Introduction
With the continuous progress in space exploration
technology, astronauts have to face the harsh physical environment
and serious physiological changes in short-term and long-term space
missions. The major physiological changes include the loss of bone
mass, muscle atrophy, arrhythmia, cognitive disorders and loss of
sense of direction (1–5). Moreover, severe lumbago, especially low
back pain, will occur in astronauts during or after space missions,
which also increases the risk of protrusion of lumbar
intervertebral disc after flight (6,7). In
addition, the intervertebral disc degeneration is also found in
animals participating in the space flight test (8).
Lumbar disc degeneration is the leading cause of low
back pain. Intervertebral disc degeneration is a complex process,
and some scholars have proposed the concept of intervertebral disc
microenvironment in recent years: The interaction among
intervertebral disc cells, extracellular matrix and biomechanics
maintain the balance of intervertebral disc microenvironment.
Intervertebral disc degeneration is the result of imbalance of
intervertebral disc microenvironment, in which the intervertebral
disc cell senescence may be one of the most important initiators
(9).
Studies have found that the cell senescence process
is mainly realized through activating the p53-p21-Rb pathway and
p16-Rb pathway (10,11). p53 is the first tumor suppressor gene
discovered, which is involved in a variety of cell biological
processes, including cell proliferation, senescence and death
(12,13). It was found that p53 has
significantly high expression in degenerative intervertebral disc
tissues (14). p16 gene is another
tumor suppressor gene involved in the regulation of cell cycle and
negative regulation of cell proliferation and division. Studies
have demonstrated that p16 is expressed in degenerative
intervertebral disc tissues, and the number of p16-positive cells
is positively correlated with the grade of intervertebral disc
degeneration (15). However, the
roles of p16 and p53 in microgravity-induced lumbar disc
degeneration remain unclear (16).
In the process of intervertebral disc degeneration
caused by senescence and biological stress, the expression levels
of pro-inflammatory factors, such as interleukin-1β (IL-1β), IL-6
and tumor necrosis factor-α (TNF-α), are significantly increased
(17), leading to severe
inflammatory response (18,19). In microgravity-induced lumbar disc
degeneration, the expression levels of these pro-inflammatory
factors deserve to be explored.
In this study, Sprague-Dawley (SD) rats were used
and randomly divided into control group and experimental group. The
degeneration model was established via simulated weightlessness
using the tail-suspension method, the messenger ribonucleic acid
(mRNA) levels of p53, p16, IL-1β, IL-6 and TNF-α were detected via
magnetic resonance imaging (MRI) examination, hematoxylin and eosin
(H&E) histopathological staining and reverse
transcription-polymerase chain reaction (RT-PCR), and p53 and p16
protein levels were detected via western blotting, so as to provide
an experimental basis for the preliminary discussion on
microgravity-induced intervertebral disc degeneration.
Materials and methods
Laboratory animals and grouping
A total of 24 healthy male specific pathogen-free
(SPF) SD rats weighing (300±30 g, 12 weeks old) were purchased from
Animal Core Facility of Nanjing Medical University. The mice were
kept in an SPF animal facility at the Laboratory of Nanjing Medical
University [health license no.: SYXK (Su) 2015-0009]. The rats were
caged separately in a room and the housing conditions wereconstant
temperature of 21°C and 12/12 h diurnal cycle. The 24 SD rats were
randomly divided into experimental group and control group of 12
rats each. In experimental group, the model was established via
simulated weightlessness using the tail-suspension method (tail
suspension for 8 weeks for each group), and rats were fed
separately. In control group, the tail was not suspended, and rats
were also fed separately. Six SD rats were randomly selected from
each group and anesthetized using 3% isoflurane gas mixed with air
in an air tight anesthesia chamber at 8 weeks. To simulate the
weightlessness, the tail of rats in experimental group was
suspended to get the posterior limbs off the ground according to
articles published previously (20).
A previous study has proven that simulated weightlessness
accelerates the intervertebral disc degeneration (21). In short, the tail was fixed with tape
first, and then linked to the top of cage through the chain. There
was a small sheave at the junction of the chain and the top of
cage, so that the rat could move freely along the chain. The
posterior limbs were approximately 0.5 cm above the ground with the
head downwards at 30°. Finally, all rats in experimental group were
euthanized under using 3% isoflurane gas mixed with air. This study
was approved by the Animal Ethics Committee of Shanghai General
Hospital of Nanjing Medical University Animal Center (Shanghai,
China).
Main reagents
Bicinchoninic acid (BCA) protein quantification kit
(C503051, Sangon Biotech, Shanghai, China), UNlQ-10 Column TRIzol
Total RNA Isolation kit (B511321, Sangon Biotech), M-MuLV One-step
RT-PCR kit (B532431, Sangon Biotech), GAPDH (D4C6R) mouse mAb (cat.
no. 97166, Cell Signaling Technology, Inc., Danvers, MA, USA), p53
(1C12) mouse mAb (cat. no. 2524, Cell Signaling Technology, Inc.),
anti-p16 (Ab-1) mouse mAb (DCS-50.1/H4) (cat. no. NA29-100UG,
Chemicon; EMD Millipore, Billerica, MA, USA) and anti-mouse IgG,
HRP-linked antibody (cat. no. 7076, Cell Signaling Technology,
Inc.).
