safely used as general anesthetics for rats (45–47). To
minimize the possible adverse effects of ketamine including
delusions and hallucinations, a lower dose (10 mg/kg, i.p.) was
selected in the present study. Using the transformation formula for
ketamine between rats and human patients, the dosage used in the
present study was equivalent to the low-dose ketamine commonly used
in clinical research for the induction of antidepressant effects in
patients (17). A previous study has
indicated that administration of 10 mg/kg, i.p. ketamine could
prevent stress-evoked dysfunctions in rats (48). Due to the administration of compound
anaesthetics, 80 mg/kg, i.p. propofol was used in the current
study. This dose has been previously demonstrated to be safe and
effective (47). No animal mortality
was observed, and, therefore, the compound dosage used in the
current study may be appropriate for MECS.
The therapeutic efficacy of ECT is associated with
the degree to which the stimulus intensity exceeds the seizure
threshold (i.e., the relative intensity) (49). The initial seizure threshold of the
stressed rats treated with the compound anesthetic was ~41 mC. The
relative intensities of 60, 120, 180 and 240 mC used in the present
study were ~1.5-, 3-, 4.5- and 6-fold the seizure threshold,
respectively. Although the highest charge was 240 mC, all rats
survived under the high intensities in the present study, which was
in line with the results reported by Luo et al (33). A previous study found that the
initial seizure threshold of solo ECS (without the administration
of anesthetics) was 10 mC on average (18), and the threshold increased to 40 mC
when ECS was administered with propofol (33), suggesting that this anaesthetic may
exhibit antiepileptic effects. Based on the result that the initial
seizure threshold of ECS co-administered with propofol and low-dose
ketamine was 41 mC in the current study, it was hypothesized that
low-dose ketamine may not interfere in the seizure effects of
ECS.
MWM test is frequently used as to study learning and
memory function of animal models (1,36,50).
However, the MWM test can lead to epigenetic alterations in the
hippocampus and influence the cognitive behavior of animals. Zots
et al (51) found that the
MWM test caused the formation of both spatial and nonspatial
memory. Carter et al (50)
demonstrated that MWM training caused distinct epigenetic and gene
expression alterations in rat hippocampal neurons (50). Therefore, repeated MWM tests may not
accurately reflect the alteration of learning and memory function
induced by ECS and anesthetics. Based on these concerns, the
current study used 8 rats from each group for the behavioural
tests, including the MWM test, and 8 rats from each group for the
biochemical assays. The MWM test test was performed only once to
prevent changes to the epigenome. This protocol prevented the
behavioral tests negatively affecting memory, which can be
associated with differential protein expression in the brain of the
stressed rats (50). Preliminary
studies indicated that the combination of ketamine and propofol
exhibited a better cognitive performance compared with the use of
propofol alone in the ECS procedure (17,52). The
present study further identified an appropriate stimulus intensity
of ECS to be applied in combination with anesthetics to improve the
cognitive function of stressed rats. The results of the present
study confirmed that the medium charge of 120 mC was the optimal
stimulus intensity for the improvement in cognitive function.
Receiving the compound anesthetics alone could not improve the
cognitive performance and depression-like behaviors in stressed
rats. The results of the current study indicated that low-dose
ketamine combined with propofol was an appropriate anesthetic
protocol for ECS, and 120 mC was the optimal charge under this
novel protocol.
A previous study suggested that ketamine, an NMDA
receptor antagonist, when administered at a low dose, may induce
rapid antidepressant effects (14).
Furthermore, low-dose ketamine could ameliorate the cognitive
impairment induced by ECS (17).
