Introduction
Idiopathic membranous nephropathy (IMN) is the most
common cause of nephrotic syndrome in adults, accounting for ~30%
of all types of renal biopsy (1). In
recent years, the proportion of IMN cases out of the number of
cases of primary glomerular disease has continued to rise (1). IMN is identified in adults by the
presence of anti-M-type phospholipase A2 receptor (PLA2R)
antibodies in the serum. M-type PLA2Ris expressed by podocytes in
patients with membranous nephropathy (MN); however, patients with
secondary or other glomerular diseases exhibit less anti-PLA2R
antibodies in the serum, which indicates that PLA2R may be a
pathogenic target antigen in adults with IMN (1). Therefore, anti-PLA2R antibodies may be
an important biomarker for the diagnosis of IMN (2,3).
Numerous studies have proposed that the anti-PLA2R antibody titers
of patients with IMN are associated with proteinuria and response
to treatment (4,5). A European research group also
identified that the anti-PLA2R antibody titer was associated with
serum creatinine, urine β2 protein microspheres and urinary IgG
(4). In addition, in 2010 Prunotto
et al (5) identified specific
IgG4 antibodies directed against the podocyte antigensaldose
reductase (AR) and superoxide dismutase (SOD) in the serum and
renal tissue of patients with MN through proteomic methods.
In the present study, anti-PLA2R, anti-SOD2 and
anti-AR antibodies in the serum of patients with IMN were detected,
in addition to the levels of other serum biochemical factors. Then,
the correlation between these factors and the response to treatment
of the patients with IMN was investigated.
Materials and methods
Study population
A total of 56 patients with a histological diagnosis
of IMN were recruited between April 2012 and August 2015 from
Qianfoshan Hospital (Jinan, China). The inclusion criteria were as
follows: i) Meet the International Classification of Diseases 10
diagnostic criteria for nephrotic syndrome (lCD-10. Geneva, World
Health Organization, 1992) [clinical manifestations of nephrotic
syndrome (proteinuria >3.5 g/d, serum albumin (Alb) <30 g/l,
and hyperlipidemia and edema) and a renal biopsy diagnosis of MN];
ii) complete clinical data; and iii) Written informed consent of
the patient was obtained prior to inclusion in the present study.
The exclusion criteria were the follows: i) nephrotic syndrome due
to infection, other autoimmune diseases, cancer, hepatitis,
diabetes, drugs or other secondary factors; ii) a sustained
creatinine level >309.4 mol, estimated glomerular filtration
rate (eGFR)<30 ml/min/1.73 m2, significantly reduced
kidney volume (long diameter <8 cm) or the presence of a severe
infection; and iii) other immunotherapy contraindications. The 2012
Kidney Disease: Improving Global Outcomes glomerulonephritis
Clinical Practice Guidelines (6)
outline the following IMN remission criteria: i) Complete
remission, 24 h urinary protein <0.3 g and normal serum Alb
(35–50 g/l); ii) partial remission, 24 h urinary protein <3.5 g
≥50% reduction in urinary protein excretion, and a normal or
elevated serum Alb level; iii) no-remission, patient does not meet
the above criteria. The present study was approved by the Ethics
Committee of Shandong University (Jinan, China).
Treatment groups
All the patients were divided into groups according
to their 24 h urinary protein excretion. Patients whose urinary
protein level was <4 g/24 h were given an angiotensin converting
enzyme inhibitor (Lotensin, 10–20 mg/day) and supportive treatment.
Patients were given Diovan in addition to Lotensin (80–160 mg/day)
if they could not tolerate coughing and other side effects.
Patients who had aurinary protein excretion of 4–6 g/24 h were
observed and treated ibid. Patients were observed for proteinuria
during drug treatment, until a urinary protein level of <4 g/24
h was reached. Close observation was continued if the proteinuria
didn't resolve within1 month. Patients with a proteinuria level
>6 g/24 h were given immunosuppressive therapy. The randomly
allocated cyclophosphamide (CTX) group of 36 patients received oral
methylprednisolone (1 mg/kg/day) and 0.8 g CTX via intravenous
injection once a month for 6 months, then once every 3 months for a
total of 12 months; the total CTX dose did not exceed 11 g. The
randomly allocated tacrolimus (FK506) group of 20 patients received
0.03–0.05 mg/kg FK506 orally twice a day (with an interval of 12 h
between doses to maintain a drug concentration in the blood of 5–10
ng/ml) for 6–12 months, while prednisone (0.5 mg/kg/day) could be
reduced following mitigation and the administration of FK506.
