Introduction
Rheumatoid arthritis (RA) is a common chronic and
autoimmune disease, which is mainly manifested as the joint
involvement, ultimately resulting in joint destruction, deformity
and limb movement disorders. RA leads to a heavy economic and
psychological burden on patients and society. Therefore, it is of
great significance to improve the early diagnosis, timely treatment
and effective control of RA progression (1). Recent studies have confirmed that the
abnormal proliferation of T-lymphocytes and fibroblast-like
synoviocytes (FLS) is the main cause of RA (2,3).
Therefore, inhibition of FLS may assist with improvement of the
treatment efficacy of RA. In addition to the stimulation of
inflammatory factors, FLS apoptosis is also an essential cause of
abnormal proliferation of RA (4).
Long non-coding RNA (lncRNA) is a kind of RNA
molecule with >200 nucleotides in length, which does not have
the function of translating proteins (5). Initially, researches on non-coding RNAs
were mainly focused on microRNAs, and lncRNAs were considered to be
only waste products of the genome. However, accumulating studies
have shown that lncRNAs are greatly involved in biological
processes. Studies have found that lncRNA expression is tissue- and
cell-specific, which is capable of regulating gene expression
levels and cellular processes at epigenetic, transcriptional and
post-transcriptional levels (6,7).
Maternally expressed gene 3 (MEG3) is a human
homologue of the mouse maternal gene Gtl2, which is highly
conserved in evolution. MEG3 is located on human chromosome 14q32.3
with ~1.6 kb in length. Genome structural analysis has revealed
that MEG3/Glt2 consists of 10 exons. Studies have confirmed that
MEG3 is not a typical antisense sequence of gene transcript, but
belongs to a kind of regulatory lncRNA with tumor suppressor
function (8). MEG3 is found to be
overexpressed in normal tissues such as the pituitary gland, brain
tissue, and placenta. Downregulated or absent MEG3 is observed in
tumors, such as liver and lung cancer. MEG3 is also associated with
tumor grade and prognosis. Functionally, MEG3 participates in cell
cycle, proliferation, metastasis and apoptosis of tumor cells
(9,10). The role of MEG3 in RA, however, needs
to be further elucidated.
Patients and methods
Cell isolation and culture of FLS
Ten RA patients who received knee arthroscopic
surgery and 10 trauma patients who received knee arthroscopic
surgery in Peking Union Medical College Hospital (Beijing, China)
were selected. The 10 RA patients were 3 males and 7 females,
51.1±12.0 years of age. The 10 trauma patients were 8 males and 2
females, 41.4±12.7 years of age. This study was approved by the
Ethics Committee of Peking Union Medical College Hospital. Signed
informed consents were obtained from the patients or the
guardians.
Synovial tissues were collected during
knee arthroscopic surgery
After grinding, digestion and centrifugation at
2,500 × g at 20°C for 10 min, FLS were cultured in Dulbecco's
modified Eagle's medium (DMEM) containing 10% fetal bovine serum
(FBS) (both from Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), 100 U/ml penicillin and 100 µg/ml streptomycin (HyClone;
GE Healthcare, Chicago, IL, USA), and were incubated in a 5%
CO2 incubator at 37°C. Cells in logarithmic growth phase
were used for the following experiments after cell passage 4–8
times.
Cell transfection
Cells were transfected with LV-Vector or LV-shMEG3,
after cell confluence was up to 60%. Polybrene was added at a final
dose of 4 µg/ml. Fresh medium was replaced every 4–6 h. All
lentiviruses were purchased from GenePharm (Shanghai, China).
TNF-α treatment
TNF-α (10 µg) was dissolved in sterile water to a
final dose of 500 ng/µl, which was then collected into 200 µl EP
tubes and preserved at −20°C. Cells were treated with 10 ng/ml
recombinant TNF-α for 24 h, followed by determination of
inflammatory factors and related genes in FLS.