Previous studies suggested that the NMDA receptor is one of the key
factors regulating the cognitive function. One study indicated that
chronic exposure to arsenic could reduce the learning and memory
function accompanied by downregulated levels of NMDA receptor
subunit 2 (NR2) and memory-associated proteins proto-oncogene c-Fos
and c-Jun (53). However, Maeng
et al (21) revealed that
both nonselective NMDA antagonists and NR2B-selective antagonists
exerted antidepressant effects by enhancing the activity of AMPA
receptors relative to NMDA receptors. Furthermore, Chen et
al (17) found that low-dose
ketamine combined with propofol could improve the cognitive
impairment induced by ECS, which may be attributed to modulating
the expression of pGluR1. GluR1 receptor subunit is expressed in
the majority of brain regions, predominantly in the hippocampus
(54). Previous studies suggested
that the phosphorylation of GluR1 was necessary for synaptic
expression, channel properties of AMPA receptors, synaptic
plasticity, spatial learning and memory function (24,54,55).
GluR1 Ser831/Ser845 double phosphomutant mice exhibited a
significant cognitive deficit (56).
Therefore, the GluR1 phosphorylation states modulated by CaMKII and
PKA may serve roles in cognitive function. The current study
indicated that the expression level of pCaMKIIα (Thr286) in group
M120 was the highest among the study groups, and was coincident
with the cognitive performance in the MWM test. However, no
significant alterations in total CaMkIIα protein levels were
observed. Therefore, these results suggested that the effects of
different charges of MECS may be associated with alterations in
CaMkIIα activity. The results of the present study are consistent
with the results of a previous study where an increase in CaMkII
activity was associated with the induction of long-term
potentiation (LTP) as a model of synaptic plasticity (57). Activation of CaMkII resulted in an
increased expression of AMPA receptors, which caused enhanced
function of AMPA and was necessary for the induction of LTP
(57). Previous studies indicated
that PKA acted in parallel with CaMKII in controlling synaptic
incorporation of GluR1 (24,58). Therefore, the activation of PKA may
also affect activity-dependent AMPA receptor trafficking (24,58). The
current study identified no marked alterations in the expression
levels of the catalytic or regulatory subunits of PKA, suggesting
that the alteration in cognitive function among the rats may not be
associated with the PKA activity. However, it has been previously
demonstrated that the activation of either PKA or CaMKII could
modulate the GluR1 phosphorylation (24). One possible explanation for the above
results was that repeated ECS, similar to single ECS, exerted its
clinical effect primarily through the modulation of AMPA activity
mediated by the GluR1 phosphorylation at Ser831 (59). The biochemical results of the present
study indicated that GluR1 was significantly phosphorylated at
Ser831 following MECS treatment, whereas the phosphorylation of
GluR1 at Ser845 was unaltered. Therefore, MECS improved the
cognitive performance in stressed rats, which may be due to the
activation of the CaMKII-mediated phosphorylation of GluR1 in the
hippocampus.
CREB is a key modulator of development, plasticity
and neuroprotection in the central nervous system. Alterations in
CREB-mediated transcription have been implicated in multiple
cognitive and psychiatric disorders including depression and
cognitive decline (60). It has been
previously demonstrated that CREB activity decreased in the brains
of depressed patients, was upregulated following administration of
a number of antidepressants, and induced antidepressant effects in
rodent hippocampi (61). Qiu et
al (62) indicated that CREB
could be phosphorylated in response to the activation of GluRs,
leading to alterations in synaptic plasticity and neuronal
structure. Therefore, the current study detected the expression of
CREB and pCREB and found that the medium charge of 120 mC
administered with the novel anesthetic protocol markedly
upregulated the level of pCREB and did not alter the expression of
CREB in the experimental animals. These results further confirmed
that the combination of ketamine, propofol and ECS affected the
cognitive function of depressed animals associated with synaptic
plasticity. The present study suggested that optimal
neuroprotective effects require administration of an appropriate
stimulus intensity for the improvement of antidepressant
activity.
In conclusion, the present study provided evidence
that the medium stimulus of 120 mC was the optimal intensity for
the treatment of stressed rats using this novel anesthetic
protocol. The improved cognitive function was associated with
CaMKII-mediated phosphorylation of GluR1 in the hippocampus.
