Introduction
Fat transplantation has become a tool not only for
augmenting tissue volume, but also for enhancing tissue
regeneration. With the development of various techniques to assist
fat transfer, fat graft retention has become more predictable but
is still less than ideal. The stromal vascular fraction (SVF) cells
assisted fat transplantation is one of the practical methods to
improve fat graft retention. However, the role of SVF in fat
grafting is still unclear. To improve these outcomes, it is
essential to elucidate the detailed mechanism underlying the
engraftment of fat tissue.
SVF is a heterogeneous cell mixture containing all
types of fat cells, with the exception of adipocytes (1). Yoshimura et al (2,3) reported
that the SVF cell-assisted facial or breast fat transplantation
presented better clinical results compared with traditional fat
grafting. In addition, our previous study revealed that SVF
promoted the fat graft retention rate through the secretion of
growth factors, promotion of angiogenesis and increasing the
density of stem cells (4). Fat
transplantation is a process accompanied by inflammatory cell
infiltration and acute inflammatory response (5). Furthermore, several previous studies on
diabetes and obesity have suggested that adipose tissue is closely
associated with inflammation (6,7).
However, the effect of SVF on the inflammatory response following
fat transplantation remains unclear.
Therefore, the present study aimed to investigate
the effect of SVF on inflammation regulation in the fat
transplanted to mice and its impact on fat graft retention in
mice.
Materials and methods
SVF-assisted fat transplantation
model
A total of 24 male and 24 female 8-week-old C57BL/6
(Southern Medical University, Guangzhou, China) weighing 20–25 g
and 5 male and 5 female green fluorescence protein (GFP) C57BL/6J
(Model Animal Research Center of Nanjing University, Nanjing,
China) mice were used in the present study. The GFP C57BL/6J mice
were 6–8 weeks old and weighed 20–25 g. The GFP C57BL/6J mice were
sacrificed to obtain SVF as previously described (8). A total of 24 C57BL/6 mice were
sacrificed, and the inguinal fat pads were harvested and dissected
into small sections (~1 mm3).
For fat transplantation, 0.5 ml dissected fat from
C57BL/6 mice was mixed with 5×106 GFP SVF cells from GFP
C57BL/6J mice and suspended in 10 µl phosphate-buffered saline
(PBS). The mixture was then injected into the subcutaneous tissues
on the left flank of C57BL/6 mice using an 1 ml syringe with
standardized blunt tipped 14 gauge infiltration cannula, and these
mice served as the SVF group (n=24). In the control group, 0.5 ml
fat mixed with 10 µl PBS was injected into the right flank of the
C57BL/6 mice (n=24). At 1, 7, 14 and 30 days after fat
transplantation, the mice were sacrificed and the grafts were
harvested and subsequently analyzed as described later. No
mortality was observed in the animals following fat transplantation
in the present study. All experiments were performed under the
approval of the Nanfang Hospital Institutional Animal Care and Use
Committee (Guangzhou, China). The volume of the grafts was measured
with a displacement method immediately after the grafts were
harvested.
Whole-mount staining
Visualization of the fat grafts was performed using
the procedure outlined by Eto et al (9). Accordingly, the grafts were cut into
0.5–1-mm sections and incubated with Hoechst 33342 (Sigma-Aldrich;
Merck AG, Darmstadt, Germany) for 30 min at room temperature in
order to stain the cell nuclei. The samples were then washed with
PBS and observed directly with a confocal microscope system (FV1000
confocal microscope; Olympus Corp., Tokyo, Japan). Hoechst and
GFP-positive cells in the SVF group were counted, and the
percentage of surviving SVF cells was defined as the number of
stained cells with respect to the total number of cells
(5×106 cells). The number of each cell population per
unit volume was then calculated. Images captured from four
different fields-of-view for each sample were analyzed.
Histological analysis
Samples were sectioned serially at 5 mm and stained
with hematoxylin and eosin, according to standard protocols. Next,
the samples were examined with an Olympus BX51 microscope and
images were captured using an Olympus DP71 digital camera (Olympus
Corp.).
Immunohistochemical staining
For immunostaining, 5-mm sections were incubated
with the rabbit anti-mouse CD31 primary antibody (cat. no.