Cell proliferation assay
Cell suspension was prepared and seeded into 96-well
plates with 4×103/well. Cell Counting Kit-8 (CCK-8)
solution (10 µl) (Dojindo, Kumamoto, Japan) was added in each well
after cells were cultured for 24, 48 and 96 h, respectively. The
absorbance of each sample at 450 nm was measured by a microplate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Cell apoptosis assay
For cell apoptosis detection, 200 µl of binding
buffer were added to prepare single cell suspension. In total 5 µl
of Annexin V-APC and 10 µl of 7-AAD (Thermo Fisher Scientific,
Inc.) were mixed in cell suspension and incubated in the dark for
15 min, followed by cell apoptosis detection using flow cytometry
(BD Biosciences, Franklin Lakes, NJ, USA). The data were analyzed
using FlowJo software (FlowJo LLC, Ashland, OR, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells by TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and then transcribed
into complementary deoxyribonucleic acid (cDNA) using the
PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd., Dalian,
China). qPCR was subsequently performed in strict accordance with
the manufacturer's instructions of SYBR Green Real-Time PCR Master
Mix (Invitrogen; Thermo Fisher Scientific, Inc.), with a total
reaction volume of 10 µl. The following thermocycling conditions
were used: denaturation at 95°C for 1 min, annealing at 95°C for 30
sec and extension at 60°C for 40 sec, for a total of 40 cycles.
GAPDH served as the internal control. The relative expression
levels of genes were expressed as 2−ΔΔCq (11). The primer sequences used in this
study were as follows: MMP1 forward, AAAATTACACGCCAGATTGCC and
reverse, GGTGTGACATTACTCCAGAGTTG; MMP2 forward, GAG
GAGCAGTTACGGTCTGTG and reverse, TCCTTTCCTTA GCTGACACTTGT; MMP3
forward, AGTCTTCCAATCCT ACTGTTGCT and reverse, TCCCCGTCACCTCCAATCC;
MMP9 forward, TGTACCGCTATGGTTACACTCG and reverse,
TGGCTTCCATAGAGTTCCTTCC; MEG3 forward, CAGCCAGAGTTAGCACAATAGG and
reverse, CTGTTG TTCCCGTCGGAGTT; GAPDH forward, AGGTCGGTG
TGAACGGATTTG and reverse, TGTAGACCATGTAGT TGAGGTCA.
Western blotting
Total protein was extracted from the treated cells
by radioimmunoprecipitation assay (RIPA) solution (Roche
Diagnostics, Basel, Switzerland). Total protein concentration was
calculated by bicinchoninic acid (BCA) Protein Assay kit (Pierce;
Thermo Fisher Scientific, Inc., Rockford, IL, USA). The protein
sample was separated by electrophoresis on 10% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and then
transferred to a polyvinylidene fluoride (PVDF) membrane (EMD
Millipore, Billerica, MA, USA). A total of 30 µg of protein were
added per lane for the electrophoresis. After the membranes were
blocked with 5% BSA at 20°C for 1 h, they were incubated with
primary antibodies overnight at 4°C. The membranes were then washed
with Tris-buffered saline with Tween-20 (TBST) and followed by
incubation of secondary antibody. Immunoreactive bands were
visualized by enhanced chemiluminescence (ECL) detection kit
(Amersham; GE Healthcare, Foster City, CA, USA). The gray value was
analyzed using ImageJ software (version 1.38; National Institutes
of Health, Bethesda, MD, USA). Primary mouse monoclonal STAT3
antibody (dilution, 1/500; cat. no. ab119352); rabbit polyclonal
PI3K antibody (dilution, 1/500; cat. no. ab70912); rabbit
polyclonal AKT antibody (dilution, 1:500; cat. no. ab8805); rabbit
monoclonal Bax antibody (dilution, 1/500; cat. no. ab32503); rabbit
polyclonal Bcl-2 antibody (dilution, 1/500; cat. no. ab59348);
rabbit polyclonal GAPDH antibody (dilution, 1:500; cat. no.
ab37168) and secondary goat anti-rabbit (HRP) IgG antibody
(dilution, 1:2,000; cat. no. ab6721) were all purchased from Abcam
(Cambridge, MA, USA).
