Introduction
Kimura's disease (KD) was first described by Kimm
and Szeto in 1937 (1) and the
definitive histological description of the disease was subsequently
reported by Kimura in 1948 (2). KD
is a rare chronic inflammatory disorder that may lead to
lymphadenopathy and subcutaneous lesions. It is characterized by
plasma and tissue eosinophilia, raised levels of immunoglobulin
(Ig)E and frequent complications of nephropathy (3). KD occurs mostly in Asian patients and
has been considered limited to the Far East (4). It has previously been reported that
12–60% of patients with KD also exhibit coexisting renal disease
(5). A number of histopathological
renal lesions have been reported in this disease, particularly
minimal change disease, membranous nephropathy and
mesangioproliferative disease (6).
However, to the best of our knowledge, IgA nephropathy in patients
with KD has rarely been reported. In the current study, a case of
steroid-responsive IgA nephropathy associated with KD is
reported.
Case study
A 45-year-old Chinese man was diagnosed with
eosinophilic hyperplasia in May 2011. In June 2012, during a
routine health examination, the patient's urine was analyzed and
the results revealed the presence of grade 3+ proteinuria.
Approximately 6 months later, the patient went to another hospital
(The First Affiliated Hospital Of Jinzhou Medical University
(Jinzhou, China) and was diagnosed with proteinuria and hematuria.
The patient was then admitted to Second Affiliated Hospital of
Dalian Medical University (Dalian, China) due to gross hematuria
and proteinuria in March 2017.
The patient also exhibited an oval-shaped
subcutaneous swelling in his right elbow that had been present for
2 months. The swelling was 2.5 cm in diameter and presented a clear
edge. In addition, the mobile swelling was neither tender nor
indurated.
Upon physical examination, the blood pressure and
heart rate of the patient were 135/80 mmHg and 72 beats/min,
respectively. The patient was also determined to be febrile.
The patient had no history of hypertension or
diabetes. Laboratory tests revealed proteinuria 3+ (normal range,
negative) and 2 consecutive 24 h urine analyses determined a
protein content of 3.9 and 3.8 g/d, respectively. Normal values
were provided previously (7). The
urinary red blood cell (RBC) count of the patient was RBC:20/HP
(normal range: 0–2/HP). Upon morphological examination
(magnification, ×40), the shape of the urine RBCs were dysmorphic,
indicating glomerular hematuria. A full blood count revealed: White
blood cell count, 13.4×109/l (normal range:
3.50–9.50×109/l); hemoglobin, 155 g/l (normal range:
130–175 g/l); and eosinophilia, 3.53×109/l (normal
range, 0.02–0.52×109/l). Furthermore, a renal function
test revealed the following: Urea, 6.10 mmol/l (normal range:
3.1–8.0 mmol/l) and serum creatinine, 115 µmol/l (normal range,
57–97 µmol/l). Serum total IgE levels were high, at 9,790.00 IU/ml
(normal range 0–100 IU/ml). N Latex IgE mono kit (Siemens AG,
Munich, Germany) was used for IgE assay. Hypersensitive c-reactive
protein [analysed using an ELISA kit from Monobind, Inc. (Lake
Forest, CA, USA); cat. no. 3125-000A] was 77.13 mg/l (normal range:
0-5 mg/l) and the erythrocyte sedimentation rate (analysed using an
automatic blood sedimentation dynamic analyzer) was 22.00 mm/h
(normal range: 0–15 mm/h). The hepatitis profile of the patient was
normal. The results for antineutrophilic cytoplasmic antibody and
antibodies associated with autoimmune hepatitis (including surface
antibodies, E antibodies and core antibodies) were determined to be
negative. Ultrasonography of the subcutaneous mass presented two
swollen lymph nodes (2.0 and 1.3 cm, respectively), and the hilus
of these nodes indicated that these swollen lymph nodes had a blood
supply. Bone marrow aspiration revealed eosinophilia hyperplasia
only and excluded the possibility of other hematological diseases.
A chest X-ray did not indicate any abnormalities. Ultrasonography
results of the liver and renal artery were normal.