Sc-1506-R; dilution, 1:400; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA). The endogenous peroxidase activity was inhibited using 1%
hydrogen peroxide (Rongbai Biological Technology Co., Ltd.,
Shanghai, China). Following the incubation, the sections were
incubated with an EnVision complex (Dako; Agilent Technologies,
Inc., Santa Clara, CA, USA) and horseradish peroxidase-conjugated
secondary antibody (cat. no. 31460; dilution, 1:5,000; goat
anti-rabbit; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Color was then developed by 3,3′-diaminobenzidine
(Sigma-Aldrich; Merck AG), and CD31-positive cells were visualized
and under a biological microscope. The images captured were
analyzed with the professional image analysis software IPP (version
6.0; Media Cybernetics, Inc., Rockville, MD, USA). The mean vessel
densities were statistically analyzed.
Immunofluorescence staining
Samples cut into 5-µm sections were incubated
overnight at 4°C with primary antibodies as follows: Rabbit
anti-mouse CD206 (cat. no. ab64693; dilution, 1:300; Abcam,
Cambridge, UK), and rat anti-mouse Mac2 (cat. no. CL8942G;
dilution, 1:200; Cedarlane Corp., Burlington, ON, Canada).
Subsequently, samples were incubated at room temperature for 1 h
with the DyLight® 488-conjugated goat anti-rabbit
immunoglobulin G (cat. no. ab96883; dilution, 1:200; Abcam) and
Alexa Fluor 647-conjugated goat anti-rat immunoglobulin G (cat. no.
4418; dilution, 1:200; Cell Signaling Technology, Inc., Danvers,
MA, USA) secondary antibodies, correspondingly. Nuclei were stained
with DAPI (dilution, 1:200; Sigma-Aldrich; Merck AG) and images
were obtained through a confocal microscope system (Zeiss GmbH,
Jena, Germany). The number of M1 macrophages
(Mac2+/CD206−) and M2 macrophages
(Mac2+/CD206+) were counted by analyzing at
least six fields of ×200 magnification for each sample by Photoshop
software (version 7.0; Adobe Systems, Inc., San Jose, CA, USA).
Data were collected by two independent, blinded observers.
Enzyme-linked immunosorbent assay
(ELISA)
Samples were ground to obtain the tissue homogenate,
and the supernatant was extracted by centrifugation at 12,000 × g
for 15 min at 4°C. The expression levels of interleukin (IL)-6
(cat. no. GTX37124; GeneTex, Inc., Irvine, CA, USA), tumor necrosis
factor-α (TNF-α; cat. no. 500850; Cayman Chemical, Ann Arbor, MI,
USA) and IL-10 (cat. no. GTX37143; GeneTex, Inc.) in the tissue
homogenates of the samples were measured using ELISA according to
the manufacturers' protocols. The absorbance of the plates was
detected on a Multiskan MK3 microplate reader (Thermo Fisher
Scientific, Inc.) set at a wavelength of 450 nm.
Statistical analysis
All data are expressed as the mean ± standard
deviations, and the statistical software SPSS version 20.0 (IBM
Corp., Armonk, NY, USA) was used for data processing. An
independent samples t-test was used to examine differences between
the two groups. Analysis of covariance, a general linear model that
combines analysis of variance and regression, was used for analysis
of different time points within one group. Tukey's test was used as
the post hoc test to detect the significant differences between the
samples in the same group following the analysis of covariance.
P<0.05 was considered to demonstrate differences that were
statistically significant.
Results
Graft volume alterations
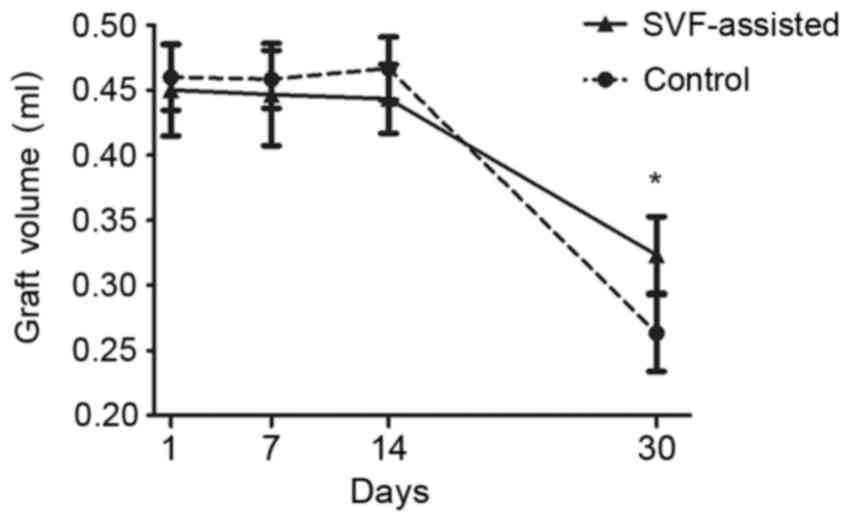
The graft volume curves of the two groups at
different time points indicated the similar volume tendency of
grafts at the initial time points, with the exception of a slight
increase in the volume of the control group at day 14 (Fig. 1). The volume presented a marked
decline from day 14 after transplantation in the two groups.