Enzyme-linked immunosorbent assay
(ELISA)
FLS in good cell growth were collected and seeded
into 6-well plates at a dose of 5×103/well, with 2
replicates in each group. Cells were treated with TNF-α for 24 h,
followed by supernatant collection. Vascular endothelial growth
factor (VEGF) expression in cell supernatant was detected according
to the manufacturer's instructions of ELISA kit (BioLegend, Inc.,
San Diego, CA, USA). Each experiment was repeated 3 times.
Transwell assay
The upper Transwell chamber was previously coated
with Matrigel (BD Biosciences, San Jose, CA, USA) and maintained in
an incubator for 2 h. A total of 20 µl of cell supernatant and 500
µl of DMEM containing 5% FBS were then added in the upper and lower
chamber, respectively. Transwell chamber was removed after 24
h-incubation, and the non-migrated cells in the chamber were gently
wiped off with a cotton swab. The chamber was fixed with methanol
for 15 min, washed with PBS twice and stained in 1% crystal violet
for 30 min. Finally, 5 randomly selected fields were captured under
an inverted microscope (magnification, ×40; type, AZ100; Nikon
Corp., Tokyo, Japan) for cell count.
Statistical analysis
All statistical analyses were conducted using
Statistical Product and Service Solutions (SPSS) 16.0 software
(SPSS, Inc., Chicago, IL, USA). Measurement data were expressed as
mean ± standard deviation. Independent-sample t-test was used to
compare the differences between two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
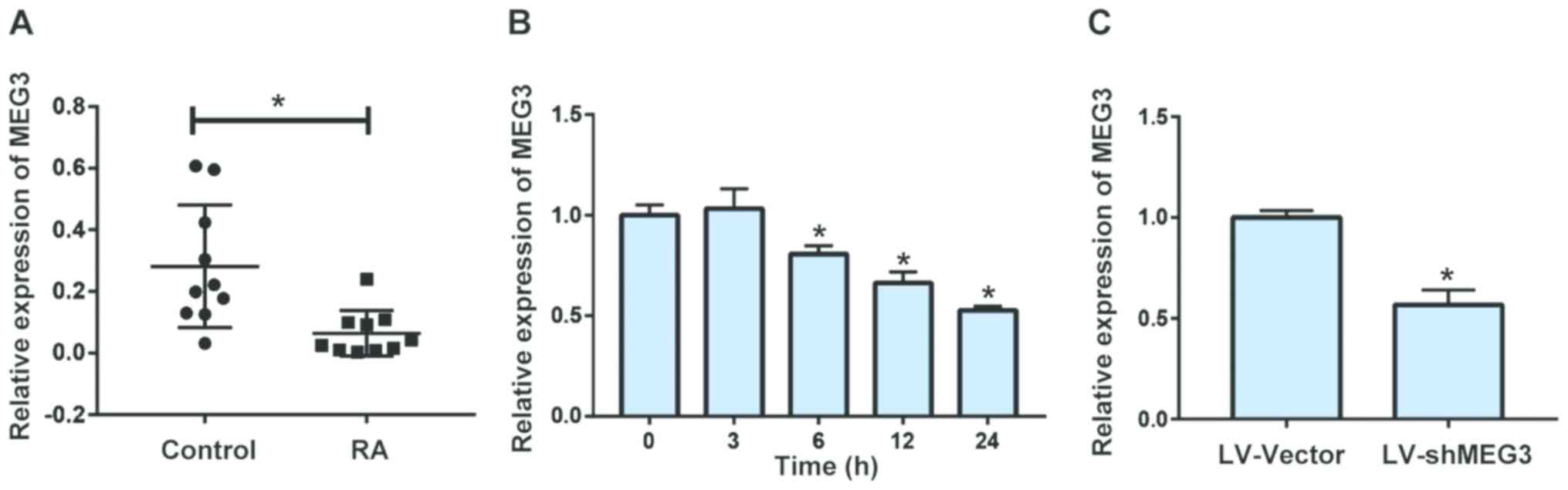
MEG3 is downregulated in FLS of RA
patients
We first extracted mRNAs of FLS from RA patients and
controls. The results indicated that the mRNA level of MEG3 was
downregulated in FLS of RA patients compared with that of controls
(Fig. 1A). In vitro
experiments also showed that TNF-α treatment could remarkably
inhibit MEG3 expression in FLS in a time-dependent manner, which
achieved the lowest level at 24 h (Fig.