MRI examination
Rats in experimental group and control group were
taken, anesthetized and fixed on the carton board. This was
followed by MRI examination. Grading evaluation was performed
according to the criteria of MRI Classification of Disc
Degeneration, Pfirrmann et al (22): Grade 1: there is partial loss and
multiple fractures in the nucleus pulposus, grade 2: The nucleus
pulposus cells are reduced, and the compensatory enlargement of
nucleus can be seen at the junction with the inner annulus
fibrosus, grade 3: The nucleus pulposus cells are significantly
reduced, and the cartilaginous metaplasia of nucleus pulposus cells
is obvious, and grade 4: Nucleus pulposus cells are rare, and more
interim cells can be seen.
H&E staining
The lumbar disc tissues in control group and
experimental group were taken, fixed in 10% formalin (20°C, 24 h),
dehydrated, embedded into paraffin and sliced into 5 µm-thick
sections, followed by H&E staining. Briefly, slides were washed
using distilled H2O and then stained with
Hematoxylin-Eosin staining kit (cat. no. E607318, Sangon Biotech)
(20°C, 5–10 min) according to the manufacturer's protocol. After
that, sections in both groups were sealed, and the pathological
differences in lumbar disc tissues between the two groups were
observed and photographed for analysis under an upright light
microscope (IX51 Olympus Corporation, Tokyo, Japan).
RT-PCR analysis
The lumbar disc tissues in control group and
experimental group were taken, added with 1 ml TRIzol, cut into
pieces and fully ground to homogenate. The samples were completely
lysed using Tissue Total Protein Lysis Buffer (cat. no. C500028,
Sangon Biotech) at room temperature for 5 min and centrifuged at
12,000 × g and 4°C for 5 min. Then the supernatant was taken
carefully, added with chloroform and mixed evenly, followed by
standing at room temperature for 5 min and centrifugation at 12,000
× g and 4°C for 15 min. The supernatant was carefully taken and
added with the same volume of isopropanol, followed by standing at
room temperature for 10 min and centrifugation at 12,000 × g and
4°C for 10 min. The precipitate was retained, added with 75%
ethanol and mixed evenly to wash the RNA precipitate. Finally,
RNase-free water was added to completely dissolve the precipitate.
The optical density (OD)260/OD280 ratio was
determined and the RNA concentration was detected. The stepwise
amplification was performed according to the instructions and the
primer sequence templates shown in Table
I, and the RT-PCR was performed for the reaction products.
 | Table I.RT-PCR primer sequences of IL-1β,
IL-6, TNF-α and β-actin mRNA. |
Table I.
RT-PCR primer sequences of IL-1β,
IL-6, TNF-α and β-actin mRNA.
| Gene name | Primer
sequence |
|---|
| IL-1β | 5′-3′:
CCCTGAACTCAACTGTGAAATAGCA |
|
| 3′-5′:
CCCAAGTCAAGGGCTTGGAA |
| IL-6 | 5′-3′:
ATTGTATGAACAGCGATGATGCAC |
|
| 3′-5′:
CCAGGTAGAAACGGAACTCCAGA |
| TNF-α | 5′-3′:
TCAGTTCCATGGCCCAGAC |
|
| 3′-5′:
GTTGTCTTTGAGATCCATGCCATT |
| p53 | 5′-3′:
GGACGACAGGCAGACTTTC |
|
| 3′-5′:
CAAGGCCTCACAGCTCTC |
| p16 | 5′-3′:
CTCACCATGGATGATGATATCGC |
|
| 3′-5′:
AGGAATCCTTCTGACCCATGC |
| β-actin | 5′-3′:
GAGCCGGGAAATCGTGCGT |
|
| 3′-5′:
GGAAGGAAGGCTGGAAGATG |
Western blot analysis
The lumbar disc tissues in control group and
experimental group were taken and washed twice with ice cold normal
saline. According to instructions of the whole protein extraction
kit (cat. no. C500028, Sangon Biotech), tissues were added with
lysis solution and homogenized for 1 min using a tissue
homogenizer, followed by centrifugation at 12,000 × g and 4°C for
10 min. The supernatant was collected as the total protein of
tissues. The protein concentration was determined using the BCA
protein concentration assay kit (C503051, Sangon Biotech). Total
protein (20 µg) was separated via a 10% SDS-PAGE gel and
transferred to PVDF membranes (Sangon Biotech). The PVDF membranes
were blocked with 1% bovine serum albumin (BSA, cat. no. A604481,
Sangon Biotech) dissolved in TBST for 1 h at room temperature and
then incubated with primary antibodies for 1 h at room temperature
or overnight at 4°C. The primary antibodies used were as follows:
p53 (1C12) mouse mAb, anti-p16 (Ab-1) mouse mAb (DCS-50.1/H4) and
GAPDH (D4C6R) mouse mAb. After washing with TBST 3 times,
anti-mouse IgG, HRP-linked antibody as secondary antibody was added
and was detected using an EasyBlot ECL kit (C506668, Sangon
Biotech). Immunoreactivity of p53 or p16 was quantified using
ImageJ software (version 1.45) [National Institutes of Health
(NIH), Rockville, MD, USA].