Serum antibody and biochemical
measurements
During treatment, renal function, serum Alb, urinary
protein, high-sensitivity C-reactive protein, and anti-PLA2R,
anti-SOD2 and anti-AR antibody titers were measured. Serum samples
were taken prior to and at 1, 3, 6, 9 and 12 months after
treatment. Anti-PLA2R, anti-AR and anti-SOD2 antibodies were
detected through ELISAs. The anti-PLA2R antibody ELISA kit was
purchased from EUROIMMUN Medizinische Labordiagnostika AG (cat. no.
FA1254; Lübeck, Germany). The anti-AR and anti-SOD2antibodyELISA
kits were from CUSABIO (Wuhan China; cat. nos. CSB-EL001975MO
andCSB-EL022398RA, respectively). The absorbances of the wells of
the ELISA plates were measured at 450 nm using a microplate reader.
The standard antibodies provided in the kits were used to make the
standard curve and fit equation in order to calculate the
concentration of the test antibodies.
Statistical analysis
All statistical analyses were performed using SPSS
software (version 20.0;IBM Corp., Armonk, NY, USA). Normally
distributed continuous variables were expressed as the mean ±
standard deviation. Count data was expressed as a percentage. The
statistical significance of differences among groups of normal
measurement data were measured using the Student's t-test between
two groups or one-way analysis of variance followed by a post hoc
Tukey's range test for multiple groups. Non-normality was examined
using the Shapiro-Wilk test and heterogeneity of variance between
data groups was examined using Levene's test. The correlation
between two normally distributed continuous variables was
investigated using the Pearson correlation coefficient and further
analyzed using the multiple linear regression equation. Serum
antibody titers were the dependent variable and all biochemical