However, the graft volume of the SVF group was significantly higher
compared with that of the control group at day 30 after
transplantation (P<0.05).
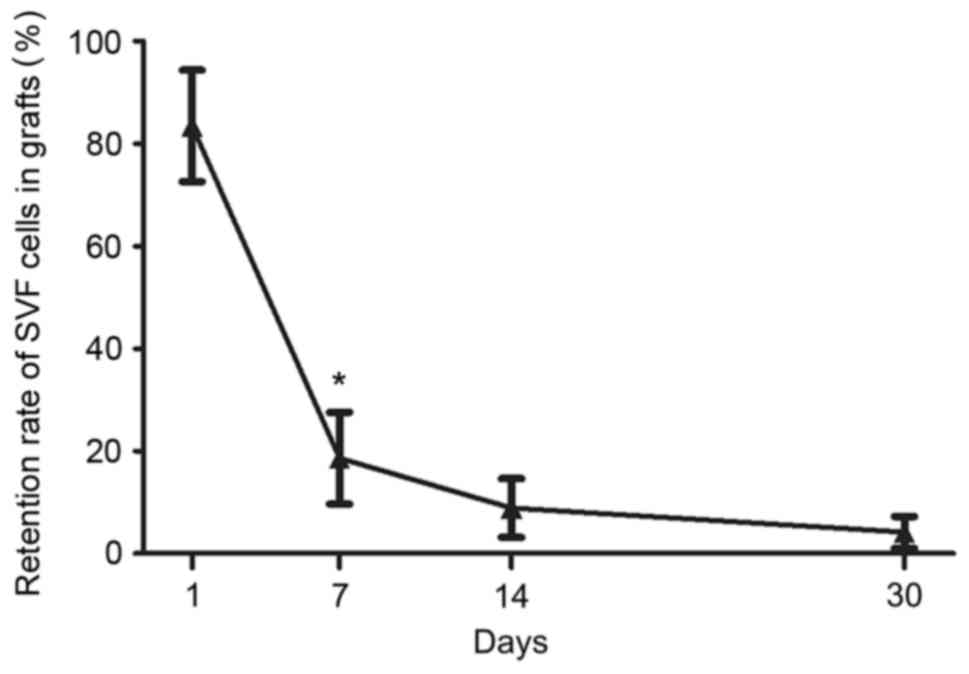
Retention rate of GFP SVF cells
The GFP fluorescence in the SVF group was detected
in order to reveal the retention rate of SVF cells in the grafts.
Quantification of the GFP of SVF cells indicated that the retention
rate significantly decreased from 83.5±10.9% on day 1 after
transplantation to 18.6±9.0% on day 7 (P<0.05), with further
decrease observed at later time points (day 14, 8.9±5.7%; day 30,
4.2±3.1%) (Fig. 2).