1B). We therefore utilized lentivirus transfection to decrease
MEG3 expression in FLS, so as to further explore the underlying
potential of MEG3 (Fig. 1C).
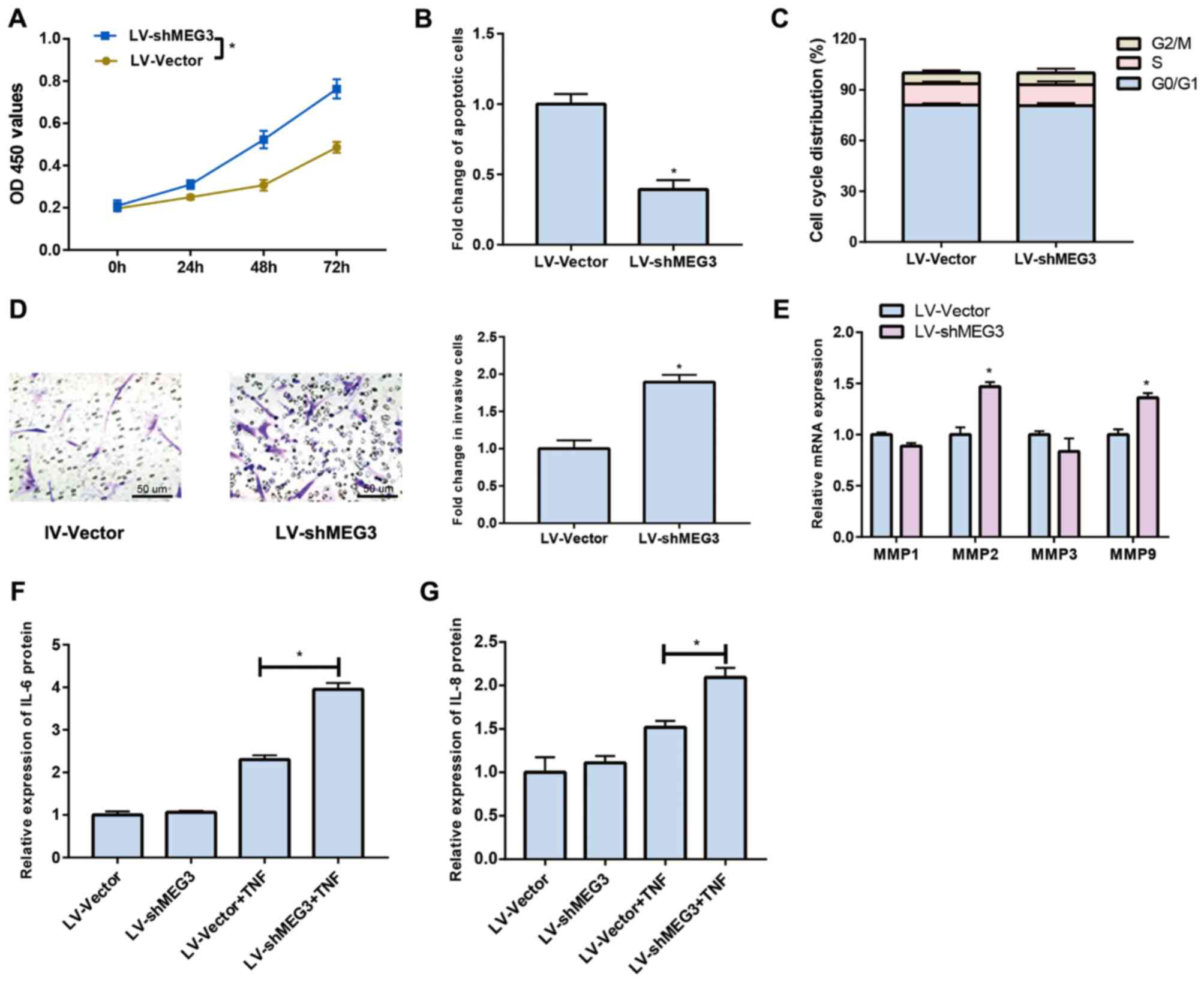
Downregulated MEG3 promotes
proliferation and invasion of FLS
The proliferative ability of FLS was remarkably
increased after MEG3 knockdown, which achieved the peak at 72 h
(Fig. 2A). Cell apoptosis results
demonstrated a decreased apoptotic rate in FLS after MEG3 was
inhibited (Fig. 2B). No significant
difference was observed in cell cycle after MEG3 knockdown
(Fig. 2C). Furthermore, the
Transwell assay elucidated that downregulated MEG3 could remarkably
promote the invasive ability of FLS compared to that of negative
(Fig. 2D). Previous studies have
pointed out that matrix metalloproteinases (MMPs) are involved in
the development and progression of RA. Hence, we speculated that
MEG3 could affect MMPs expression levels. Our data demonstrated
that downregulated MEG3 could lead to increased expression levels
of MMP2 and MMP9, but there were no significant differences in MMP1
and MMP3 (Fig. 2E). Cytokine
secretion was also detected and the results revealed that
downregulated MEG3 could remarkably promote the secretion of IL-6
(Fig. 2F) and IL-8 (Fig. 2G) after TNF-α treatment.