Statistical analysis
The experimental data are expressed as mean ±
standard error of mean (mean ± SEM), and SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA) was used for the statistical analysis of
experimental results. The difference between two groups was
calculated by t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
MRI examination results
MRI examination was performed for rats in control
group and experimental group. Results revealed that the
intervertebral disc in control group was normal, while there was
significant intervertebral disc injury in experimental group
(Table II).
 | Table II.MRI results in control group and
experimental group. |
Table II.
MRI results in control group and
experimental group.
| Group | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|
| Control | 12 | 0 | 0 | 0 |
| Experimental | 0 | 1 | 3 | 8 |
H&E staining results
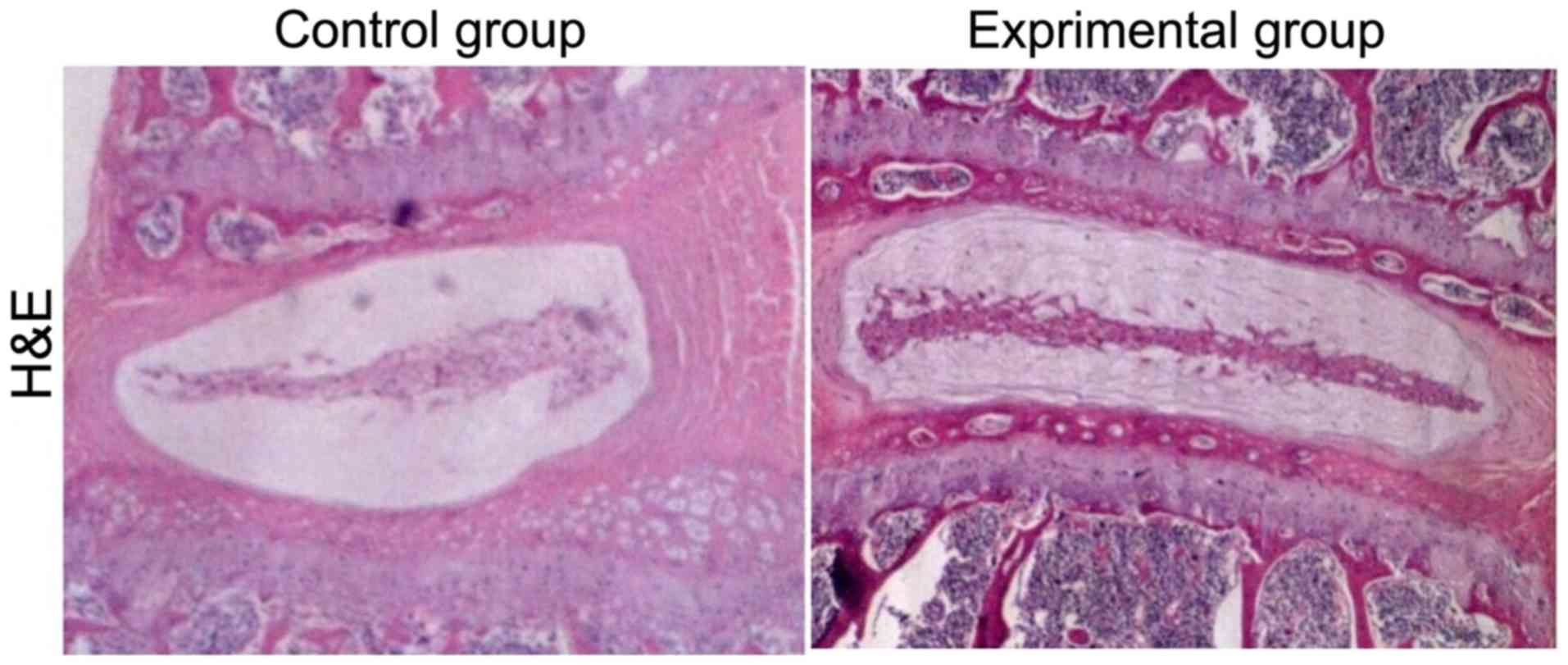
Compared with those in control group, the L4/5 and
L5/6 intervertebral discs had obvious signs of degeneration after
intervertebral disc injury with disc height loss, nucleus pulposus
structure disorder in annulus fibrosus and inflammatory cell
infiltration in experimental group (Fig.
1).
