Introduction
Fibroblasts are the primary source of most
extracellular matrix components, are abundant in skin,
participating in the turnover of extracellular matrix as one of the
main events in wound healing (1).
The density of the fibroblasts varies in different areas of skin as
well as the collagen and elastin quantity varies within anatomic
location (2). The role of
fibroblasts is complex; they intervene in fibroplasias and
angiogenesis (3) but also in
inflammation, granulation tissue formation and scar remodeling
(4,5). Their involvement in regeneration was
revisited by many recent studies related to scar-free wound healing
process in oral mucosa, an area distinct from regular skin with
respect to fibroblasts phenotype (6–8). The
mechanisms of scar formation following skin damage are still to be
elucidated although several reports indicate that wound healing in
oral mucosa is faster and trigger a reduced inflammation compared
with regular skin fibroblasts. These differences were attributed to
a specific phenotype of fibroblasts from human gingiva (GF) which
is different from other skin fibroblasts (SF). For instance, GF
express high levels of molecules involved in regulation of
inflammation and MMPs favorable for rapid resolution of
inflammation and ECM remodeling, which is typical for scar-free
wound healing, while SF exhibit a profibrotic, scar-prone phenotype
(9). Moreover, recent studies claim
involvement of some cell surface specific molecules in sustaining
the profibrotic phenotype. Thus, studies in mice have linked a high
expression of CD26 to a profibrotic phenotype of fibroblasts also
associated with an increased level of transforming growth factor-β
signaling. Therefore, it is possible that CD26-positive fibroblast
population that is abundant in human skin but not in gingiva, may
differently drive the profibrotic response, scar formation and
wound healing (10). A complex
meta-analysis regarding the efficacy of platelet concentrates in
pulpotomy of human teeth was recently published (11). The enrolled were in the range of 4–25
years and autologous platelet-rich fibrin (PRF) allogeneic
lyophilized freeze-dried platelet was used as pulpotomy material
compared to calcium hydroxide and mineral trioxide aggregate. Two
of the three investigated studies have shown 100% success of
pulpotomy with PRF and the rest of the studies more than 80%.
Interestingly when assessing the control groups the differences in
regeneration were not statistically significant. This meta-analysis
pointed out the need for further studies of the mechanisms that
reside in this regeneration (11).
Thus, in the light of gathered information we are focusing on an
in vitro experimental model to test the proliferation and
migration capacity of standard fibroblasts in the presence of
normal alveolar blood clots (ABCs), platelets or platelets lysates
as the main source of pro-regeneration molecules.
Materials and methods
ABCs were harvested from normal
subjects who underwent elective dental extractions
In each case, a full thickness flap was performed in
the area by giving an intra sulcular incision. Hemostasis was
obtained and postsurgical instructions were given to the patients.
Instructions included applying pressure for 2 h and a warning not
to manipulate the surgical site or attempt to retract the lip to
visualize the surgical area, and 20 min later, the periodontal flap
was reflected to visualize alveolar sockets; blood clots were noted
in relation to the surgical site and were removed with a curette,
in sterile conditions. The site was irrigated with saline solution
and flaps were secured back to its original position with 3-0 silk
suture.
This study was approved by the Ethics Committee of
Colentina University Hospital (no. 63/31.10.2016; Bucharest,
Romania), and all patients gave their informed written consent for
this study complying with the Declaration of Helsinki.
Four different clots were tested after re-suspending
them in 1 ml of cell culture medium without any additional
supplements. To remove all cells and cellular debris all the
resuspended clots were filtered through 0.22-µm sterile filters
(EMD Millipore, Billerica, MA, USA). Afterwards, serial dilutions
were made in plain cell culture medium as follows: 1:4, 1:8, 1:16,
1:48. The PRF scaffolds were prepared according to the following
protocol: ten healthy volunteers in an age range between 18 to 60
years participated in this study. For each individual, 4 tubes of
peripheral blood were collected and immediately placed in a
pre-programmed centrifuge. Centrifugation was performed according
to the following protocol: advanced PRF, sterile plain glass-based
vacuum tubes (A-PRF10 tube) (10 ml; centrifuged at 200 × g for 14
min). To produce PRGF we harvested blood from other 10 healthy
donors into 9-ml collection tubes containing 0.9 ml of 3.8% (wt/v)
trisodium citrate. Blood samples were centrifuged at 580 × g for 8
min at room temperature in the PRGF-Endoret System IV centrifuge
(BTI Biotechnology Institute, S.L., Álava, Spain). Afterward, the
whole plasma column over the buffy coat was collected using Endoret
kit (BTI Biotechnology Institute, S.L.) avoiding the layer
containing leukocytes.
L929 standard skin fibroblast cell
line
L929 standard skin fibroblast cell line was
purchased from European Collection of Authenticated Cell Cultures
(ECACC) and kept in ‘Victor Babes’ National Institute of Pathology
Biobank (Bucharest, Romania). L929 (ECACC; cat. no. 85011425) was
maintained in culture according the supplier specification.