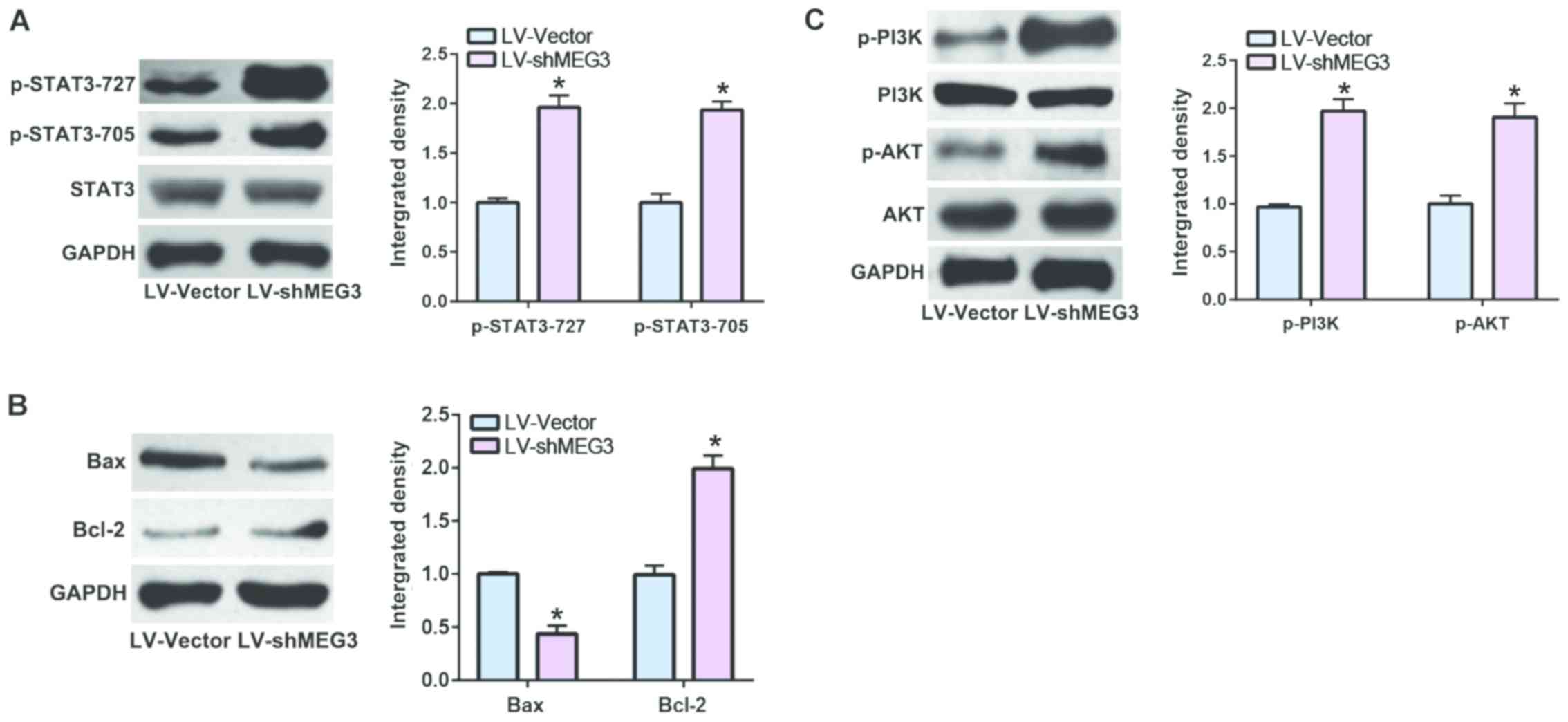
Downregulated MEG3 stimulates STAT3
pathway
It has been reported that STAT3 is greatly involved
in cell apoptosis of RA (12). As a
consequence, we speculated that MEG3 could regulate RA via STAT3
pathway. In the present study, our data indicated that
downregulated MEG3 could not only promote the phosphorylation of
STAT3 (Fig. 3A), but also regulate
the expression levels of the downstream factors of STAT3 (Fig. 3B). Considering the crucial role of
PI3K/AKT in cell proliferation, we detected the key factors in this
pathway and found that downregulated MEG3 was also capable of
stimulating the PI3K/AKT pathway (Fig.
3C).
Discussion
RA is a systemic autoimmune disease, which is
characterized by synovial hyperplasia, articular cartilage
destruction and subchondral bone erosion (13). Interaction of various immune cells
and inflammatory factors leads to FLS activation when they are
under various stimuli, such as the environment change, smoking and
sex hormones alteration. Activated FLS in turn secrete a variety of
chemokines and inflammatory factors, which further stimulate FLS
proliferation from the original 4 layers to 10 layers. The
continuously proliferating and activating FLS gradually transform
into RA-FLS, which present the characteristics of tumor cells such