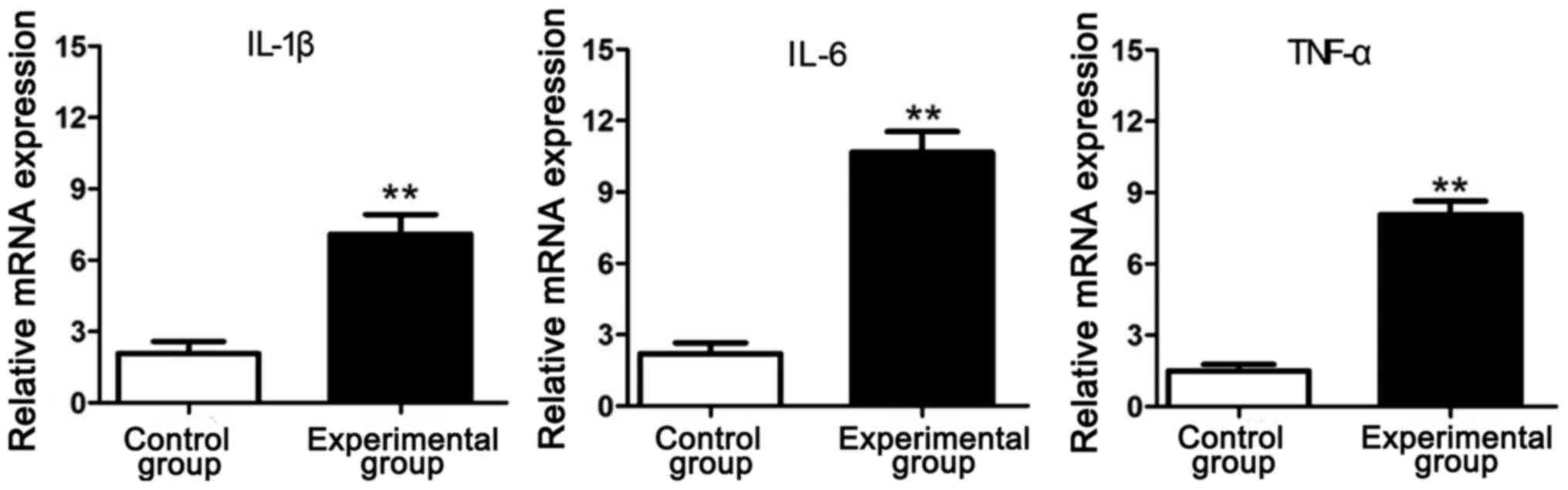
RT-PCR results of inflammatory
factors
The total RNA was extracted from the intervertebral
disc tissue samples in experimental group and control group and
detected via RT-PCR. Results showed that the mRNA expression levels
of inflammatory factors (IL-1β, IL-6 and TNF-α) in experimental
group were significantly increased compared with those in control
group, indicating that the lumbar disc degeneration of rats in
simulated weightlessness is closely related to inflammation
(Fig. 2).
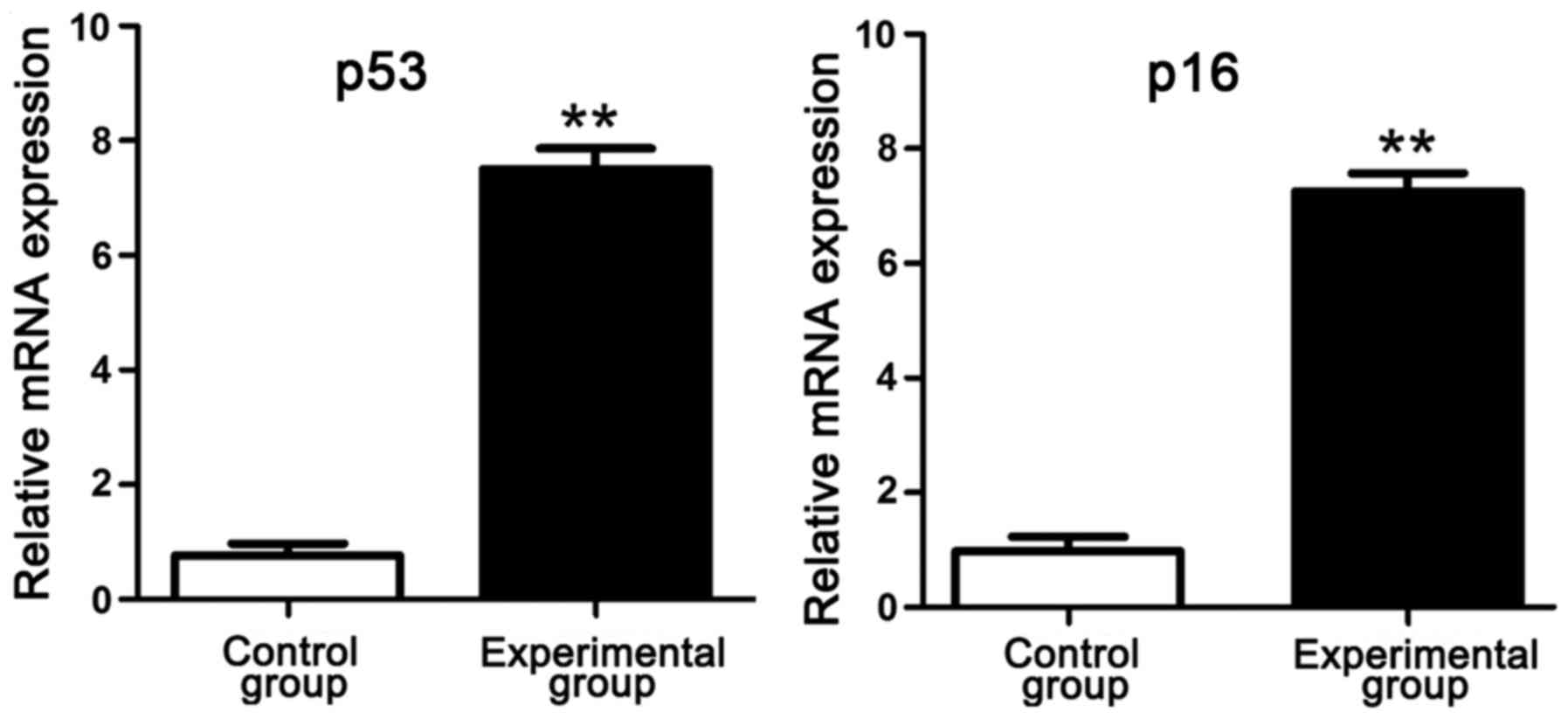
RT-PCR results of p53 and p16
mRNA
RT-PCR results manifested that compared with those
in control group, p53 and p16 mRNA levels in intervertebral disc
tissues in experimental group were obviously increased, suggesting
that p53 and p16 genes have an association with the degree of
lumbar disc degeneration of rats in simulated weightlessness
(Fig. 3).
