Introduction
Myocardial infarction is a common presentation of
coronary artery disease. Each year, >3 million cases of
ST-elevated myocardial infarction (STEMI) and 4 million cases of
non-STEMI (NSTEMI) occur worldwide (1,2).
Reperfusion therapies, including primary percutaneous coronary
intervention and fibrinolytic therapy, promptly restore the blood
flow to the ischemic myocardium and limit the infarct size.
However, the return of blood flow and oxygen may result in
additional cardiac damage and complications, which is referred to
as ischemia/reperfusion injury, or hypoxia/reoxygenation injury.
The reperfusion and reoxygenation injury increases cell apoptosis
and the production of reactive oxygen species (3). Despite an improved understanding of the
pathophysiology of the process and encouraging results of
pre-clinical trials for multiple agents, effective therapies to
reduce or prevent reperfusion and reoxygenation injury have
remained elusive. Most clinical trials to prevent reperfusion
injury have been disappointing (4–6).
Therapies to limit reperfusion and reoxygenation injury require
further investigation.
Loperamide is an opioid-receptor agonist that acts
on µ-opioid receptors in the myenteric plexus. It acts in a similar
manner to morphine, decreasing the activity of the myenteric
plexus, which decreases the tone of longitudinal and circular
smooth muscles of the intestinal wall (7,8).
Loperamide is used to decrease the frequency of diarrhea in
gastroenteritis, inflammatory bowel disease and short bowel
syndrome (9). Morphine, another
µ-opioid receptor agonist, was reported to protect against
myocardial ischemia/reperfusion injury in rabbits (10). However, the effect of loperamide
against hypoxia/reoxygenation injury of cardiomyocytes has remained
elusive.
MicroRNAs (miRNAs/miRs) are a class of short
non-coding RNAs that have important regulatory roles on gene
expression by sequence-specific base pairing with the
3′-untranslated region (3′-UTR) of target mRNAs, promoting mRNA
degradation or inhibiting their translation (11). miR-21 has been identified to be
overexpressed in numerous types of solid tumor. Altered miR-21
expression reported to be associated with the proliferation,
invasion and apoptosis of malignant cells via targeting and
downregulating of various tumor suppressors, including programmed
cell death 4 (12,13), phosphatase and tensin homologue
(PTEN) (14), B-cell lymphoma 2 and
tropomyosin l (15,16). In addition, miR-21 was revealed to
attenuate hepatocyte hypoxia/reoxygenation injury via inhibiting
the PTEN/phosphoinositide-3 kinase (PI3K)/AKT signaling pathway
(17). Rapamycin suppressed
hypoxia/reoxygenation-induced islet injury by upregulating miR-21
via the PI3K/AKT signaling pathway (18). However, the role of miR-21 in
hypoxia/reoxygenation injury of cardiomyocytes remains elusive.
Therefore, the present study aimed to investigate
the role of loperamide and miR-21 in hypoxia/reoxygenation injury
of cardiomyocytes, and to explore the underlying molecular
mechanisms.
Materials and methods
Reagents
Loperamide hydrochloride (cat. no. S2480) was
purchased from Selleck Chemicals (Houston, TX, USA). Dulbecco's
modified Eagle's medium (DMEM) was purchased from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Trypsin (Invitrogen; Thermo
Fisher Scientific, Inc.), the Annexin V-phycoerythrin
(PE)/7-aminoactinomycin D (7-AAD) apoptosis assay kit (KGA1017;
Keygentec Inc., Jiangsu, China) and the reactive oxygen species
assay kit (S0033; Beyotime Institute of Biotechnology, Haimen,
China) were used in the present study. Primers and probes, TRIzol
reagent, SuperScript III Reverse Transcriptase, SYBR-Green I and
diethylpyrocarbonate-treated H2O were from Invitrogen
(Thermo Fisher Scientific, Inc.). RNase inhibitor was purchased
from Fermentas (Thermo Fisher Scientific, Inc.). SYBR qPCR mix kit
was purchased from Invitrogen (4309155; Thermo Fisher Scientific,
Inc.).
Cell hypoxia/reoxygenation model
H9c2 rat cardiomyocytes (cat. no. CRL-1446; American
Type Culture Collection, Manassas, VA, USA) were cultured in DMEM
supplemented with glucose, 10% fetal bovine serum (GE Healthcare,
Chicago, IL, USA) and 1% penicillin/streptomycin at 37°C in a
humidified atmosphere containing air with 5% CO2 to a
cell confluence of 50%. Hypoxia was induced by culturing H9c2 cells
in DMEM without glucose in an atmosphere of 100% N2.
Following hypoxia treatment for 6 h, the H9c2 cells were returned
to normoxic conditions (air with 5% CO2) for 3 h.
Experiments were performed using cells that were to be subjected to
this hypoxia and reoxygenation treatment.
Cell viability assay
H9c2 rat cardiomyocytes were pre-treated with 0, 10,
50, 100 or 200 mM loperamide for 24 h prior to hypoxia and
reoxygenation treatment. H9c2 cells without loperamide or
hypoxia/reoxygenation treatment served as a control. The viability
of H9c2 cells was measured by using a cell counting kit 8 (CCK-8)
cell viability assay (C0038; Beyotime Institute of Biotechnology).
CCK-8 reagent was added into each well, followed by incubation for
4 h. The absorbance was measured utilizing a microplate reader at
490 nm. Experiments were performed in triplicate.
Transfection of miR-21 mimics and
inhibitor
Transfection was performed prior to hypoxia
treatment. miR-21 mimics and miR-21 inhibitor were designed and
chemically synthesized (Shanghai GenePharma Co., Ltd., Shanghai,
China), with homo sapiens (hsa)-miR-21 mimics: Sense,
5′-UAGCUUAUCAGACUGAUGUUGA-3′ and anti-sense,
5′-AACAUCAGUCUGAUAAGCUAUU-3′; and hsa-miR-21 inhibitor,
5′-UCAACAUCAGUCUGAUAAGCUA-3′. Carboxyfluorescein (FAM)-labeled
negative control siRNA was used as control. H9c2 cells were seeded
in 6-well plates at 5×105 cells/well and cultured in
antibiotic-free medium for 48 h to achieve a confluence of >70%
on the day of transfection. The miR-21 mimics and inhibitor (40 nm)
were transfected into cells using Lipofectamine™ 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) under serum-free
conditions for 6 h prior to replacing the culture supernatant with
complete medium. The efficiency of transfection was determined
using a Leica fluorescence microscope (Leica Microsystems, Wetzlar,
Germany) for the FAM-labeled negative control siRNA. The
transfection efficiency was also determined by assessing the
effective upregulation and downregulation using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
Flow cytometry
H9c2 rat cardiomyocytes were divided into 5 groups:
i) No loperamide, no hypoxia/reoxygenation (Control group), ii)
hypoxia/reoxygenation (H/R group), iii) cells were pre-treated with
50 nm loperamide for 24 h prior to hypoxia/reoxygenation (H/R +
loperamide group), iv) cells were pre-treated with 50 nm loperamide
and transfected with miR-21 inhibitor prior to
hypoxia/reoxygenation (H/R+loperamide+miR-21 inhibitor group) and
v) cells were pre-treated with 50 nm loperamide and transfected
with miR-21 mimics prior to hypoxia/reoxygenation
(H/R+loperamide+miR-21 mimics group). Cell apoptosis was detected
with the Annexin V-PE/7-AAD apoptosis assay kit using flow
cytometry. The cells from each of the 5 groups were washed with PBS
twice and incubated with trypsin at 37°C for 1 min. Following
digestion, the cell suspension was centrifuged at 400 × g at room
temperature for 5 min. The cell pellet was resuspended with PBS and
the centrifugation and resuspension steps were repeated twice. The
cells were blocked with 2% bovine serum albumin (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) for 30 min at room temperature.