After informed consent was obtained from the
patient, a renal biopsy was performed. The tissue was prefixed with
ethanol:formaldehyde:PBS (7:3:1) at 4°C for 10 min. Following
prefixing, the tissues was fixed with Bouine stationary liquid (25
ml 80% ethanol saturated picric acid solution + 120 ml formaldehyde
+ 220 ml anhydrous ethanol + 60 ml glacial acetic acid + 50 ml
water) at 4°C for 6 h. Then the tissues were dehydrated at
different ethanol concentrations (75% ethanol, 85% ethanol, 95%
ethanol, absolute ethyl alcohol) for 20 min ateach concentration.
The environmental friendly transparent dewaxing liquid was on the
tissue for 30 min, then specimens were embedded in paraffin at 65°C
for 40 min.
The specimens were embedded in paraffin and sliced
to: i) 3-µm thickness and for immunohistochemistry and Congo red
staining, ii) 2-µm sections for hematoxylin and eosin (H&E),
periodic acid-Schiff and Masson staining, iii) 1.5-µm sections for
periodic acid silver methenamine (PASM) staining, and iv) 1-µm
sections for PASM + HE staining.
For H&E staining, the nucleus was stained with
hematoxylin at 25°C for 10 min, then differentiated with 1%
hydrochloric acid for 1 sec, and counterstained with Scott's bluing
reagent (0.35 g sodium bicarbonate + 2 g magnesium sulfate + 100 ml
water) at 25°C for 10 sec. The cytoplasm was then stained with 5%
eosin at 25°C for 5 min.
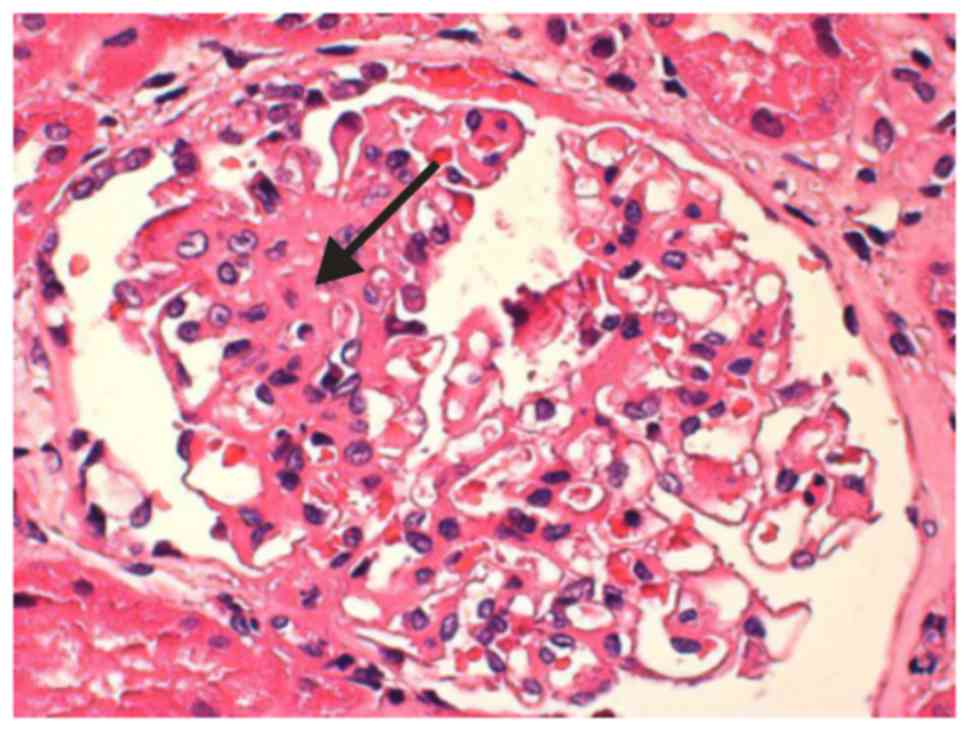