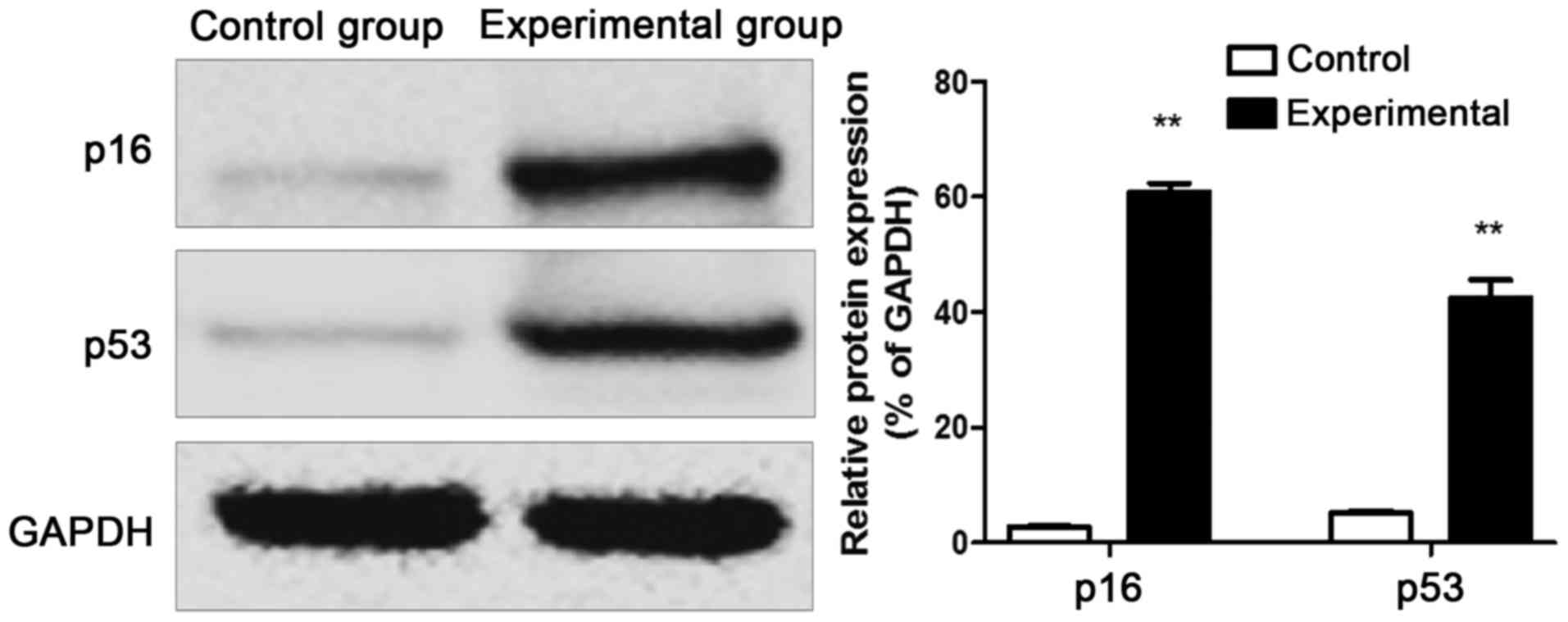
Western blot results of p53 and p16
proteins
The protein was extracted from the intervertebral
disc tissue samples of rats in control group and experimental
group, and western blotting results revealed that the p53 and p16
protein expression levels in intervertebral disc tissues of rats in
experimental group were remarkably increased compared with those in
control group (Fig. 4).
Discussion
It is reported that lumbago occurs in more than 50%
of astronauts during or after space missions (8,23).