7-AAD (5 µl) and Annexin V-PE (1 µl) reagents were added to 100 µl
cell suspension, followed by incubation at room temperature for 10
min. Cells were centrifuged at 400 × g at room temperature for 5
min and re-suspended with PBS three times. Cell fluorescence was
then detected by flow cytometry. Data were acquired on an LSRII
flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and
analyzed with FlowJo software (FlowJo, LLC, Ashland, OR, USA).
Experiments were performed in triplicates.
Detection of reactive oxygen
species
H9c2 rat cardiomyocytes were divided into 5 groups
as mentioned above. Reactive oxygen species were detected with the
reactive oxygen species assay kit using dichlorofluorescein
diacetate (DCFDA), following the manufacturer's protocol. DCFDA (10
µM) was added to the cell suspension (106 cells/ml),
with subsequent incubation at 37°C for 20 min. After being washed
with culture medium 3 times, cells were observed using a
fluorescence microscope. The integrated optical density (IOD) was
calculated by multiplying the area (size) and average density of
fluorescence (19). Samples were
evaluated using Image-Pro Plus 7 software (Media Cybernetics Inc.,
Rockville, MD, USA), and 6 fields of view (magnification, ×200)
were assessed for each sample. Three repeats were performed.
mRNA chip assay and RT-qPCR
H9c2 rat cardiomyocytes were divided into 5 groups
as mentioned above. After loperamide pretreatment and the following
H/R treatment, genes regulated by miR-21 were screened by using an
mRNA chip assay (Affymetrix GeneChip Rat Gene 1.0 ST Array;
Affymetrix; Thermo Fisher Scientific, Inc.). Genes associated with
cardiomyocyte apoptosis were screened out by pathway enrichment
analysis. Pathway analysis was used to identify the significant
pathways of differentially expressed genes according to the Kyoto
Encyclopedia of Genes and Genomes, Biocarta and Reactome. Fisher's
exact test was used to identify the pathways of genes that are
significantly differentially expressed. The threshold of
significance was defined by the P-value and false discovery rate,
and the enrichment was calculated (20–22). The
results of the mRNA chip assay were confirmed by RT-qPCR.
Diethylpyrocarbonate-treated water was used when handling RNA, to
reduce the risk of degradation by RNases. Total RNA was extracted
from cells using TRIzol reagent according to the manufacturer's
protocol. A universal complementary DNA synthesis kit (Invitrogen;
Thermo Fisher Scientific, Inc.) was utilized for RT. Each reaction
mixture contained 0.5 µl random primers (0.2 µg/µl) and 1 µl
SuperScript III reverse transcriptase (200 U/µl). The specific
primers used are listed in Table I.
PCR was performed using the SYBR qPCR mix kit. The PCR conditions
were as follows: Initial denaturation at 95°C for 2 min, followed
by 40 cycles of denaturation at 95°C for 10 sec, annealing at 60°C
for 30 sec and elongation at 70°C for 45 sec. PCR was performed
using a CFX96 Touch™ Real-Time PCR Detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Gene expression was
determined and normalized to β-actin. The primers for rat β-actin
were forward, 5′-AGGGAAATCGTGCGTGAC-3′ and reverse,
5′-CGCTCATTGCCGATAGTG-3′. The 2−ΔΔCq method was utilized
to determine the relative gene expression (23).
 | Table I.Primers used for polymerase chain
reaction. |
Table I.
Primers used for polymerase chain
reaction.
| Gene | Primer name | Sequence
(5′-3′) |
|---|
| Brip1 | Q-RAT-Brip1-F |
CCCGTGCCGTCATAACCATA |
|
| Q-RAT-Brip1-R |
GCAAAGGTTGAGTGGTGCTG |
| Rad51b | Q-RAT-Rad51b-F |
TACGACCCATCTGAGTGGAGC |
|
| Q-RAT-Rad51b-R |
GGGGACTTGGCGATGAGAAT |
| Hspa14 | Q-RAT-Hspa14-F |
TGGGCTCAGATGCAAACGAT |
|
| Q-RAT-Hspa14-R |
CCGATACATCCCGCTGTTCA |
| Bard1 | Q-RAT-Bard1-F |
CTTGCCCGTCTGGAGAAGTT |
|
| Q-RAT-Bard1-R |
GGGCATCCTGATCCAACACA |
| Akap8 | Q-RAT-Akap8-F |
ACTACAATGCCCAGAACACCA |
|
| Q-RAT-Akap8-R |
CTTGGCAATGAGCGAGTCAGA |
| MXD4 | Q-RAT-MXD4-F |
GAGTACCTGGAGCGTAGGGA |
|
| Q-RAT-MXD4-R |
GTTTAGCTCGTCGTCGAAGG |
| MTHFD1 | Q-RAT-MTHFD1-F |
GTCACGACGTCATTCCGGT |
|
| Q-RAT-MTHFD1-R |
CCCGGGGAAACTCAGTCAAT |
3′-UTR luciferase reporter assay
The transfection efficiency for delivering miRNA
mimics to H9c2 cardiomyocytes was low when the luciferase reporter
assay was first performed, potentially as a result of
co-transfection of miRNA with luciferase vectors. Therefore,
luciferase reporter assays were then performed with 293 cells
(CRL-1573, American Type Culture Collection, Manassas, VA, USA)
according to protocols of previous studies (24–26).
Luciferase reporter vectors driven by the respective putative
miR-21 binding sequence in the 3′-UTR of A-kinase anchoring protein
8 (Akap8; CL853-Gluc-Cluc-AKAP8-3′UTR) and BRCA1 associated RING
domain 1 (Bard1; CL852-Gluc-Cluc-BARD1-3′UTR) were constructed. The
293 cells were divided into 7 experimental groups (5×105
cells/well): i) Control (untreated); ii) Akap8-3′UTR+miR-21
negative control (NC); iii) Akap8-3′UTR+miR-21 mimics; iv)
Akap8-3′UTR+miR-21 inhibitor; v) Bard1-3′UTR+miR-NC; vi)
Bard1-3′UTR+miR-21 mimics; and vii) Bard1-3′UTR+miR-21 inhibitor.
miR-21 mimics or miR-21 inhibitor and luciferase vector driven by
Bard1 3′-UTR or luciferase vector driven by Akap8 3′-UTR were
co-transfected into 293 cells according to the group design using
Lipofectamine 2000 reagent. Luciferase activity in the culture
supernatant was measured using a Dual-Luciferase Reporter Assay
system (cat. no. E1910; Promega Corp., Madison, WI, USA) at 36 h
after transfection following manufacturer's protocols. The firefly
luciferase activity, expressed relative light units was normalized
to Renilla luciferase activity for each sample. Samples were
analyzed using TargetScan (www.targetscan.org). Experiments were performed in
triplicate.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 5.0 (GraphPad Software Inc., La Jolla, CA, USA). The
results are expressed as the mean ± standard error of mean.