as reduced apoptosis, enhanced invasiveness, and secretion of
cytokines, MMPs and proteoglyases, thereby destroying structures of
articular cartilage and bone (14).
In this experiment, we found that MEG3 was downregulated in FLS of
RA patients. In vitro experiments showed that TNF-α
negatively regulates MEG3 expression. Also, downregulated MEG3 was
found to lead to FLS proliferation, secretion of IL-6 and IL-8,
increased expression levels of MMP2 and MM9, and to inhibite FLS
apoptosis.
Our results also showed that downregulated MEG3
activates STAT3 pathway. It has been reported that STAT3 possesses
two phosphorylation sites that are related to cellular function,
namely STAT3 (Y705) and STAT3 (S727) (15). STAT3 is presented in the cytoplasm in
an inactive form. Once the tyrosine residue of STAT3 (Tyr705) is
phosphorylated, STAT3 induces the phosphorylation of tyrosine-SH2
region to form a STAT3 dimer. The STAT3 dimer further translocates
into the nucleus and binds to STAT-specific DNA reaction fragments,
thus activating a series of downstream gene expression levels
(16,17). Our experiment confirmed that MEG3
results in increased expression levels of RA-FLS, pSTAT3-705 and
pSTAT3-727 in vitro. Moreover, downregulated MEG3 activates
STAT3 pathway in RA-FLS.