Although there is no definite evidence proving that intervertebral
disc degeneration is the cause of lumbago in astronauts currently,
it is a major cause of low back pain. On the contrary to the
sustained stable pressure on intervertebral disc under the standard
gravity on the earth, the compressive force against intervertebral
disc under weightless environment in the space flight significantly
declines. In addition, the decline in the hydrostatic pressure on
intervertebral disc cells under the weightless condition is
considered as the core link in the pathophysiology of
intervertebral disc degeneration of astronauts (24). The latest research shows that
intervertebral disc degeneration is a constantly developing vicious
circle. The homeostasis of intervertebral disc microenvironment is
the result of interaction among intervertebral disc cells,
extracellular matrix and biomechanics in intervertebral disc. If
such a homeostasis is broken, intervertebral disc cells will stop
secreting proteoglycan and extracellular matrix components will be
changed. Due to the water-gathering effect of proteoglycan, the
hydrostatic pressure in intervertebral disc will be reduced, while
the shear force will be increased. Such biomechanical changes will
further affect intervertebral disc cells, so that they will secrete
less proteoglycan. Finally, the vicious circle is formed, and
intervertebral disc degeneration will develop constantly (9). It can be seen that the decline in
hydrostatic pressure in the intervertebral disc affects the
extracellular matrix through affecting intervertebral disc cells
under the weightless condition, ultimately breaking the homeostasis
of intervertebral disc microenvironment and accelerating the
intervertebral disc degeneration. The above results are consistent
with those in this experiment that the number and grade of
intervertebral disc degeneration in experimental group were
significantly larger and higher than those in control group.
Intervertebral disc degeneration has been studied
using different animal models in a large number of literature in
recent years. Jin et al reported that the culture of
intervertebral disc tissues in vitro under simulated
weightlessness accelerates the intervertebral disc degeneration
(21). However, it is argued that
the microenvironment of intervertebral disc in vitro has
been destroyed, and whether intervertebral disc degeneration is
caused by simulated weightlessness cannot be determined. Therefore,
the live rats were fed under simulated weightlessness to study the
pathophysiological process of intervertebral disc degeneration in
this experiment.
It was found in this study that the p53 and p16 mRNA
levels in intervertebral disc tissues of rats in experimental group
were significantly increased, and the p53 and p16 protein
expression levels were also obviously increased. The molecular
mechanism of cell senescence in intervertebral disc degeneration
includes two parts. The p53-p21-Rb pathway and p16-Rb pathway play
important roles in cell cycle arrest. In telomere damage and DNA
damage response, the p53-p21-Rb pathway is generally activated,
leading to replicative senescence (13,25).
p53, as a tumor suppressor gene, is involved in various cell
biological processes, including cell proliferation, senescence and
death. p53 responds to telomere shortening or DNA damage, and
initiates the irreversible cell cycle arrest (26,27).
Some experiments reveal that the expression levels of p53, p21 and
Rb in senescent nucleus pulposus cells in degenerative nucleus
pulposus tissue samples are increased accompanied by telomere
shortening and reduced telomerase activity (28). With the extension of culture cycle of
nucleus pulposus cells, the p53-p21-Rb pathway is activated in
human nucleus pulposus cells, leading to the replicative senescence
of intervertebral disc cells (29).
At the same time, the p53 expression is significantly up-regulated
in senescent annulus fibrosus cells in degenerative intervertebral
disc tissue samples (14). According
to the latest research, the ATMChk2-p53-p21-Rb pathway is activated
in the oxidative stress of human nucleus pulposus cells. Unlike the
p53-dependent pathway, the non-p53-dependent pathway, p16 pathway,
is generally activated by various stimuli, especially oxidative
stress, which leads to stress-induced premature senescence (SIPS).
p16, as an inhibitor of CDK4 and CDK6, blocks the cell cycle
progression when activated by oxidative stress (30,31).
Previous studies have found that the p16 gene expression is
increased in senescent nucleus pulposus cells in degenerative
intervertebral disc tissues (15,28,32), and
the number of p16-positive cells is correlated with the grade of
intervertebral disc degeneration (15). The p16-Rb pathway is also activated
in intervertebral disc cells with the extension of culture cycle,
which is consistent with the p53-p21-Rb pathway (29). It has been found in recent studies
that the p16-Rb pathway mediates the high glucose-induced
senescence of intervertebral disc cells. The aggregation of high
glucose produces excessive reactive oxygen species through damaging
the mitochondria, so that the p16-Rb pathway is activated and
induces SIPS in intervertebral disc cells (33–35). In
summary, the senescence of intervertebral disc cells is mainly
mediated by the p53-p21-Rb pathway and p16-Rb pathway.
In this study, RT-PCR results showed that the mRNA
levels of inflammatory factors (IL-1β, IL-6 and TNF-α) in
experimental group were significantly increased compared with those
in control group. According to previous studies, senescent nucleus
pulposus cells may change the secretion mode to alter the
intervertebral disc microenvironment (13,36). In
the intervertebral disc, these cells reduce the production of
extracellular matrix and enhance the degradation of extracellular
matrix. In addition, senescent intervertebral disc cells secrete
pro-inflammatory factors (IL-1β, IL-6 and TNF-α), all of which may
accelerate the senescence of adjacent intervertebral disc cells and
promote the autoimmune cell infiltration, thereby strengthening the
inflammatory response of nucleus pulposus cells in degenerative
intervertebral disc (17,25,36,37). In
recent years, increasingly more studies have demonstrated that the
secretion level of cytokines in the body can be significantly
changed in simulated weightlessness, and cytokines exert an
efficient regulatory effect in local tissues mainly in autocrine
and paracrine manner (38,39). Jin et al found using H&E
staining that degeneration occurs in intervertebral disc tissues of
rats cultured under simulated weightlessness, and the expression of
MMP-3 and apoptosis of nucleus pulposus cells are increased
(21).