Differences between 2 groups were assessed using Student's t-test.
Differences among ≥3 groups were compared by one-way analysis of
variance followed by the Bonferroni post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
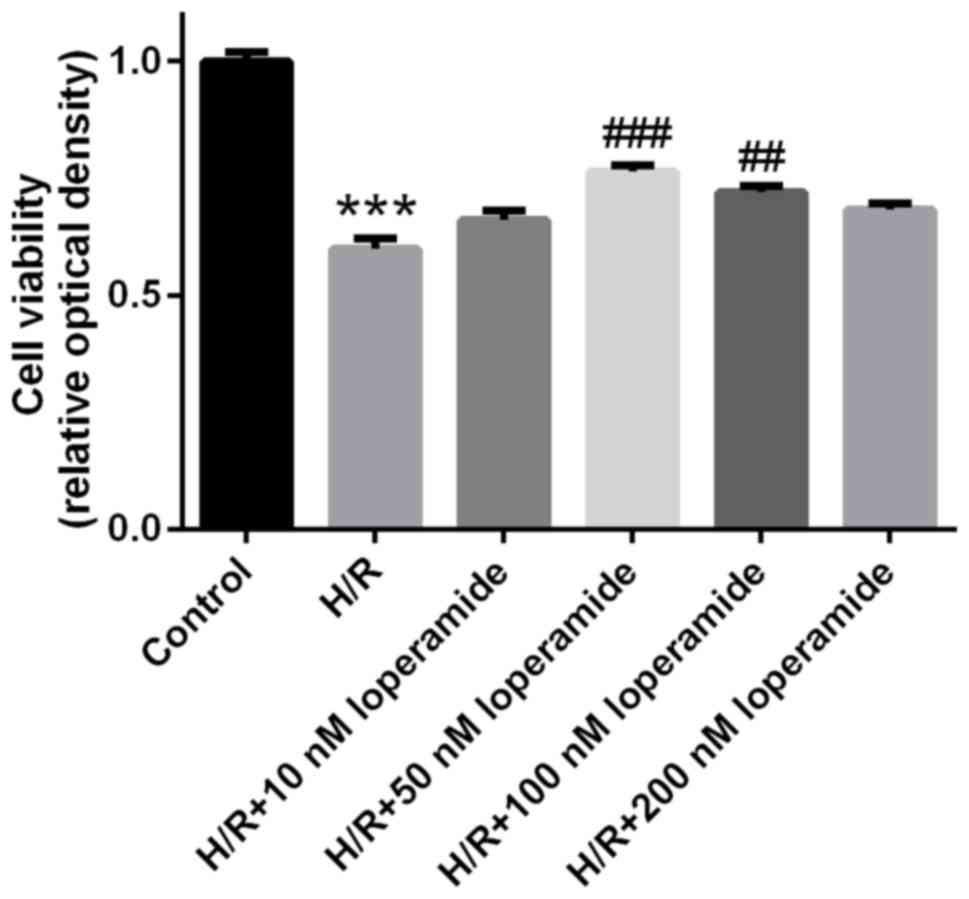
Loperamide pre-treatment at 50 and 100
nm increases the viability of rat cardiomyocytes after
hypoxia/reoxygenation
Compared with that in the control group, the
viability of H9c2 cells was significantly decreased after
hypoxia/reoxygenation treatment (P<0.001; Fig. 1). However, the viability of H9c2
cells treated with 50 or 100 nm loperamide prior to
hypoxia/reoxygenation was markedly increased compared with that in
the H/R group. Cells treated with 50 nm loperamide had the highest
viability. Therefore, 50 nm loperamide was used in the subsequent
experiments.
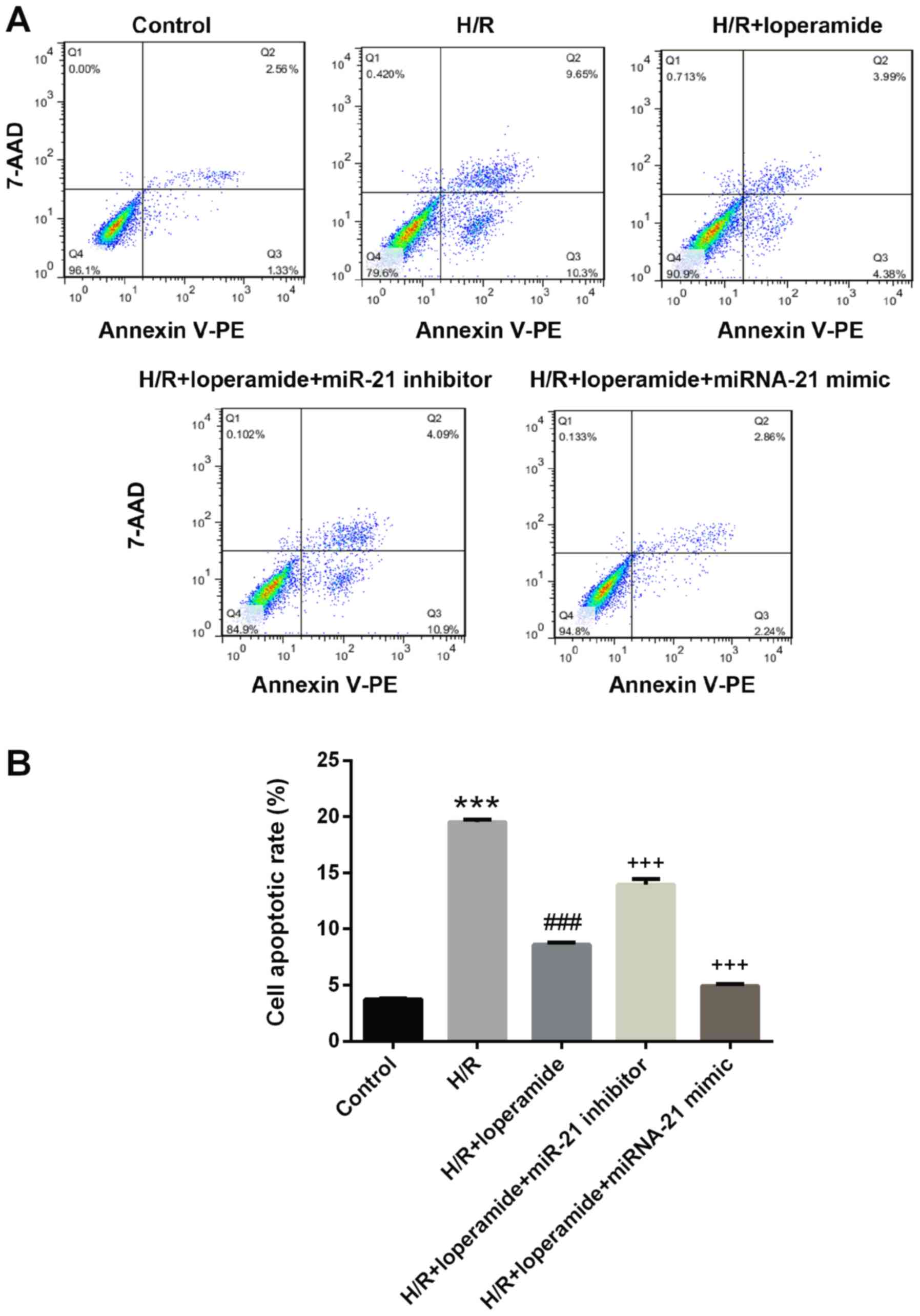
The protective effect of loperamide on
rat cardiomyocytes against hypoxia/reoxygenation-induced apoptosis
is markedly decreased by miR-21 inhibitor and enhanced by miR-21
mimics
The transfection efficiency of miR-21 mimics and
inhibitor was good for these experiments. The apoptotic cells were
determined by quantifying the early (quadrant 3) and late (quadrant
2) apoptotic cells. The apoptotic rate of rat cardiomyocytes was
significantly increased after hypoxia/reoxygenation treatment as
compared with that in the control group (P<0.001; Fig. 2). Loperamide pre-treatment
significantly protected H9c2 cells against apoptosis after
hypoxia/reoxygenation (P<0.001). The protective effect of
loperamide was markedly decreased by miR-21 inhibitor (P<0.001)
and enhanced by miR-21 mimics (P<0.001; Fig. 2).
