Introduction
Cardiac failure, a complex clinical syndrome,
represents the serious stage of several heart diseases (1). It has a high incidence, with a 5-year
mortality rate of >50% (1). The
incidence of cardiac failure has continued to increase in recent
years, posing a serious threat to human health in the 21st century.
As myocardial infarction is a serious threat to human health
(2), how to effectively reduce the
cell apoptosis caused by myocardial injury and protect cardiac
function remains an important question in the clinical
cardiovascular field (2). Myocardial
infarction leads to markedly increased myocardial ischemia hypoxia,
inflammation, oxidative stress, and myocardial cell necrosis and
apoptosis (3). As a result, it
causes myocardial collagen hyperplasia, alternative fibrosis and
cardiac function disorder (3).
Cell apoptosis occupies an important position in the
process of the pathological evolution of myocardial ischemia injury
(4). In early acute ischemia,
apoptosis is the main form of myocardial infarction (5). It is associated with the entire
pathological evolution process of myocardial injury. Inhibiting
myocardial cell apoptosis following acute heart ischemia, and
increasing the quantity of surviving myocardial cells is
undoubtedly conducive to the prevention and treatment of myocardial
ischemia (6). In addition, it can
promote the recovery of cardiac function, and the long-term
prognosis of patients (5).
The phosphoinositide 3-kinase (PI3K)/akt pathway is
an important signal transduction pathway in organisms. It has been
confirmed to exert important biological functions in activities
including cell survival, apoptosis and proliferation (7). PI3K can phosphorylate the 3′ hydrogen
base on the inositol ring to produce phosphatidylinositol 3,4,5
trisphosphate (PIP3), which can be activated following the
phosphorylation of AKT as a secondary messenger (8). The phosphorylated AKT can activate
endothelial nitric oxide synthase, glycogen synthase kinase-3β and
heat shock protein. Therefore, it can exert myocardial protective
effects (9). It has been verified in
experiments in vivo and in vitro that the PI3K/AKT
signal transduction pathway is important in protecting against
myocardial ischemia reperfusion injury (9). Ischemic preconditioning or active drug
stimulation can activate the PI3K/AKT signaling pathway in
myocardial cells. The activation of such a signal pathway has a
decisive role in the protective mechanism of myocardial ischemia
reperfusion injury (10).
As an important nuclear transcription factor,
nuclear factor (NF)-κB is a transcription factor for fast response
nucleated cells commonly distributed in the cytoplasm. It locates
at the hub location of the Toll-like receptor downstream signal
pathway. In addition, it can regulate the cascade reaction between
immunity and inflammation associated factors and inflammatory
transmitters. Therefore, it can synthesize and release inflammatory
cytokines, cyclooyxgenase-2, inducible nitric oxide synthase,
chemotaxis granulocytes and macrophages. Subsequently, it can
increase capillary permeability, induce lymphocyte infiltration,
and complete the transmission of inflammatory signals. In addition,
it can exert the early immune response effects and is pivotal in
inflammatory and immune responses. Myocardial ischemia and hypoxia
can induce myocardial inflammation, whereas the phosphorylation and
degradation of NF-κB subunit inhibitor of NF-κB (IκB) leads to the
nuclear translocation of NF-κB. This accounts for the initiation
mechanism of the genesis and development of acute inflammation.
NF-κB is a type of transcription factor with a multi-directional
regulatory effect. It can regulate the expression of multiple
inflammatory cytokines, therefore, it is closely associated with
the inflammatory response (11).
Among the numerous signal transduction pathways, the
phosphatase and tensin homolog (PTEN) pathway is closely correlated
with PI3K/AKT (12). PI3K/AKT can be
gradually phosphorylated through activating the enzyme system
(13). Therefore, it can activate
pathway regulatory cytokines, including vascular endothelial growth
factors. PTEN is the tumor suppressor gene, phosphatase and tensin
homolog deleted on chromosome 10, and is the negative regulatory
factor of the PI3K/AKT pathway (13). PTEN can suppress activation of the
PI3K/AKT pathway and catalyze the dephosphorylation of PIP3. Thus,
it can antagonize the activity of the PI3K/AKT pathway (14). Therefore, PTEN is important in
regulating embryonic development, cell growth, differentiation,
apoptosis and migration (14).
Ramirez-Moya et al showed that microRNA-146b promoted
PI3K/AKT pathway hyperactivation and thyroid cancer progression by
targeting PTEN (15). Hendgen-Cotta
et al (16) showed that
microRNA-146b was inhibited in acute myocardial
ischemia/reperfusion injury in vivo. In the present study,
the function of microRNA-146b in myocardial infarction and the
underlying mechanism were evaluated.
Materials and methods
Animals and acute myocardial I/R (AMI)
injury
Sprague-Dawley rats (male; 5–6 weeks; 160–200 g)
were housed at 22–23°C and a humidity of 55–60%, with a 12 h
light/dark cycle and free access to food and water. All rats were
obtained from the Experiment Animal Center of Shandong University
(Shandong, China) and were divided into two groups randomly:
Control (n=6) and AMI (n=6) groups. The rats were anesthetized by
intraperitoneal injection of 35 mg/kg pentobarbital sodium.
Thoracotomy was performed and the left anterior descending coronary
artery was ligated 2–3 mm away for 30 min. All experimental
manipulations were undertaken in accordance with the Guide for the
Care and Use of Laboratory Animals by the National Institutes of
Health, with the approval of the Animal Experimental Ethics
Committee of Jining No. 1 People's Hospital (Jining, China).
Hematoxylin and eosin (HE) staining
assay
Following induction for 30 min, the rats were
anesthetized by intraperitoneal injection of 35 mg/kg pentobarbital
sodium and sacrificed via decollation. Heart samples were acquired
and fixed with 4% paraformaldehyde for 24 h. The heart samples were
then embedded in plastic and sectioned at 10-µM. The samples were
stained with an HE assay for 15 min and observed using a confocal
microscope (magnification, ×100; Leica Microsystems GmbH, Wetzlar,
Germany).
Reverse transcription-polymerase chain
reaction (RT-PCR) and quantitative PCR (qPCR) analysis
Total RNA was extracted from the transfected cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and total RNA was used for the synthesis of
first-strand cDNA using the PrimeScript RT reagent kit (Takara Bio,
Inc., Shiga, Japan). The RT-PCR analysis was performed using a 7500
Fast real-time PCR system and SYBR Premix Ex Taq kit (Takara Bio,
Inc.). The primers utilized were as follows: U6 forward,
5′GCTTCGGCAGCACATATACTAAAAT3′ and reverse,
5′CGCTTCACGAATTTGCGTGTCAT3′; miR-146b-5p forward,
5′-TGACCCATCCTGGGCCTCAA-3′ and reverse,
5′-CCAGTGGGCAAGATGTGGGCC-3′. The amplification conditions were as
follows: 95°C for 10 min, 40 cycles of denaturation at 95°C for 30
sec, followed by annealing and extension at 58°C for 10 sec, 72°C
for 10 sec. The 2−ΔΔCq method was used to calculate the
relative gene expression (17).
GeneChip miRNA array
Total RNA (500 ng) was isolated using Cyanine-5-CTP
and hybridized into the SurePrint G3 Mouse Whole Genome GE 8×_60 K
Microarray G4852A platform (Stratagene; Agilent Technologies, Inc.,
Santa Clara, CA, USA). The results were quantified using Agilent
Feature Extraction software (version A.10.7.3.1).
Cell culture and transfection
H9c2 cells were purchased from Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China) and
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a 95% air/5%
CO2 atmosphere. The H9c2 cells were incubated by
continuously flushing a chamber with 5% CO2 and 95% N2
for 3 h at 37°C. MicroRNA-146b mimics, si-microRNA-146b and
negative control mimics were transfected into cells; transfection
was performed with Lipofectamine™ 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). Following 4 h of transfection, VO-Ohpic
trihydrate (10 µM; PTEN inhibitor) was added to cells for 44 h.