A total of 34 glomeruli were detected via light
microscopy (magnification, ×100): Five glomeruli exhibited global
sclerosis, one glomerulus exhibited segmental sclerosis, and two
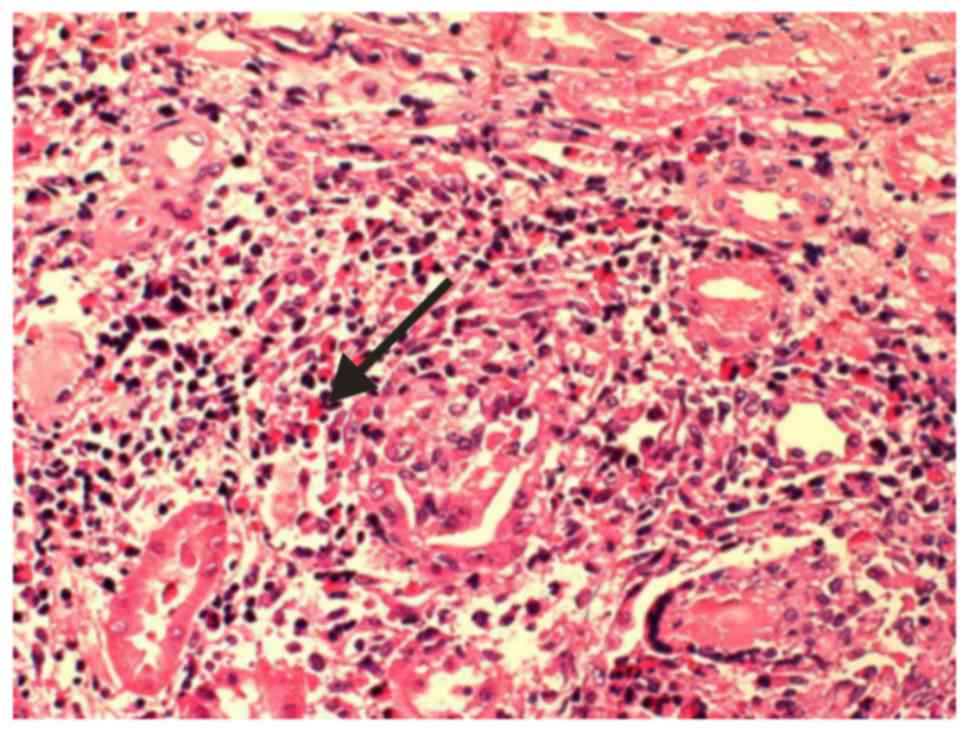
glomeruli presented with a cellular segmental crescent (Fig. 1). Renal tubules exhibited localized
atrophy and tubule cells presented granular degeneration. A small
quantity of protein casts were identified in the lumen and
inflammatory cell infiltration was observed in the interstitium.
Most infiltrating cells were eosinophils and several regions of the
interstitium exhibited marked pathological changes to granulomas
(Fig. 2). Glomerular basement
membrane was normal. Immunofluorescence was performed as follows:
Tissues were frozen on a glacial table and cut into 4-µm frozen
sections for immunofluorescence. Antibodies were diluted to working
solution according to the appropriate dilution factor (IgA, IgM,
IgG, C3 and C4 were diluted to 1:40, and C1q was diluted to 1:50).
Tissues were incubated with the antibodies at 37°C for 30 min.
Tissues were then washed and differentiated with absolute ethyl
alcohol. After drying, they were sealed with glycerin. Fluorescence
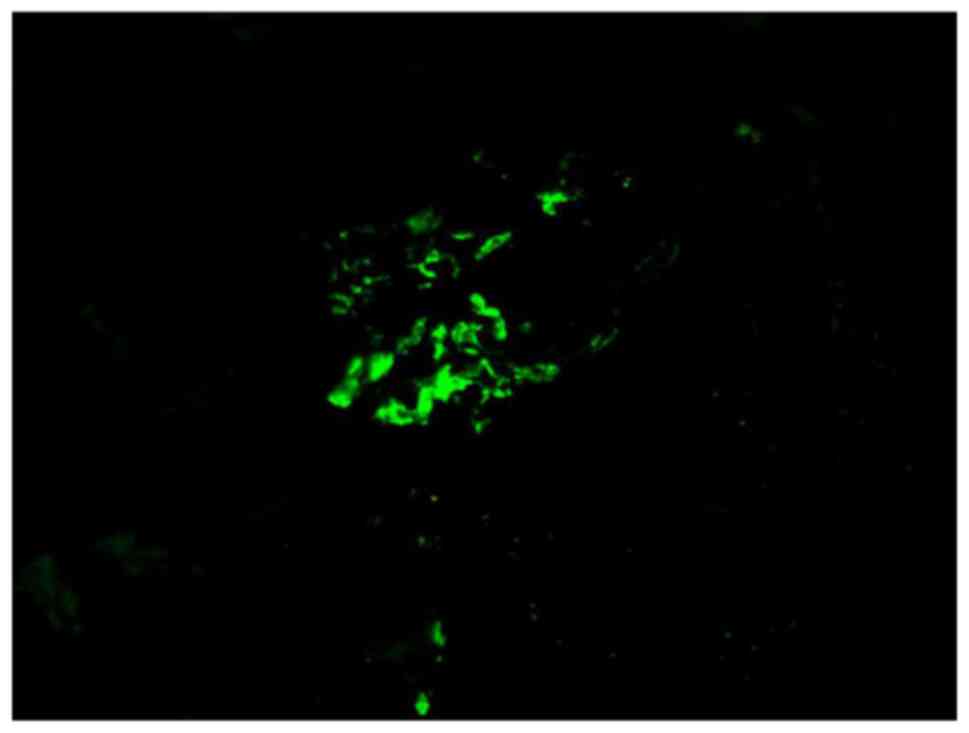
microscopy was used to observe the tissues. It was revealed that
several coarse granular deposits of IgA(3+) and C3(2+) were present
in several mesangial areas (Fig.