Briefly, seeding was done at 10,000 cells/cm3 in DMEM
cell culture medium supplemented with 2 mM glutamine and 10% fetal
bovine serum (FBS); cultures were maintained in 5% CO2
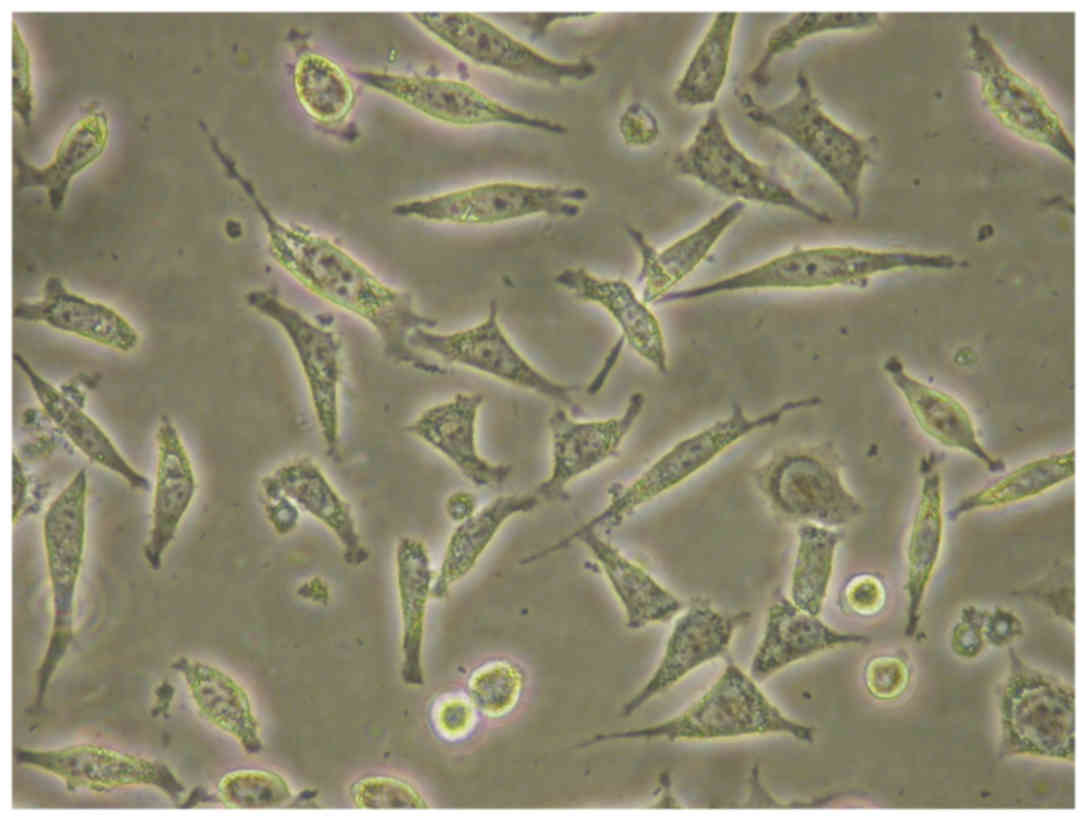
at 37°C. Cells displayed specific spindle shape morphology
(Fig. 1). When reaching 80%
confluence, adherent cells were detached with 0.25% trypsin/EDTA,
cell suspension was counted in an automated Cell Counter Countess
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) using Trypan
blue exclusion test. Cell culture viability was 100% and their
doubling time for L929 fibroblasts was 14 h. When cell cultures
reached 80% confluence we seeded the fibroblasts in E16 plates at
2,500 cells/well. After 2 h for controls plain cell culture medium
was added (control 1), cell culture medium supplemented with 10%
horse serum (control 2). For testing the ABCs the tested clots were
added in serial dilution without any additional serum in the
mentioned dilutions. Triplicates of each experimental system were
registered. For migration experiments CIM plates were treated with
fibronectin 1 µg/ml in sterile PBS (75 µl/well overnight incubation
at 4°C) to facilitate cell migration. After incubation fibronectin
was totally removed and plates gently washed with sterile PBS
twice. Fibroblasts cultivated in medium without horse serum were
seeded in the upper chamber at 1×105/ml; in the lower
chamber B the compounds were added in triplicates along with
controls horse serum 10% and plain medium.
Real-time monitoring of cell response
using impedance technology
We have used this technology to provide the
quantification of cell proliferation as previously reported by us
(12,13). Briefly, experiments were performed on
non-coated E-16 plates, compatible with RCTA-DP system (both from
Roche Applied Science, Penzberg, Upper Bavaria, Germany). Cells
were left to adhere for 2 h in the RTCA DP device at 37°C and 5%
CO2. Readings were collected at 1 min intervals for 72 h
and the results reported as normalized cellular index (CI) to the
time before addition of the compounds. The assay system expresses
cell's impedance in arbitrary CI units; results are presented as
overall CI for each sample and time-dynamic evaluation of CI. The
same technology was used to investigate the migratory capacity of
the tested fibroblasts in the presence of compounds. For the
overall CI the software delivers automatically at pre-established
time interval the values, in our case at 2, 24 and 48 h. For CI
time-dynamic evaluation graphs are also automatically displayed and
we have presented the general patterns of cell's evolution.
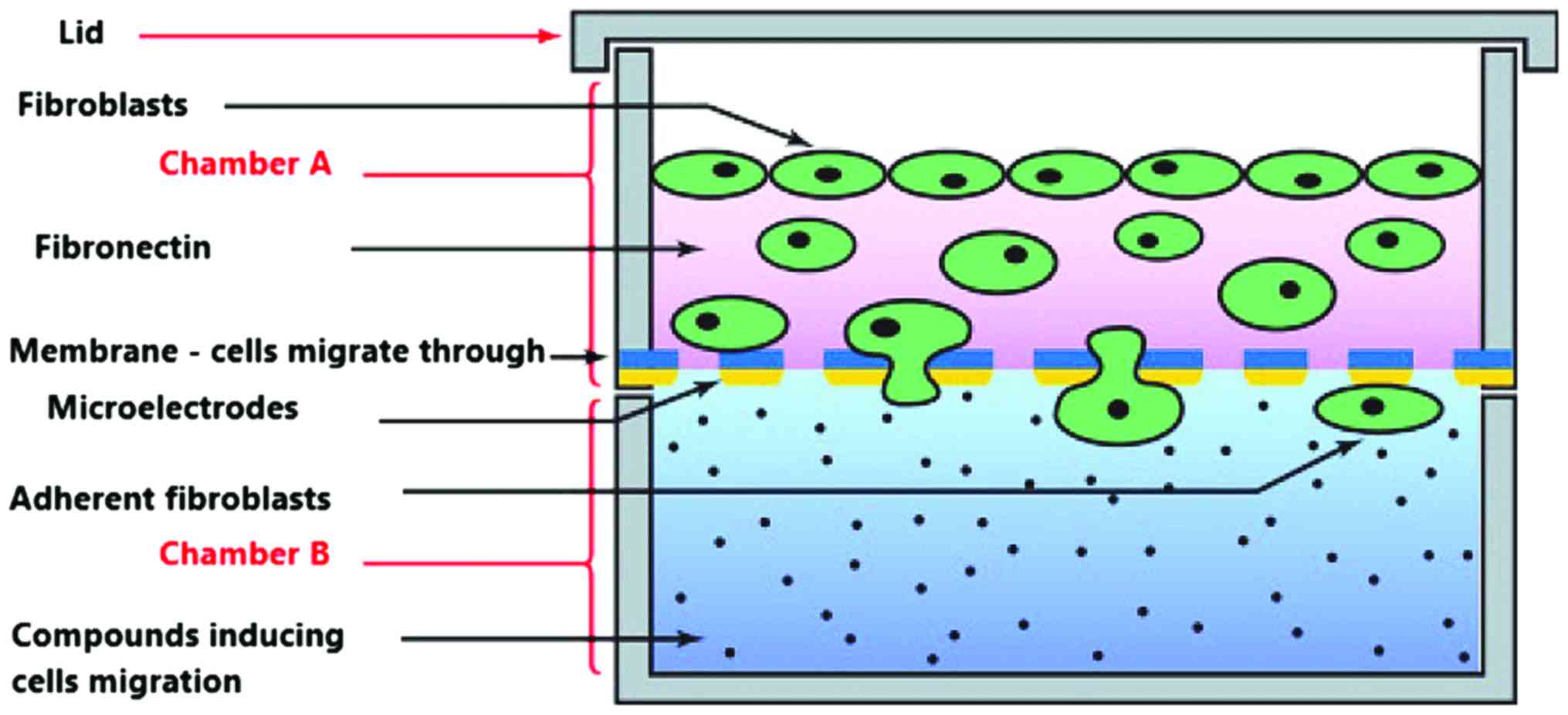
Migration was quantified using CIM-Plate 16 (Roche Applied
Science), compatible with RCTA-DP system. The plates are actually a
Boyden chamber with an upper and a lower chamber as presented in
Fig. 2. Polyethylene terephatalate
(PET) membrane has specific pores that accomodate only elongated
migratory cells. The lower part of the membrane accomodates the
microelectrodes for cellular impedance measurement.