Cell viability assay and Annexin
V/propidium iodide (PI) apoptosis assay
To the transfected cells, 20 µl of MTT assay was
added for 4 h at 37°C and DMSO was added to the cell and shaken at
37°C for 20 min. The cell viability was measured using a VERSAmax
microplate reader (Molecular Devices LLC; Sunnyvale, CA, USA) at
492 nm. The transfected cells were washed with PBS, and stained
with Annexin V-FITC and PI fluorescence (all 5 µl, BD Biosciences,
Franklin Lakes, NJ, USA) for 15 min in the dark. Apoptosis was
analyzed with a FACSCalibur flow cytometer (BD Biosciences).
ELISA
The transfected cells were lysed with modified
radioimmunoprecipitation assay (RIPA) buffer at 4°C for 30 min.
Protein concentration was determined using the BCA protein
determination kit (Beyotime Institute of Biotechnology, Shanghai,
China), and 10 µg was used to measure the levels of TNF-α, IL-1β,
IL-6 and IL-18 using ELISA kits. The absorbance was measured using
a VERSAmax microplate reader (Molecular Devices, LLC) at 405
nm.
Luciferase assay
The potential binding sites of miR146b in the
3′-untranslated region (UTR) of PTEN were determined using
TargetScan (http://www.targetscan.org/vert_71). The
pGL3-PTEN-3′-UTR and microRNA-146b and negative control mimics were
added to cells, with transfection performed with Lipofectamine™
2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Following transfection 48 h, the cells were analyzed with the
Dual-Luciferase Reporter Assay kit (Promega Corporation, Madison,
WI, USA).
Western blot analysis and caspase-3/8
activity assay
The transfected cells were lysed with modified RIPA
buffer at 4°C for 30 min. Protein concentration was determined
using the BCA protein determination kit (Beyotime Institute of
Biotechnology), and 30–50 µg of the protein samples were
electrophoresed on 8–12% SDS-PAGE gels and transferred onto PVDF
membranes (EMD Millipore, Billerica, MA, USA). Subsequently, 5%
milk was used to block the membranes in a shaker at 37°C for 1 h.
Antibodies for Bax (cat. no. sc-6236, 1:1,000), phosphorylated Akt
(cat. no. sc-7985-R, 1:1,000), PI3K (cat. no. sc-7174, 1:1,000),
NF-κB (p65, cat. no. sc-109, 1:1,000) and GAPDH (cat. no. sc-25778,
1:5,000) antibodies, all from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA) were used for incubation at 4°C overnight. The
membranes were then washed with TBST buffer and incubated with
horseradish peroxidase-labeled goat anti-rabbit IgG (cat. no.
sc-2004, 1:5,000, Santa Cruz Biotechnology) at 37°C for 1 h. The
protein bands were visualized by ECL (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
For the analysis of caspase activity, total protein
(3–5 µg) was incubated with caspase-3 and caspase-8 activity kits
(Beyotime Institute of Biotechnology) at 37°C for 1 h. Caspase-3/8
activity was measured using a VERSAmax microplate reader (Molecular
Devices, LLC) at 405 nm.
Statistical analysis
The data are presented as the mean ± standard
deviation using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). All data
were analyzed for significance using Student's t-test for two
groups or one-way analysis of variance with Tukey's post hoc test
(three groups). P<0.05 was considered to indicate a
statistically different difference.
Results
Expression of microRNA-146b in the AMI
rat
To determine whether the expression of microRNAs
regulates myocardial infarction in the AMI rat model, changes in
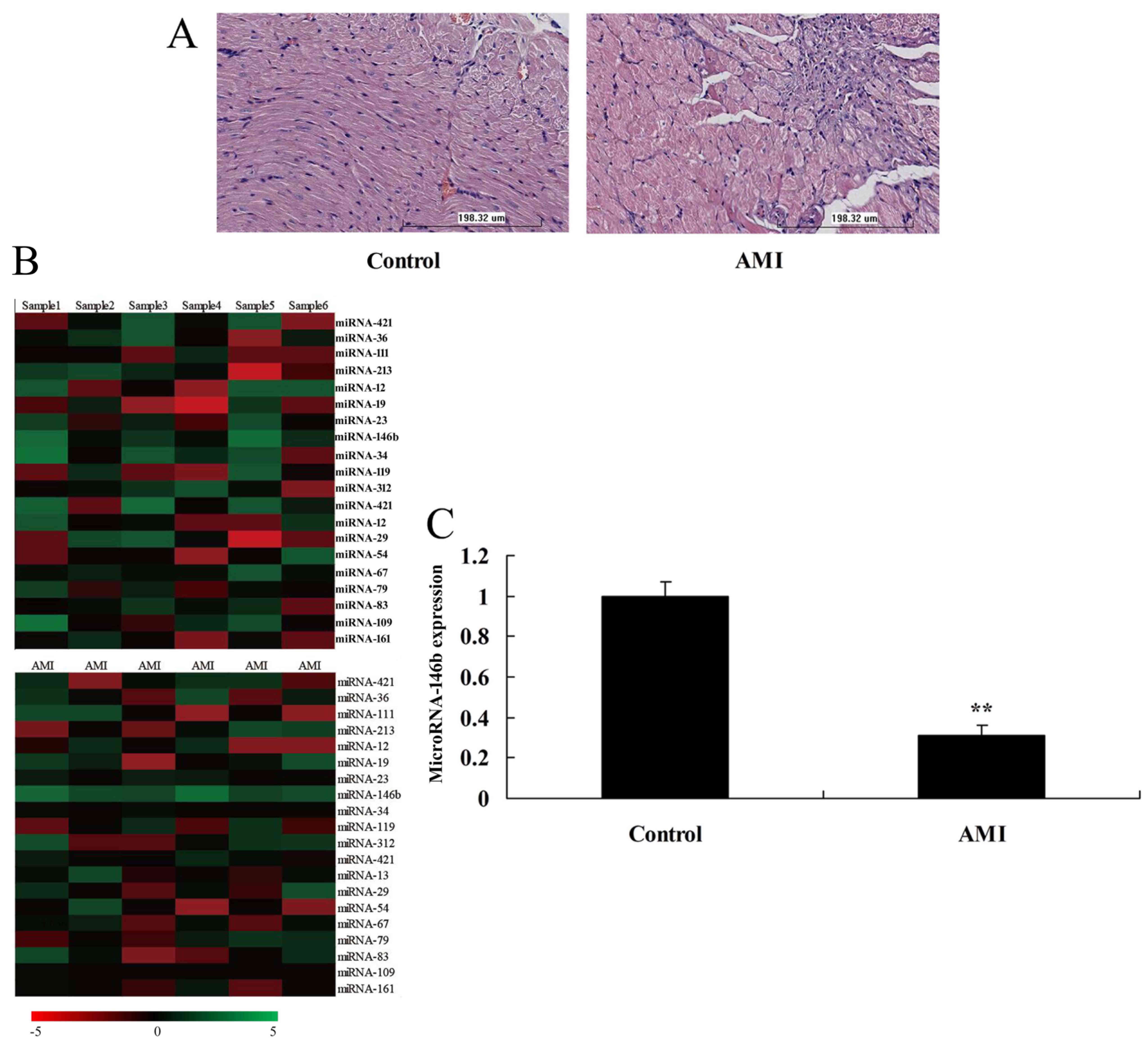
the expression of microRNAs were analyzed. The HE staining showed
myocardial damage in the AMI rat model, compared with the sham
control group (Fig. 1A). The
expression of microRNA-146b was downregulated in the myocardial
infarction rat, compared with that in the control group (Fig. 1B and C).