Acknowledgements
The authors would like to thank Dr Jie Luo
(Department of Anesthesiology, The First Affiliated Hospital of
Chongqing Medical University, Chongqing, China) for her help in
correcting the manuscript.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81760257), the
Science and Technology Department of Sichuan Province (grant no.
2018JY0351) and the Science and the Science and Technology
Department of Hubei Province (grant no. 2016CFB368).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FZ was performed the experiments, data analysis and
manuscript preparation. GH and XZ wrote and revised the manuscript,
and produced the experimental design.
Ethics approval and consent to
participate
The experiment protocols were approved by the
Ethical Committee of Chongqing Medical University (reference no.
2017-004).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of
interests regarding the publication of this paper.
References
|
1
|
Han J, Wang LU, Bian H, Zhou X and Ruan C:
Effects of paroxetine on spatial memory function and protein kinase
C expression in a rat model of depression. Exp Ther Med.
10:1489–1492. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pittenger C and Duman RS: Stress,
depression, and neuroplasticity: A convergence of mechanisms.
Neuropsychopharmacology. 33:88–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pagnin D, de Queiroz V, Pini S and Cassano
GB: Efficacy of ECT in depression: A meta-analytic review. J ECT.
20:13–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lisanby SH: Electroconvulsive therapy for
depression. N Engl J Med. 357:1939–1945. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patel AS, Gorst-Unsworth C, Venn RM,
Kelley K and Jacob Y: Anesthesia and electroconvulsive therapy: A
retrospective study comparing etomidate and propofol. J ECT.
22:179–183. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo J, Min S, Wei K, Cao J, Wang B, Li P,
Dong J and Liu Y: Propofol prevents electroconvulsive-shock-induced
memory impairment through regulation of hippocampal synaptic
plasticity in a rat model of depression. Neuropsychiatr Dis Treat.
10:1847–1859. 2014.PubMed/NCBI
|
|
7
|
Zhu X, Hao X, Luo J, Min S, Xie F and
Zhang F: Propofol inhibits inflammatory cytokine-mediated glutamate
uptake dysfunction to alleviate learning/memory impairment in
depressed rats undergoing electroconvulsive shock. Brain Res.
1595:101–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rampton A, Griffin R, Durcan J and Stuart
C: Propofol and electroconvulsive therapy. Lancet. 331:296–297.
1988. View Article : Google Scholar
|
|
9
|
Warnell RL, Swartz CM and Thomson A:
Propofol interruption of ECT seizure to reduce side-effects: A
pilot study. Psychiatry Res. 175:184–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Naughton M, Clarke G, O'Leary OF, Cryan JF
and Dinan TG: A review of ketamine in affective disorders: Current
evidence of clinical efficacy, limitations of use and pre-clinical
evidence on proposed mechanisms of action. J Affect Disord.
156:24–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
White PF, Way WL and Trevor AJ:
Ketamine-its pharmacology and therapeutic uses. Anesthesiology.
56:119–136. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kranaster L, Kammerer-Ciernioch J, Hoyer C
and Sartorius A: Clinically favourable effects of ketamine as an
anaesthetic for electroconvulsive therapy: A retrospective study.
Eur Arch Psychiatry Clin Neurosci. 261:575–582. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okamoto N, Nakai T, Sakamoto K, Nagafusa
Y, Higuchi T and Nishikawa T: Rapid antidepressant effect of
ketamine anesthesia during electroconvulsive therapy of
treatment-resistant depression: Comparing ketamine and propofol
anesthesia. J ECT. 26:223–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zarate CA, Singh JB, Carlson PJ, Brutsche
NE, Ameli R, Luckenbaugh DA, Charney DS and Manji HK: A randomized
trial of an N-methyl-D-aspartate antagonist in treatment-resistant
major depression. Arch Gen Psychiatry. 63:856–864. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Corlett PR, Honey GD, Aitken MR, Dickinson
A, Shanks DR, Absalom AR, Lee M, Pomarol-Clotet E, Murray GK,
McKenna PJ, et al: Frontal responses during learning predict
vulnerability to the psychotogenic effects of ketamine: Linking
cognition, brain activity and psychosis. Arch Gen Psychiatry.