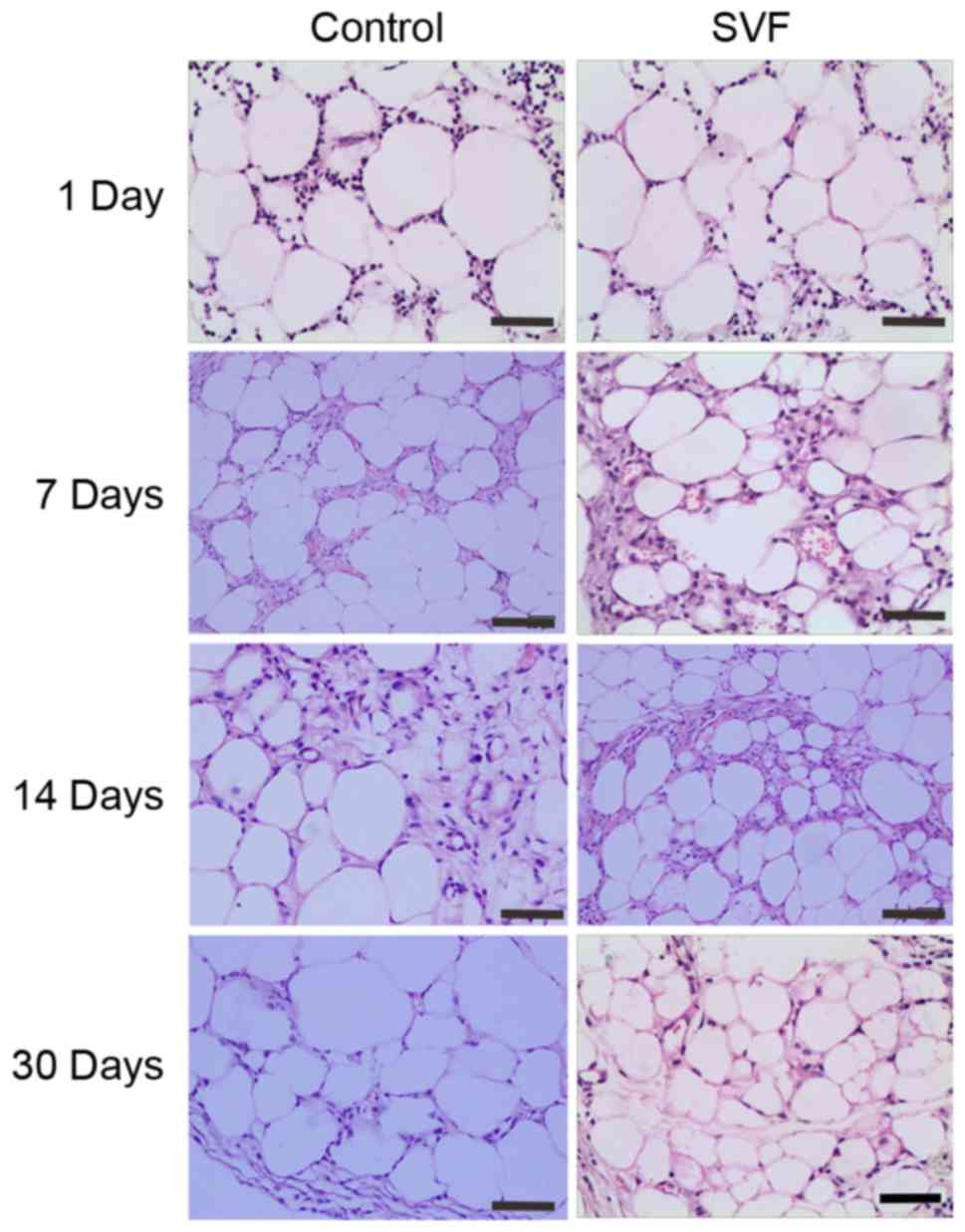
Tissue structure analysis of
grafts
Histological observation by hematoxylin and eosin
staining demonstrated that an increased number of inflammatory
cells infiltrated into the graft in the control group when compared
with the SVF group at day 1 after transplantation (Fig. 3). At days 7 and 14, the number of
inflammatory cells decreased, however, the number of new vessels
increased in the two groups. The SVF group presented increased
number of small-sized adipocytes compared with the control group at
days 7 and 14, as well as a more mature structure of the adipose
tissue at day 30 (Fig. 3).