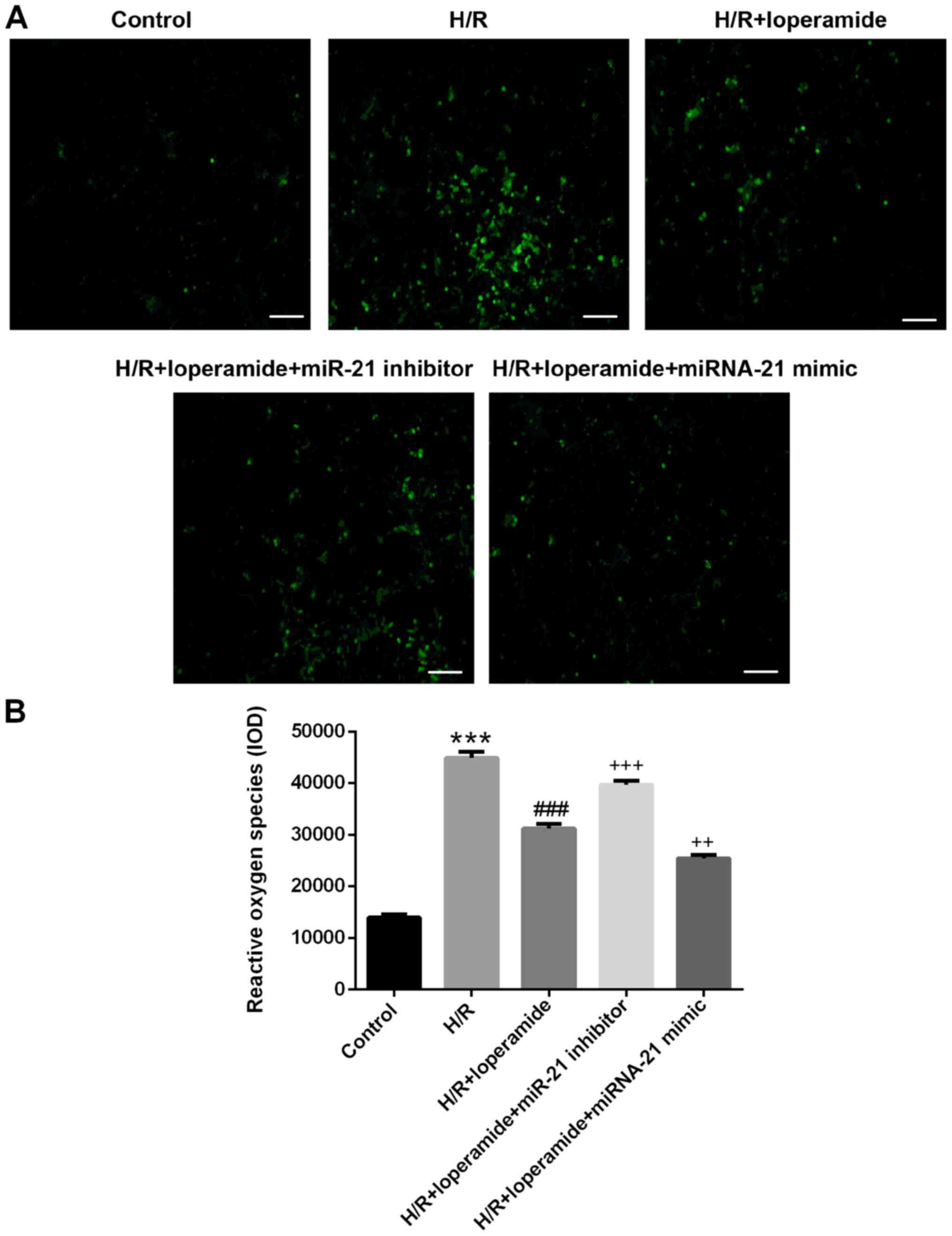
The protective effect of loperamide on
rat cardiomyocytes against hypoxia/reoxygenation-induced reactive
oxygen species production is markedly decreased by miR-21 inhibitor
and enhanced by miR-21 mimics
The reactive oxygen species levels in rat
cardiomyocytes increased significantly after hypoxia/reoxygenation
treatment as compared with those in the control group (P<0.001;
Fig. 3). Compared with those in the
H/R group, pre-treatment with loperamide markedly decreased the
reactive oxygen species levels in H9c2 cells after
hypoxia/reoxygenation (P<0.001). The decrease was markedly
attenuated by miR-21 inhibitor (P<0.001) and enhanced by miR-21
mimics (P<0.01; Fig. 3).
miR-21 regulates the expression of
Akap8 and Bard1, and enhances the inhibitory effects of loperamide
on Akap8 and Bard1 expression in rat cardiomyocytes after
hypoxia/reoxygenation
Among the deregulated genes identified using the
mRNA chip assay, those associated with cardiomyocyte apoptosis were
screened out by pathway enrichment analysis: Akap8, BRCA1
interacting protein C-terminal helicase 1 (Brip1), heat shock
protein family A (Hsp70) member 14 (Hspa14), RAD51 paralog B (Rad
51b); MAX dimerization protein 4, methylenetetrahydrofolate
dehydrogenase, cyclohydrolase and formyltetrahydrofolate synthetase
and Bard1. The results of the mRNA chip assay were confirmed by
RT-qPCR. The relative expression of Akap8 and Bard1 was consistent
with the experimental results on apoptosis and reactive oxygen
species in the 5 groups (Fig. 4).
The expression of Brip1, Akap8, Rad51b, Hspa14 and Bard1 in rat
cardiomyocytes increased significantly after hypoxia/reoxygenation
treatment as compared with that in the control group (P<0.001;
Fig. 4). Compared with that in the
H/R group, pre-treatment with loperamide markedly decreased the
expression of Brip1, Akap8 and Bard1 in H9c2 cells after
hypoxia/reoxygenation (P<0.001). The decrease in the expression
of Akap8 and Bard1 was markedly attenuated by miR-21 inhibitor
(P<0.001 for Akap8; P<0.01 for Bard1) and enhanced by miR-21
mimics (P<0.001; Fig. 4).