indicators were the independent variables. The multiple linear
regression equations were analyzed using the stepwise method.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical characteristics of the study
population
The present study included 56 patients with IMN.
There were 36 males and 20 females, with a mean age of 46.85 years
(range, 13–66 years). The follow-up time was 9.21 months on average
(range, 6–24 months). On average, the 24 h urinary protein levels
were 6.40±3.65 g, serum Alb was 24.21±6.08 g/l, the eGFR was
113.33±36.35 ml/min/1.73 m2, total cholesterol was
9.43±6.82 mmol/l, systolic blood pressure was 131.7±16.09 mmHg and
diastolic blood pressure was 81.3±12.05 mmHg. The clinical
characteristics of the study population and the two treatment
groups are presented in Table I. The
CTX and FK506 groups exhibited no significant difference in any
MN-associated serum biochemical indicators or antibodies prior to
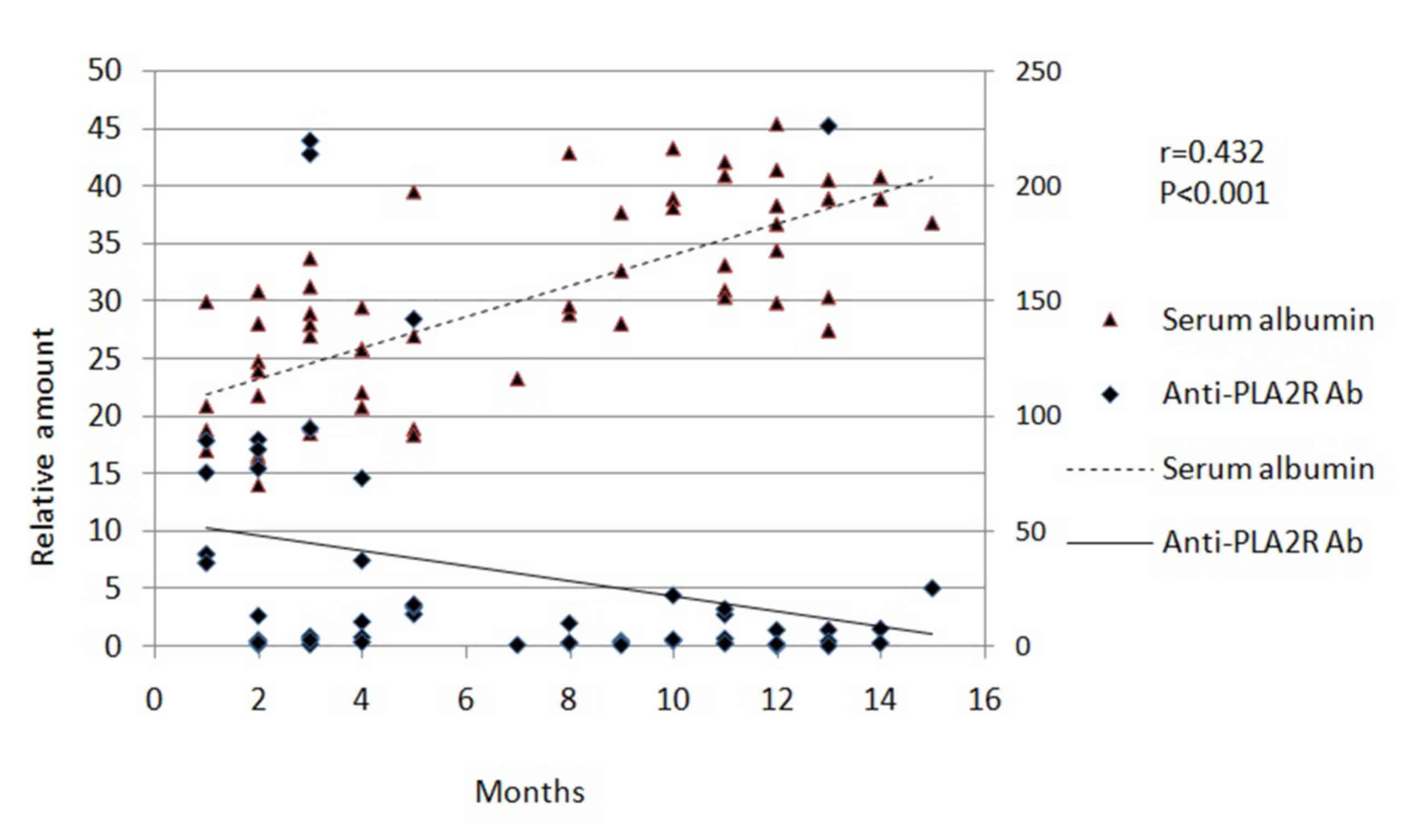
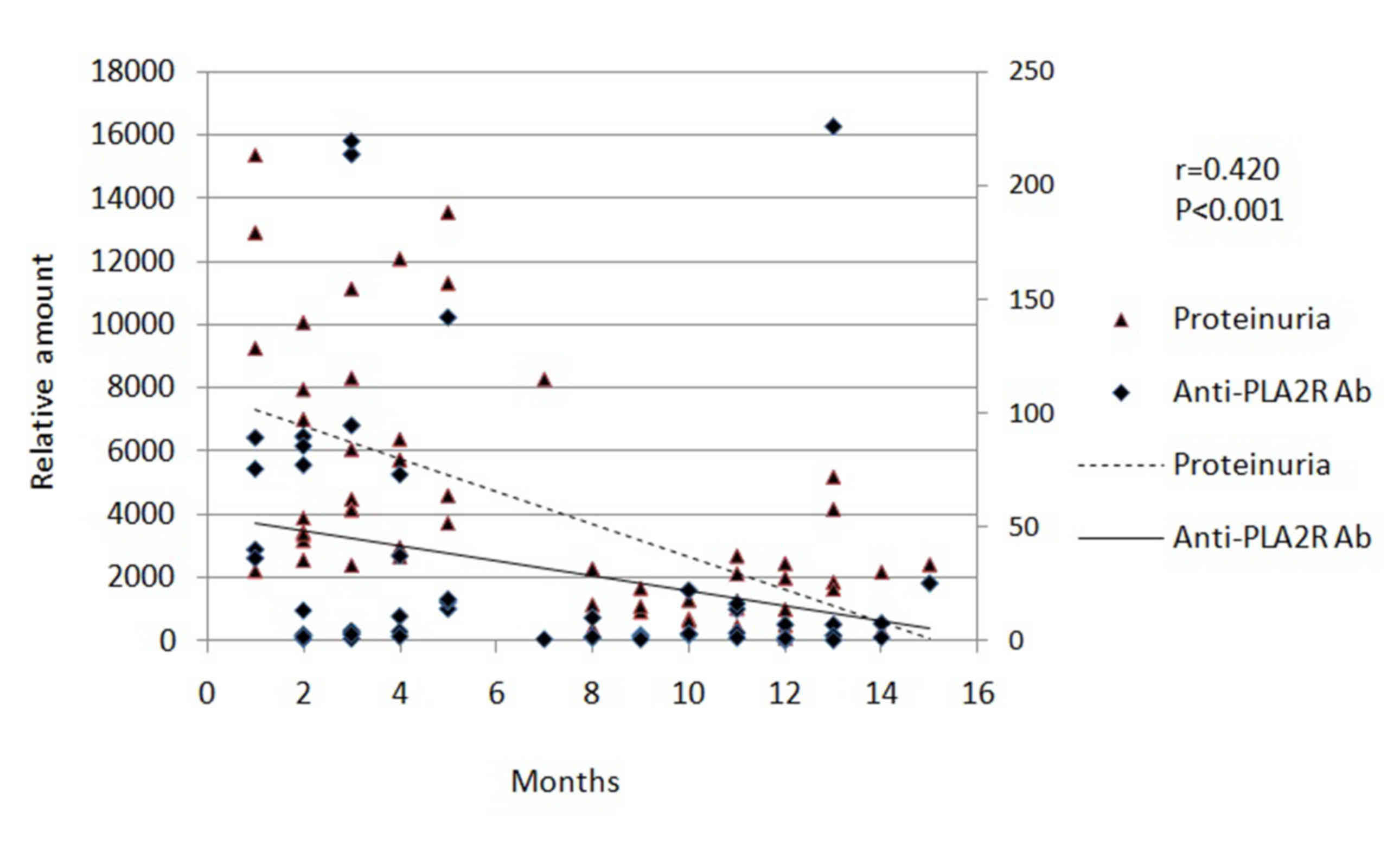
treatment. During the experiment, serum Alb levels increased
(Fig. 1) and proteinuria decreased
(Fig. 2) with immunosuppressive
therapy.
 | Table I.Clinical characteristics of the study
population. |
Table I.
Clinical characteristics of the study
population.
|
|
| Group |
|
|---|
|
|
|
|
|
|---|
| Characteristics | All patients
(n=56) | CTX (n=36) | FK506 (n=20) | P-value (CTX vs.
FK506) |
|---|
| Sex, ratio
(male/female) | 1.8 (36/20) | 2.0 (24/12) | 1.5 (12/8) | – |
| Age (years) | 46.9±13.62 | 48.6±10.29 | 43.2±17.21 | 0.22 |
| Proteinuria (g/24
h) | 6.40±3.65 | 6.02±3.54 | 7.13±3.83 | 0.28 |
| Serum albumin
(g/l) | 24.21±6.08 | 25.31±6.11 | 22.24±5.64 | 0.07 |
| eGFR (ml/min/1.73
m2) | 113.33±36.35 | 112.72±29.35 | 114.44±47.28 | 0.88 |
