Introduction
Central retinal vein occlusion (CRVO), a common
retinal vascular disease, is one of the most frequent causes of
vision loss in individuals aged >60 years due to retinal
ischemia, intra-retinal hemorrhage and edema. CRVO may be
classified into two types, including the ischemic (hemorrhagic)
type and the non-ischemic (partial) type. The two types vary in
fundus appearance, with the non-ischemic type accounting for an
estimated 60–70% of cases. Each of the two types presents with
thrombosis at the level of the lamina cribrosa (1). However, ischemic CRVO is associated
with a higher incidence of developing ocular neovascularization
(NV) (2,3), and its prognosis is usually worse
compared with that of partial CRVO. Complications of NV, including
neovascular glaucoma (NVG) and vitreous hemorrhage (VH), may cause
severe visual morbidity and blindness (4). Hence, there is an urgent requirement to
identify novel effective prevention and treatment strategies for
ischemic CRVO.
Current treatments for ischemic CRVO include
anti-vascular endothelial growth factor (VEGF) drugs, steroids,
anti-coagulants, laser treatments and a range of surgical
interventions to minimize or delay the onset of complications
associated with CRVO, including ME and NV (5). Laser photocoagulation is considered to
be the first-line therapy for treating the complications of retinal
vascular disease. Panretinal photocoagulation (PRP) is universally
considered to be an established treatment for the prevention of
ocular NV, particularly NVG, in ischemic CRVO. The beneficial
effects of PRP in preventing and/or treating ocular NV in CRVO have
been confirmed by multiple studies (6,7). The
risk of NVG, VH and NV of the iris is decreased after receiving PRP
(8). Similarly, the Central Vein
Occlusion Study (CVOS), demonstrated that the risk of anterior
segment NV is decreased but not eliminated in eyes with ischemic
CRVO after receiving prophylactic PRP (9).
However, in several other trials, no beneficial
effects of PRP have been identified in prospective settings
(10,11). Consequently, it is necessary to
perform a systematic study evaluating the role of PRP treatment in
eyes with ischemic CRVO. In the present study, all relevant studies
were systematically reviewed in order to identify whether PRP
prevents or reduces the incidence of ocular NV, particularly NVG,
and whether it influences the ultimate visual outcome in affected
eyes.
Materials and methods
Search strategy and selection
criteria
A comprehensive search was performed in four
electronic medical databases: the PubMed, Embase, Chinese
Biomedical Literature Database and, Chinese Science and Technology
Periodicals databases. Articles published until April 2017 were
included. The search terms were as follows: ‘Central retinal vein
obstruction’ OR ‘CRVO’ (MeSH terms) AND ‘laser’ OR ‘panretinal
photocoagulation’ (MeSH terms) AND English OR Chinese (language).
In order to identify additional studies, the references of
retrieved articles and relevant reviews were searched manually.
Articles were selected according to the set criteria. The inclusion
criteria were as follows: i) Articles published in English or
Chinese; ii) studies with ≥20 subjects; and iii) articles
evaluating the efficacy of laser therapy for ischemic CRVO. The
exclusion criteria were as follows: i) Articles evaluating the
efficacy of laser therapy for CRVO, but not specifically ischemic
CRVO; ii) patients with obvious cataract or other ocular symptoms
that affect visual acuity; and iii) reviews or case reports.
Study selection and data
extraction
Two independent reviewers screened all titles and
abstracts for eligibility and performed full-text reviews in
duplicate. Any disagreements between the two reviewers were
resolved by consensus.
The following information was extracted and
collected from each of the included articles: Author, publication
year, location, diagnostic information, number of participants and
eyes, mean age of participants, treatment protocols and follow-up
time. Clinical outcomes at the final follow-up after laser therapy
were also reviewed using a standardized data collection form. Data
on visual acuity (VA) were extracted from the studies if available.
In addition, other clinical outcomes, including the overall
effectiveness, average papillary retinal nerve fiber layer (RNFL)
thickness, corneal nerve plexus parameters, upper temporal retinal
blood flow (RBF), and macular RBF were recorded. All temporary and
permanent complications were also collected, including
complications of neovascularization of the retina (NVR), optic disc
neovascularization (NVD) and iris neovascularization (NVI),
neovascular glaucoma (NVG), vitreous hemorrhage (VH), changes in
the visual field, ME, macular thickness and intraocular pressure
(IOP).