In this study, 24 male SD rats were selected and
randomly divided into control group and experimental group. The
model of lumbar disc degeneration was established via simulated
weightlessness in experimental group. MRI examination was performed
for rats in control group and experimental group to observe the
lumbar intervertebral disc. MRI results showed that the lumbar
intervertebral disc of rats in control group was normal, while
there was significant intervertebral disc injury in experimental
group. The intervertebral disc was detected and analyzed in both
groups via H&E histopathological staining, and it was found
that there were obvious signs of degeneration in the intervertebral
disc in the experimental group. Moreover, the expression levels of
inflammatory factors in both groups were detected via RT-PCR, and
results manifested that the expression levels of IL-1β, IL-6 and
TNF-α in experimental group were significantly increased compared
with those in control group. Besides, RT-PCR and western blotting
proved that both p53 and p16 mRNA and protein expression levels in
experimental group were obviously increased. To sum up, the
abnormal expression levels of p53 and p16 genes are closely related
to the lumbar disc degeneration in rats in simulated
weightlessness, which may lead to the high expression levels of
inflammatory factors. This experiment provides a live model for
further study on the mechanism of intervertebral disc cell
senescence in intervertebral disc degeneration, and lays a
foundation for further study on human intervertebral disc
degeneration. In the future, more experiments are needed to deeply
study the mechanism of cell senescence in intervertebral disc
degeneration.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YL wrote the manuscript. YL and LC were responsible
for PCR. JL and ZS analysed and interpreted MRI result. HL and DW
contributed to western blot analysis. CL and JT helped with HE
staining and statistical analysis. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Animal Ethics
Committee of Shanghai General Hospital of Nanjing Medical
University Animal Center (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
|
1
|
Morey ER and Baylink DJ: Inhibition of
bone formation during space flight. Science. 201:1138–1141. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vico L, Collet P, Guignandon A,
Lafage-Proust MH, Thomas T, Rehaillia M and Alexandre C: Effects of
long-term microgravity exposure on cancellous and cortical
weight-bearing bones of cosmonauts. Lancet. 355:1607–1611. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hargens AR and Watenpaugh DE:
Cardiovascular adaptation to spaceflight. Med Sci Sports Exerc.
28:977–982. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vico L, Novikov VE, Very JM, Chappard D
and Alexandre C: Effects of a 40 day tail-suspension on rat
weight-bearing bones. Physiologist. 33 Suppl 1:S96–S97.
1990.PubMed/NCBI
|
|
5
|
Globus RK, Bikle DD, Halloran B and
Morey-Holton E: Skeletal response to dietary calcium in a rat model
simulating weightlessness. J Bone Miner Res. 1:191–197. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sayson JV and Hargens AR: Pathophysiology
of low back pain during exposure to microgravity. Aviat Space
Environ Med. 79:365–373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Johnston SL, Campbell MR, Scheuring R and
Feiveson AH: Risk of herniated nucleus pulposus among U.S.
astronauts. Aviat Space Environ Med. 81:566–574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Földes I, Kern M, Szilágyi T and Oganov
VS: Histology and histochemistry of intervertebral discs of rats
participated in spaceflight. Acta Biol Hung. 47:145–156.
1996.PubMed/NCBI
|
|
9
|
Vergroesen PP, Kingma I, Emanuel KS,
Hoogendoorn RJ, Welting TJ, van Royen BJ, van Dieën JH and Smit TH:
Mechanics and biology in intervertebral disc degeneration: A
vicious circle. Osteoarthritis Cartilage. 23:1057–1070. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Webley K, Bond JA, Jones CJ, Blaydes JP,
Craig A, Hupp T and Wynford-Thomas D: Posttranslational
modifications of p53 in replicative senescence overlapping but
distinct from those induced by DNA damage. Mol Cell Biol.
20:2803–2808. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmitt CA, Fridman JS, Yang M, Lee S,
Baranov E, Hoffman RM and Lowe SW: A senescence program controlled
by p53 and p16INK4a contributes to the outcome of cancer therapy.
Cell. 109:335–346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Toussaint O, Medrano EE and von Zglinicki
T: Cellular and molecular mechanisms of stress-induced premature
senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp
Gerontol. 35:927–945. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ben-Porath I and Weinberg RA: The signals
and pathways activating cellular senescence. Int J Biochem Cell
Biol. 37:961–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gruber HE, Watts JA, Hoelscher GL, Bethea
SF, Ingram JA, Zinchenko NS and Hanley EN Jr: Mitochondrial gene
expression in the human annulus: In vivo data from annulus cells
and selectively harvested senescent annulus cells. Spine J.