3).
Based on the results, the patient was diagnosed with
lgA nephropathy and interstitial nephritis.
The patient was administered methylprednisone pulse
therapy (intravenous administration, 500 mg/day for 3 days),
followed by oral 24 mg/day methylprednisone and 0.8 mg/kg/day
cyclophosphamide (the body weight of the patient was 72 kg) for 4
weeks. Following 10 days of treatment, the swelling situated on the
elbow disappeared while the peripheral eosinophil count returned to
normal (0.06×109/l). However, the levels of total serum
IgE (9,030.00 IU/ml) and proteinuria (2.8 g/day) did not show any
marked reduction. Following 3 months, the level of serum creatinine
dropped to 91 µmol/l, while the level of lgE dropped to 5,653
IU/ml. The patient was discharged in March 2017 following 20 days
of hospitalization. During the follow-up visit conducted 6 months
following discharge, lgE levels dropped to 2,810 IU/ml and 24 h
proteinuria reduced to 0.4 g/day with no evidence of hematuria. In
April 2018, the dose of methylprednisone was reduced to 8 mg/day
and there was no evidence of relapse, although the patient is still
being followed-up.
Discussion
KD was first described by Kimm and Szeto (1) in 1937 and the definitive histological
description of the disease was reported by Kimura et al in
1948 (2). The majority of KD cases
in Asian adults are associated with a protracted course (4,8). KD is
characterized by obvious peripheral blood eosinophilia and an
elevated level of IgE, with subcutaneous swelling around the head
and neck, particularly in the periauricular region (9). In addition, epitrochlear lymph nodes,
inguinal lymph nodes and submandibular salivary glands are sites
that are commonly affected (10).
However, in cases of relapse, the disease may present over the
entirety of the patient's body surface. Among previously reported
cases, 1% of swellings were observed on the elbow, >50% of
patients exhibited a single lesion and 46.6% of patients exhibited
multiple lesions (11). In the
majority of cases, a definitive diagnosis can only be made
following biopsy of the subcutaneous swelling (6). Although the patient in the current
study refused to undergo a biopsy of subcutaneous swelling, his
demographics and symptoms were typical of KD.
The association between KD and renal diseases
including minimal change disease, membranous nephropathy and
mesangioproliferative disease is well recognized (10). Minimal change disease and membranous
nephropathy are the primary symptoms observed in patients from
Asia, the majority of whom exhibit nephrotic syndromes (12). However, IgA nephropathy in patients
with KD is rarely reported. To the best of our knowledge, the
association between KD and IgA nephropathy was first reported in
1998 (13). Since then, only one
more article (9) reported a case of
KD associated IgA nephropathy. In each case, the patients also
exhibited nephrotic syndrome. The renal histology results of the
patient in the current study revealed granular and mesangial
deposits of IgA and complement as detected by immunofluorescence.
Therefore, the manifestation of nephritic syndrome in this patient
strongly indicated the presence of IgA nephropathy.