Downregulation of microRNA-146b
increases inflammatory factors and apoptosis in vitro
The function of microRNA-146b in myocardial
infarction was then examined using anti-microRNA-146b mimics to
decrease the expression of microRNA-146b in the in vitro
model. As shown in Fig. 2A, there
was significant inhibition of the expression of microRNA-146b in
the in vitro model in the anti-microRNA-146b mimics group,
compared with that in the negative control group. The
downregulation of microRNA-146b inhibited cell viability, induced
apoptosis, increased levels of inflammatory factors TNF-α, IL-1β,
IL-6 and IL-18, and increased apoptosis in the in vitro
model, compared with the negative control group (Fig. 2B-H).
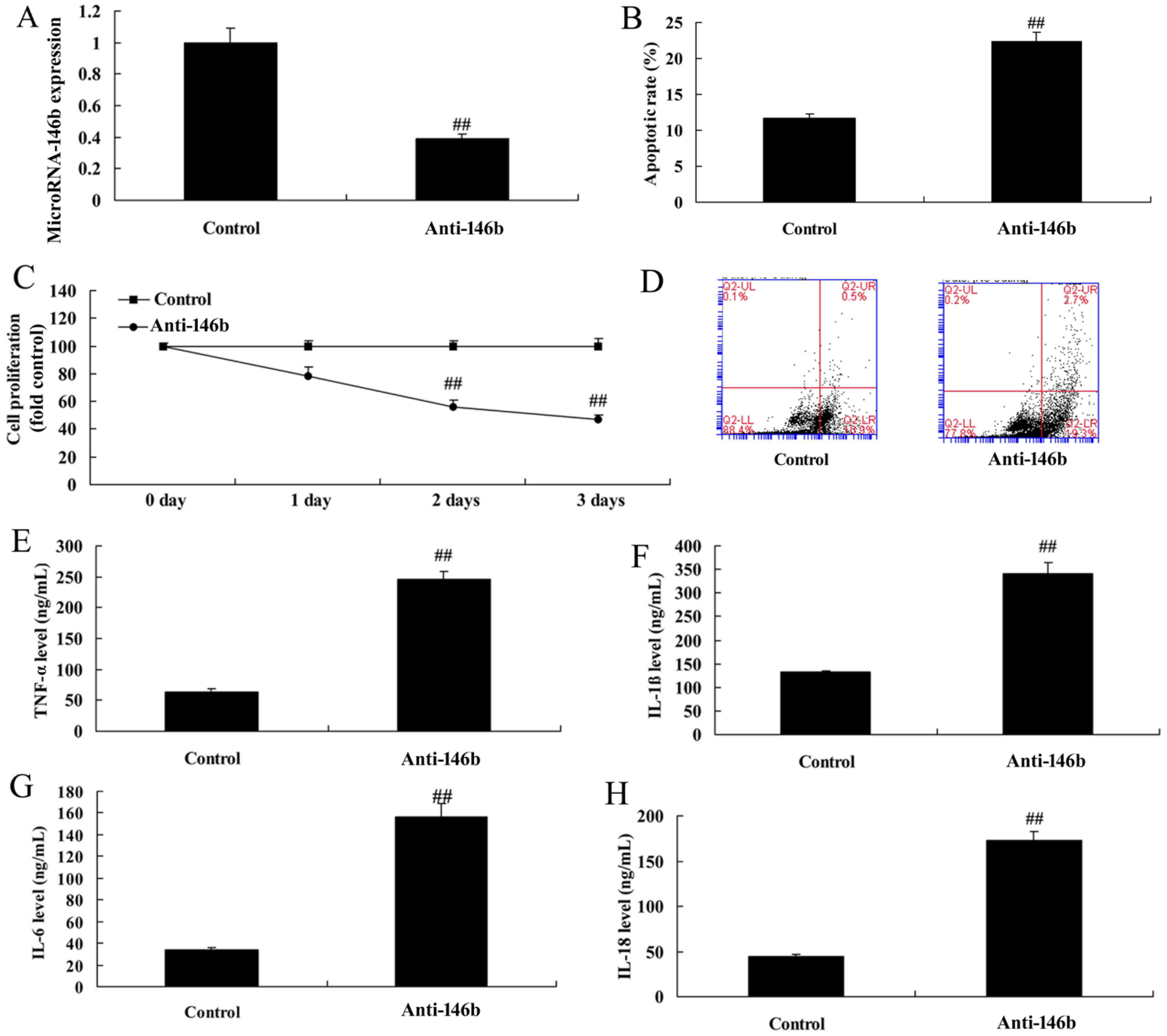 | Figure 2.Downregulation of microRNA-146b
increases inflammatory factors and apoptosis in an in vitro
model. (A) Expression of microRNA-146b, (B) vascular apoptotic
rate, (C) cell viability, and (D) vascular apoptosis, detected
using flow cytometry, of myocardial infarction. Levels of (E)
TNF-α, (F) IL-1β, (G) IL-6 and (H) IL-18 in the in vitro
model. ##P<0.01, vs. Control group. Control, negative
control group; Anti-146b, microRNA-146b downregulation group;
TNF-α, tumor necrosis factor-α; IL, interleukin. |
Upregulation of microRNA-146b
decreases inflammatory factors and apoptosis in the in vitro
model
There was a significant increase in the expression
of microRNA-146b in the in vitro model following
transfection with the microRNA-146b mimics, compared with the
negative control group (Fig. 3A).
The upregulation of microRNA-146b promoted cell viability, reduced
apoptosis, decreased levels of inflammatory factors TNF-α, IL-1β,
IL-6 and IL-18, and decreased apoptosis in the in vitro
model, compared with the negative control group (Fig. 3B-H).
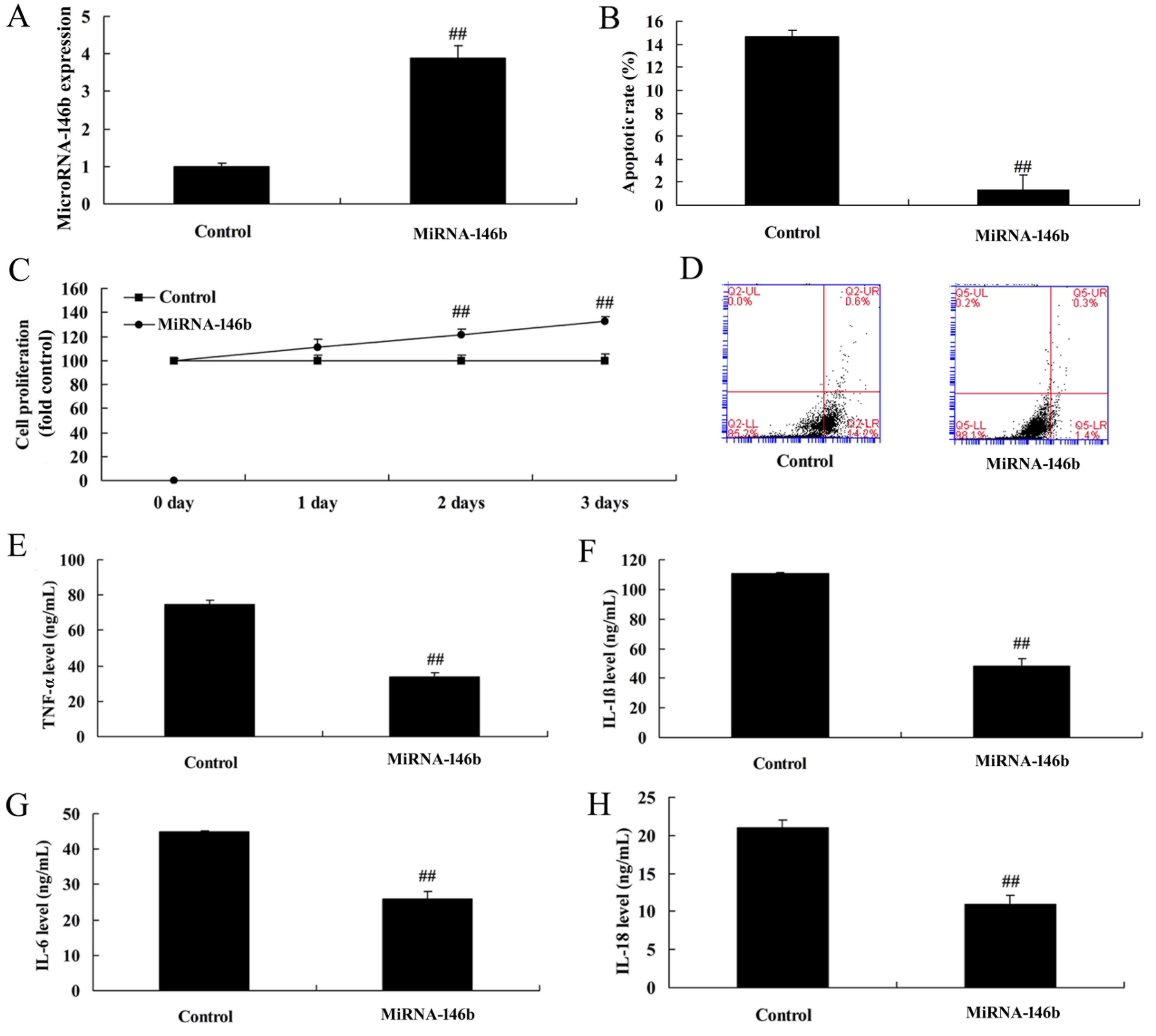 | Figure 3.Upregulation of miRNA-146b decreases
inflammatory factors and apoptosis in an in vitro model. (A)
Expression of miRNA-146b, (B) vascular apoptotic rate, (C), cell
viability, and (D) vascular apoptosis, detected using flow
cytometry, of myocardial infarction. Levels of (E) TNF-α, (F)
IL-1β, (G) IL-6 and (H) IL-18 in the in vitro model.