| TC (mmol/l) | 9.43±6.82 | 8.17±2.49 | 11.70±10.72 | 0.16 |
| SBP (mmHg) | 133.5±16.98 | 131.4±17.65 | 132.4±12.65 | 0.79 |
| DBP (mmHg) | 81.3±12.05 | 81.8±13.22 | 80.2±9.40 | 0.51 |
| PLA2R antibody level
(mU/ml) | 1.25±0.72 | 1.35±0.66 | 1.06±0.79 | 0.17 |
| AR antibody level
(mU/ml) | 16.88±14.10 | 16.15±12.77 | 18.34±16.79 | 0.65 |
| SOD2 antibody level
(mU/ml) | 3.73±6.15 | 2.87±5.78 | 5.27±6.65 | 0.19 |
Anti-PLA2R antibody expression in
patients with IMN over the course of treatment
The level of serum anti-PLA2R antibodies were
compared in 56 patients with IMN prior to treatment with normal
ranges. A total of 40 patients with IMN (71.43%) tested positive
for serum anti-PLA2R antibodies. Patients were separated into a CTX
group and a FK506 group, which received two different treatment
regimens. Among the 40 patients that tested positive for serum
anti-PLA2R antibodies, 26 were from the CTX group and 14 were from
the FK506 group. Of the 16 patients that tested negative for serum
anti-PLA2R antibodies, 10 patients were from CTX group and the
remainder was from the FK506 group. Prior to treatment, all
patients were positive for anti-PLA2R antibodies. Comparing the
anti-PLA2R antibody level prior to and after treatment (Table II), out of the 36 patients in the
CTX group, 21 patients tested negative following treatment, while
15 patients remained positive, although the titers decreased after
treatment. Out of the 20 patients in the FK506 group, 14 patients
tested negative for serum anti-PLA2R antibodies following
treatment, while 6 patients remained positive, although again the
titers decreased following treatment.
 | Table II.Biochemical measurements prior to and
following immunosuppressive therapy in patients with IMN. |
Table II.