Quality assessment
The studies included in the present systematic
review were primarily non-randomized (comparative or
non-comparative studies). Therefore, the quality of the studies
included was assessed using a revised version of the Methodological
Index for Non-Randomized Studies (MINORS) (1), which contained 12 items: i) A stated
aim of the study; ii) inclusion of consecutive patients; iii)
prospective collection of data; iv) an endpoint appropriate
regarding the study aim; v) unbiased evaluation of end-points; vi)
follow-up period appropriate regarding the major end-point; vii)
loss to follow-up not exceeding 5%; viii) prospective calculation
of the sample size; ix) a control group having the gold standard
intervention; x) all group were managed during the same time
period; xi) baseline equivalence of groups; xii) statistical
analyses adapted to the study design. The items i-viii are
associated with non-comparative studies, whereas items ix-xii are
also relevant to comparative studies. The score of each item ranged
from 0–2: 0 suggested it was not reported, 1 suggested the item was
reported but not sufficiently, and 2 suggested that the item was
reported and the information was sufficient. The ideal global score
was 16 for the non-comparative studies and 24 for the comparative
studies.
The quality of evidence regarding early laser
therapy for ischemic CRVO was assessed based on the Grading of
Recommendations, Assessment, Development, and Evaluation (GRADE)
method (12,13). The articles were evaluated
independently by the authors according to the GRADE criteria. The
quality of the evidence from each article was evaluated as high,
moderate, low or very low.
Results
Literature search
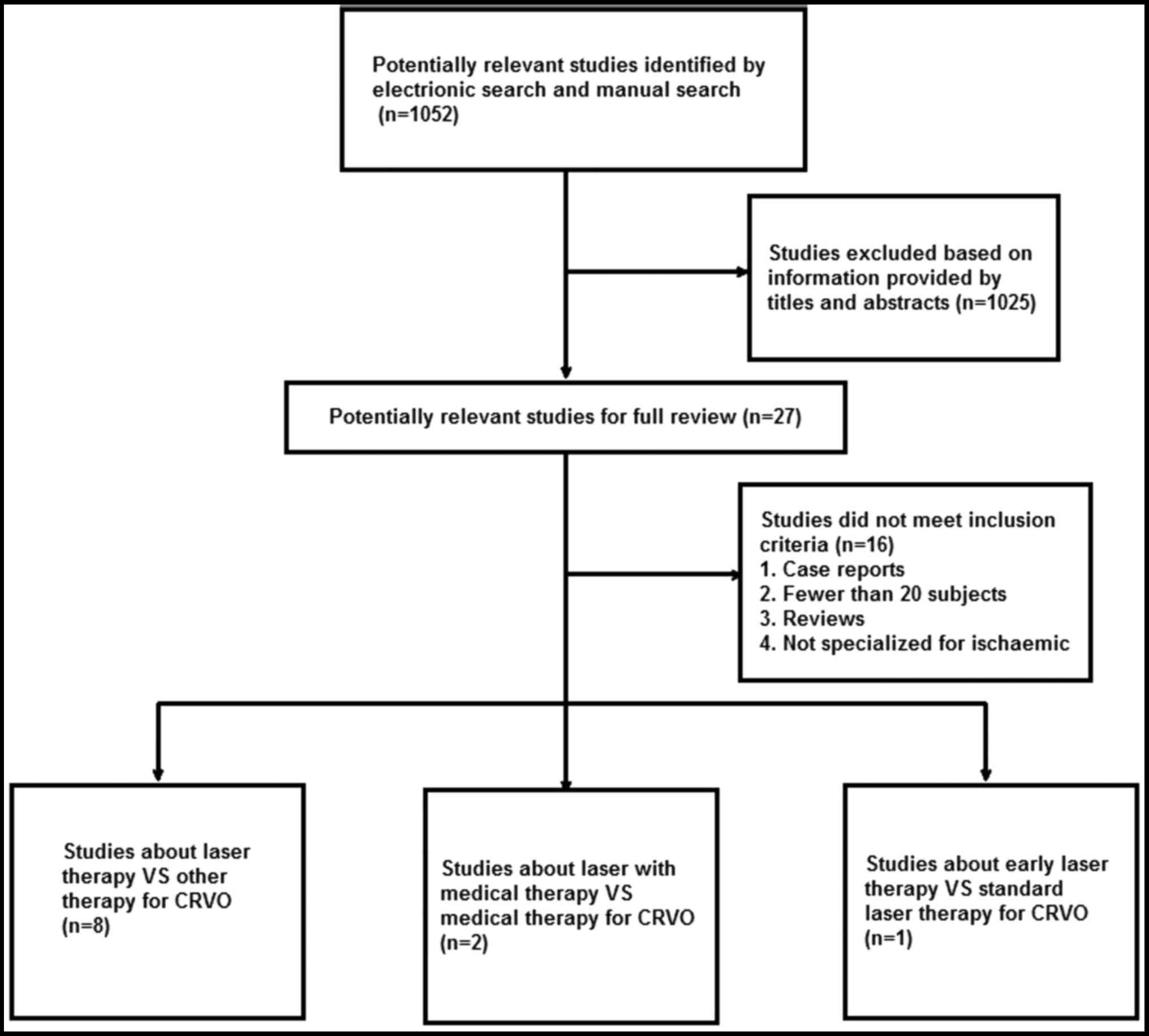
The search yielded 1,052 articles (411 from PubMed,
234 from Embase, 215 from the Chinese Biomedical Literature
Database and 192 from the Chinese Science and Technology
Periodicals Database), of which 1,025 articles (including
duplicates) were excluded based on information provided by titles
and abstracts. A further 16 articles were excluded after full-text
review. A total of 11 studies, including seven studies in English
(8,12–17) and
four Chinese studies (18–21), were finally included for the present
systematic review (Fig. 1). A total
of 8 studies compared the efficacy of laser therapy with other
therapies for CRVO (drug treatment or no treatment). Furthermore,
two studies compared the efficacy of laser and drug treatment with
medical treatment alone for CRVO. The remaining study compared the
efficacy of early laser therapy with standard laser therapy
(regular examinations and laser therapy as soon as NV is
identified) for CRVO. The details of the studies included are
presented in Table I.
 | Table I.Characteristics of the 11 studies
included. |
Table I.
Characteristics of the 11 studies
included.
| First author
(year) | Country | Diagnostic