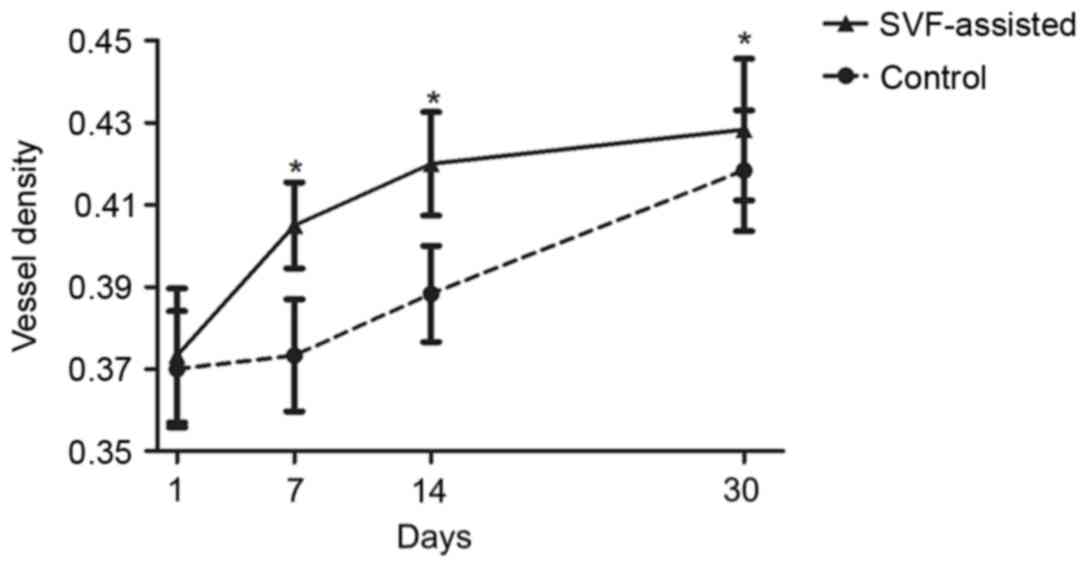
Vascular alterations of the
grafts
In order to observe the vascular alterations of the
fat grafts in more detail, immunostaining of vascular endothelial
cells with CD31 antibody was conducted. As shown in Fig. 4, the vessel density of the SVF group
was significantly higher compared with that in the control group at
days 7, 14 and 30 after transplantation (P<0.05).
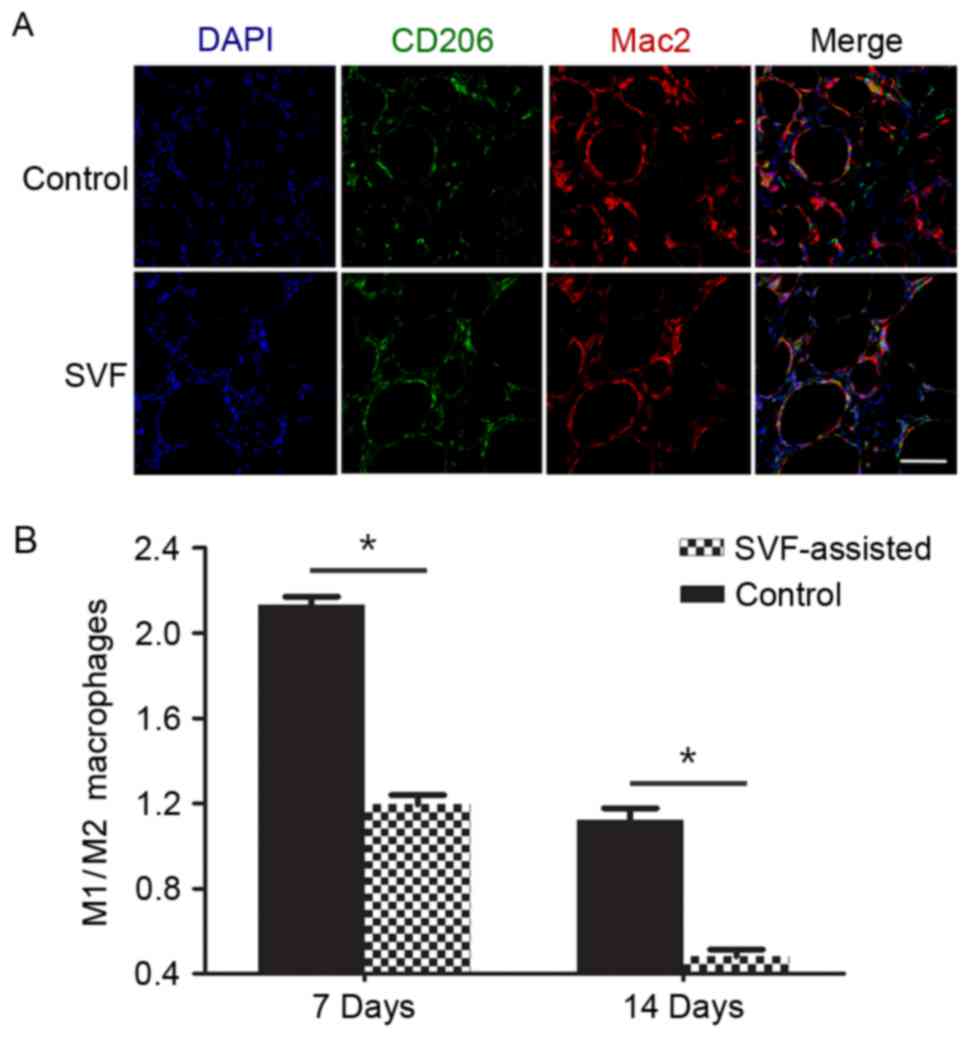
Infiltration of M2 macrophages
The immunofluorescence analysis demonstrated the
infiltration of M1 macrophages (Mac2+/CD206−;
red fluorescence) and M2 macrophages
(Mac2+/CD206+; purple fluorescence) in the
two groups days 7 after transplantation (Fig. 5A). The ratio of M1/M2 was
significantly higher in the control group at day 7 compared with
the SVF group (P<0.05) (Fig. 5B).