Biochemical measurements prior to and
following immunosuppressive therapy in patients with IMN.
|
|
| Time point |
|
|---|
|
|
|
|
|
|---|
| Factor measured | Group | Prior to
treatment | After treatment | P-value |
|---|
| Proteinuria (g/24
h) | All patients | 6.40±3.65 | 1.81±1.70 | <0.001 |
|
| CTX | 6.02±3.54 | 1.54±1.34 | <0.001 |
|
| FK506 | 7.14±3.83 | 2.25±2.17 | <0.001 |
| Serum albumin
(g/l) | All patients | 24.21±6.08 | 35.81±5.74 | <0.001 |
|
| CTX | 25.31±6.11 | 36.31±5.47 | <0.001 |
|
| FK506 | 22.24±5.56 | 34.90±6.24 | <0.001 |
| eGFR (ml/min/1.73
m2) | All patients | 113.33±36.35 | 113.27±37.59 | 0.84 |
|
| CTX | 112.72±29.35 | 108.53±27.97 | 0.44 |
|
| FK506 | 114.44±47.28 | 121.34±49.75 | 0.23 |
| PLA2R antibody level
(mU/ml) | All patients | 1.25±0.72 | 0.58±0.59 | <0.001 |
|
| CTX | 1.35±0.66 | 0.82±0.54 | <0.001 |
|
| FK506 | 1.05±0.79 | 0.12±0.37 | <0.001 |
| AR antibody level
(mU/ml) | All patients | 16.88±14.10 | 16.45±15.93 | 0.78 |
|
| CTX | 16.15±12.77 | 14.71±13.88 | 0.42 |
|
| FK506 | 18.34±16.80 | 19.94±19.42 | 0.53 |
| SOD2 antibody level
(mU/ml) | All patients | 3.73±6.15 | 4.82±9.63 | 0.92 |
|
| CTX | 2.87±5.78 | 4.81±11.09 | 0.32 |
|
| FK506 | 5.26±6.64 | 4.85±6.44 | 0.23 |
Correlation between anti-PLA2R
antibody titers and blood biochemical indexes in patients with IMN
over the course of treatment
Table II lists the
first and last measurements of urinary protein, serum Alb, the
eGFR, and anti-PLA2R, anti-AR and anti-SOD2 antibody levels. After
immune suppression treatment, the anti-PLA2R antibody titers of
patients with IMN significantly decreased compared to those prior
to treatment (P<0.001). In addition, compared to levels prior to
treatment, proteinuria levels significantly decreased and serum Alb
levels significantly increased (P<0.001). The analysis of the
correlation for these data revealed that there was a significant
positive correlation between the reduction in anti-PLA2R antibody
titers and the decrease in proteinuria (r=0.420; P<0.001;
Fig. 2). By contrast, there was a
significant negative correlation between the reduction in
anti-PLA2R antibody titers and the increase in serum Alb (r=0.432;
P<0.001; Fig. 1). However, the
reduction of eGFR (r=−0.103, P=0.294) and serum cholesterol
(r=−0.078, P=0.437) had no significant association with the
reduction in anti-PLA2R antibody titers (data not shown).
Correlation between anti-AR and
anti-SOD2 antibody titers and blood biochemical indexes in patients
with IMN over the course of treatment
Out of the 56 patients with IMN, 26 tested positive
for serum anti-AR antibodies. The number of patients who tested
positive for serum anti-AR antibodies was not significantly
different compared with the normal controls. For the patients who
tested negative for anti-PLA2R antibodies following treatment,
54.4% tested positive for anti-AR antibodies, although this
difference was not statistically significant when compared with
samples taken prior to treatment (data not shown). Correlation
analysis revealed that the serum anti-AR antibody titers were
significantly positively correlated with urinary protein levels
(r=0.204; P<0.05) and the eGFR (r=0.338; P<0.01) (data not
shown). Of the 56 patients with IMN, 38 tested positive for serum
anti-SOD2 antibodies. Serum anti-SOD2 antibody titers were
significantly positively correlated with urinary protein (r=0.223;
P<0.05) and serum Alb (r=−0.194; P<0.05) levels; however,
there was no correlation between anti-SOD2 antibody titers and the
eGFR or total cholesterol (data not shown).