information | Eyes (n) | Males/females
(n) | Age (years) | Treatment in Study
group vs. Control group | Time of follow-up
after treatment (mean) | Quality
classification of evidence | (Refs.) |
|---|
| Qu (2014) | China | Ischemic CRVO | 63 (Treatment group,
33; Control, 30) | Total, 38:25 | Total, 59.3±11.1
(32–64) | Argon ion laser | 3 months therapy vs.
drug treatment | Very low | (19) |
| Fan (2011) | China | Ischemic CRVO | 68 (Treatment group,
40; Control, 28) | Treatment group,
18:22; Control, 12:16 | Treatment group,
57.23±11.2 (39–69); Control, 55.43±12.20 (40–67) | PRP vs. no
treatment | >8 (11±2.3)
months | Moderate | (18) |
| Cao (2006) | China | Ischemic CRVO | 40 | 20:20 | 56 (25–75) | Krypton laser PRP
(control treatment not stated) | 6–36 months | Very low | (20) |
| Bitirgen (2017) | USA | Unilateral ischemic
CRVO | 32 | 19:13 | 63.56±10.74
(45–85) | PRP vs. fellow eyes
with no treatment | 6–156 months | Moderate | (14) |
| Hayreh (1990) | USA | Ischemic CRVO | 123 (Treatment group,
47; Control, 76) | Treatment group,
27:20; Control, 39:39 | Treatment group,
72.7a; Control,
69.5b | Argon laser PRP vs.
no treatment | Every 6 months | Moderate | (15) |
| Laatikainen
(1977) | UK | Ischemic CRVO | 23 (Treatment group,
12; Control, 11) | – | – | PRP vs. no
treatment | ≥12 months | Low | (16) |
| Magargal
(1982) | USA | Ischemic CRVO | 100 | 64:36 | 71 (39–86) | Argon laser PRP
(control treatment not stated) | ≥6 months | Very low | (8) |
| Arvas (2002) | Turkey | Ischemic CRVO | 24 (Treatment
group, 12; Control, 12) | Treatment group,
8:4; Control, 6:6 | Treatment group,
59.8±7.6; Control, 58.7±6.9 | Laser vs. normal
eyes | 1 month | Moderate | (13) |
| Cai (2009) | China | Ischemic CRVO | 60 (Treatment
group, 30; Control, 30) | Treatment group,
10:20; Control, 12:18 | Treatment group,
70.3; Control, 71.4 | Photocoagulation
Krypton yellow laser (control treatment not stated) | 2 weeks | Moderate | (21) |
| Pikkel (2016) | Israel | Ischemic CRVO with
ME | 65 (D1,
23; D2, 21; D3, 21) | – | D1,
64.0±9.1; D2, 62.9±10.0; D3, 66.9±8.8 | Medical vs. PRP +
Medical. D1: Anti-VEGF injections; D2: Laser
grid + PRP; D3: Anti-VEGF injections + laser grid +
PRP | 12 months | Moderate | (17) |
| Kjeka (2013) | Norway | Ischemic CRVO with
NV | 36 (Treatment
group, 18; Control, 18) | Treatment group,
7:11; Control, 12:6 | Treatment group,
76.1; Control, 79.8 | Early laser group
vs. standard laser group | 48 months
(Treatment group; 41 months; Control, 30 months) | Moderate | (12) |
Quality assessment
The quality of all 11 studies included was assessed
according to the items of MINORS. Of all of the studies, nine had a
control group, whereas two studies had no control group. The MINORS
mean score was 10.5 (range, 10–11) for studies without a control
group, and 18.9 (range, 18–20) for studies with a control group;
thus, the quality of the articles included was generally low
(Table II).