11:782–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Le Maitre CL, Freemont AJ and Hoyland JA:
Accelerated cellular senescence in degenerate intervertebral discs:
A possible role in the pathogenesis of intervertebral disc
degeneration. Arthritis Res Ther. 9:R452007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gruber HE, Ingram JA, Norton HJ and Hanley
EN Jr: Senescence in cells of the aging and degenerating
intervertebral disc: Immunolocalization of senescence-associated
beta-galactosidase in human and sand rat discs. Spine. 32:321–327.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Risbud MV and Shapiro IM: Role of
cytokines in intervertebral disc degeneration: Pain and disc
content. Nat Rev Rheumatol. 10:44–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Acosta JC, O'loghlen A, Banito A, Guijarro
MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N,
et al: Chemokine signaling via the CXCR2 receptor reinforces
senescence. Cell. 133:1006–1018. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Acosta JC, Banito A, Wuestefeld T,
Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka
F, Andrulis M, et al: A complex secretory program orchestrated by
the inflammasome controls paracrine senescence. Nat Cell Biol.
15:978–990. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang R, Ran HH, Cai LL, Zhu L, Sun JF,
Peng L, Liu XJ, Zhang LN, Fang Z, Fan YY, et al: Simulated
microgravity-induced mitochondrial dysfunction in rat cerebral
arteries. FASEB J. 28:2715–2724. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin L, Feng G, Reames DL, Shimer AL, Shen
FH and Li X: The effects of simulated microgravity on
intervertebral disc degeneration. Spine J. 13:235–242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pfirrmann CW, Metzdorf A, Zanetti M,
Hodler J and Boos N: Magnetic resonance classification of lumbar
intervertebral disc degeneration. Spine (Phila Pa 1976).
26:1873–1878. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wing PC, Tsang IK, Susak L, Gagnon F,
Gagnon R and Potts JE: Back pain and spinal changes in
microgravity. Orthop Clin North Am. 22:255–262. 1991.PubMed/NCBI
|
|
24
|
Hutton WC, Elmer WA, Boden SD, Hyon S,
Toribatake Y, Tomita K and Hair GA: The effect of hydrostatic
pressure on intervertebral disc metabolism. Spine. 24:1507–1515.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Muller M: Cellular senescence: Molecular
mechanisms, in vivo significance, and redox considerations.
Antioxid Redox Signal. 11:59–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gire V, Roux P, Wynford-Thomas D,
Brondello JM and Dulic V: DNA damage checkpoint kinase Chk2
triggers replicative senescence. EMBO J. 23:2554–2563. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Herbig U, Jobling WA, Chen BP, Chen DJ and
Sedivy JM: Telomere shortening triggers senescence of human cells
through a pathway involving ATM, p53, and p21(CIP1), but not
p16(INK4a). Mol Cell. 14:501–513. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim KW, Chung HN, Ha KY, Lee JS and Kim
YY: Senescence mechanisms of nucleus pulposus chondrocytes in human
intervertebral discs. Spine J. 9:658–666. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jeong SW, Lee JS and Kim KW: In vitro
lifespan and senescence mechanisms of human nucleus pulposus
chondrocytes. Spine J. 14:499–504. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Itahana K, Zou Y, Itahana Y, Martinez JL,
Beausejour C, Jacobs JJ, Van Lohuizen M, Band V, Campisi J and
Dimri GP: Control of the replicative life span of human fibroblasts
by p16 and the polycomb protein Bmi-1. Mol Cell Biol. 23:389–401.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ressler S, Bartkova J, Niederegger H,
Bartek J, Scharffetter-Kochanek K, Jansen-Dürr P and Wlaschek M:
p16INK4A is a robust in vivo biomarker of cellular aging in human
skin. Aging Cell. 5:379–389. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Heathfield SK, Le Maitre CL and Hoyland
JA: Caveolin-1 expression and stress-induced premature senescence
in human intervertebral disc degeneration. Arthritis Res Ther.
10:R872008. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park JS, Park JB, Park IJ and Park EY:
Accelerated premature stress-induced senescence of young annulus
fibrosus cells of rats by high glucose-induced oxidative stress.
Int Orthop. 38:1311–1320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kong JG, Park JB, Lee D and Park EY:
Effect of high glucose on stress-induced senescence of nucleus
pulposus cells of adult rats. Asian Spine J. 9:155–161. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park JB, Byun CH and Park EY: Rat
notochordal cells undergo premature stress-induced senescence by
high glucose. Asian Spine J. 9:495–502. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
van Deursen JM: The role of senescent
cells in ageing. Nature. 509:439–446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Muñoz-Espín D and Serrano M: Cellular
senescence: From physiology to pathology. Nat Rev Mol Cell Biol.
15:482–496. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han C, Jiang C, Yu C and Shen H:
Differentiation of transforming growth factor β1-induced
mesenchymal stem cells into nucleus pulposus-like cells under
simulated microgravity conditions. Cell Mol Biol (Noisy-le-grand).
61:50–55. 2015.PubMed/NCBI
|
|
39
|
Luo W, Xiong W, Qiu M, Lv Y, Li Y and Li
F: Differentiation of mesenchymal stem cells towards a nucleus
pulposus-like phenotype utilizing simulated microgravity In vitro.
J Huazhong Univ Sci Technolog Med Sci. 31:1992011. View Article : Google Scholar : PubMed/NCBI
|