Scratch test
Scratch test for registering the capacity of PRGF
and PRF compounds to induce wound healing was used the test for
monitoring the actual fibroblasts migration on a standard gap
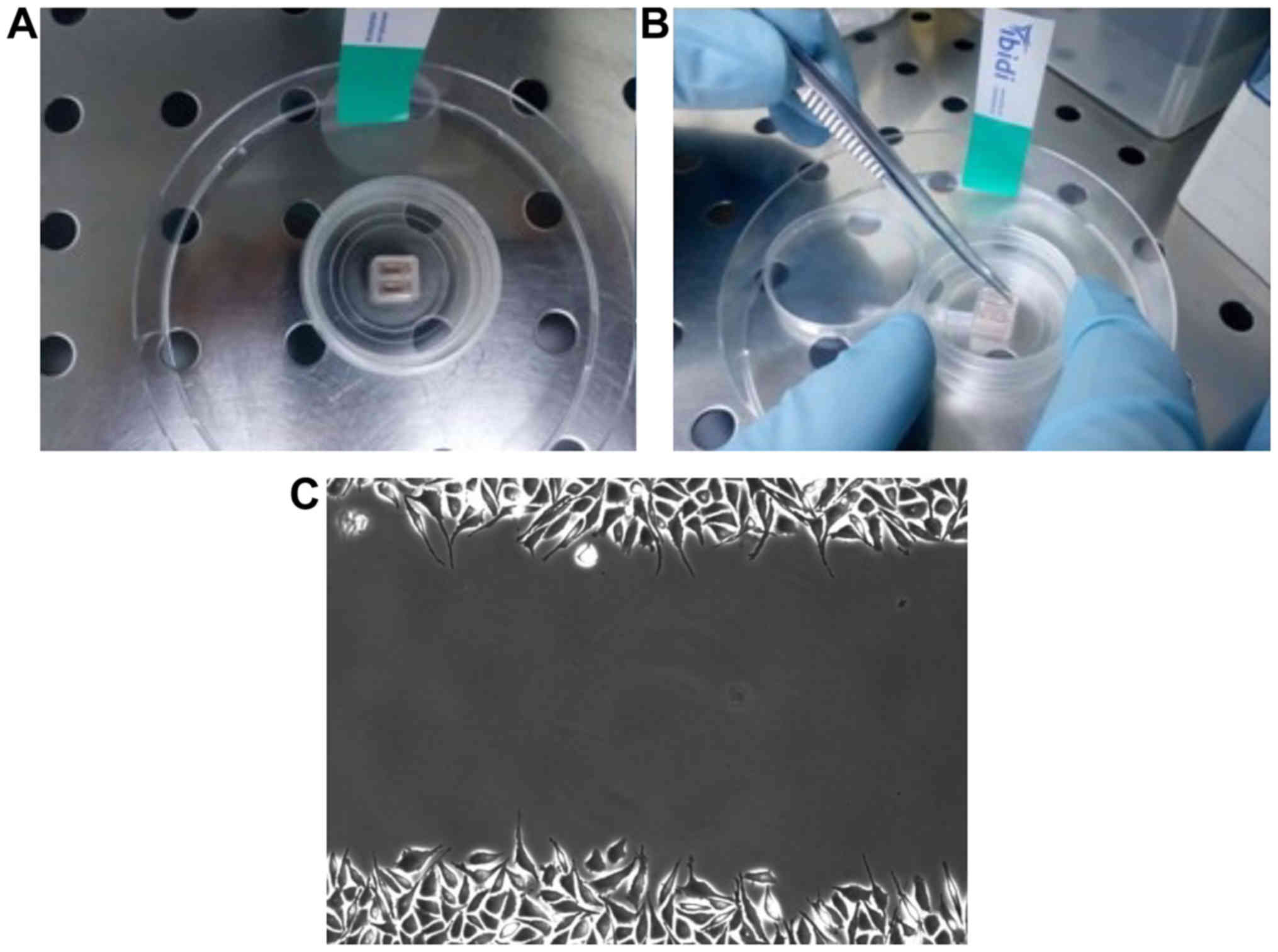
(14). Fibroblasts were seeded at a
density of 0.33×106 cells/IBIDI plate and 24 h of
cultivation at 37°C in 5% CO2, the silicon frame was
removed displaying a standard gap of 500 µm (Fig. 3). The plates were washed thoroughly
with PBS to remove any debris. Fibroblasts in the presence of 3 ml
complete medium with/without 10% serum (controls) or in the
presence of the tested compounds were incubated and migration
registered by video capture in BioStation (Nikon Corporation,
Tokyo, Japan).
Statistical analysis
All the experiments were performed three times and
each system comprised at least triplicates as stated. CI was
automatically registered by the software (RTCA 2.1.0 Software; ACEA
Biosciences Inc., San Diego, CA, USA) and mean ± SD is represented
in the figures. For individual representation of the experiments
the mean value of the triplicates was presented as directly
registered by the equipment.