##P<0.01, vs. Control group. Control, control
negative group; miRNA-146b, microRNA-146b upregulation group;
TNF-α, tumor necrosis factor-α; IL, interleukin. |
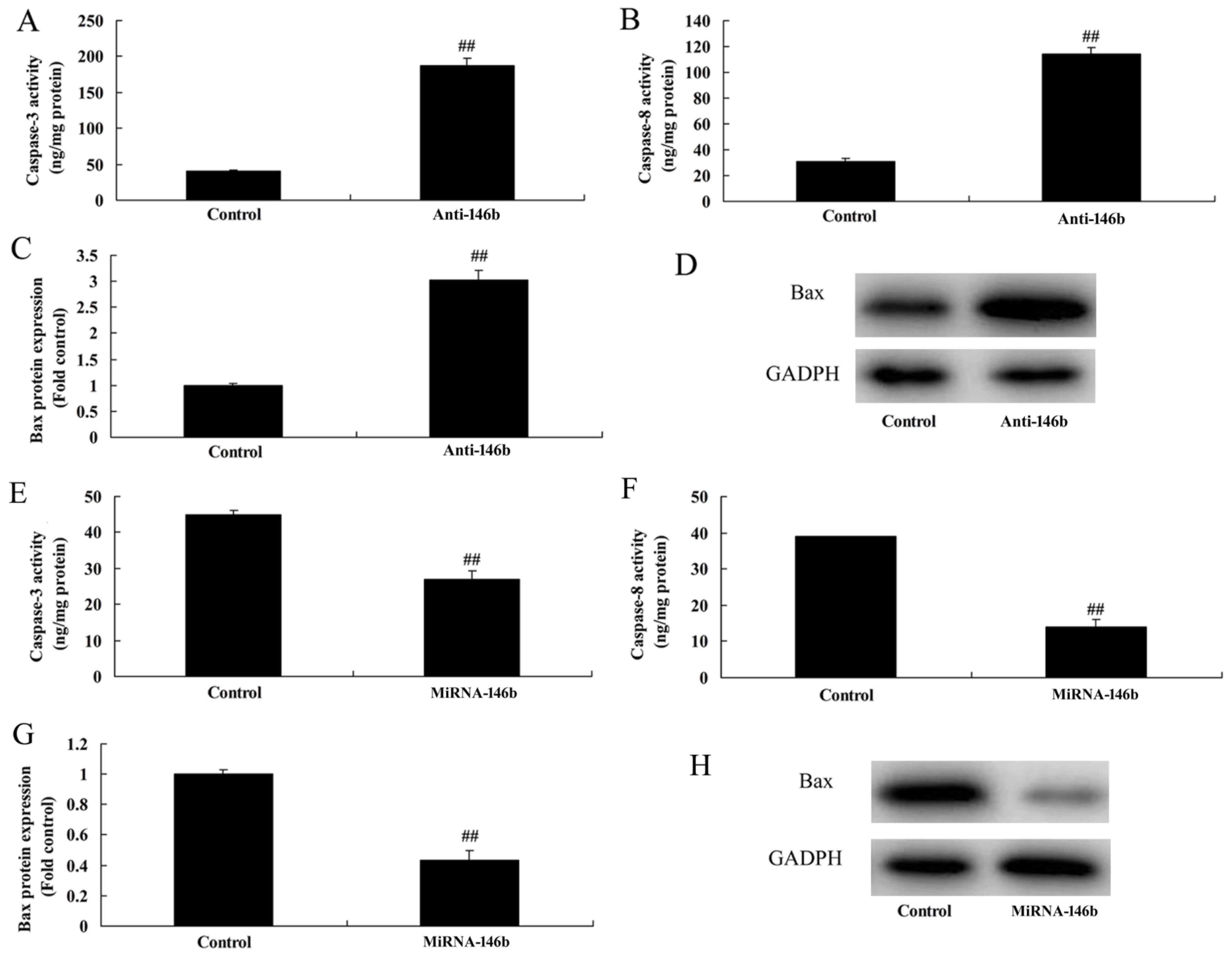
MicroRNA-146b regulates the
Bax/caspase-8/caspase-3 signaling pathway in vitro
The present study also assessed whether
microRNA-146b regulated the Bax/caspase-3/caspase-9 signaling
pathway in the in vitro model. As shown in Fig. 4A-D, the downregulation of
microRNA-146b significantly induced caspase-3/8 activity and the
protein expression of Bax in the in vitro model, compared
with the negative control group. The upregulation of microRNA-146b
significantly suppressed the protein expression of Bax and activity
of caspase-3/8 in the in vitro model, compared with levels
in the negative control group (Fig.
4E-H).
MicroRNA-146b regulates the PI3K/Akt/
NF-κB signaling pathway in vitro by PTEN
To evaluate the mechanism of microRNA-146b in
myocardial infarction, the potential binding sites of microRNA-146b
on the 3′-UTR of PTEN mRNA were examined (Fig. 5A). Luciferase activity levels were
reduced in the group overexpressing microRNA-146b, compared with
those in the negative group (Fig.
5B). The downregulation of microRNA-146b significantly induced
the protein expression of PTEN and NF-κB, and suppressed the
protein expression of PI3K and p-Akt in the in vitro model,
compared with the levels in the negative control group (Fig. 5C-G). The upregulation of
microRNA-146b significantly suppressed the protein expression of
PTEN and NF-κB, and induced the protein expression of PI3K and
p-Akt in the in vitro model, compared with the levels in the
negative control group (Fig.
5H-L).