Bcl-2 is a member of the Bcl-2 family with
anti-apoptotic capacity, which is an essential downstream of STAT3
pathway (18,19). Herein, we found that Bcl-2 is related
to RA-FLS apoptosis. In vivo studies showed a higher Bcl-2
expression in the synovium of RA patients than that of
osteoarthritis patients. Previous studies have confirmed that the
stability of mitochondrial RA-FLS is regulated by Bcl-2, which
exerts a crucial role in cell viability. Downregulated Bcl-2 can
increase mitochondrial permeability and induce apoptosis (20). In vitro studies have shown
that TNF and IL-1 can induce Bcl-2 expression in RA-FLS, which
protects RA-FLS in the inflammatory microenvironment (21). In this study, downregulated MEG3 led
to an increased expression of Bcl-2 and decreased expression of Bax
that promotes cell apoptosis of RA-FLS.
In addition to the excessive proliferation of FLS,
degradation of articular cartilage is also one of the most serious
pathological features of RA, which may be explained by the
overactivation of proteolytic enzyme system. Among them, MMPs are
widely expressed in RA-FLS. MMPs are a group of zinc-dependent
endopeptidases that degrade various components of the extracellular
matrix and are major proteases involved in cell migration and
invasion (22,23). Multiple members of the MMPs family
have already been reported to be associated with RA, such as
collagenase MMP1 and matrix lysin MMP3. The gelatinase subfamily
includes two members, MMP2 and MMP9, which can digest
collagenase-induced denatured collagen. MMP2 and MMP9 can also
digest other matrix components, including fibrillar collagen I and
II, and proteoglycans, thus participating in collagen degradation
(24,25). Studies have confirmed that MMP2 and
MMP9 are remarkably upregulated in RA and are closely related to
the erosion of articular cartilage (26). This experiment elucidated that
downregulated MEG3 remarkably increases the expression levels of
MMP2 and MMP9, but has no significant effect on MMP1 and MMP3.
In conclusion, downregulated MEG3 promotes
proliferation and invasion, and inhibits apoptosis of FLS via STAT3
pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XL and JQ designed the study, performed the
experiments, collected and analyzed the data. XL prepared the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Peking Union Medical College Hospital (Beijing, China). Signed
informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Di Spigna G, Del Puente A, Covelli B,
Abete E, Varriale E, Salzano S and Postiglione L: Vitamin D
receptor polymorphisms as tool for early screening of severe bone
loss in women patients with rheumatoid arthritis. Eur Rev Med
Pharmacol Sci. 20:4664–4669. 2016.PubMed/NCBI
|
|
2
|
Calabrò A, Caterino AL, Elefante E,
Valentini V, Vitale A, Talarico R, Cantarini L and Frediani B: One
year in review 2016: Novelties in the treatment of rheumatoid
arthritis. Clin Exp Rheumatol. 34:357–372. 2016.PubMed/NCBI
|
|
3
|
Bellucci E, Terenzi R, La Paglia GM,
Gentileschi S, Tripoli A, Tani C and Alunno A: One year in review
2016: Pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol.
34:793–801. 2016.PubMed/NCBI
|
|
4
|
Flurey CA, Hewlett S, Rodham K, White A,
Noddings R and Kirwan J: Men, rheumatoid arthritis, psychosocial
impact and self-management: A narrative review. J Health Psychol.
21:2168–2182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang C, Wang L, Ding Y, Lu X, Zhang G,
Yang J, Zheng H, Wang H, Jiang Y and Xu L: LncRNA structural
characteristics in epigenetic regulation. Int J Mol Sci.
18:26592017. View Article : Google Scholar
|
|
6
|
Peng WX, Koirala P and Mo YY:
LncRNA-mediated regulation of cell signaling in cancer. Oncogene.