63:611–621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bowdle AT, Radant AD, Cowley DS, Kharasch
ED, Strassman RJ and Roy-Byrne PP: Psychedelic effects of ketamine
in healthy volunteers relationship to steady-state plasma
concentrations. J Am Soc Anesthesiologists. 88:82–88. 1998.
|
|
17
|
Chen J, Peng LH, Luo J, Liu L, Lv F, Li P,
Ao L, Hao XC and Min S: Effects of low-dose ketamine combined with
propofol on phosphorylation of AMPA receptor GluR1 subunit and
GABAA receptor in hippocampus of stressed rats receiving
electroconvulsive shock. J ECT. 31:50–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andrade C, Kurinji S, Sudha S and Chandra
JS: Effects of pulse amplitude, pulse frequency and stimulus
duration on seizure threshold: A laboratory investigation. J ECT.
18:144–148. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu Y, Li X, Wang X, Yao J and Zhuang S:
miR-34a deficiency in APP/PS1 mice promotes cognitive function by
increasing synaptic plasticity via AMPA and NMDA receptors.
Neurosci Lett. 670:94–104. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Treccani G, Kristian GDJ, Wegener G and
Müller HK: Differential expression of postsynaptic NMDA and AMPA
receptor subunits in the hippocampus and prefrontal cortex of the
flinders sensitive line rat model of depression. Synapse.
70:471–474. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maeng S, Zarate CA Jr, Du J, Schloesser
RJ, McCammon J, Chen G and Manji HK: Cellular mechanisms underlying
the antidepressant effects of ketamine: role of
alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors.
Biol Psychiatry. 63:349–352. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Greger IH, Ziff EB and Penn AC: Molecular
determinants of AMPA receptor subunit assembly. Trends Neurosci.
30:407–416. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nordgren M, Karlsson T, Svensson M, Koczy
J, Josephson A, Olson L, Tingström A and Brené S: Orchestrated
regulation of Nogo receptors, LOTUS, AMPA receptors and BDNF in an
ECT model suggests opening and closure of a window of synaptic
plasticity. PLoS One. 8:e787782013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Keifer J and Zheng Z: AMPA receptor
trafficking and learning. Eur J Neurosci. 32:269–277. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bracey JM, Kurz JE, Low B and Churn SB:
Prolonged seizure activity leads to increased protein kinase A
activation in the rat pilocarpine model of status epilepticus.
Brain Res. 1283:167–176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Coultrap SJ, Zaegel V and Bayer KU: CaMKII
isoforms differ in their specific requirements for regulation by
nitric oxide. FEBS Lett. 588:4672–4676. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ren L, Zhang F, Min S, Hao X, Qin P and
Zhu X: Propofol ameliorates electroconvulsive shock-induced
learning and memory impairment by regulation of synaptic
metaplasticity via autophosphorylation of CaMKIIa at Thr 305 in
stressed rats. Psychiatry Res. 240:123–130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Banasr M, Valentine GW, Li XY, Gourley SL,
Taylor JR and Duman RS: Chronic unpredictable stress decreases cell
proliferation in the cerebral cortex of the adult rat. Biol
Psychiatry. 62:496–504. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Karolina P, Barbara B and Grazyna B:
Utility of the chronic unpredictable mild stress model in research
on new antidepressants. Curr Issue Pharm Med Sci. 27:97–101. 2014.
View Article : Google Scholar
|
|
30
|
Luo KR, Hong CJ, Liou YJ, Hou SJ, Huang YH
and Tsai SJ: Differential regulation of neurotrophin S100B and BDNF
in two rat models of depression. Prog Neuropsychopharmacol Biol
Psychiatry. 34:1433–1439. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie F, Min S, Liu L, Peng L, Hao X and Zhu
X: Advanced age enhances the sepsis-induced up-regulation of the γ-
and α7-nicotinic acetylcholine receptors in different parts of the
skeletal muscles. Arch Gerontology Geriatr. 65:1–8. 2016.