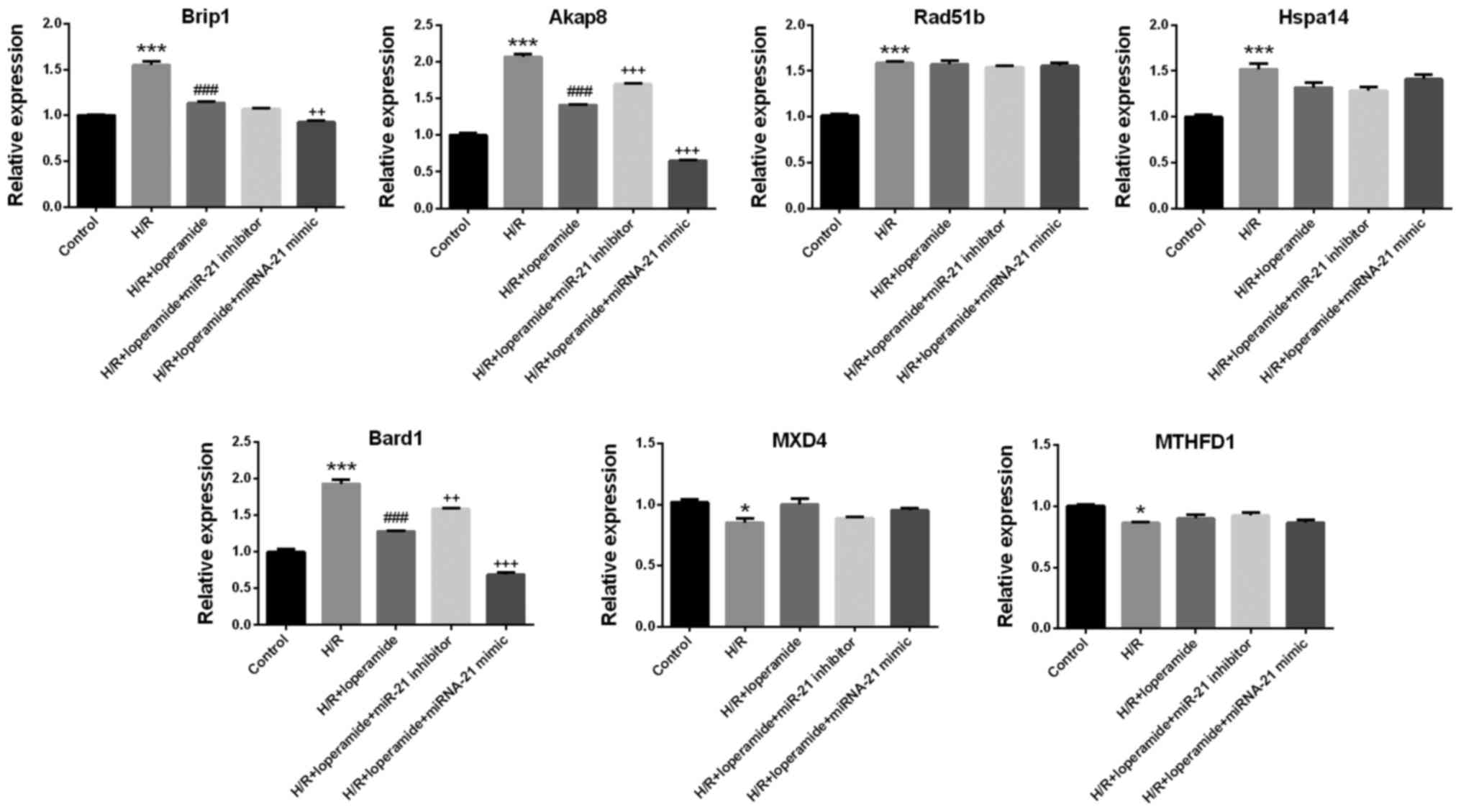 | Figure 4.Akap8 and Bard1 expression is
regulated by miR-21, and the inhibitory effects of loperamide on
Akap8 and Bard1 expression in H9c2 rat cardiomyocytes after H/R are
enhanced by miR-21. Following loperamide pre-treatment and H/R,
miR-21-regulated genes were screened by mRNA chip. A pathway
enrichment analysis was applied to screen out genes associated with
cardiomyocyte apoptosis: Brip1, Akap8, Rad51b, Hspa14, Bard1, MXD4
and MTHFD1. The results of the mRNA chip analysis were confirmed by
reverse transcription quantitative polymerase chain reaction. The
relative expression of Akap8 and Bard1 was consistent with the
results on apoptosis and reactive oxygen species in the
experimental groups. The expression of Brip1, Akap8, Rad51b, Hspa14
and Bard1 in rat cardiomyocytes was significantly increased after
H/R treatment, as compared with that in the control group. Compared
with the group treated with H/R alone, pre-treatment with
loperamide markedly decreased the expression of Brip1, Akap8 and
Bard1 in H9c2 cells after H/R. The decrease in expression of Akap8
and Bard1 was markedly alleviated by miR-21 inhibitor and enhanced
by miR-21 mimics. Values are expressed as the mean ± standard error
of the mean (n=3/group). *P<0.05, ***P<0.001 vs. control
group; ###P<0.001 vs. H/R group;
++P<0.01, +++P<0.001 vs. H/R +
loperamide group. H/R, hypoxia/reoxygenation; Akap8, A-kinase
anchoring protein 8; Bard1, BRCA1-associated RING domain 1; Brip1,