36:5661–5667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frank S, Aguirre A, Hescheler J and Kurian
L: A lncRNA perspective into (re)building the heart. Front Cell Dev
Biol. 4:1282016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Y, Zhang X and Klibanski A: MEG3
noncoding RNA: A tumor suppressor. J Mol Endocrinol. 48:R45–R53.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Balik V, Srovnal J, Sulla I, Kalita O,
Foltanova T, Vaverka M, Hrabalek L and Hajduch M: MEG3: A novel
long noncoding potentially tumour-suppressing RNA in meningiomas. J
Neurooncol. 112:1–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Benetatos L, Voulgaris E and Vartholomatos
G: DLK1-MEG3 imprinted domain microRNAs in cancer biology. Crit Rev
Eukaryot Gene Expr. 22:1–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krause A, Scaletta N, Ji JD and Ivashkiv
LB: Rheumatoid arthritis synoviocyte survival is dependent on
Stat3. J Immunol. 169:6610–6616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deane KD, Demoruelle MK, Kelmenson LB,
Kuhn KA, Norris JM and Holers VM: Genetic and environmental risk
factors for rheumatoid arthritis. Best Pract Res Clin Rheumatol.
31:3–18. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fu H, Hu D, Zhang L and Tang P: Role of
extracellular vesicles in rheumatoid arthritis. Mol Immunol.
93:125–132. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sehgal PB: Paradigm shifts in the cell
biology of STAT signaling. Semin Cell Dev Biol. 19:329–340. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tebbutt NC, Giraud AS, Inglese M, Jenkins
B, Waring P, Clay FJ, Malki S, Alderman BM, Grail D, Hollande F, et
al: Reciprocal regulation of gastrointestinal homeostasis by SHP2
and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat
Med. 8:1089–1097. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ranger JJ, Levy DE, Shahalizadeh S,
Hallett M and Muller WJ: Identification of a Stat3-dependent
transcription regulatory network involved in metastatic
progression. Cancer Res. 69:6823–6830. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsumoto S, Müller-Ladner U, Gay RE,
Nishioka K and Gay S: Ultrastructural demonstration of apoptosis,
Fas and Bcl-2 expression of rheumatoid synovial fibroblasts. J
Rheumatol. 23:1345–1352. 1996.PubMed/NCBI
|
|
19
|
Yu H and Jove R: The STATs of cancer - new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Perlman H, Georganas C, Pagliari LJ, Koch
AE, Haines K III and Pope RM: Bcl-2 expression in synovial
fibroblasts is essential for maintaining mitochondrial homeostasis
and cell viability. J Immunol. 164:5227–5235. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Korb A, Pavenstädt H and Pap T: Cell death
in rheumatoid arthritis. Apoptosis. 14:447–454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tolboom TC, van der Helm-Van Mil AH,
Nelissen RG, Breedveld FC, Toes RE and Huizinga TW: Invasiveness of
fibroblast-like synoviocytes is an individual patient
characteristic associated with the rate of joint destruction in
patients with rheumatoid arthritis. Arthritis Rheum. 52:1999–2002.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ishiguro N: Cartilage degradation in
rheumatoid arthritis. Clin Calcium. 19:347–354. 2009.(In Japanese).
PubMed/NCBI
|
|
24
|
Eck SM, Blackburn JS, Schmucker AC,
Burrage PS and Brinckerhoff CE: Matrix metalloproteinase and G
protein coupled receptors: Co-conspirators in the pathogenesis of
autoimmune disease and cancer. J Autoimmun. 33:214–221. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kotake S, Nanke Y, Yago T and Yamanaka H:
Serum markers of synovitis and bone metabolism in rheumatoid
arthritis. Clin Calcium. 19:362–371. 2009.(In Japanese). PubMed/NCBI
|
|
26
|
Chen Q, Jin M, Yang F, Zhu J, Xiao Q and
Zhang L: Matrix metalloproteinases: Inflammatory regulators of cell
behaviors in vascular formation and remodeling. Mediators Inflamm.
2013:9283152013. View Article : Google Scholar : PubMed/NCBI
|