By contrast, the number of M1 and M2 macrophages was relatively
similar in the SVF group at day 7, as well as in the control group
at day 14, as observed by the ratio of M1/M2. In addition, the SVF
group presented a significantly lower ratio of M1/M2 at day 14
compared with the control group (P<0.05).
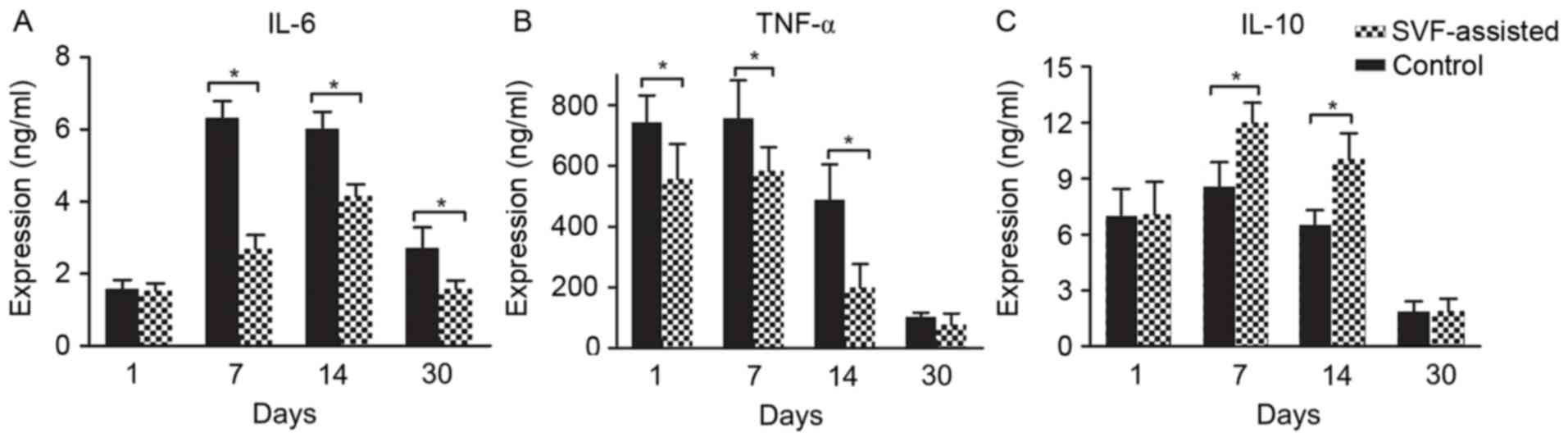
Expression levels of pro-inflammatory
(IL-6 and TNF-α) and anti-inflammatory (IL-10) cytokines
ELISA was used to detect the expression levels of
the pro-inflammatory IL-6 and TNF-α cytokines, as well as of the
anti-inflammatory IL-10 cytokine. The expression of IL-6 in the two
groups increased between days 1 and 14, and then decreased on day
30, with the exception of the IL-6 level in the control group that
presented a slight decrease between days 7 and 14 (Fig. 6A). In addition, the expression of
TNF-α in the two groups was maintained at a high level prior to day
7 and then decreased on days 14 and 30 (Fig. 6B). Notably, statistical analysis
demonstrated that the SVF group had a significantly lower
expression level of IL-6 on days 7, 14 and 30 compared with the
control, while the TNF-α levels were also significantly decreased
in the SVF group on days 1, 7 and 14 (P<0.05) (Fig. 6A and B). By contrast, compared with
the control group, the SVF group presented a markedly higher
expression level of IL-10 at days 7 and 14 (P<0.05) (Fig. 6C).