BRCA1 interacting protein C-terminal helicase 1; Hspa14, heat shock
protein family A (Hsp70) member 14; Rad 51b, RAD51 paralog B; MXD4,
MAX dimerization protein 4; MTHFD1, methylenetetrahydrofolate
dehydrogenase, cyclohydrolase and formyltetrahydrofolate synthetase
1; miR, microRNA. |
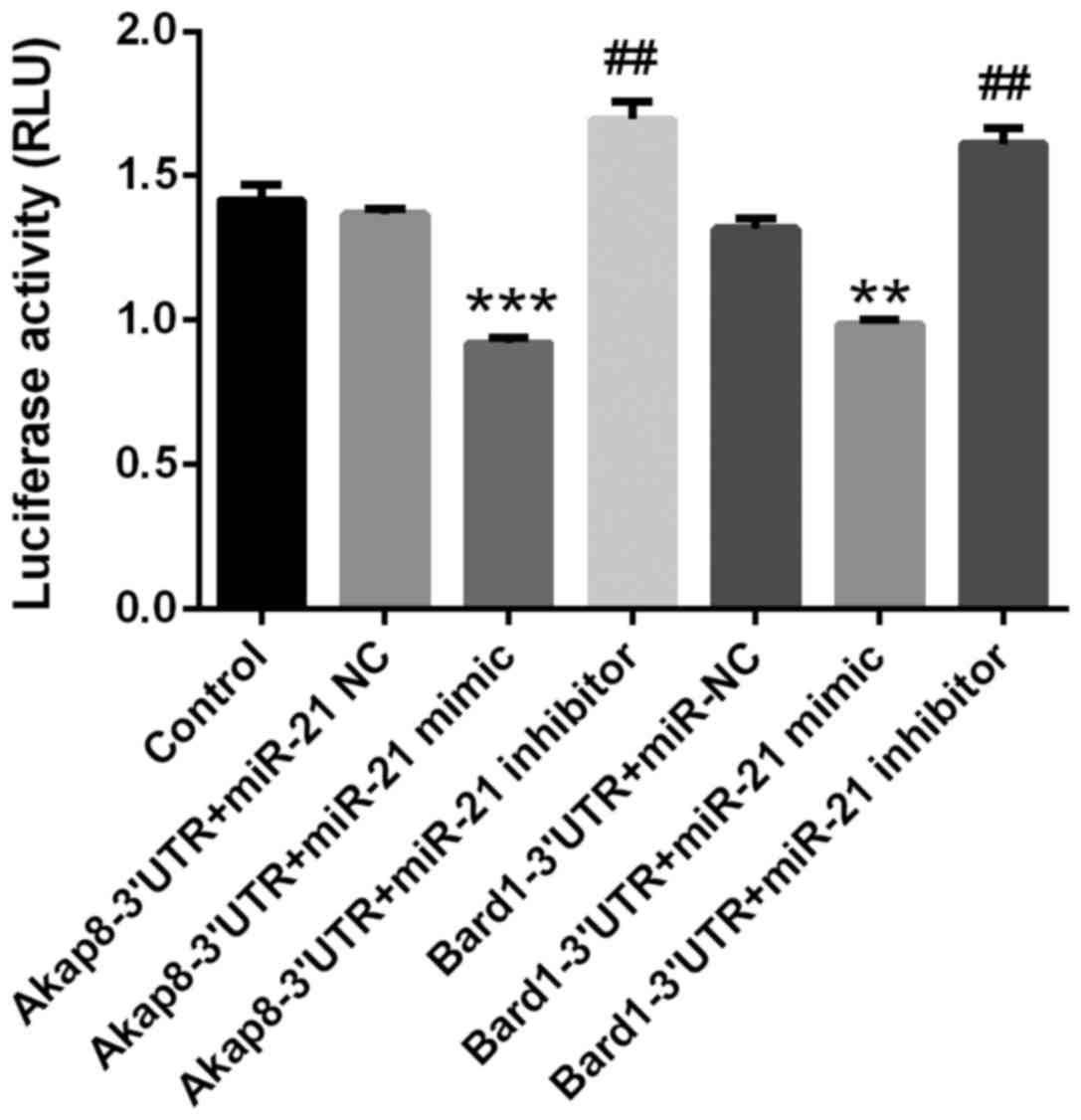
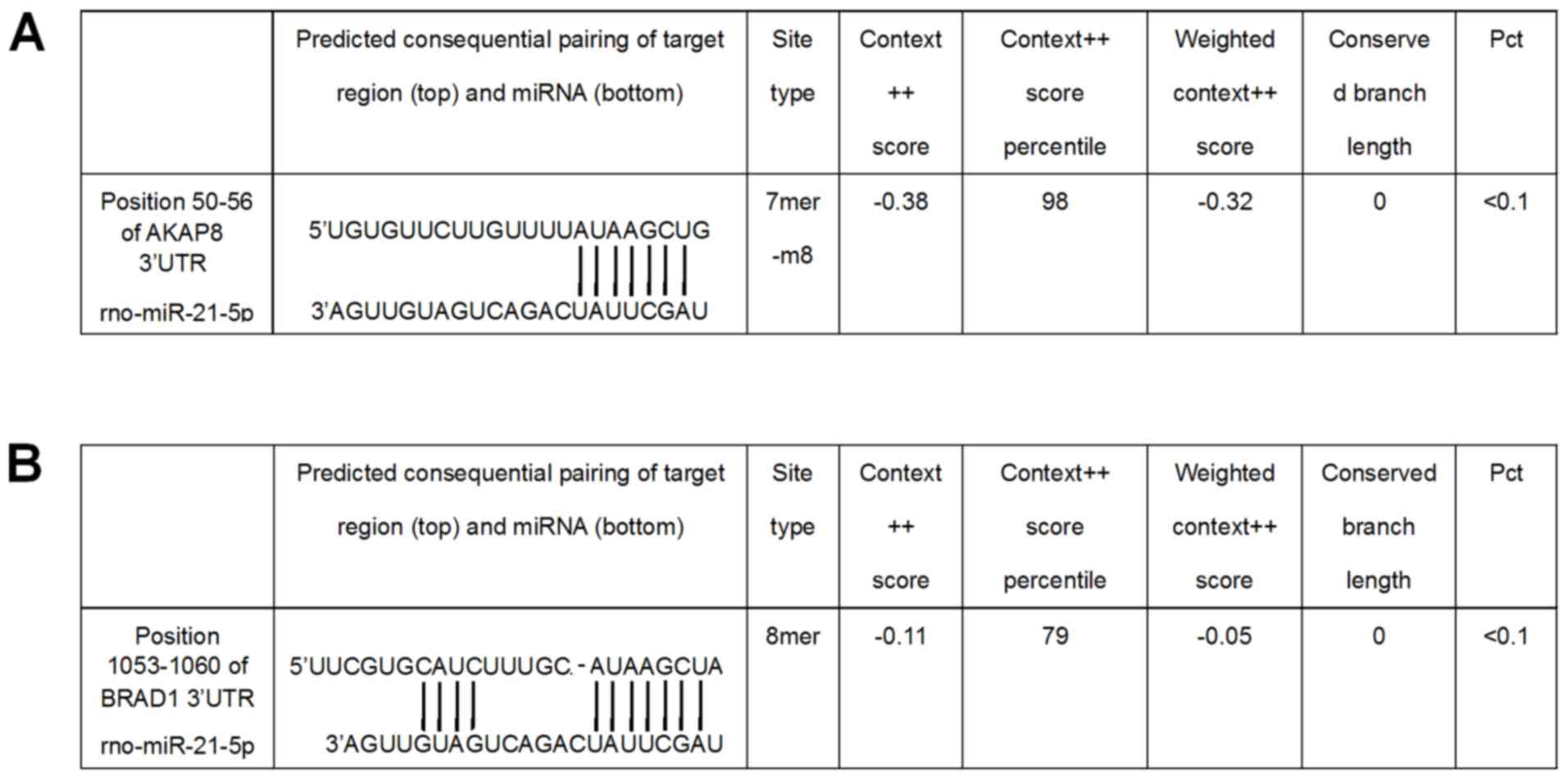
miR-21 directly targets the 3′-UTR of
Akap8 and Bard1 mRNA
The direct binding of miR-21 to its putative binding
sequences in the 3′-UTR of Akap8 and Bard1 was demonstrated using a
Dual-Luciferase Reporter Assay system. At 36 h after
co-transfection with reporter vectors driven by the binding regions
in the 3′-UTR of Akap8 or Bard1 and miR-21 mimics or inhibitor, the
luciferase activity in the different groups was determined. miR-21
mimics decreased the expression of Akap8 (P<0.001) and Bard1
(P<0.01), whereas miR-21 inhibitor increased the expression of
Adap8 and Bard1 (P<0.01; Fig. 5).
The putative miR-21 binding sites in the 3′-UTR of Akap8 and Bard1
mRNA are presented in Fig. 6.
Discussion
In the present study, it was demonstrated that the
protective effect of loperamide on rat cardiomyocytes against
hypoxia/reoxygenation-induced apoptosis and reactive oxygen species
production was markedly enhanced by miR-21. miR-21 directly targets
the 3′-UTR of Akap8 and Bard1 mRNA, and enhances the inhibitory
effects of loperamide on the expression of Akap8 and Bard1 in rat
cardiomyocytes after hypoxia/reoxygenation.