Results
Fibroblasts proliferation
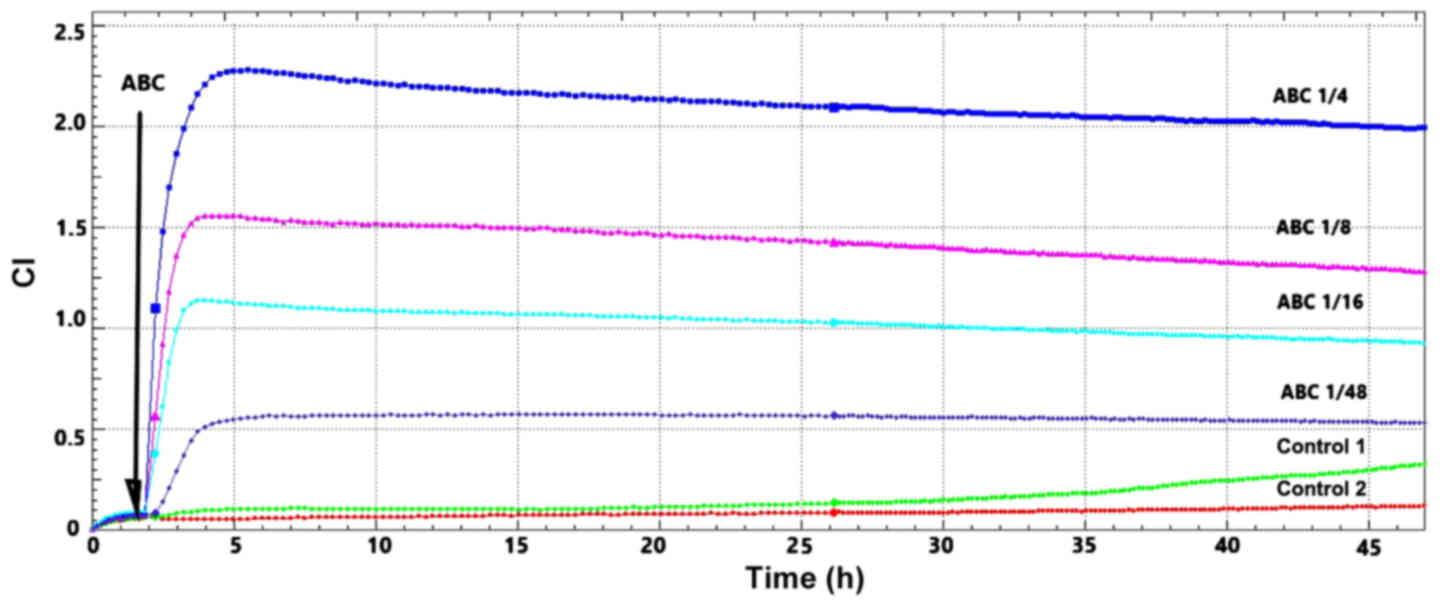
After the first 2 h of L929 cell cultivation in E16
plates we introduced serial dilution of different ABCs along with
the appropriate controls. Registering the CI for 47 h we noted the
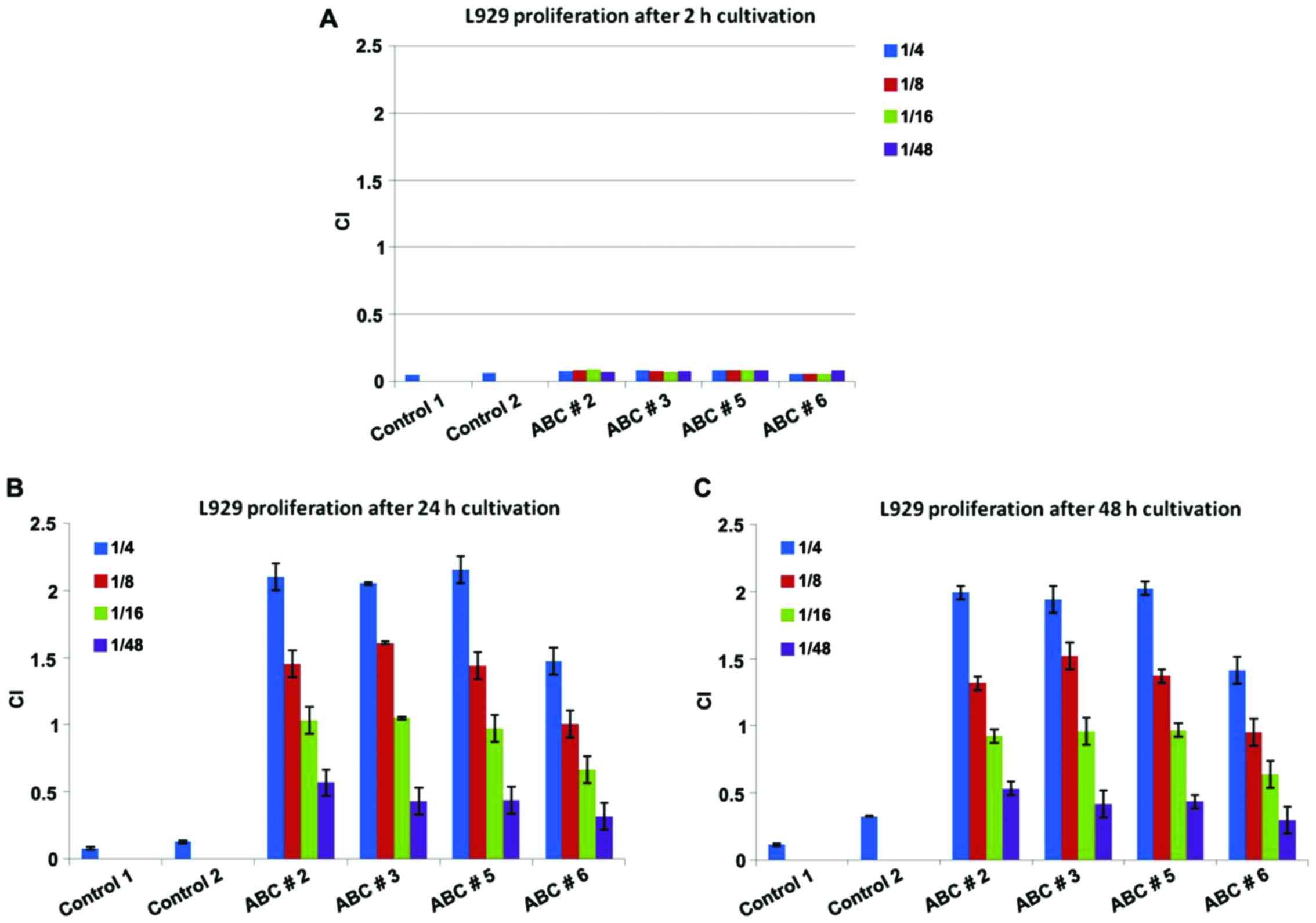
same pattern for all the tested compounds (Fig. 4). It can be observed that the
proliferation capacity is dose-dependent and perfectly matches the
tested ABC dilutions. Moreover, 48 h cultivation in the highest
dilution (1:48) of ABCS, displayed a pattern matching one of the
control cells.
The RCTA-DP system comprises the RTCA software 2.1.0
that automatically displays impedance measurements in arbitrary
units and calculates automatically CI for each well. When we
individually represented the obtained results (Fig. 5) some interesting findings occurred.
Thus, no matter the tested dilutions, all individual ABCs have the
same proliferation capacity after the first 2 h (Fig. 5A). Representation depicted in
Fig. 5B and C was performed with the
same Y axis to show the differences between CI registered at 2 h
compared to further cultivation.