View Article : Google Scholar
|
|
32
|
Segi-Nishida E, Warner-Schmidt JL and
Duman RS: Electroconvulsive seizure and VEGF increase the
proliferation of neural stem-like cells in rat hippocampus. Proc
Natl Acad Sci USA. 105:11352–11357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Luo J, Min S, Wei K, Zhang J and Liu Y:
Propofol interacts with stimulus intensities of electroconvulsive
shock to regulate behavior and hippocampal BDNF in a rat model of
depression. Psychiatry Res. 198:300–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ding L, Zhang X, Guo H, Yuan J, Li S, Hu
W, Golden T and Wu N: The functional study of a chinese herbal
compounded antidepressant medicine-jie yu chu fan capsule on
chronic unpredictable mild stress mouse model. PLoS One.
10:e01334052015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cao XZ, Ma H, Wang JK, Liu F, Wu BY, Tian
AY, Wang LL and Tan WF: Postoperative cognitive deficits and
neuroinflammation in the hippocampus triggered by surgical trauma
are exacerbated in aged rats. Prog Neuropsychopharmacology Biol
Psychiatry. 34:1426–1432. 2010. View Article : Google Scholar
|
|
36
|
Liao WT, Xiao XY, Zhu Y and Zhou SP: The
effect of celastrol on learning and memory in diabetic rats after
sevoflurane inhalation. Arch Med Sci. 14:370–380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tianzhu Z, Shihai Y and Juan D:
Antidepressant-like effects of cordycepin in a mice model of
chronic unpredictable mild stress. Evid Based Complement Alternat
Med. 2014:4385062014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Willner P, Towell A, Sampson D,
Sophokleous S and Muscat R: Reduction of sucrose preference by
chronic unpredictable mild stress and its restoration by a
tricyclic antidepressant. Psychopharmacology (Berl). 93:358–364.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Willner P: Validity, reliability and
utility of the chronic mild stress model of depression: A 10-year
review and evaluation. Psychopharmacology (Berl). 134:319–329.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xinxing W, Wei L, Lei W, Rui Z, Baoying J
and Lingjia Q: A neuroendocrine mechanism of co-morbidity of
depression-like behavior and myocardial injury in rats. PLoS One.
9:e884272014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Garcia LS, Comim CM, Valvassori SS, Réus
GZ, Stertz L, Kapczinski F, Gavioli EC and Quevedo J: Ketamine
treatment reverses behavioral and physiological alterations induced
by chronic mild stress in rats. Prog Neuropsychopharmacol Biol
Psychiatry. 33:450–455. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Caligiuri MP and Ellwanger J: Motor and
cognitive aspects of motor retardation in depression. J Affect
Disord. 57:83–93. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Frey R, Heiden A, Scharfetter J,
Schreinzer D, Blasbichler T, Tauscher J, Felleiter P and Kasper S:
Inverse relation between stimulus intensity and seizure duration:
Implications for ECT procedure. J ECT. 17:102–108. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang X, Yang Y, Zhou X, Qu Q, Ou C, Liu L
and Zhou S: Propofol pretreatment increases antidepressant-like
effects induced by acute administration of ketamine in rats
receiving forced swimming test. Psychiatry Res. 185:248–253. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Alves HN, da Silva AL, Olsson IA, Orden JM
and Antunes LM: Anesthesia with intraperitoneal propofol,
medetomidine, and fentanyl in rats. J Am Assoc Lab Anim Sci.
49:454–459. 2010.PubMed/NCBI
|
|
46
|
Taşkara E, Gör A, Kutlu O, Karagüzel E,
Topbaş M and Şenel AC: Does propofol prevent testicular
ischemia-reperfusion injury due to torsion in the long term?