 | Table II.Evaluation of the methodological
quality of the studies included according to the Methodological
Index for Non-Randomized Studies. |
Table II.
Evaluation of the methodological
quality of the studies included according to the Methodological
Index for Non-Randomized Studies.
| First author
(year) | Aim of the study is
stated | Inclusion of
consecutive patients | Prospective
collection of data | Endpoint
appropriate regarding the study aim | Unbiased evaluation
of endpoints | Follow-up period
appropriate regarding the major endpoint | Loss to follow-up
not exceeding 5% | Prospective
calculation of the sample size | Control group as
the gold standard of intervention | Groups assessed
together | Equivalence of
groups at baseline | Statistical
analyses adapted to the study design | Total score | (Refs.) |
|---|
| Qu (2014) | 2 | 2 | 2 | 2 | 0 | 0 | 2 | 0 | 2 | 2 | 2 | 2 | 18a | (19) |
| Fan (2011) | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 20a | (18) |
| Cao (2006) | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 0 | N/A | N/A | N/A | N/A | 11 | (20) |
| Bitirgen
(2017) | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 18a | (14) |
| Hayreh (1990) | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 1 | 2 | 20a | (15) |
| Latikainen
(1977) | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 18a | (16) |
| Magargal
(1982) | 2 | 2 | 2 | 2 | 0 | 0 | 2 | 0 | N/A | N/A | N/A | N/A | 10 | (8) |
| Arvas (2002) | 2 | 2 | 2 | 2 | 0 | 0 | 2 | 0 | 2 | 2 | 2 | 2 | 18a | (13) |
| Cai (2009) | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 20a | (21) |
| Pikkel (2016) | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 20a | (17) |
| Kjeka (2011) | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 2 | 2 | 18a | (12) |
VA
VA is an important outcome of laser treatment for
ischemic CRVO. A total of eight articles reported on the index of
VA after laser therapy at the final follow-up. Of these, five
articles had a control group, two articles had no control group and
1 article compared the standard PRP treatment (regular examinations
and PRP as soon as NV is identified) with early PRP treatment
(Table III). Five articles, which
had a control group, reported that there was no significant
difference in VA between the treated group and the control group
(P>0.05) (15–18,21). The
quality was rated as moderate for four studies (15,18,17,21) and
low for one study (16). The two
studies that had no control group reported that the VA was improved
after laser treatment in the majority of cases, but the quality
classification was low for each of these two studies. The logMAR
for patients undergoing early PRP treatment (performed as soon as
possible after the electroretinography examination) was lower
compared with that after standard PRP treatment, suggesting that
ischemic CRVO should be treated early (P=0.003), and the quality of
this study was determined to be moderate. These results suggest
that laser therapy may not improve the VA of ischemic CRVO, but it
is crucially important to further verify this with high-quality
clinical studies.
 | Table III.Clinical outcomes of laser treatment
at the final follow-up. |
Table III.