This effect becomes statistically different after 24
h of cultivation (Fig. 5B;
p<0.001) and different activation potential was registered
between individual ABCs. There is a clear dose-dependent
proliferation capacity induced by ABCs, with 1:48 dilution having
the lowest capacity for all the tested compounds. After 48 h of
cultivation we register an activated proliferation, but slightly
decreased compared to the 24 h profile (Fig. 5C; p<0.001). At 48 h, the lowest
dilution of ABCs matches the fibroblast proliferation induced by
complete medium. The ABC particularities are reflected by the ABC
no. 6 that, from the beginning, induces the lowest proliferative
capacity in fibroblasts, while ABC nos. 2, 3 and 5 have similar
effects. We chose to represent the same CI units on the y-axis to
underline approximately 8-fold increase in the proliferation
capacity of fibroblasts when using 1:4 dilution. Moreover, the
registered effect has a clear dose-effect pattern decreasing with
the increased dilution of the compounds. This effect is sustained
by the soluble components of ABC as cell/cell debris were removed.
An identical pattern for ABC testing was obtained in the migration
tests of fibroblasts (data not shown).
Fibroblasts migration
The migration capacity of fibroblasts is strictly
linked to the capacity of wound healing. More interesting results
were obtained in the migratory tests for the gel compounds (PRGF
and PRF). In our system we have noted that PRGF 1 and 2 compounds
develop a good migratory capacity in the first 24 h of cultivation,
in contrast to the tested PRFs that display no proliferative action
upon in vitro fibroblasts (Fig.
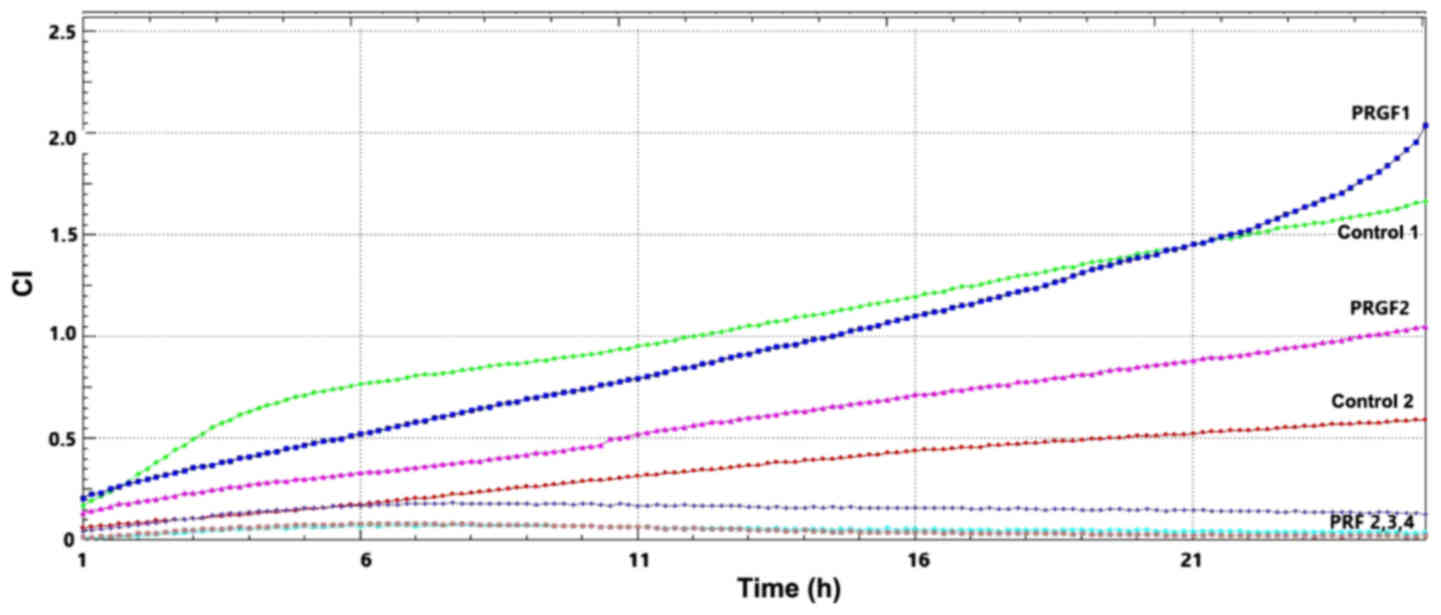
6). When testing further this capacity, we observed that PRGF1
and 2 diminish in their action that is surpassed by the control 1,
namely the positive control in complete cultivation medium
(Fig. 7).
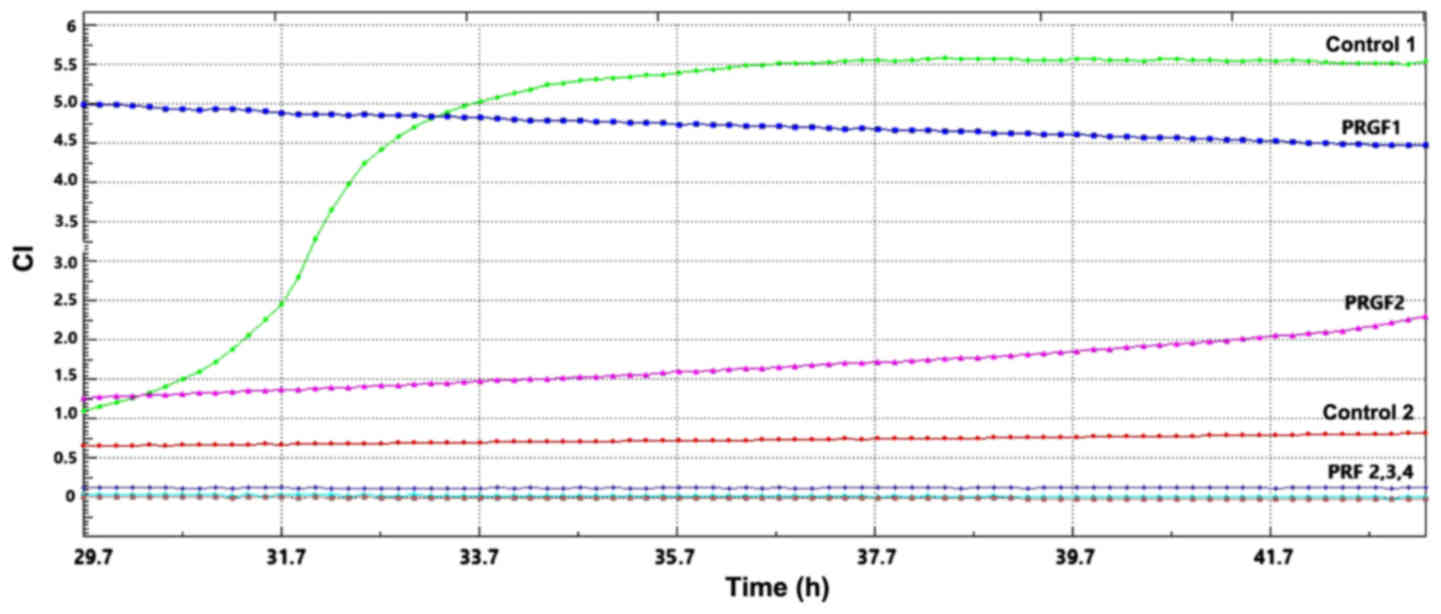
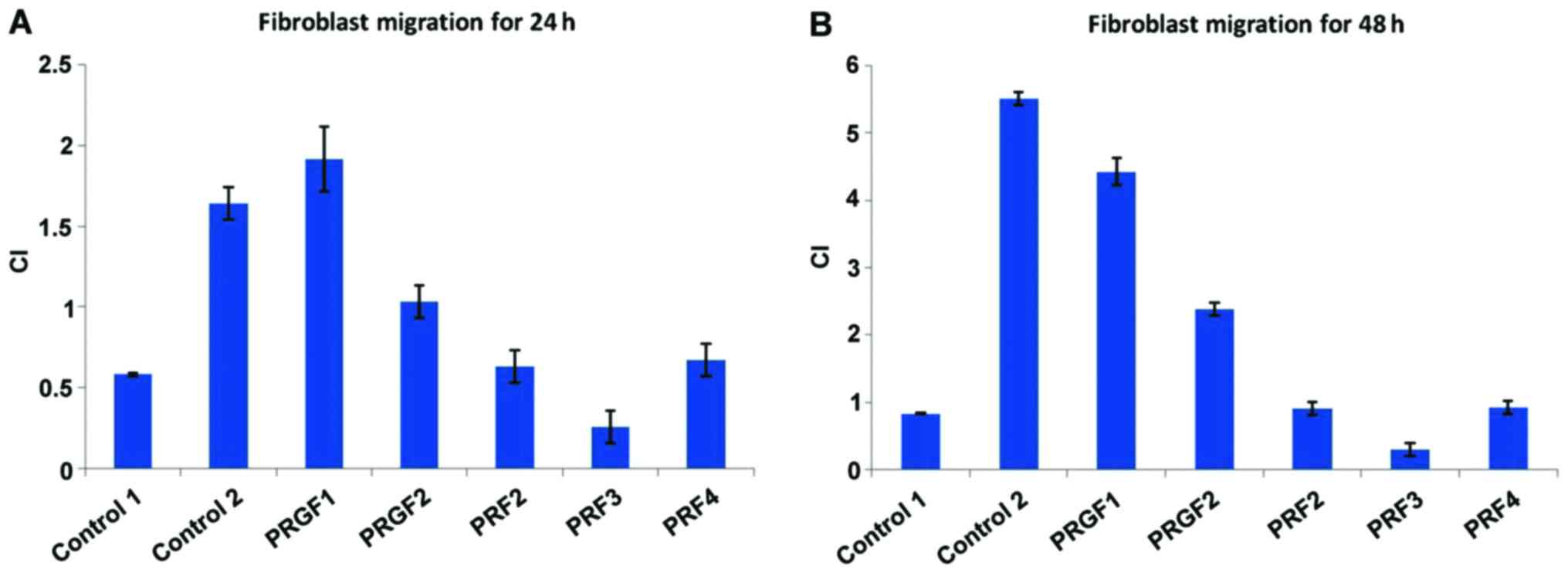
When investigating the automated CI performed by the
software there is clear statistically different action upon
fibroblasts migration. Thus, within the first 24 h of migration,
cells display a statistically identical effect with the positive
control (PRGF1), while a 50% migration capacity from the positive
control for PRGF2 (Fig. 8A). After
48 h the migratory capacity of PRGF1 and PRGF2 are surpassed by the
positive control (Fig. 8B).
Interestingly, all the tested PRFs are in the range of control 1,
whether registered at 24 or 48 h, this being the case for PRF2 and
4. PRF3 registers from the beginning an inhibition of migration
capacity inflicted upon fibroblasts.
Wound healing capacity
To reinforce the migration capacity of PRGFs and
PRFs we performed also a scratch test upon fibroblasts and
registered their capacity to cover a 500 µm gap. The highest rate
of ‘wound healing’ was registered by the control 2, closely