KD is an allergic and inflammatory disorder with
unknown cause. It is believed that KD is caused by a type I
hypersensitivity reaction or via a T cell-associated immune
disorder (3). Ohta et al
(14) demonstrated that Th2 cells (a
subset of T helper cells), and Tc1 cells (a subset of
CD8+ T cells), may contribute to the pathogenesis of KD.
Furthermore, Yamazaki et al (15) demonstrated that an increased level of
Th2 cells may serve important roles in the pathogenesis of KD by
increasing the synthesis of certain Th2-type cytokines, including
interleukin (IL)-4 and IL-5, which consequently increase
eosinophilic infiltration and IgE synthesis. In addition, several
previous studies have indicated that T cell-mediated tissue damage
serves an important role in the immunopathogenesis of lgA
nephropathy (16). The involvement
of various novel subsets of T cells in the immune responses of IgA
nephropathy, including Th17 and Th1 cells, have also been confirmed
(17). Previous studies have also
determined that, compared with Iga nepropathy (IgAN) patients
without proteinuria, a higher percentage of Th22 cells were present
in IgAN patients with proteinuria (18). In addition, Th22 cells were revealed
to be involved in the immune responses of IgAN (19). During the patient's hospitalization
the current study, he presented massive and relatively infrequent
proteinuria, which we hypothesize may have been caused by an
abnormality in T cell subsets. Taken together, the changes of T
cells in patients with concurrent IgA nephropathy and KD remain
unclear and should be further investigated.
There is no consensus regarding the optimal
treatment for KD. However, Surgical excision and systemic steroid
therapy are the preferred treatments. Recurrence of KD is frequent
after the cessation of treatment (20). A previous study has demonstrated that
KD with renal recurrence is affected by sex, age and history of
hypertension, and it usually presents a good prognosis (12). Nephrotic syndrome in patients with KD
is usually treated with the oral administration of steroid
hormones, such as methylprednisone, but patient response to the
treatment varies (21). The
occurrence of relapse was also revealed to depend on the results of
renal histology (22–23). In the case of the present study, the
patient responded well to methylprednisone treatment and there was
no recurrence of either elbow lesions or nephritis syndrome during
follow-up.
A rare case of KD associated with IgA nephropathy
was described in the current study. The case also exhibited
hematuresis and massive proteinuria. Short-term, the patient was
successfully treated with methylprednisone combined with
cyclophosphamide, while the prevention of KD relapse was achieved
via the long-term administration of methylprednisone at a
maintenance dose (8 mg/d). However, it cannot be concluded whether
the long-term administration of methylprednisolone may be used as
an effective strategy to prevent KD relapse and further studies are
required for confirmation.
Acknowledgments
Not applicable.
Funding
The present study was supported by The technology
Plan Projects for Liaoning Province, China (grant no.
20170540279).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WZ put forward the concepts of the present study,
was responsible for designing and data analysis, and was a major
contributor in writing the manuscript. CL provided the histological
examination of the kidney, and contributed to the manuscript
preparation and editing. AP contributed to the manuscript review
and editing, and performed the pathological and immunohistochemical
interpretation of the renal tissue.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Second Affiliated Hospital of Dalian Medical
University.
Patient consent for publication
Concent for publication has been provided.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kimm HT and Szeto C: Eosinophilic
hyperplastic lymphogranuloma, comparison with Mikulicz's disease.
Proc Chin Med Soc. 1:3291937.
|
|
2
|
Kimura T, Yoshimura S and Ishikaura E: On
the unusual granulation combined with hyperplastic changes of
lymphatic tissue. Trans Soc Pathol Jpn. 37:179–180. 1948.
|
|
3
|
Rajpoot DK, Pahl M and Clark J: Nephrotic
syndrome associated with Kimura disease. Pediatr Nephrol.
14:486–488. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sorbello M, Laudini A, Morello G, Sidoti
MT, Maugeri JG, Giaquinta A, Tallarita T, Corona D, Zerbo D,
Cappellani A, et al: Anaesthesiological implications of Kimura's
disease: A case report. J Med Case Rep. 3:73162009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fouda MA, Gheith O, Refaie A, El-Saeed M,
Bakr A, Wafa E, Abdelraheem M and Sobh M: Kimura Disease: A case
report and review of the literature with a new management protocol.