Ischemia/reperfusion injury or hypoxia/reoxygenation
injury is the tissue and cell damage caused when blood supply
and/or oxygen returns to tissue and cells after a period of
ischemia or lack of oxygen. The restoration of the circulation and
oxygen supply results in inflammation and oxidative damage via the
induction of oxidative stress rather than restoration of normal
function. Activated endothelial cells and other cell types produce
more reactive oxygen species following reperfusion and
reoxygenation, and the imbalance results in a subsequent
inflammatory response (27). The
restored blood flow and oxygen within cells and their associated
free radicals damage cellular proteins, DNA and the plasma
membrane. Damage to the cell membrane may in turn cause the release
of more free radicals (28). In
addition, ischemic tissue and hypoxic cells have a decreased
reactive oxygen species scavenger function due cell injury
(29). Such reactive oxygen species
may also act in redox signaling to turn on apoptosis.
The present study revealed that loperamide protected
rat cardiomyocytes against hypoxia/reoxygenation-induced apoptosis
and reactive oxygen species production. Morphine, another
µ-receptor agonist, was reported to protect against acute
myocardial ischemia/reperfusion injury in rats (30,31).
Morphine post-conditioning protected against reperfusion injury via
inhibiting c-Jun N-terminal kinase/p38 mitogen-activated protein
kinase (MAPK) and mitochondrial permeability transition pore
signaling pathways (32).
Furthermore, morphine administration at reperfusion failed to
improve post-ischemic cardiac function but limited myocardial
injury via phosphorylation of HSP27 (33). Morphine pre-conditioning in the
delayed phase was reported to exert protective effects on
myocardial ischemia/reperfusion injury by inhibiting myocardial p38
MAPK activity and decreasing tumor necrosis factor-α production in
rabbits (34). Evidence for the
effects of loperamide on hypoxia/reoxygenation injury of
cardiomyocytes is scarce. To the best of our knowledge, the present
study was the first to demonstrate the protective role of
loperamide against hypoxia/reoxygenation-induced apoptosis and
reactive oxygen species production in rat cardiomyocytes.
In addition, it was demonstrated that the protection
of rat cardiomyocytes against hypoxia/reoxygenation-induced injury
by loperamide was markedly enhanced by miR-21. It was also unveiled
that miR-21 directly targeted the 3′-UTR of Akap8 and Bard1 mRNA,
and enhanced the inhibitory effects of loperamide on Akap8 and
Bard1 expression in rat cardiomyocytes after hypoxia/reoxygenation.
miR-1 and −21 were reported to exert synergistic effects against
hypoxia-induced cardiomyocyte apoptosis by activating AKT and
blocking hypoxia-induced upregulation of p53 (35). miR-21 was revealed to attenuate
hepatocyte hypoxia/reoxygenation injury by inhibiting the
PTEN/PI3K/AKT signaling pathway (17). Rapamycin was reported to suppress
hypoxia/reoxygenation-induced islet injury by upregulating miR-21
via the PI3K/AKT signaling pathway (18). Furthermore, the Akaps are a group of
structurally diverse proteins that bind to the regulatory subunit
of protein kinase A and confine it to discrete locations within
cells (36). Akap8 is involved in
the regulation of structural changes of chromatin through nuclear
tyrosine phosphorylation. Akap8/Akap95 has been reported to mediate
apoptosis, and to serve as a carrier to transport caspase 3 from
the cytoplasm to the nucleus to induce morphological changes in the
nucleus (37). Akap8 was also
reported to have an important role in the modulation of head size
and may contribute to the risk of autism (38). Furthermore, Bard1 is involved in
apoptosis through binding and stabilizing p53 independently of
BRCA1 (39). The expression of Bard1
is upregulated by genotoxic stress. Bard1 is also vital in the
rapid relocation of BRCA1 to DNA damage sites (40). In the present study, it was revealed
that pre-treatment with loperamide and transfection of miR-21
mimics decreased the expression of the pro-apoptotic genes Akap8
and Bard1 in rat cardiomyocytes after hypoxia/reoxygenation. miR-21
was demonstrated to directly target the 3′-UTR of Akap8 and Bard1
mRNA. Despite of minor limitation that we did not construct vectors
containing a mutated 3′UTR sequence, these results provide an
explanation for the protective role of loperamide and miR-21
against hypoxia/reoxygenation-induced apoptosis and reactive oxygen
species production in cardiomyocytes.
In conclusion, the present study demonstrated that
the protection of rat cardiomyocytes against
hypoxia/reoxygenation-induced apoptosis and reactive oxygen species
production by loperamide is markedly enhanced by miR-21. miR-21
directly targets the 3′-UTR of Akap8 and Bard1 mRNA, and enhances
the inhibitory effects of loperamide on Akap8 and Bard1 expression
in rat cardiomyocytes after hypoxia/reoxygenation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key Program
of Health and Family Planning Commission of Shanghai, China (grant
no. 201440024) and Key Project of Shanghai Municipal Health Bureau
(grant no. 2016ZB0202).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
HS designed experiments and drafted manuscript. ZY
analyzed and interpreted data. WZ and YZ collected experimental
data. CY interpreted the data, provided experimental support and
critically revised manuscript. CT designed experiments and
critically revised manuscript. The final version of the manuscript
has been read and approved by all authors and each author believes
that the manuscript represents honest work.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
White HD and Chew DP: Acute myocardial
infarction. Lancet. 372:570–584. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Gara PT, Kushner FG, Ascheim DD, Casey
DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM,
Franklin BA, et al: 2013 ACCF/AHA guideline for the management of
ST-elevation myocardial infarction: A report of the American
College of Cardiology Foundation/American Heart Association Task
Force on Practice Guidelines. Circulation. 127:e362–e425.
2013.PubMed/NCBI
|
|
3
|
Fernández-Jiménez R, García-Prieto J,
Sánchez-González J, Agüero J, López-Martín GJ, Galán-Arriola C,
Molina-Iracheta A, Doohan R, Fuster V and Ibáñez B: Pathophysiology
underlying the bimodal edema phenomenon after myocardial
ischemia/reperfusion. J Am Coll Cardiol. 66:816–828. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bolli R, Becker L, Gross G, Mentzer R Jr,
Balshaw D and Lathrop DA: NHLBI Working Group on the Translation of
Therapies for Protecting the Heart from Ischemia: Myocardial
protection at a crossroads: The need for translation into clinical
therapy. Circ Res. 95:125–134. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cannon RO III: Mechanisms, management and
future directions for reperfusion injury after acute myocardial
infarction. Nat Clin Pract Cardiovasc Med. 2:88–94. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ibáñez B, Heusch G, Ovize M and Van de
Werf F: Evolving therapies for myocardial ischemia/reperfusion
injury. J Am Coll Cardiol. 65:1454–1471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
MacDonald R, Heiner J, Villarreal J and
Strote J: Loperamide dependence and abuse. BMJ Case Rep.
2015:bcr20152097052015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baker DE: Loperamide: A pharmacological
review. Rev Gastroenterol Disord. 7 Suppl 3:S11–S18.