Clinical outcomes of laser treatment
at the final follow-up.
| First author
(year) | Testing index | VA | Complications | Other means of
evaluation of clinical efficacy | (Refs.) |
|---|
| Qu (2014) | VA Size of ME
Retinal thickness Retinal perfusion | The visual acuity
of the patients in the experimental group was significantly
restored. The difference was significant. |
| i) Excellent, ii)
effective, iii) ineffectivea. Treatment group: i) 24; ii) 6; iii)
3; total effective rate, 90.9%. Control: i) 14; ii) 7; iii 9; total
effective rate, 70.0%; P<0.05 | (19) |
| Fan (2011) | VA
Complications | Treatment group:
Improved, 1; no change, 20; worsened, 19 Control: Improved, 1; no
change, 12; worsened, 15 Treatment vs. control group:
P>0.05 | Treatment group:
18/40 (45%); Control: 14/28 (50%); P>0.05 |
| (18) |
| Cao (2006) | VA | Improved, 23; no
change, 9; worsened, 4 |
| i) Excellent, ii)
effective, iii) ineffectivea. i) 27; ii), 6; iii) 3 | (20) |
| Bitirgen
(2017) | IOP, average RNFL
thickness; corneal nerve plexus parameters |
| IOP (mmHg)
Treatment group: 13.41±2.43; Control: 13.47±2.66; P=0.823 | i) RNFL thickness
(µm). Treatment group: 88.78±13.98; Control: 95.06±13.46; P=0.007
ii) Corneal nerve plexus parameters. a) NFD
(fibers/mm2). Treatment group: 18.74 (12.49–24.99);
Control: 31.24 (18.75–35.93); P<0.001. b) NBD
(branches/mm2). Treatment group: 21.87 (12.49–42.17);
Control: 43.74 (24.99–60.93) P<0.001. c) NFL
(mm/mm2). Treatment group: 11.89±4.83; Control:
16.97±3.25; P<0.001 | (14) |
| Hayreh (1990) | VA, complications
(development of NVG, development of iris and angle NV, development
of NVR and/or NVD, development of VH, visual field) | Category I:
P=0.981; category II: P=0.538; category III: P=0.806 (treatment vs.
control group) | i) Development of
NVG. Category I: P=0.295; category II: P=1.000 (treatment vs.
control group). ii) Development of iris and angle NV. Category I:
P=0.040 (good in treatment group); category II: P=0.329 (treatment
vs. control group). iii) Development of NVR and/or NVD. Category I:
P=0.308; category II, P=1.000 (treatment vs. control group). iv)
Development of VH. Category I: P=0. 210; category II: P=1.000
(treatment vs. control group). Visual field. Category I: P=0.002
(treatment group worse than Control); category II: P=1.000;
category III: P=0.371 (treatment vs. control group). |
| (15) |
| Laatikainen
(1977) | VA, visual field,
iris NV, NVG, neovascular complications, macular appearance | Treatment group:
Improved, 2; no change, 4; worsened, 6. Control: Improved, 2; no
change, 5; worsened, 4. (P>0.05, treatment vs. control
group) | i) Visual field: no
difference between the two groups. ii) Iris NV: Treatment better
than control group. Treatment: Decreased ordisappeared, n=5 (42%);
developed, n=2 (17%). Contol: Decreased or disappeared, n=0 (0%);
developed, n=5 (45%). iii) NVG: Treatment better than control group
Treatment group: NVG developed, n=0; Control: NVG developed, n=2
(18%). iv) Neovascular complications: Treatment better than control
group. Treatment group: n=8 (67%); Control group: n=13 (118%). v)
Macular appearance: No difference between the two groups. NVG,
n=0. |
| (16) |
| Magargal
(1982) | VA, NVG |
| NVG |
| (8) |
| Arvas (2002) | Upper temporal RBF,
macular RBF | No change, 98;
worsened, 2. | Two subjects
developed NVG | i) Upper temporal
RBF (arbitrary units). a) Baseline vs. after treatment in Treatment
group: Volume, 8.86±2.26 vs. 11.48±1.86 (P<0.05); flow,
172.87±28.33 vs. 214.96±17.51 (P<0.05); velocity, 0.78±0.13 vs.
0.93±0.09 (P<0.05). b) Treatment group (after treatment) vs.
Control: Volume, 11.48±1.86 vs. 24.32±2.15 (P<0.05); flow,
214.96±17.51 vs. 308.62±13.35 (P<0.05); velocity, 0.93±0.09 vs.
1.48±0.18 (P<0.05). ii) Macular RBF (arbitrary units). a)
Treatment: Baseline vs. after treatment in Treatment group: Volume,
13.06±1.49 vs. 13.63±1.22 (P>0.05); flow, 238.8±28.46 vs.