Effect of CTX and FK506on IMN
remission
The56 patients with IMN were followed up after
treatment for 9.21 months on average (Table III). In the CTX group, 12 patients
(33.3%) achieved clinical complete remission, 20 patients (55.6%)
exhibited partial remission and4 patients (11.1%) had no remission.
In the FK506 group, all patients achieved complete or partial
remission; 4 patients (20%) exhibited complete remission and 16
patients (80%) achieved partial remission. After treatment, serum
anti-PLA2R antibody titers in the two groups were significantly
decreased compared with prior to treatment (data not shown).
Between the two groups, the complete and partial remission rates
were not significantly different (Table III), which suggests that CTX and
FK506 do not have significantly different effects on IMN
remission.
 | Table III.Remission rates of patients with IMN
following immunosuppressive therapy. |
Table III.
Remission rates of patients with IMN
following immunosuppressive therapy.
|
| Remission status,
no. of patients (%) |
|
|---|
|
|
|
|
|---|
| Group | Complete
remission | Partial
remission | No remission |
|---|
| CTX (n=36) | 12 (33.3) | 20 (55.6) | 4 (11.1) |
| FK506 (n=20) | 4
(20.0) | 16 (80.0) | 0 (0.0) |
| All patients
(n=56) | 16 (28.6) | 36 (64.3) | 4 (7.1) |
Discussion
IMN is a chronic disease that can exhibit
spontaneous remission and relapse; typically in the first 2 years
after onset ~40% of patients go into spontaneous remission
(7,8). Predictive factors for spontaneous
remission include a proteinuria level<8 g/24 h at baseline,
being female, an age of<50 years and good renal function at the
time of the disease onset (9). In
addition, 2/3 patients with IMN can exhibit persistent proteinuria
but maintain good renal function long-term; although, despite
receiving immunosuppressive therapy, the majority of patients
progress to end-stage renal disease (ESRD) (10). MN is a common cause of primary
glomerulonephritis, which leads to ESRD (10).
Currently, the diagnosis of IMN mainly relies upon
renal pathology, which is invasive, as there is a lack of sensitive
biomarkers to predict the disease effectively (1). A noninvasive examination or serum
biomarkers would be preferable, as they can effectively reduce the
risk of bleeding, infection and other side effects from invasive
procedures. The present study investigated the significance of
serum autoantibodies in the diagnosis of MN, which could replace
traditional biopsy diagnosis and serve a role in treatment
strategies. A previous meta-analysis evaluated the diagnostic value
of serum anti-PLA2R antibodies in the detection in IMN, which
revealed that its sensitivity and specificity were 78 and 99%,
respectively (11).
The present study examined the levels ofanti-PLA2R
antibodies in the serum of 56 patients with IMN, which demonstrated
that 71.43% of the patients exhibited expression of these
antibodies. This is similar to the results of previous studies that
reported a positive rate for PLA2R antibodies of 71.0–77.8% in
patients with IMN (12,13). The present study also identified a
correlation between the level of serum anti-PLA2R antibodies and
serum biochemical indicators of IMN. Correlation analysis revealed
that serum anti-PLA2R antibody titers were significantly positively
correlated with proteinuria over the course of treatment, which was
consistent with the findings of several previous studies (13–15). IMN
diagnosis and staging relies on pathology, however, the
pathological stage has no clear association with clinical disease
activity or drug efficacy (16).
Clinicians monitor the activity of IMN and determine drug efficacy
primarily through monitoring proteinuria and serum Alb levels
(16). The results of the current
study demonstrated that the level of anti-PLA2R, anti-AR and
anti-SOD2antibody titers were significantly positively correlated
with proteinuria, which is an indicator of renal function. In
patients with IMN who respond to drugs, achieving complete or
partial remission, these serusm antibody titers may decrease or
disappear (16). Previous studies
have demonstrated that serum anti-PLA2R antibody titer decline
occurs prior to the decrease of urinary protein and to the other
laboratory parameters of remission (13–15).