Int J Nephrol. 2011:6739082010.
|
|
6
|
Liu C, Hu W, Chen H, Tang Z, Zeng C, Liu Z
and Li L: Clinical and pathological study of Kimura's disease with
renal involvement. J Nephrol. 21:517–525. 2008.PubMed/NCBI
|
|
7
|
Boege F: Urinary proteins. Clinical
Laboratory Diagnostics. Thomas L: 1st. TH-Books
Verlagsgesellschaft; Frankfurt: pp. 382–400. 1998
|
|
8
|
Kung IT, Gibson JB and Bannatyne PM:
Kimura's disease: A clinico-pathological study of 21 cases and its
distinction from angiolymphoid hyperplasia with eosinophils.
Pathology. 16:39–44. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu YC, Wang R and Lv XA: Kimura disease
associated with IgA nephropathy. Kaohsiung J Med Sci. 30:213–214.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun QF, Xu DZ, Pan SH, Ding JG, Xue ZQ,
Miao CS, Cao GJ and Jin DJ: Kimura disease: Review of literature.
Intern Med J. 38:668–674. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adler BL, Krausz AE, Minuti A, Silverberg
JI and Lev-Tov H: Epidemiology and treatment of angiolymphoid
hyperplasia with eosinophilia (ALHE): A systematic review. J Am
Acad Dermatol. 74:506–512. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y, Wang J, Xu F, Zeng C and Liu Z:
Clinicopathological features and prognosis of Kimura's disease with
renal involvement in Chinese patients. Clin Nephrol. 85:332–339.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Natov SN, Strom JA and Ucci A: Relapsing
nephrotic syndrome in a patient with Kimura's disease and IgA
glomerulonephritis. Nephrol Dial Transplant. 13:2358–2363. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ohta N, Sakurai S, Yoshitake H and Aoyagi
M: Analysis of Th1, Th2, Tc1 and Tc2 cells in patients with
allergic rhinitis. Clin Exp All Rev. 5:68–71. 2005. View Article : Google Scholar
|
|
15
|
Yamazaki K, Kawashima H, Sato S, Tsunoda
H, Yoshimura Y, Higuchi M, Hokibara S, Yamazaki T and Agematsu K:
Increased CD45RO+ CD62L+ CD4+ T-cell subpopulation responsible for
Th2 response in Kimura's disease. Hum Immunol. 74:1097–1102. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng Z, Tian J, Cui X, Xian W, Sun H, Li
E, Geng L, Zhang L and Zhao P: Increased number of Th22 cells and
correlation with Th17 cells in peripheral blood of patients with
IgA nephropathy. Hum Immunol. 74:1586–1591. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kurts C, Panzer U, Anders HJ and Rees AJ:
The immune system and kidney disease: Basic concepts and clinical
implications. Nat Rev Immunol. 13:738–753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duhen T, Geiger R, Jarrossay D,
Lanzavecchia A and Sallusto F: Production of interleukin 22 but not
interleukin 17 by a subset of human skin-homing memory T cells. Nat
Immunol. 10:857–863. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miyagaki T, Sugaya M, Suga H, Kamata M,
Ohmatsu H, Fujita H, Asano Y, Tada Y, Kadono T and Sato S: IL-22,
but not IL-17, dominant environment in cutaneous T-cell lymphoma.
Clin Cancer Res. 17:7529–7538. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu XK, Ren J, Wang XH, Li XS, Zhang HP
and Zeng K: Angiolymphoid hyperplasia with eosinophilia and
Kimura's disease coexisting in the same patient: Evidence for a
spectrum of disease. Australas J Dermatol. 53:e47–e50. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang DY, Mao JH, Zhang Y, Gu WZ, Zhao SA,
Chen YF and Liu AM: Kimura disease: A case report and review of the
Chinese literature. Nephron Clin Pract. 111:c55–c61. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsuda O, Makiguchi K, Ishibashi K, Chida
Y, Ida T, Matsuda K, Tomita K, Marumo F and Hiruma M: Long-term
effects of steroid treatment on nephrotic syndrome associated with
Kimura's disease and a review of the literature. Clin Nephrol.
37:119–123. 1992.PubMed/NCBI
|
|
23
|
Chartapisak W and Opastirakul S:
Steroid-resistant nephrotic syndrome associated with Kimura's
disease. Am J Nephrol. 22:381–384. 2002. View Article : Google Scholar : PubMed/NCBI
|