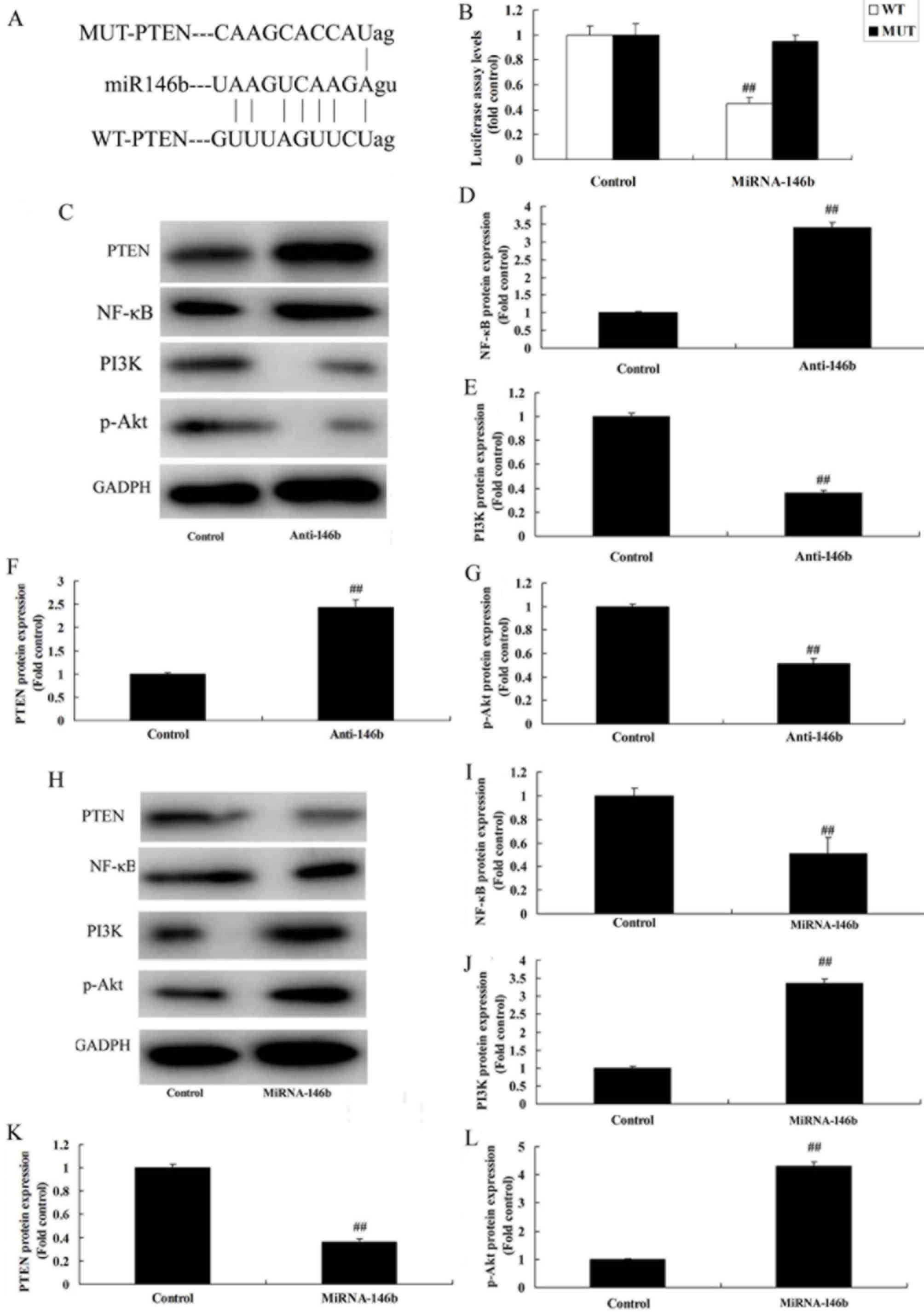 | Figure 5.miRNA-146b regulates the
PI3K/Akt/NF-κB signaling pathway in an in vitro model by
PTEN. (A) miRNA-146b potential binding sites on the 3′-untranslated
region of PTEN mRNA. (B) Luciferase activity levels. (C) Western
blot analysis of PTEN, PI3K, p-Akt and NF-κB, and statistical
analysis of (D) NF-κB, (E) PI3K, (F) PTEN and (G) p-Akt under
miRNA-146b downregulation. (H) Western blot analysis of PTEN, PI3K,
p-Akt and NF-κB, and statistical analysis of (I) NF-κB, (J) PI3K,
(K) PTEN and (L) p-Akt under miRNA-146b upregulation.
##P<0.01, vs. Control group. Control, negative
control group; Anti-146b, microRNA-146b downregulation group;
miRNA-146b, microRNA-146b upregulation group; PI3K,
phosphoinositide 3-kinase; NF-κB, nuclear factor-κB; PTEN,
phosphatase and tensin homolog; p-Akt, phosphorylated Akt. |
Inhibition of PTEN reduces the effect
of microRNA-146b in myocardial infarction
In order to determine whether the PI3K is involved
in the effect of microRNA-146b in myocardial infarction, the PTEN
inhibitor, VO-Ohpic trihydrate (10 µM), was added to cells
following microRNA-146b. As shown in Fig. 6, the PTEN inhibitor induced the
protein expression of PI3K and p-Akt, and suppressed the protein
expression of PTEN and NF-κB in the in vitro model following
microRNA-146b, compared with the microRNA-146b only group. The
inhibition of PTEN reduced the effect of microRNA-146b on the
promotion of myocardial cell viability, the suppression of
apoptosis, and inhibition of the levels of TNF-α, IL-1β, IL-6 and
IL-18 in the in vitro model following microRNA-146b,
compared with the microRNA-146b only group (Fig. 7A-G).
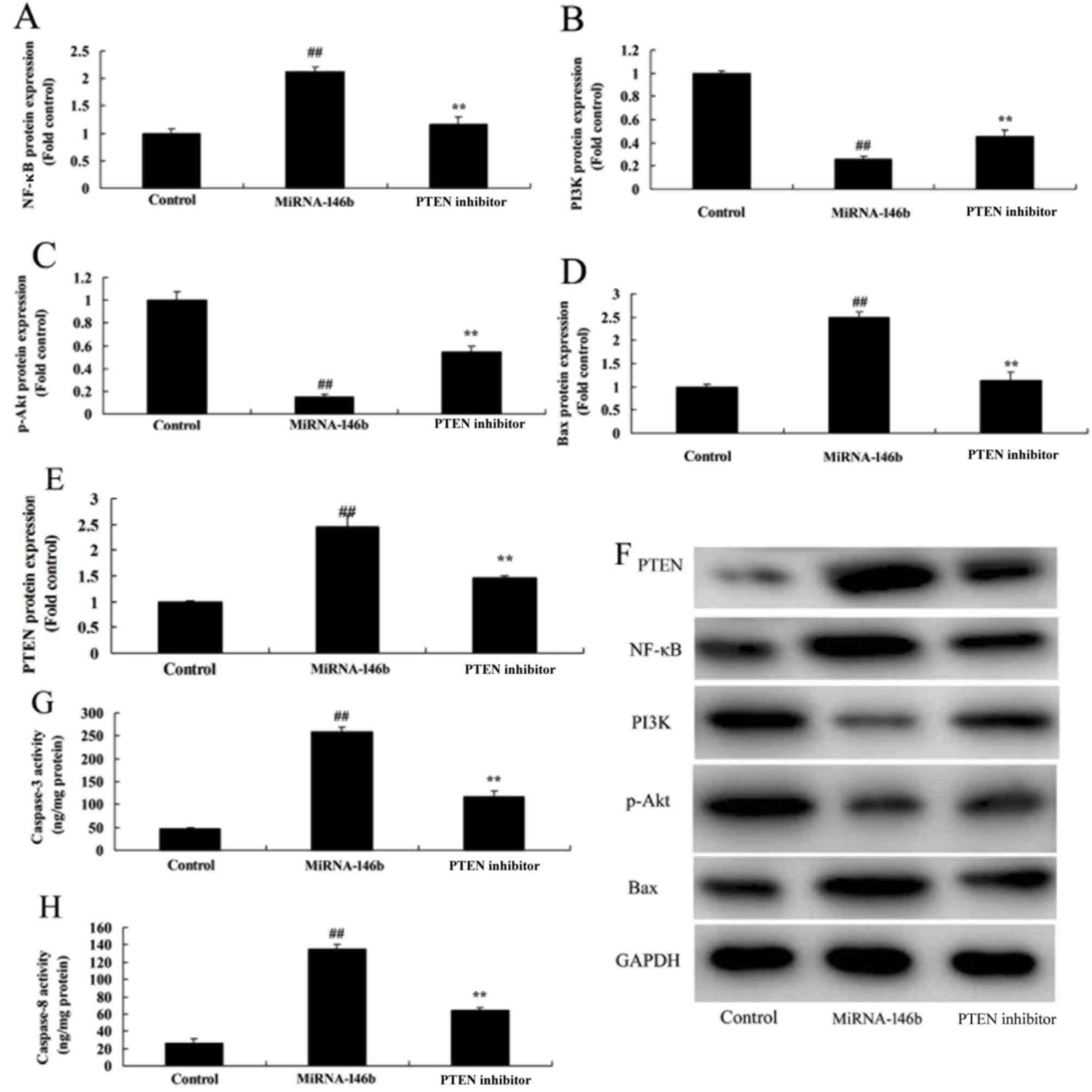 | Figure 6.Inhibition of PI3K reduces the effect
of miRNA-146b on the PI3K/Akt/NF-κB signaling pathway. Statistical
analysis of (A) NF-κB, (B) PI3K, (C) p-Akt, (D) Bax and (E) PTEN
from the (F) western blot results of PI3K, p-Akt, NF-κB, Bax and
PTEN. Activity of (G) Caspase-3 and (H) Caspase-8.