followed by PRGF1.
Discussion
It is known that blood components are key players in
the wound healing process. Fibrin matrix binds bioactive molecules
and regulates in time and space the microenvironment that will
induce the healing process. As a biomimetic approach, the use of
blood derivatives in regenerative strategies has awakened as a
source of multiple therapeutic biomolecules. Nevertheless, and
despite their clinical relevance, blood derivatives have been
showing inconsistent therapeutic results due to several factors,
including proper control over their delivery mechanisms. Herein, we
highlight recent trends on the use biomaterials to protect,
sequester and deliver these pools of biomolecules in tissue
engineering and regenerative medicine approaches. Particular
emphasis is given to strategies that enable to control their
spatiotemporal delivery and improve the selectivity of presentation
profiles of the biomolecules derived from blood derivatives rich in
platelets. Finally, we discussed possible directions for
biomaterials design to potentiate the aimed regenerative effects of
blood derivatives and achieve efficient therapies (14). Our data confirm the knowledge that
presence of the blood clot are involved in the regenerative
processes; routine observations show that hematoma within the
fracture site has outmost importance for callus formation and
proper bone repair (15). The
chemical mediators present within the blood clot, either produced
by inflammatory cells captive within or by endothelial or
mesenchymal cells (such as platelet-derived growth factor (PDGF),
epidermal growth factor (EGF), vascular endothelial growth factor
(VEGF), stromal cell-derived factor-1 (SDF-1), insulin-like growth
factor-1 (IGF-1), fibroblast growth factor basic (bFGF),
transforming growth factor-β (TGF-β), hepatocyte growth factor
(HGF), IL-1β, IL-6, IL-8 or monocyte chemoattractant protein 1
(MCP-1) induce fibroblasts proliferation and subsequent collagen
deposition (1,16–19). We
demonstrated the in vitro proliferative effect of the
constituents of the blood clot on the fibroblasts in culture in a
dose-dependent manner. Also we emphasize that the blood clots used
in our experiments are ABCs; there are recent studies showing that
there are differences between the composition of the blood clot
obtained from peripheral blood and that from bone marrow, the
latter being richer in growth factors (20).
In daily practice, some clinicians used to retain
ABC within alveolar socket after extraction; however, this
procedure has no evidence-based support to date; to our knowledge,
our work is the first study addressing the biological arguments in
favor of this issue. PRF clots are reported in regenerative
dentistry, and recently it was reported that the actual method for
obtaining PRFs drives the quality and numbers of platelets within.