Investigations into the association between serum
anti-PLA2R antibody levels and the development of IMN has primarily
been on small sample populations and over a short-term period;
thus, the reliability of antibody titers for monitoring disease
progression and treatment efficacy still requires further
verification. It has been reported that the anti-PLA2R antibody
titer can predict the recurrence of MN at the end of
immunosuppressive therapy. After 5 years of immunosuppressive
therapy, ~58% of patients with IMN who are negative for anti-PLA2R
antibodies do not suffer recurrence, while patients who are
positive for these antibodies at the end of treatment are more
likely to relapse (12). Thus, the
presence or absence of anti-PLA2R antibodies can be used as a
strong predictor for the clinical remission of PLA2R-associated MN.
In addition, monitoring serum antibodies regularly prior to and
during treatment can help predict the efficacy of therapy (9). Certain studies have suggested that
prior to starting immunosuppressive treatment, antibody titers
should be measured once every 2 months in order to avoid
unnecessary treatment (12,16). Another study reported that in the
first 6 months of immunosuppressive therapy, antibody titers should
be tested once a month to assess the effect of drug treatment
(17). In the present study, certain
patients continued to express anti-PLA2R antibodies during and
after treatment; however, the levels decreased significantly after
treatment, so the potential long-term beneficial effects of
immunosuppressive therapy cannot be excluded in these patients. If
a long-term period of follow-up observation was performed, these
patients may stop expressing anti-PLA2R antibodies.
At present, the impact of anti-AR and anti-SOD2
antibodies on the diagnosis and treatment response of patients with
IMN remains unclear. Previous studies have identified AR and SOD2
in IMN biopsy specimens (5). Anti-AR
and anti-SOD2 antibodies and C5b-9 are co-localized in the
electron-dense material of podocytes in patients with IMN (5). In the present study, anti-AR antibody
levels in the serum were significantly positively correlated with
proteinuria and the eGFR in patients with IMN, but had no
significant effect on the dynamics of disease progression (as
determined by urinary protein and serum Alb levels). Particularly,
the anti-SOD2 antibodies are similar to the inflammatory markers
for the oxidative stress reaction in the body, and there is no
specific or sensitive method for monitoring the progress of IMN
(5). In the clinic, anti-AR and
anti-SOD2antibodies cannot be used as specific monitoring
indicators.
In conclusion, the expression of anti-PLA2R
antibodies in patients with IMN is associated with the efficacy of
immunosuppressive therapy. Immunosuppressive therapy alone or
combined with hormone therapy has been proven to relieve the
symptoms of IMN effectively in clinical practice, but not to
influence clinical remission (9). In
the present study, two different immunosuppressive treatment
regimens, CTX and FK506, were used, and it was revealed that
anti-PLA2R antibody levels and progress of the disease were not
significantly different between the two groups, which is in
agreement with the findings of previous studies (18,19).
However, the present study was limited by a small sample size and
so the influence of this cannot be ruled out. The results of the
present study determined that the specificity of anti-AR and
anti-SOD2 antibodies for the diagnosis of MN is low, thus their use
in clinical diagnosis is limited. In addition, the present study
had a relatively short follow-up time and did not include the
patients with secondary MN as a control. Therefore, future research
with a larger sample size, an extended follow-up period and
including patients with secondary MN is required to validate the
results of the current study.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
technology Research Project of Shandong (grant no. 2013GSF11818)
and the Science and Technology Development Projects of Jinan (grant
no. 201503002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WWH and DMX conceived and designed the experiments.
WWH, HY and XLK collected the data. WWH and XLK were involved in
the analysis of data. WWH and LJT performed the experiments. WWH
edited the manuscript. DMX revised the manuscript. All authors have
read and approved this article.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shandong University (Jinan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ponticelli C: Membranous nephropathy. J
Nephrol. 20:268–287. 2007.PubMed/NCBI
|
|
2
|
Beck LH Jr, Bonegio RG, Lambeau G, Beck
DM, Powell DW, Cummins TD, Klein JB and Salant DJ: M-type
phospholipase A2 receptor as target antigen in idiopathic
membranous nephropathy. N Engl J Med. 361:11–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qin W, Beck LH Jr, Zeng C, Chen Z, Li S,
Zuo K, Salant DJ and Liu Z.: Anti-phospholipase A2 receptor
antibody in membranous nephropathy. J Am Soc Nephrol. 22:1137–1143.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hofstra JM, Beck LH Jr, Beck DM, Wetzels
JF and Salant DJ: Anti-phospholipase A2 receptor antibodies
correlate with clinical status in idiopathic membranous
nephropathy. Clin J Am Soc Nephrol. 6:1286–1291. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prunotto M, Carnevali ML, Candiano G,
Murtas C, Bruschi M, Corradini E, Trivelli A, Magnasco A, Petretto
A, Santucci L, et al: Autoimmunity in membranous nephropathy
targets aldose reductase and SOD2. J Am Soc Nephrol. 21:507–519.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cattran DC, Feehally J, Cook HT, Liu ZH,
Fervenza FC, Mezzano SA, Floege J, Nachman PH, Gipson DS, Praga M,
et al: Kidney Disease: Improving Global Outcomes (KDIGO)
Glomerulonephritis Work Group. KDIGO clinical practice guideline
for glomerulonephritis. Kidney Int Suppl. 2:186–197. 2012.