##P<0.01, vs. Control group; **P<0.01, vs.
miRNA-146b group. Control, negative control group; miRNA-146b,
microRNA-146b upregulation group; PTEN inhibitor, microRNA-146b
upregulation and PTEN inhibitor group; PI3K, phosphoinositide
3-kinase; NF-κB, nuclear factor-κB; Bax, B-cell lymphoma
2-associated X protein; PTEN, phosphatase and tensin homolog;
p-Akt, phosphorylated Akt. |
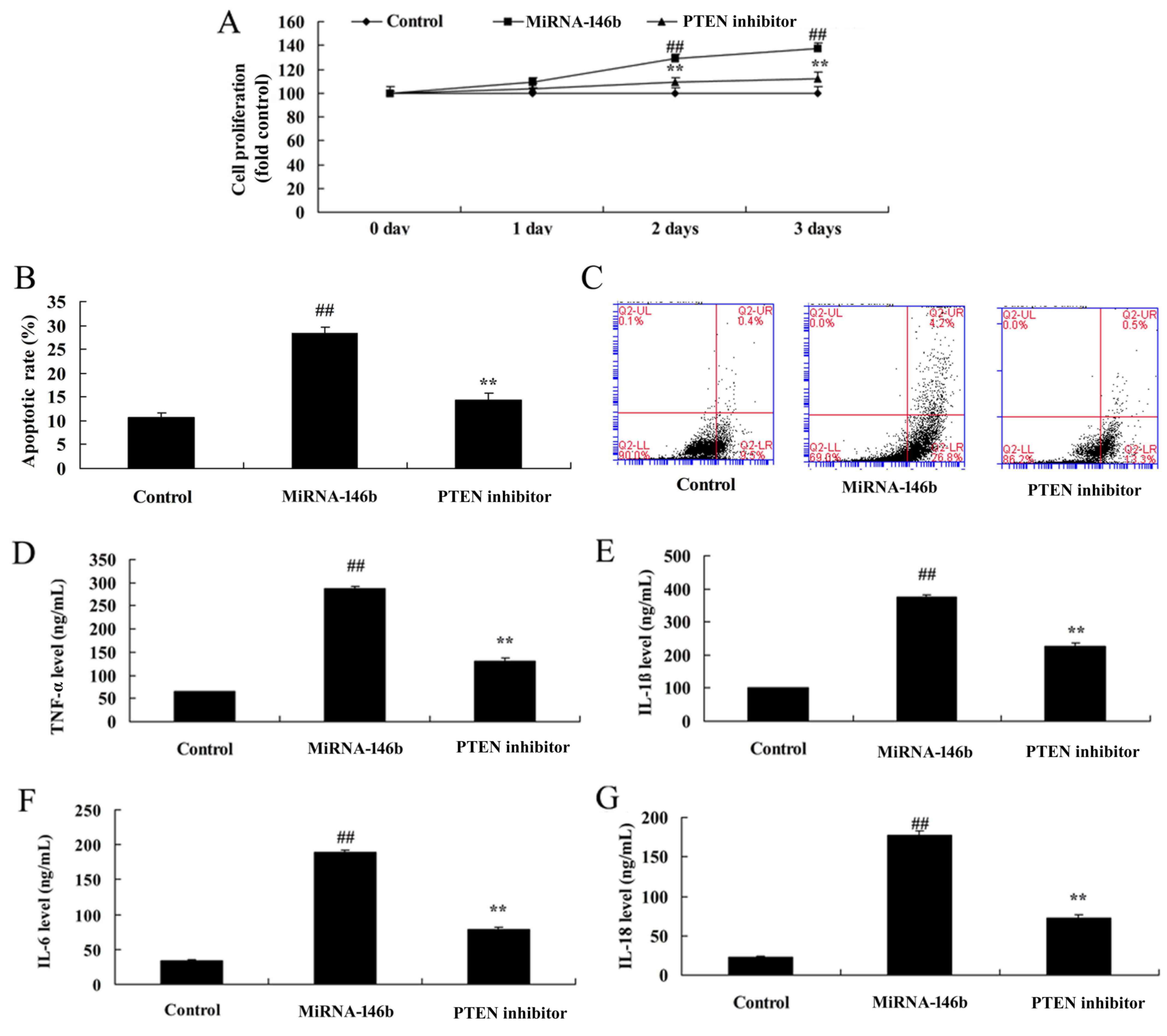 | Figure 7.Inhibition of PTEN reduces the effect
of miRNA-146b in myocardial infarction. (A) Cell viability, (B)
vascular apoptotic rate, and (C) vascular apoptosis (determined
using flow cytometry) of myocardial infarction. Levels of (D)
TNF-α, (E) IL-1β, (F) IL-6 and (G) IL-18 in the in vitro
model. ##P<0.01, vs. Control group; **P<0.01, vs.
miRNA-146b up-regulation group. Control, negative control group;
miRNA-146b, microRNA-146b upregulation group; PTEN inhibitor,
miRNA-146b upregulation and PTEN inhibitor group; PI3K,
phosphoinositide 3-kinase; TNF-α, tumor necrosis factor-α; IL,
interleukin. |
Discussion
Myocardial infarction is a common pathological
process. It is also the core link in the pathogenesis of myocardial
infarction, and in the myocardial revascularization treatment
process (18). How the
identification of effective myocardial protection measures to
reduce myocardial ischemia/reperfusion injury has had remained a
focus of interest for investigations (19). Studies have found that implementing a
few brief ischemia/reperfusion events in a canine myocardial
infarction model at the beginning of the reperfusion cycle can
markedly reduce edema and inflammation in regions of
post-reperfusion myocardial infarction in myocardial tissue. The
concept of ischemic post-conditioning myocardial protection has
also been proposed (18). A series
of subsequent studies have shown that ischemic post-conditioning
offers myocardial protection (5,20).
Additionally, it can markedly reduce myocardial infarction in
myocardial infarction models in mice, rats, rabbits, dogs and other
species (21). In previous years,
several studies have found that isoflurane inhalation anesthetic
post-conditioning can simulate ischemic post-conditioning, protect
against myocardial infarction, and function through a series of
mechanisms. The present study showed that the expression of
microRNA-146b was downregulated in a myocardial infarction rat
model. Hendgen-Cotta et al (16) showed that miR-146b was inhibited in
ischemia/reperfusion of acute myocardial injury in vivo.
Previous studies have shown the presence of
myocardial cell apoptosis in myocardial infarction (20,22).
Adjusting the marker protein of myocardial cell apoptosis can
alleviate myocardial cell apoptosis and improve the left
ventricular ejection fraction (20).
These finding suggests that reducing myocardial cell apoptosis can
improve cardiac function in heart failure.