232.24±25.18 (P>0.05); velocity, 0.83±0.09 vs. 0.87±0.12
(P>0.05). b) Treatment group (after treatment) vs. Control:
Volume, 13.63±1.22 vs. 14.41±1.91 (P>0.05); flow: 232.24±25.18
vs. 233.10±26.39 (P>0.05); velocity, 0.87±0.12 vs. 0.87±0.08
(P>0.05). | (13) |
| Cai (2009) | VA, incidence rate
of NVG | Treatment group:
Baseline VA, 0.094±0.034; post-treatment VA, 0.101±0.043
(P>0.05). Control group: Baseline VA, 0.097±0.038;
post-treatment VA, 0.102±0.066 (P>0.05). Post-VAT vs.
Post-VAC, P>0.05. | Incidence rate of
NVG: Treatment group, 4.8%; Control group, 30.4%. |
| (21) |
| Pikkel (2016) | ΔVA (final
VA-baseline VA), macular thickness assessed by optical coherence
tomography, ΔOCT (baseline OCT-final OCT), ME [n (%)]§ | ΔVA in group D1,
0.128±0.077; D2, 0.088±0.057; D3, 0.095±0.065
(P=0.110) | i) ΔOCT (µm): D1,
131.5±41.2; D2, 108.6±29.2; D3, 121.1±34.5
(P=0.111). ii) ME: D1, n=6 (26.1%); D2, n=6
(26.8%); D3, n=3 (14.3%) (P=0.499) |
| (17) |
| Kjeka (2013) | VA (logMAR), ∆IOP
(mmHg; final-baseline), ocular NV, NVG |
| i) VA: Early group,
1.85; standard group, 2.74 (P=0.003). ii) IOP (after treatment.)
[mmHg; mean (range)]: Early group, 13.8 (10–18; within normal
range); Standard group, 19.8 (10–40; higher than normal range) iii)
∆IOP: Early group: −1.2; Standard group, 3.3 (P=0.045). iv)
Incidence of ocular NV: Early group: n=1; Standard group: n=18
(P<0.0001). v) Incidence of NVG: Early group, n=0; Standard
group, n=12 (P<0.0001). |
| (12) |
Complications
Information on complications was available for seven
studies (8,12,15,16,18,19,21),
which was used for evaluating the adverse effects of laser
treatment. This included six articles (12,15,16,18,19,21) with
a control group and one article (8)
without a control group. Neovascular complications, including NVG,
NVR, NVD and NVI, were documented in six articles (8,12,15,16,19,21).
NVG was reported in five articles (8,12,15,16,21).
Laser therapy achieved a good outcome for NVG in three articles
that had a control group (12,16,21).
However, there was no difference between the argon laser PRP
treatment group and the control group (untreated), and the quality
was rated as low. The study that had no control group reported that
only 2 out of 100 eyes developed NVG at the final follow-up after
argon laser PRP treatment (2). These
results indicate that it was possible to prevent the development of
NVG in patients with ischemic CRVO by laser treatment, but further
high-quality research should be performed to confirm this. In the
study by Hayreh et al (15),
there was no difference in the development of NVR and/or NVD
between the argon laser PRP treatment and the untreated group.
However, NVR and neovascular complications were lower in the PRP
treatment group compared with those in the untreated group in the
study by Laatikainen et al (16). Furthermore, compared with the
standard laser PRP group, the occurrence of ocular NV was less
frequent in the early laser PRP group. In the study by Fan and Pan
(18), the rate of complications,
comprising NVR and secondary glaucoma, was comparable between the
PRP-treated group and the untreated group. The visual field was
assessed in two articles, which had control groups. The study by
Laatikainen et al (16)
reported that there was no difference between the laser PRP-treated
group and the untreated group. However, the study by Hayreh et
al (15) indicated that the
laser PRP treatment group suffered a significantly greater loss of
visual field compared with the non-laser treated group, but this
was only for the group in which the onset time of ischemic CRVO was
<90 days.
Other outcomes
The IOP was reported by two studies (12,14).
There was no difference in IOP between the PRP treatment group and
the control (fellow eyes) in patients with unilateral ischemic CRVO
(14). The study by Kjeka et
al (12) reported that the value
of IOP remained within the normal range in the early laser PRP
group, while it was above the normal range in the standard laser
PRP group. ME, macular thickness and VH were comparable in the
treated and untreated groups, and each of them was reported in one
study. Other outcomes, including average RNFL thickness, NFD, NBD
and upper temporal RBF, were also reported, and all of these
outcomes were better in the laser-treated vs. the control groups.
Hence, it was indicated that laser treatment is an effective
surgical method for ischemic CRVO.