|
|
7
|
Polanco N, Gutiérrez E, Covarsí A, Ariza
F, Carreño A, Vigil A, Baltar J, Fernández-Fresnedo G, Martín C,
Pons S, et al: Grupo de Estudio de las Enfermedades Glomerulares de
la Sociedad Española de Nefrología: Spontaneous remission of
nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc
Nephrol. 21:697–704. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Polanco N, Gutiérrez E, Rivera F,
Castellanos I, Baltar J, Lorenzo D and Praga M: Grupo de Estudio de
las Enfermedades Glomerulares de la Sociedad Española de Nefrología
(GLOSEN): Spontaneous remission of nephritic syndrome in membranous
nephropathy with chronic renal impairment. Nephrol Dial Transplant.
27:231–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cattran D: Management of membranous
nephropathy: When and what for treatment. J Am Soc Nephrol.
16:1188–1194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Glassock RJ: Diagnosis and natural course
of membranous nephropathy. Semin Nephrol. 23:324–332. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Du Y, Li J, He F, Lv Y, Liu W, Wu P, Huang
J, Wei S and Gao H: The diagnosis accuracy of PLA2R-AB in the
diagnosis of idiopathic membranous nephropathy: A meta-analysis.
PLoS One. 9:e1049362014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bech AP, Hofstra JM, Brenchley PE and
Wetzels JF: Association of anti-PLA2R antibodies with outcomes
after immunosuppressive therapy in idiopathic membranous
nephropathy. Clin J Am Soc Nephrol. 9:1386–1392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanigicherla D, Gummadova J, McKenzie EA,
Roberts SA, Harris S, Nikam M, Poulton K, McWilliam L, Short CD,
Venning M and Brenchley PE: Anti-PLA2R antibodies measured by ELISA
predict long-term outcome in a prevalent population of patients
with idiopathic membranous nephropathy. Kidney Int. 83:940–948.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Segarra-Medrano A, Jatem-Escalante E,
Carnicer-Cáceres C, Agraz-Pamplona I, Salcedo MT, Valtierra N,
Ostos-Roldán E, Arredondo KV and Jaramillo J: Evolution of antibody
titre against the M-type phospholipase A2 receptor and clinical
response in idiopathic membranous nephropathy patients treated with
tacrolimus. Nefrologia. 34:491–497. 2014.(In English, Spanish).
PubMed/NCBI
|
|
15
|
Hofstra JM, Debiec H, Short CD, Pellé T,
Kleta R, Mathieson PW, Ronco P, Brenchley PE and Wetzels JF:
Antiphospholipase A2 receptor antibody titer and subclass in
idiopathic membranous nephropathy. J Am Soc Nephrol. 23:1735–1743.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ponticelli C and Passerini P: Can
prognostic factors assist therapeutic decisions in idiopathic
membranous nephropathy. J Nephrol. 23:156–163. 2010.PubMed/NCBI
|
|
17
|
Ronco P and Debiec H: Pathophysiological
advances in membranous nephropathy: Time for a shift in patient's
care. Lancet. 385:1983–1992. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ruggenenti P, Debiec H, Ruggiero B,
Chianca A, Pellé T, Gaspari F, Suardi F, Gagliardini E, Orisio S,
Benigni A, et al: Anti phospholipase A2 receptor antibody titer
predicts post-rituximab outcome of membranous nephropathy. J Am Soc
Nephrol. 26:2545–2558. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu H and Luo W: GONG Shaomin Detection
and the clinical significance of phospholipase A2 receptor in
idiopathic membranous nephropathy tissues. Fudan University Journal
of Medical Sciences. 42:2015.
|