Apoptosis is a form of programmed cell death, which
is also an energy consumption process that can be regulated. The
Caspase family and Bcl-2, to a certain extent, are important in the
regulation of apoptosis (22). The
activation of Caspase 3 can cause myocardial cell apoptosis, and
the activation of PI3K signaling pathways can inhibit the
activation of Caspase-3. Therefore, inhibiting the occurrence of
apoptosis and providing a specific inhibitor of Caspase 3 can
inhibit apoptosis and reduce cardiac remodeling (11). Lin and An (23), reported that the inhibition of
miRNA-146b-5p promoted inflammation by targeting TNF receptor
associated factor 6 in atherosclerosis-associated foam cell
formation. In the present study, only the H9c2 cell line was used,
which is a limitation of the study; another cell line is to be used
to verify the results in further investigations.
NF-κB is a key transcription factor mediating the
release of inflammatory factors, which is distributed in vascular
endothelial cells, vascular smooth muscle cells and myocardial
cells, and is involved in the genesis and development of
cardiovascular disease (11). Under
normal physiological conditions, it binds with the inhibitory
protein, IκB, exists in the form of homodimer, and is under an
inactive status (24). In the case
of stimulation by multiple pathological factors, the bind between
NF-κB and IκB breaks. NF-κB and IκB are activated by
phosphorylation and transferred to the cell nucleus, bind with
specific target genes, regulate target gene transcription, and
release relevant inflammatory factors, including IL-6 and TNF-α
(25). In this regard, the
downregulation of microRNA-146b significantly induced the protein
expression of PTEN and NF-κB, and suppressed the protein expression
of PI3K and p-Akt in the in vitro model. Jiang et al
(26) showed that miRNA-146b
ameliorated high-fat diet-induced non-alcoholic fatty liver disease
by directly suppressing NF-κB.
The PI3K/AKT signal pathway is an important
intracellular signal transduction pathway, which exerts important
biological functions in activities including cell apoptosis,
survival and proliferation (7). It
has been confirmed in experiments in vivo and in
vitro that the PI3K/AKT signal transduction pathway is
important in protecting from myocardial ischemia reperfusion injury
(27) As is indicated in a number of
studies, ischemic preconditioning or pharmacological
preconditioning can activate the PI3K/AKT signal pathway in
myocardial cells. This can induce a series of subsequent reactions,
including alleviating cell apoptosis, eliminating intracellular
reactive oxygen species, inhibiting activation and aggregation of
neutrophils, and protecting mitochondrial functions. Therefore, it
is decisive in the protective mechanism of myocardial ischemia
reperfusion injury (28). The data
in the present study provided support that the downregulation of
microRNA-146b significantly suppressed the protein expression of
PI3K and p-Akt in the in vitro model. Ramírez-Moya et
al (15), showed that
microRNA-146b promoted the PI3K/AKT pathway by targeting PTEN in
hyperactivation and thyroid cancer progression.
PTEN is the negatively regulatory factor of the
PI3K/AKT signaling pathway (14). It
is a tumor suppressor factor that downregulates the expression of
phosphatidyl triphosphate in multiple systems (14). In addition, it is vital in regulating
embryonic development, cell growth, differentiation, apoptosis and
migration. PTEN can antagonize the action of PI3K and promote
myocardial apoptosis (12). In the
present study, it found that the inhibition of PTEN reduced the
effect of microRNA-146b on myocardial infarction. Ramirez-Moya
et al (15), showed that
microRNA-146b promoted PI3K/AKT pathway hyperactivation and thyroid
cancer progression by targeting PTEN. The present study is the
first, to the best of our knowledge, to report that microRNA-146b
regulates the PI3K/AKT pathway to reduce vascular inflammation and
apoptosis in myocardial infarction.
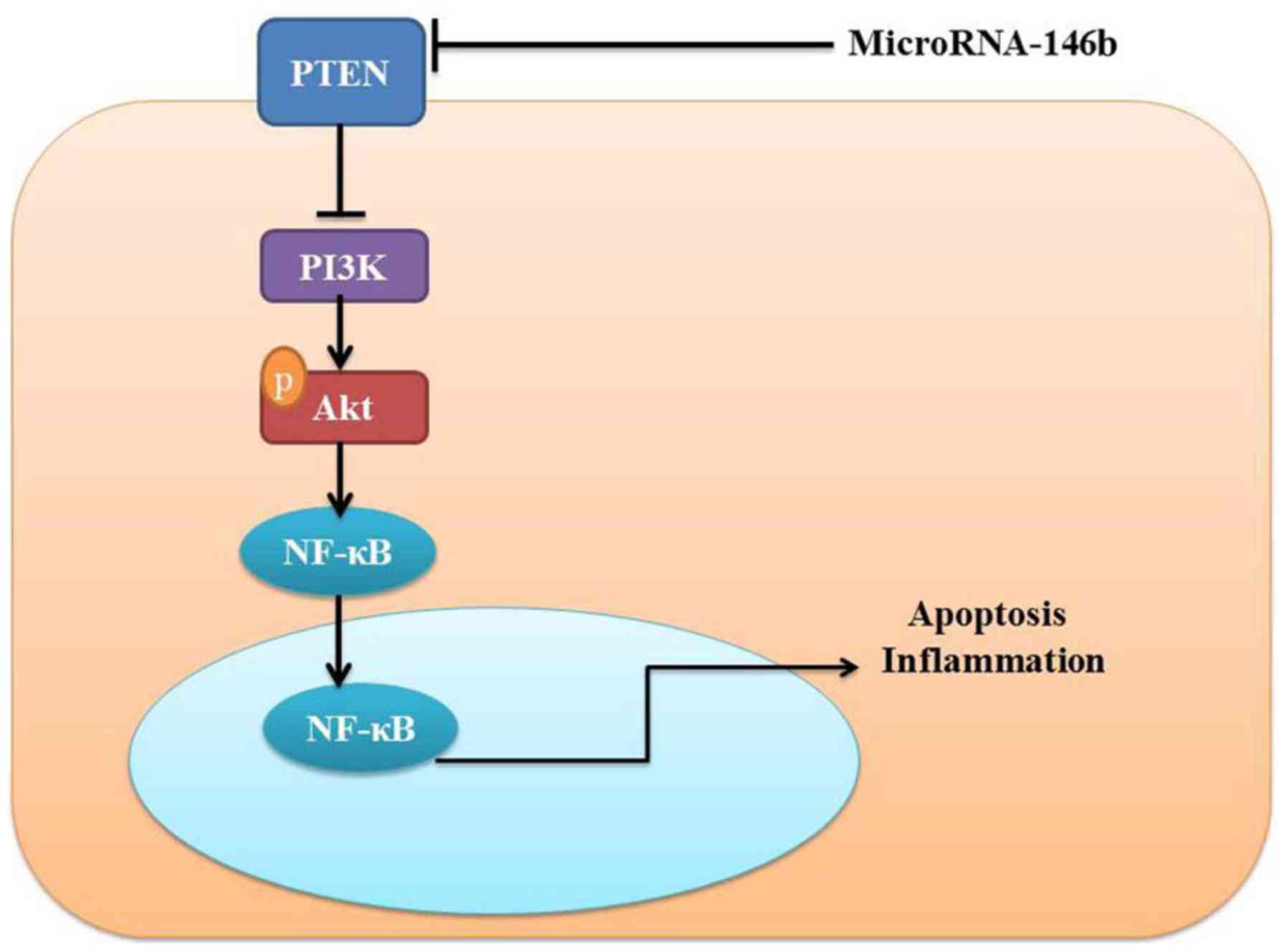
In conclusion, the present study demonstrated that
microRNA-146b mediated vascular inflammation and apoptosis in
patients with myocardial infarction, which may be associated with
activatsion of the PI3K/Akt/NF-κB signaling pathway by PTEN
(Fig. 8). Therefore, microRNA-146b
may be a promising therapeutic target for the treatment of
myocardial infarction, cardiac injury and heart failure.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
Not applicable.