Estimating the number of platelets entrapped in the fibrin matrix
and their distribution in PRF clots and red thrombi is a measure of
the thrombi quality (21). They also
represent a source of growth factors such as PDGF-BB, TGFβ-1, and
IGF-I (22). PRGF contains important
quantities of TGF-β1, PDGF-BB, VEGF but in lower quantities than
PRF while pro-inflammatory cytokines such as IL-6 and IL-1β are
barely present (IL-6 is present in low levels and IL-1β is
undetectable in PRGF) (23,24). The PRF compounds that were tested by
us did not induce migratory action upon fibroblasts, while PRGF
displayed a clear statistically significant pro-regenerative
effect. The differences are related to the biologic composition of
these growth factor sources: while both PRF and PRGF function as
growth factor reservoirs due to platelets enrichment with slow
release of the biomolecules, PRGF contains a very small amount (if
any) of white blood cells and subsequently very few
pro-inflammatory cytokines (24,25). Of
these cytokines with pro-inflammatory effects IL-1β has major role
in leucocytic diapedesis in the acute phase of inflammation by
stimulating expression of adhesion molecules within endothelial
cells; IL-6 induces matrix degradation via matrix
metalloproteinases (26,27) interfere with the effect of
biologically active growth factors, thus explaining the evident
proliferative effect of PRGF when compared with PRP. Several other
studies showed that white blood cells have negative effects on
tissue regeneration, data supporting our findings (27–29).
However, it is interesting that other authors identified
significantly less evident effects of PRGF than PRF on human
periosteal cells as inducers of cell proliferation in culture
(25,30,31).
In conclusion, cellular impedance technology reveals
fibroblasts of skin origin as a perfect model to evaluate in
vitro the dynamic of proliferation and migration following
exposure to the tested compounds and further monitoring the
efficacy in individual cases. Within this preliminary study we are
highlighting the importance of investigating the
cellular/fibroblasts functionality in a network comprising both
ABCs and platelet rich fibrin compounds in the tissue regeneration
process. Moreover, studies should be expanded to other cellular
models relevant for wound healing especially that oral
keratinocytes could enlarge the cell population panels specific for
tissue regeneration evaluation due to their interesting behavior in
biomedical applications such as biotechnology of dental or
orthopedic implants (32). Besides
cellular models, omics tools should be additionally involved in
this attempt as cell function viewed through omics lens constitute
also a good barometer of tissue healing (33). Regeneration is a very complex process
still insufficiently studied and both cell populations and soluble
molecules should be intertwined and further connected to various
additional factors, e.g., neuroendocrine factors (34,35) to
comprehensively decipher the tissue remodeling background.
Acknowledgements
The presented study will be integrated in the
original part of PhD thesis of author Mihai Bucur.
Funding
This study was partially supported by a grant of
Romanian Ministry of Research and Innovation, CCCDI-UEFISCDI,
project no. 61PCCDI⁄2018, code PN-III-P1-1.2-PCCDI-2017-0341,
within PNCDI-III.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MB, CC, MN and SZ were responsible for the research
creation and design, data acquisition, analysis and interpretation
of data, statistical analysis, manuscript drafting, and critical
revision of the manuscript for important intellectual content. OD
and CV were responsible for the data acquisition, analysis and
interpretation of data, manuscript drafting, critical revision of
the manuscript for important intellectual content. MC, CP and LN
were responsible for analysis and interpretation of data,
statistical analysis, manuscript drafting, critical revision of the
manuscript for important intellectual content. EI was responsible
for the research creation and design, analysis and interpretation
of data, manuscript drafting, critical revision of the manuscript
for important intellectual content. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Colentina University Hospital (no. 63/31.10.2016; Bucharest,
Romania). All the enrolled subjects gave their informed consent for
the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PRF
|
platelet-rich fibrin
|
|
PRGFs
|
plasma rich in growth factors
|
|
GF
|
fibroblasts from human gingiva
|
|
SFs
|
skin fibroblasts
|
|
MMP
|
matrix metalloproteinase
|
|
ECM
|
extracellular matrix
|
|
PDGF
|
platelet-derived growth factor
|
|
EGF
|
epidermal growth factor
|
|
VEGF
|
vascular endothelial growth factor
|
|
SDF-1
|
stromal cell-derived factor-1
|
|
IGF-1
|
insulin-like growth factor-1
|
|
bFGF
|
fibroblast growth factor basic
|
|
TGF-β
|
transforming growth factor-β
|
|
HGF
|
hepatocyte growth factor
|
|
IL
|
interleukin
|
|
MCP-1
|
monocyte chemoattractant protein-1
|
References
|
1
|
Perez RL and Roman J: Fibrin enhances the
expression of IL-1 beta by human peripheral blood mononuclear
cells. Implications in pulmonary inflammation. J Immunol.
154:1879–1887. 1995.PubMed/NCBI
|
|
2
|
Butzelaar L, Niessen FB, Talhout W,
Schooneman DPM, Ulrich MM, Beelen RHJ and Mink van der Molen AB:
Different properties of skin of different body sites: The root of
keloid formation? Wound Repair Regen. 25:758–766. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Greaves NS, Ashcroft KJ, Baguneid M and
Bayat A: Current understanding of molecular and cellular mechanisms
in fibroplasia and angiogenesis during acute wound healing. J
Dermatol Sci. 72:206–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Driskell RR and Watt FM: Understanding
fibroblast heterogeneity in the skin. Trends Cell Biol. 25:92–99.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Häkkinen L, Larjava H and Koivisto L:
Granulation tissue formation and remodeling. Endod Topics.
24:94–129. 2011. View Article : Google Scholar
|
|
6
|
Glim JE, van Egmond M, Niessen FB, Everts
V and Beelen RH: Detrimental dermal wound healing: What can we
learn from the oral mucosa? Wound Repair Regen. 21:648–660. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Larjava H, Wiebe C, Gallant-Behm C, Hart
DA, Heino J and Häkkinen L: Exploring scarless healing of oral soft
tissues. J Can Dent Assoc. 77:b182011.PubMed/NCBI
|
|
8
|
Wong JW, Gallant-Behm C, Wiebe C, Mak K,
Hart DA, Larjava H and Häkkinen L: Wound healing in oral mucosa
results in reduced scar formation as compared with skin: Evidence
from the red Duroc pig model and humans. Wound Repair Regen.
17:717–729. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mah W, Jiang G, Olver D, Cheung G, Kim B,
Larjava H and Häkkinen L: Human gingival fibroblasts display a
non-fibrotic phenotype distinct from skin fibroblasts in
three-dimensional cultures. PLoS One. 9:e907152014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mah W, Jiang G, Olver D, Gallant-Behm C,
Wiebe C, Hart DA, Koivisto L, Larjava H and Häkkinen L: Elevated
CD26 expression by skin fibroblasts distinguishes a profibrotic
phenotype involved in scar formation compared to gingival
fibroblasts. Am J Pathol. 187:1717–1735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noor Mohamed R, Basha S and Al-Thomali Y:
Efficacy of platelet concentrates in pulpotomy - a systematic
review. Platelets. 29:440–445. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Neagu M, Constantin C, Tampa M, Matei C,
Lupu A, Manole E, Ion RM, Fenga C and Tsatsakis AM: Toxicological
and efficacy assessment of post-transition metal (Indium)
phthalocyanine for photodynamic therapy in neuroblastoma.