2007.PubMed/NCBI
|
|
9
|
Kalgutkar AS and Nguyen HT: Identification
of an N-methyl-4-phenylpyridinium-like metabolite of the
antidiarrheal agent loperamide in human liver microsomes:
Underlying reason(s) for the lack of neurotoxicity despite the
bioactivation event. Drug Metab Dispos. 32:943–952. 2004.PubMed/NCBI
|
|
10
|
Wang XH, Zeng JF, Lin C and Chen SB:
Effects of morphine and sufentanil preconditioning against
myocardial ischemic-reperfusion injury in rabbits. Int J Clin Exp
Med. 8:15692–15699. 2015.PubMed/NCBI
|
|
11
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Asangani IA, Rasheed SA, Nikolova DA,
Leupold JH, Colburn NH, Post S and Allgayer H: MicroRNA-21 (miR-21)
post-transcriptionally downregulates tumor suppressor Pdcd4 and
stimulates invasion, intravasation and metastasis in colorectal
cancer. Oncogene. 27:2128–2136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Frankel LB, Christoffersen NR, Jacobsen A,
Lindow M, Krogh A and Lund AH: Programmed cell death 4 (PDCD4) is
an important functional target of the microRNA miR-21 in breast
cancer cells. J Biol Chem. 283:1026–1033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Si ML, Zhu S, Wu H, Lu Z, Wu F and Mo YY:
miR-21-mediated tumor growth. Oncogene. 26:2799–2803. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu S, Si ML, Wu H and Mo YY: MicroRNA-21
targets the tumor suppressor gene tropomyosin 1 (TPM1). J Biol
Chem. 282:14328–14336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu X, Sun C, Zheng D, Liu R, Wei X and Wu
Z: [The miR-21 attenuates hepatocyte hypoxia/reoxygenation injury
via inhibiting PTEN/PI3K/AKT signaling pathway]. Xi Bao Yu Fen Zi
Mian Yi Xue Za Zhi. 33:497–502. 2017.(In Chinese). PubMed/NCBI
|
|
18
|
Zhang Y, He S, Du X, Jiang Y, Tian B and
Xu S: Rapamycin suppresses hypoxia/reoxygenation-induced islet
injury by up-regulation of miR-21 via PI3K/Akt signalling pathway.
Cell Prolif. 50:2017. View Article : Google Scholar
|
|
19
|
Tee YT, Han CP, Ko JL, Chen GD, Yang SF,
Chen SC, Tsai HJ, Lin LY and Wang PH: Evaluation of matrix
metalloproteinase 2 expression in cervical carcinogenesis using
tissue array and integrated optical density for immunoreactivity.
Reprod Sci. 14:719–726. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanehisa M, Goto S, Kawashima S, Okuno Y
and Hattori M: The KEGG resource for deciphering the genome.
Nucleic Acids Res. 32:(Database Issue). D277–D280. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yi M, Horton JD, Cohen JC, Hobbs HH and
Stephens RM: WholePathwayScope: A comprehensive pathway-based
analysis tool for high-throughput data. BMC Bioinformatics.
7:302006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Draghici S, Khatri P, Tarca AL, Amin K,
Done A, Voichita C, Georgescu C and Romero R: A systems biology
approach for pathway level analysis. Genome Res. 17:1537–1545.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xia M, Huang R, Guo V, Southall N, Cho MH,
Inglese J, Austin CP and Nirenberg M: Identification of compounds
that potentiate CREB signaling as possible enhancers of long-term
memory. Proc Natl Acad Sci USA. 106:2412–2417. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brunzell DH, Mineur YS, Neve RL and
Picciotto MR: Nucleus accumbens CREB activity is necessary for
nicotine conditioned place preference. Neuropsychopharmacology.
34:1993–2001. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cali JJ, Niles A, Valley MP, O'Brien MA,
Riss TL and Shultz J: Bioluminescent assays for ADMET. Expert Opin
Drug Metab Toxicol. 4:103–120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carden DL and Granger DN: Pathophysiology
of ischaemia-reperfusion injury. J Pathol. 190:255–266. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Caraceni P, VAN Thiel DH and Borle AB:
Time course of free radicals formation, lipid peroxidation, and
reoxygenation injury in perfused rat hepatocytes. Ann N Y Acad Sci.
723:356–359. 1994. View Article : Google Scholar
|
|
29
|
Burn BR and Varner KJ: Environmentally
persistent free radicals compromise left ventricular function
during ischemia/reperfusion injury. Am J Physiol Heart Circ
Physiol. 308:H998–H1006. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang ZY, Li PJ, Chen TD, Yang L, Zhao JH,
Na T and Tang XQ: Protective effect and mechanism of morphine on
acute myocardial ischemia/reperfusion injury in rats. Zhongguo Wei
Zhong Bing Ji Jiu Yi Xue. 16:656–659. 2004.(In Chinese). PubMed/NCBI
|
|
31
|
Chang WL, Lee SS and Su MJ: Attenuation of
post-ischemia reperfusion injury by thaliporphine and morphine in
rat hearts. J Biomed Sci. 12:611–619. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen Z, Zhang X, Liu Y and Liu Z: Morphine
postconditioning protects against reperfusion injury via inhibiting
JNK/p38 MAPK and mitochondrial permeability transition pores
signaling pathways. Cell Physiol Biochem. 39:61–70. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mourouzis I, Saranteas T, Perimenis P,
Tesseromatis C, Kostopanagiotou G, Pantos C and Cokkinos DV:
Morphine administration at reperfusion fails to improve
postischaemic cardiac function but limits myocardial injury
probably via heat-shock protein 27 phosphorylation. Eur J
Anaesthesiol. 26:572–581. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ran K, Gong ZX, Yang DL, Chang YT, Duan KM
and Ou YW: Effect of morphine preconditioning in the delayed phase
on the expression of p38 mitogen-activated protein kinase in a
rabbit model of myocardial ischemia-reperfusion injury. Genet Mol
Res. 14:6642–6648. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu Y, Zhu W, Wang Z, Yuan W, Sun Y, Liu H
and Du Z: Combinatorial microRNAs suppress hypoxia-induced
cardiomyocytes apoptosis. Cell Physiol Biochem. 37:921–932. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Eide T, Taskén KA, Carlson C, Williams G,
Jahnsen T, Taskén K and Collas P: Protein kinase A-anchoring
protein AKAP95 interacts with MCM2, a regulator of DNA replication.
J Biol Chem. 278:26750–26756. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kubota S, Morii M, Yuki R and Yamaguchi N,
Yamaguchi H, Aoyama K, Kuga T, Tomonaga T and Yamaguchi N: Role for
tyrosine phosphorylation of A-kinase anchoring protein 8 (AKAP8) in
its dissociation from chromatin and the nuclear matrix. J Biol
Chem. 290:10891–10904. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nebel RA, Kirschen J, Cai J, Woo YJ,
Cherian K and Abrahams BS: Reciprocal relationship between head
size, an autism endophenotype, and gene dosage at 19p13.12 points
to AKAP8 and AKAP8L. PLoS One. 10:e01292702015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Irminger-Finger I, Leung WC, Li J,
Dubois-Dauphin M, Harb J, Feki A, Jefford CE, Soriano JV, Jaconi M,
Montesano R and Krause KH: Identification of BARD1 as mediator
between proapoptotic stress and p53-dependent apoptosis. Mol Cell.
8:1255–1266. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li M and Yu X: Function of BRCA1 in the
DNA damage response is mediated by ADP-ribosylation. Cancer Cell.
23:693–704. 2013. View Article : Google Scholar : PubMed/NCBI
|