Pediatr Surg Int. 27:1003–1007. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kushikata T, Sawada M, Niwa H, Kudo T,
Kudo M, Tonosaki M and Hirota K: Ketamine and propofol have
opposite effects on postanesthetic sleep architecture in rats:
relevance to the endogenous sleep-wakefulness substances orexin and
melanin-concentrating hormone. J Anesth. 30:437–443. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nikiforuk A and Popik P: Ketamine prevents
stress-induced cognitive inflexibility in rats.
Psychoneuroendocrinology. 40:119–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sackeim HA, Devanand DP and Prudic J:
Stimulus intensity, seizure threshold and seizure duration: Impact
on the efficacy and safety of electroconvulsive therapy. Psychiatr
Clin North Am. 14:803–843. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Carter SD, Mifsud KR and Reul JM: Distinct
epigenetic and gene expression changes in rat hippocampal neurons
after Morris water maze training. Front Behav Neurosci. 9:1562015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zots MA, Ivashkina OI, Ivanova AA and
Anokhin KV: Formation of spatial and nonspatial memory in different
condensed versions of short-term learning in morris water maze.
Bull Exp Biol Med. 156:602–604. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Erdogan Kayhan G, Yucel A, Colak YZ, Ozgul
U, Yologlu S, Karlıdag R and Ersoy MO: Ketofol (mixture of ketamine
and propofol) administration in electroconvulsive therapy. Anaesth
Intensive Care. 40:305–310. 2012.PubMed/NCBI
|
|
53
|
Wang D, Wang X, Liu X, Jiang L, Yang G,
Shi X, Zhang C and Piao F: Inhibition of miR-219 alleviates
arsenic-induced learning and memory impairments and synaptic damage
through up-regulating CaMKII in the hippocampus. Neurochem Res.
43:948–958. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Matsuzaki K, Miyazaki K, Sakai S, Yawo H,
Nakata N, Moriguchi S, Fukunaga K, Yokosuka A, Sashida Y, Mimaki Y,
et al: Nobiletin, a citrus flavonoid with neurotrophic action,
augments protein kinase A-mediated phosphorylation of the AMPA
receptor subunit, GluR1 and the postsynaptic receptor response to
glutamate in murine hippocampus. Eur J Pharmacol. 578:194–200.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lee HK, Barbarosie M, Kameyama K, Bear MF
and Huganir RL: Regulation of distinct AMPA receptor
phosphorylation sites during bidirectional synaptic plasticity.
Nature. 405:955–959. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lee HK, Takamiya K, Han JS, Man H, Kim CH,
Rumbaugh G, Yu S, Ding L, He C, Petralia RS, et al: Phosphorylation
of the AMPA receptor GluR1 subunit is required for synaptic
plasticity and retention of spatial memory. Cell. 112:631–643.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Singleton MW, Holbert WH II, Lee AT,
Bracey JM and Churn SB: Modulation of CaM kinase II activity is
coincident with induction of status epilepticus in the rat
pilocarpine model. Epilepsia. 46:1389–1400. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wu LJ, Ren M, Wang H, Kim SS, Cao X and
Zhuo M: Neurabin contributes to hippocampal long-term potentiation
and contextual fear memory. PLoS One. 3:e14072008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Fumagalli F, Pasini M, Sartorius A,
Scherer R, Racagni G, Riva MA and Gass P: Repeated
electroconvulsive shock (ECS) alters the phosphorylation of
glutamate receptor subunits in the rat hippocampus. Int J
Neuropsychopharmacol. 13:1255–1260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tanis KQ, Duman RS and Newton SS: CREB
binding and activity in brain: Regional specificity and induction
by electroconvulsive seizure. Biol Psychiatry. 63:710–720. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Carlezon WA Jr, Duman RS and Nestler EJ:
The many faces of CREB. Trends Neurosci. 28:436–445. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Qiu S and Currás-Collazo MC:
Histopathological and molecular changes produced by hippocampal
microinjection of domoic acid. Neurotoxicol Teratology. 28:354–362.
2006. View Article : Google Scholar
|