Ethics approval and consent to
participate
All experimental manipulations were undertaken in
accordance with the Guide for the Care and Use of Laboratory
Animals by the National Institutes of Health, with the approval of
the Animal Experimental Ethics Committee of Jining No. 1 People's
Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lavi S, Alemayehu M, McCarty D, Warrington
J and Lavi R: One-year outcome of the sevoflurane in acute
myocardial infarction randomized trial. Can J Anaesth.
62:1279–1286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galea N, Francone M, Zaccagna F, Ciolina
F, Cannata D, Algeri E, Agati L, Catalano C and Carbone I: Ultra
low-dose of gadobenate dimeglumine for late gadolinium enhancement
(LGE) imaging in acute myocardial infarction: A feasibility study.
Eur J Radiol. 83:2151–2158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu L, Cai Z, Xiong M, Li Y, Li G, Deng Y,
Hau WK, Li S, Huang W and Qiu J: Efficacy of an early home-based
cardiac rehabilitation program for patients after acute myocardial
infarction: A three-dimensional speckle tracking echocardiography
randomized trial. Medicine (Baltimore). 95:e56382016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu M, Mao C, Li J, Han F and Yang P:
Effects of the Activin A-Follistatin System on myocardial cell
apoptosis through the endoplasmic reticulum stress pathway in heart
failure. Int J Mol Sci. 18:2017.
|
|
5
|
Chen TL, Zhu GL, He XL, Wang JA, Wang Y
and Qi GA: Short-term pretreatment with atorvastatin attenuates
left ventricular dysfunction, reduces infarct size and apoptosis in
acute myocardial infarction rats. Int J Clin Exp Med. 7:4799–4808.
2014.PubMed/NCBI
|
|
6
|
Boshra V and Atwa A: Effect of
cerebrolysin on oxidative stress-induced apoptosis in an
experimental rat model of myocardial ischemia. Physiol Int.
103:310–320. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng XY, Gu XY, Gao Q, Zong QF, Li XH and
Zhang Y: Effects of dexmedetomidine postconditioning on myocardial
ischemia and the role of the PI3K/Akt-dependent signaling pathway
in reperfusion injury. Mol Med Rep. 14:797–803. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li CM, Shen SW, Wang T and Zhang XH:
Myocardial ischemic post-conditioning attenuates ischemia
reperfusion injury via PTEN/Akt signal pathway. Int J Clin Exp Med.
8:15801–15807. 2015.PubMed/NCBI
|
|
9
|
Lu C, Wang X, Ha T, Hu Y, Liu L, Zhang X,
Yu H, Miao J, Kao R, Kalbfleisch J, et al: Attenuation of cardiac
dysfunction and remodeling of myocardial infarction by
microRNA-130a are mediated by suppression of PTEN and activation of
PI3K dependent signaling. J Mol Cell Cardiol. 89:87–97. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li H, Song F, Duan LR, Sheng JJ, Xie YH,
Yang Q, Chen Y, Dong QQ, Zhang BL and Wang SW: Paeonol and
danshensu combination attenuates apoptosis in myocardial infarcted
rats by inhibiting oxidative stress: Roles of Nrf2/HO-1 and
PI3K/Akt pathway. Sci Rep. 6:236932016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raish M: Momordica charantia
polysaccharides ameliorate oxidative stress, hyperlipidemia,
inflammation, and apoptosis during myocardial infarction by
inhibiting the NF-kappaB signaling pathway. Int J Biol Macromol.
97:544–551. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Xing R, Zhang X, Dong W, Zhang J,
Yan Z, Li W, Cui J and Lu Y: miR-375 targets the p53 gene to
regulate cellular response to ionizing radiation and etoposide in
gastric cancer cells. DNA Repair (Amst). 12:741–750. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhuo SM, Li SC, Lin YQ, Yu HB and Li N:
The effects of anti-Fas ribozyme on T lymphocyte apoptosis in mice
model with chronic obstructive pulmonary disease. Iran J Basic Med
Sci. 20:1102–1108. 2017.PubMed/NCBI
|
|
14
|
Zhang MJ, Su H, Yan JY, Li N, Song ZY,
Wang HJ, Huo LG, Wang F, Ji WS, Qu XJ and Qu MH: Chemopreventive
effect of Myricetin, a natural occurring compound, on colonic
chronic inflammation and inflammation-driven tumorigenesis in mice.
Biomed Pharmacother. 97:1131–1137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ramirez-Moya J, Wert-Lamas L and
Santisteban P: MicroRNA-146b promotes PI3K/AKT pathway
hyperactivation and thyroid cancer progression by targeting PTEN.
Oncogene. 37:3369–3383. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hendgen-Cotta UB, Messiha D, Esfeld S,
Deenen R, Rassaf T and Totzeck M: Inorganic nitrite modulates miRNA
signatures in acute myocardial in vivo ischemia/reperfusion. Free
Radic Res. 51:91–102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boghdady A and Elbadry MI: Comparison of
successful myocardial reperfusion and adverse events in patients
with ST-Elevation myocardial infarction who underwent rescue
percutaneous coronary intervention after failed fibrinolytic
therapy with vs. without manual coronary thrombus aspiration. Am J
Cardiol. 116:1185–1192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tanaka S, Masuda T, Kamiya K, Hamazaki N,
Akiyama A, Kamada Y, Maekawa E, Noda C, Yamaoka-Tojo M and Ako J: A
single session of neuromuscular electrical stimulation enhances
vascular endothelial function and peripheral blood circulation in
patients with acute myocardial infarction. Int Heart J. 57:676–681.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song XJ, Yang CY, Liu B, Wei Q, Korkor MT,
Liu JY and Yang P: Atorvastatin inhibits myocardial cell apoptosis
in a rat model with post-myocardial infarction heart failure by
downregulating ER stress response. Int J Med Sci. 8:564–572. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Poss J, Desch S, Eitel C, de Waha S,
Thiele H and Eitel I: Left ventricular thrombus formation after
ST-segment-elevation myocardial infarction: Insights from a cardiac
magnetic resonance multicenter study. Circ Cardiovasc Imaging.
8:e0034172015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roubille F, Combes S, Leal-Sanchez J,
Barrère C, Cransac F, Sportouch-Dukhan C, Gahide G, Serre I, Kupfer
E, Richard S, et al: Myocardial expression of a dominant-negative
form of Daxx decreases infarct size and attenuates apoptosis in an
in vivo mouse model of ischemia/reperfusion injury. Circulation.
116:2709–2717. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin N and An Y: Blockade of 146b-5p
promotes inflammation in atherosclerosis-associated foam cell
formation by targeting TRAF6. Exp Ther Med. 14:5087–5092.
2017.PubMed/NCBI
|
|
24
|
Sun Y, Huang J and Song K: BET protein
inhibition mitigates acute myocardial infarction damage in rats via
the TLR4/TRAF6/NF-kappaB pathway. Exp Ther Med. 10:2319–2324. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin JL, Lv RG, Guo J, Liu XH, Liang YW,
Wei JR and Wang L: Improvement of left ventricular remodelling by
inhibition of NF-kappaB in a rat model of myocardial infarction.
Heart Lung Circ. 25:1007–1012. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang W, Liu J, Dai Y, Zhou N, Ji C and Li
X: MiR-146b attenuates high-fat diet-induced non-alcoholic
steatohepatitis in mice. J Gastroenterol Hepatol. 30:933–943. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee TM, Chang NC and Lin SZ: Inhibition of
infarction-induced sympathetic innervation with endothelin receptor
antagonism via a PI3K/GSK-3beta-dependent pathway. Lab Invest.
97:243–255. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu S, Ai Q, Feng K, Li Y and Liu X: The
cardioprotective effect of dihydromyricetin prevents
ischemia-reperfusion-induced apoptosis in vivo and in vitro via the
PI3K/Akt and HIF-1alpha signaling pathways. Apoptosis.
21:1366–1385. 2016. View Article : Google Scholar : PubMed/NCBI
|