Discussion
Retinal photocoagulation is an established treatment
for numerous types of retinal disease, as well as complications of
retinal vascular disease (22).
Retinal photocoagulation exerts its therapeutic effect secondary to
direct thermal injury of the ciliary nerves and causes changes in
the anterior segment (23). The
therapeutic value of retinal photocoagulation, as well as its side
effects of the deterioration of visual field sensitivity, have been
extensively investigated (24). The
CVOS, a large, multicenter prospective randomized controlled trial
(25), indicated that the risk of
anterior segment NV was decreased but not eliminated in eyes with
ischemic CRVO undergoing prophylactic PRP. In the CVOS, when PRP
treatment was applied, NV had regressed in 90% of cases at 1 year,
and the risk of NVG was lowered to 1%. Furthermore, grid macular
photocoagulation was proposed to confer a small benefit in patients
under the age of 65 years for improving VA in eyes with ME
secondary to perfused CRVO, but this result was not significant
(25). Wald (26) suggested that the risk of anterior
segment NV may be prevented in eyes with ischemic CRVO undergoing
prophylactic scatter photocoagulation when they present with ≥75
disc diameters of ischemia.
Although numerous studies have indicated beneficial
effects of PRP against ischemic CRVO, no comprehensive evaluation
of the effects of PRP on retinal outcomes has been previously
provided, to the best of our knowledge. In addition, earlier
studies did not differentiate between the well-established ischemic
and non-ischemic types. If there was a differentiation, ischemia in
CRVO was defined by various criteria based on examinations or test
results. Since PRP has no application in non-ischemic cases, and is
associated with adverse effect of reducing the visual field
according to Goldmann perimetry, including non-ischemic cases in a
PRP study produces a marked bias. There is also a requirement for a
comprehensive systematic review documenting the effects of PRP in
patients with ischemic CRVO. The objective of the present
systematic literature review was to comprehensively document the
current clinical outcomes associated with laser treatment
interventions in ischemic CRVO, in terms of VA, NV complications,
NVG, VH, changes in the visual field, ME, macular thickness and
IOP.
The present systematic review indicated laser
photocoagulation did not appear to be effective in improving the
VA, but in preventing complications. Laatikainen et al
(16) reported that photocoagulation
should be used to prevent complications in the ischaemic type of
central retinal vein occlusion. The risk of NVG, VH and NV of the
iris is decreased after PRP. CRVO is a major cause of NVG, which in
turn is a major cause of blindness leading to enucleation; of note,
PRP in eyes with an ischemic CRVO pattern virtually eliminates the
severe complications of NVG (8). In
addition, in the eyes of patients receiving PRP for the treatment
of ischemic CRVO, significant reductions in corneal sub-basal nerve
plexus parameters and average peripapillary RNFL thickness were
observed. Laser photocoagulation increased RBF in eyes with
ischemic CRVO. It is worth noting that in future clinical studies,
longer follow-up periods are necessary to further clarify these
effects.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data are included in the manuscript.
Authors' contributions
CL and GL designed the manuscript. RW, GL and ZG
collected the data. CL, DJ and YM analyzed the results. CL wrote
the manuscript and GL submitted the study. All authors read and
approved the final manuscript.
Ethics approval and informed consent
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hayreh SS: Classification of central
retinal vein occlusion. Ophthalmology. 90:458–474. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hayreh SS, Klugman MR, Beri M, Kimura AE
and Podhajsky P: Differentiation of ischemic from non-ischemic
central retinal vein occlusion during the early acute phase.
Graefes Arch Clin Exp Ophthalmol. 228:201–217. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hayreh SS, Rojas P, Podhajsky P, Montague
P and Woolson RF: Ocular neovascularization with retinal vascular
occlusion-III. Incidence of ocular neovascularization with retinal
vein occlusion. Ophthalmology. 90:488–506. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McIntosh RL, Rogers SL, Lim L, Cheung N,
Wang JJ, Mitchell P, Kowalski JW, Nguyen HP and Wong TY: Natural
history of central retinal vein occlusion: An evidence-based
systematic review. Ophthalmology. 117:1113–1123, e1115. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bradshaw SE, Gala S, Nanavaty M, Shah A,
Mwamburi M and Kefalas P: Systematic literature review of
treatments for management of complications of ischemic central
retinal vein occlusion. BMC Ophthalmol. 16:1042016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
No authors listed: Argon laser scatter
photocoagulation for prevention of neovascularization and vitreous
hemorrhage in branch vein occlusion. A randomized clinical trial.