Oncotarget. 7:69718–69732. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tampa M, Matei C, Caruntu C, Poteca T,
Mihaila D, Paunescu C, Pitigoi G, Georgescu SR, Constantin C and
Neagu M: Cellular impedance measurement - novel method for in vitro
investigation of drug efficacy. Farmacia. 64:430–434. 2016.
|
|
14
|
Beyeler J, Schnyder I, Katsaros C and
Chiquet M: Accelerated wound closure in vitro by fibroblasts from a
subgroup of cleft lip/palate patients: Role of transforming growth
factor-α. PLoS One. 9:e1117522014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mendes BB, Gómez-Florit M, Babo PS,
Domingues RM, Reis RL and Gomes ME: Blood derivatives awaken in
regenerative medicine strategies to modulate wound healing. Adv
Drug Deliv Rev. 129:376–393. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghiasi MS, Chen J, Vaziri A, Rodriguez EK
and Nazarian A: Bone fracture healing in mechanobiological
modeling: A review of principles and methods. Bone Rep. 6:87–100.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Campbell RA, Vieira-de-Abreu A, Rowley JW,
Franks ZG, Manne BK, Rondina MT, Kraiss LW, Majersik JJ, Zimmerman
GA and Weyrich AS: Clots are potent triggers of inflammatory cell
gene expression: Indications for timely fibrinolysis. Arterioscler
Thromb Vasc Biol. 37:1819–1827. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Distler O, Pap T, Kowal-Bielecka O,
Meyringer R, Guiducci S, Landthaler M, Schölmerich J, Michel BA,
Gay RE, Matucci-Cerinic M, et al: Overexpression of monocyte
chemoattractant protein 1 in systemic sclerosis: Role of
platelet-derived growth factor and effects on monocyte chemotaxis
and collagen synthesis. Arthritis Rheum. 44:2665–2678. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee ME, Rhee KJ and Nham SU: Fragment E
derived from both fibrin and fibrinogen stimulates interleukin-6
production in rat peritoneal macrophages. Mol Cells. 9:7–13.
1999.PubMed/NCBI
|
|
20
|
Szaba FM and Smiley ST: Roles for thrombin
and fibrin(ogen) in cytokine/chemokine production and macrophage
adhesion in vivo. Blood. 99:1053–1059. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tezono K, Sarker KP, Kikuchi H, Nasu M,
Kitajima I and Maruyama I: Bioactivity of the vascular endothelial
growth factor trapped in fibrin clots: Production of IL-6 and IL-8
in monocytes by fibrin clots. Haemostasis. 31:71–79.
2001.PubMed/NCBI
|
|
22
|
Shoji T, Nakasa T, Yoshizuka M, Yamasaki
T, Yasunaga Y, Adachi N and Ochi M: Comparison of fibrin clots
derived from peripheral blood and bone marrow. Connect Tissue Res.
58:208–214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kitamura Y, Watanabe T, Nakamura M, Isobe
K, Kawabata H, Uematsu K, Okuda K, Nakata K, Tanaka T and Kawase T:
Platelet counts in insoluble platelet-rich fibrin clots: A direct
method for accurate determination. Front Bioeng Biotechnol.
6:42018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dohan DM, Choukroun J, Diss A, Dohan SL,
Dohan AJ, Mouhyi J and Gogly B: Platelet-rich fibrin (PRF): a
second-generation platelet concentrate. Part II: platelet-related
biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 101:e45–e50. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Masuki H, Okudera T, Watanebe T, Suzuki M,
Nishiyama K, Okudera H, Nakata K, Uematsu K, Su CY and Kawase T:
Growth factor and pro-inflammatory cytokine contents in
platelet-rich plasma (PRP), plasma rich in growth factors (PRGF),
advanced platelet-rich fibrin (A-PRF), and concentrated growth
factors (CGF). Int J Implant Dent. 2:192016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nishiyama K, Okudera T, Watanabe T, Isobe
K, Suzuki M, Masuki H, Okudera H, Uematsu K, Nakata K and Kawase T:
Basic characteristics of plasma rich in growth factors (PRGF):
Blood cell components and biological effects. Clin Exp Dent Res.
2:96–103. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Anitua E, Zalduendo M, Troya M, Padilla S
and Orive G: Leukocyte inclusion within a platelet rich
plasma-derived fibrin scaffold stimulates a more pro-inflammatory
environment and alters fibrin properties. PLoS One.
10:e01217132015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Contassot E, Beer HD and French LE:
Interleukin-1, inflammasomes, autoinflammation and the skin. Swiss
Med Wkly. 142:w135902012.PubMed/NCBI
|
|
29
|
Sundararaj KP, Samuvel DJ, Li Y, Sanders
JJ, Lopes-Virella MF and Huang Y: Interleukin-6 released from
fibroblasts is essential for up-regulation of matrix
metalloproteinase-1 expression by U937 macrophages in coculture:
Cross-talking between fibroblasts and U937 macrophages exposed to
high glucose. J Biol Chem. 284:13714–13724. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abnave P and Ghigo E: Role of the immune
system in regeneration and its dynamic interplay with adult stem
cells. Semin Cell Dev Biol. Apr 9–2018.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nguyen HX, Hooshmand MJ, Saiwai H, Maddox
J, Salehi A, Lakatos A, Nishi RA, Salazar D, Uchida N and Anderson
AJ: Systemic neutrophil depletion modulates the migration and fate
of transplanted human neural stem cells to rescue functional
repair. J Neurosci. 37:9269–9287. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Calenic B, Greabu M, Caruntu C, Nicolescu
MI, Moraru L, Surdu-Bob CC, Badulescu M, Anghel A, Logofatu C and
Boda D: Oral keratinocyte stem cells behavior on diamond like
carbon films. Rom Biotechnol Lett. 21:11914–11922. 2016.
|
|
33
|
Boda D: Cellomics as integrative omics for
cancer. Curr Proteomics. 10:237–245. 2013. View Article : Google Scholar
|
|
34
|
Lupu M, Caruntu A, Caruntu C, Papagheorghe
LML, Ilie MA, Voiculescu V, Boda D, Constantin C, Tanase C, Sifaki
M, et al: Neuroendocrine factors: The missing link in non-melanoma
skin cancer (Review). Oncol Rep. 38:1327–1340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Caruntu C, Boda D, Constantin C, Caruntu A
and Neagu M: Catecholamines increase in vitro proliferation of
murine B16F10 melanoma cells. Acta Endocrinol (Buc). 10:545–558.
2014. View Article : Google Scholar
|