Discussion
At the early stage of fat transplantation, acute
ischemia and inflammation occur due to inadequate blood supply and
tissue injury, which leads to tissue edema (10). It is hypothesized that this may cause
the increase in the graft volume observed in the control group at
day 14 in the present study. By contrast, the SVF group did not
present a graft volume increase, indicating that there was a lower
level of edema as a result of better blood supply and lower
inflammation compared with the control group.
SVF has been applied in the treatment of ischemia
diseases and burn wounds due to its ability to promote angiogenesis
and neovascularization (11,12). In our previous animal study it was
demonstrated that SVF cell-assisted fat transplantation in nude
mice resulted in a higher graft retention rate with enhanced
angiogenesis (4). In the present
study, it was noted that the vascular network exhibited more rapid
growth in the SVF group in comparison with the control group, which
gradually grew into the center of the graft from the peripheral
region in the SVF group. Furthermore, the SVF group demonstrated
higher vessel density compared with the control group. This
sufficient blood supply in the SVF group significantly contributed
to the improved transplantation results.
The process of angiogenesis is regulated by various
growth factors, including vascular endothelial growth factor
(VEGF), basic fibroblast growth factor (bFGF), hepatocyte growth
factor and transforming growth factor-β. Our previous study
indicated a significant increase of VEGF and bFGF levels in the SVF
group, and suggested that SVF cells had a strong paracrine function
(4). Several other studies
demonstrated that the SVF cells, including endothelial progenitor
cells (EPCs), adipose-derived stem cells (ASCs), macrophages and
fibroblasts, are able to secret various angiogenic growth factors
(1–3,9).
Besides the paracrine effect, the cellular
components of the SVF, such as the endothelial cells and pericytes,
directly reassemble to form new vessels (4). SVF is a source of progenitor and stem
cells, which have the potential to differentiate along different
lineages (4). The key component of
the SVF is ASCs, identified as
CD34+CD31−CD45−, which
differentiate into osteogenic, adipogenic and chondrogenic cell
types (4). Other important subtypes
with progenitor ability include the EPCs and pericytes (9). These cells possess a high proliferative
potential and are also able to differentiate into vascular cells,
participating in the formation of the new vascular network
(1).
In the present study, the SVF group demonstrated
reduced level of inflammation compared with the control group, as
observed by the lower expression of inflammatory cytokines,
including IL-6 and TNF-α, as well as the higher expression of
anti-inflammatory cytokines, such as IL-10. Furthermore, an
increased number of M2 macrophages were detected in the SVF group
in comparison with the control group. Tiemessen et al
(13) indicated that T-regulatory
cells contained in the SVF maintained the M2 phenotype of the
macrophages in the SVF. A previous study on ischemia heart failure
also revealed that SVF transplantation served an anti-inflammation
role in cell therapy through the decrease of myocardial mRNA
expression of the inflammatory cytokines TNF-α, IL-6, matrix
metalloproteinase (MMP-1) and tissue inhibitor of MMP (TIMP-1)
(11). Therefore, in the current
study, it is hypothesized that SVF presented anti-inflammatory
properties following fat transplantation through the expression and
suppression of various cytokines, as well as the conversion of the
macrophage phenotype.
The improved graft results observed in the SVF group
were due to not only the earlier and sufficient angiogenesis, but
also the lower level of inflammation. The damage and ischemia
subsequent to fat transplantation lead to inflammatory response,
which results in increased cell death, although inflammation also
contributes to angiogenesis (10).
However, the improved angiogenesis in the SVF group in the current
study demonstrated that the effect of SVF in directly promoting
angiogenesis counteracted its indirect inhibition of inflammation
that suppressed angiogenesis.