Branch vein occlusion study group. Arch Ophthalmol. 104:34–41.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Newcomb EW, Madonia WJ, Pisharody S, Lang
FF, Koslow M and Miller DC: A correlative study of p53 protein
alteration and p53 gene mutation in glioblastoma multiforme. Brain
Pathol. 3:229–235. 1983. View Article : Google Scholar
|
|
8
|
Magargal LE, Brown GC, Augsburger JJ and
Donoso LA: Efficacy of panretinal photocoagulation in preventing
neovascular glaucoma following ischemic central retinal vein
obstruction. Ophthalmology. 89:780–784. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
No authors listed: Baseline and early
natural history report. The central vein occlusion study. Arch
Ophthalmol. 111:1087–1095. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Evans K, Wishart PK and McGalliard JN:
Neovascular complications after central retinal vein occlusion. Eye
(Lond). 7:520–524. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
No authors listed: A randomized clinical
trial of early panretinal photocoagulation for ischemic central
vein occlusion. The central vein occlusion study group n report.
Ophthalmology. 102:1434–1444. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kjeka O, Jansson RW, Bredrup C and Krohn
J: Early panretinal photocoagulation for ERG-verified ischaemic
central retinal vein occlusion. Acta Ophthalmol. 91:37–41. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arvas S, Ocakoglu O and Ozkan S: The
capillary blood flow in ischaemic type central retinal vein
occlusion: The effect of laser photocoagulation. Acta Ophthalmol
Scand. 80:490–494. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bitirgen G, Belviranli S, Malik RA,
Kerimoglu H and Ozkagnici A: Effects of panretinal laser
photocoagulation on the corneal nerve plexus and retinal nerve
fiber layer in retinal vein occlusion. Eur J Ophthalmol.
27:591–595. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hayreh SS, Klugman MR, Podhajsky P,
Servais GE and Perkins ES: Argon laser panretinal photocoagulation
in ischemic central retinal vein occlusion. A 10-year prospective
study. Graefes Arch Clin Exp Ophthalmol. 228:281–296. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Laatikainen L, Kohner EM, Khoury D and
Blach RK: Panretinal photocoagulation in central retinal vein
occlusion: A randomised controlled clinical study. Br J Ophthalmol.
61:741–753. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pikkel YY, Sharabi-Nov A, Beiran I and
Pikkel J: Comparison of anti-vascular endothelial growth factors,
laser treatments and a combination of the both for treatment of
central retinal vein occlusion. Int J Ophthalmol. 9:431–433.
2016.PubMed/NCBI
|
|
18
|
Fan F and Pan SX: The clinical efficacy of
panretinal photocoagulation in the treatmnent for ischemic central
retinal vein occlusion. J Prac Prev Blind. 4:156–158. 2011.(In
Chinese).
|
|
19
|
Qu Q, Hong T and Zhou L: Argon-ion laser
treatment of ischemic central retinal vein occlusion: Analysis of
63 cases. World Health Digest. 8:113. 2014.(In Chinese).
|
|
20
|
Cao Y: Therapeutic effect of holmium laser
on 35 cases of central retinal vein occlusion. Shandong Med.
26(47)2006.(In Chinese).
|
|
21
|
Cai J, Cheng J, Li Y and Wei R: Laser
photocoagulation for prevention of neovascular glaucoma due to
central retinal vein occlusion. Chin J Prac Ophthalmol. 1:24–26.
2009.(In Chinese).
|
|
22
|
Muqit MM, Sanghvi C, McLauchlan R, Delgado
C, Young LB, Charles SJ, Marcellino GR and Stanga PE: Study of
clinical applications and safety for Pascal® laser
photocoagulation in retinal vascular disorders. Acta Ophthalmol.
90:155–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mellerio J: The thermal nature of retinal
laser photocoagulation. Exp Eye Res. 5:2421996. View Article : Google Scholar
|
|
24
|
Fong DS, Girach A and Boney A: Visual side
effects of successful scatter laser photocoagulation surgery for
proliferative diabetic retinopathy: A literature review. Retina.
27:816–824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Central vein occlusion study of
photocoagulation therapy. Baseline findings. Central vein occlusion
study group. Online J Curr Clin Trials. 95:1993.
|
|
26
|
Wald KJ: The CVOS Group M and N reports.
Ophthalmology. 103:352–354. 1996. View Article : Google Scholar : PubMed/NCBI
|