In conclusion, the results of the present study
indicated that SVF promoted the retention rate of grafts following
fat transplantation through the well-known pro-angiogenic mechanism
(paracrine function and involvement in the formation of new
vessels), as well as through the anti-inflammatory properties of
SVF, which was observed by the expression and suppression of
various cytokines, and the conversion of the macrophage
phenotype.
Acknowledgements
Not applicable.
Funding
The current study was funded by the Nature Science
Foundation of Guangdong Province (grant no. 2014A030310458).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MZ, JX, SL, YY and YL designed the study, performed
the experiments, analyzed the data, and wrote the paper. JQ, CL and
QL collected the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experiments were performed under the approval of
the Nanfang Hospital Institutional Animal Care and Use
Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors have declared no conflicting
interests.
Glossary
Abbreviations
Abbreviations:
|
SVF
|
stromal vascular fraction
|
|
GFP
|
green fluorescent protein
|
|
TNF-α
|
tumor necrosis factor-α
|
References
|
1
|
Yoshimura K, Suga H and Eto H:
Adipose-derived stem/progenitor cells: Roles in adipose tissue
remodeling and potential use for soft tissue augmentation. Regen
Med. 4:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoshimura K, Sato K, Aoi N, Kurita M,
Inoue K, Suga H, Eto H, Kato H, Hirohi T and Harii K: Cell-assisted
lipotransfer for facial lipoatrophy: Efficacy of clinical use of
adipose-derived stem cells. Dermatol Surg. 34:1178–1185. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoshimura K, Sato K, Aoi N, Kurita M,
Hirohi T and Harii K: Cell-assisted lipotransfer for cosmetic
breast augmentation: Supportive use of adipose-derived stem/stromal
cells. Aesthetic Plast Surg. 32:48–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu M, Dong Z, Gao J, Liao Y, Xue J, Yuan
Y, Liu L, Chang Q and Lu F: Adipocyte regeneration after free fat
transplantation: Promotion by stromal vascular fraction cells. Cell
Transplant. 24:49–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dong Z, Fu R, Liu L and Lu F: Stromal
vascular fraction (SVF) cells enhance long-term survival of
autologous fat grafting through the facilitation of M2 macrophages.
Cell Biol Int. 37:855–859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hocking SL, Stewart RL, Brandon AE,
Suryana E, Stuart E, Baldwin EM, Kolumam GA, Modrusan Z, Junutula
JR, Gunton JE, et al: Subcutaneous fat transplantation alleviates
diet-induced glucose intolerance and inflammation in mice.
Diabetologia. 58:1587–1600. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tran TT, Yamamoto Y, Gesta S and Kahn CR:
Transplantation of subcutaneous fat to the visceral cavity induced
protective metabolic effects: Evidence for intrinsic properties of
subcutaneous fat. Diabetes. 56:A5–A6. 2007.
|
|
8
|
Zhang S, Dong Z, Peng Z and Lu F:
Anti-aging effect of adipose-derived stem cells in a mouse model of
skin aging induced by D-galactose. PLoS One. 9:e975732014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eto H, Suga H, Matsumoto D, Inoue K, Aoi
N, Kato H, Araki J and Yoshimura K: Characterization of structure
and cellular components of aspirated and excised adipose tissue.
Plast Reconstr Surg. 124:1087–1097. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eto H, Kato H, Suga H, Aoi N, Doi K, Kuno
S and Yoshimura K: The fate of adipocytes after nonvascularized fat
grafting: Evidence of early death and replacement of adipocytes.
Plast Reconstr Surg. 129:1081–1092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Premaratne GU, Ma LP, Fujita M, Lin X,
Bollano E and Fu M: Stromal vascular fraction transplantation as an
alternative therapy for ischemic heart failure: Anti-inflammatory
role. J Cardiothorac Surg. 6:432011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ceserani V, Ferri A, Berenzi A, Benetti A,
Ciusani E, Pascucci L, Bazzucchi C, Coccè V, Bonomi A, Pessina A,
et al: Angiogenic and anti-inflammatory properties of
micro-fragmented fat tissue and its derived mesenchymal stromal
cells. Vasc Cell. 8:32016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tiemessen MM, Jagger AL, Evans HG, van
Herwijnen MJ, John S and Taams LS: CD4+CD25+Foxp3+ regulatory T
cells induce alternative activation of human monocytes/macrophages.
Proc Natl Acad Sci USA. 104:19446–19451. 2007. View Article : Google Scholar : PubMed/NCBI
|