Introduction
Retinal vein occlusion (RVO) has been reported to be
the second most common retinal vascular disease following diabetic
retinopathy (1), which commonly
leads to vision loss in the elderly population and is frequently
associated with arteriosclerotic diseases and glaucoma. It is
traditionally divided into central retinal vein occlusion (CRVO)
and branch retinal vein occlusion (BRVO) (2). The present study focused on CRVO that
has been reported widely to cause blinding fundus lesions mainly
characterized by retinal hemorrhage, exudation and cystoid macular
edema. The occurrence of CRVO is associated with age, which is
mostly accompanied with chronic systemic diseases, including
hypertension, diabetes and arteriosclerosis (3). According to fundus fluorescein
angiography (FFA), CRVO can be divided into ischemic and
non-ischemic types. The non-ischemic type is common in the clinic
and occurs in the early stage of disease, so the visual prognosis
is relatively good. Occlusion may occur in the central trunk,
branch or hemiretinal vein (hemi) and its duration is divided into
≤1 month, 1–3 months and >3 months.
The important risk factors for CRVO in patients
older than 50 years mainly include systemic hypertension and
vascular disease. In addition, diabetes mellitus and
hyperlipidemia, African-descent ethinicity, male gender, peripheral
artery disease, stroke, hypercoagulable state, ocular hypertension
and primary open-angle glaucoma also add to the prevalance of CRVO
(4). On the other hand,
hyperlipidemia has been reported to be the predominant medical
condition associated with CRVO in young patients (5). All these pathological conditions
utlimately result in systemic diseases leading to inflammatory
alterations in the blood vessels of the retina, which in turn cause
occlusion of the central retinal vein (6).
The clinical treatment used widely for CRVO is the
combination of intravitreal injection of ranibizumab with argon ion
laser photocoagulation (7).
Combination therapy has already demonstrated higher safety and
better efficacy, in comparison with the single therapy (8,9).
However, there is paucity of information with regard to the
efficacy of this combination at variable degrees of CRVO.
Therefore, in the present study, the clinical efficacy of
ranibizumab combined with argon ion laser photocoagulation therapy
on CRVO in different degrees was analyzed.
Materials and methods
Study subjects
A total of 112 patients continuously diagnosed as
CRVO in the Huizhou Municipal Central Hospital (Huizhou, China)
from June 2014 to October 2016 were selected. Written informed
consent was provided by the patients. Inclusion criteria: i)
Patients with monocular lesions and the best corrected visual
acuity (BCVA) <0.3; ii) patients with optic disc edema, blurred
edges, cystoid macular edema, hemorrhage, smaller arterial
diameter, venous tortuous dilatation, retinal edema and flame
bleeding in fundus demonstrated by fundus photography, and with
prolonged retinal circulation time, aneurysmal dilatation of blood
capillaries, fluorescein leakage, large non-perfusion area, stained
venous wall and diffuse fluorescein leakage of macula lutea
demonstrated by FFA, and with retinal thickening, edema and
hemorrhage demonstrated by optical coherence tomography (OCT) and
damaged retinal pigment epithelium; iii) patients who did not
receive the intravitreal injection, laser photocoagulation and drug
therapy promoting circulation and removing stasis; iv) patients
with clinical data and informed consent right obtained. Exclusion
criteria: i) Patients with glaucoma, cataract, proliferative
vitreoretinopathy, macular ischemia, diabetic retinopathy,
age-associated macular degeneration, eye traumas or other eye
diseases; ii) patients with severe hypertension, diabetes,
atherosclerosis or cardiovascular and cerebrovascular diseases;
iii) patients who participated in other studies at the same time
and quit the study voluntarily. The study was approved by the
Ethics Committee of Huizhou Municipal Central Hospital. Patient
characteristics are presented in Table
I.
 | Table I.Baseline data of patients in the three
groups. |
Table I.
Baseline data of patients in the three
groups.
| Group | Trunk occlusion
(n=25) | Branch occlusion
(n=50) | Hemi-occlusion
(n=37) | F/χ2
value | P-value |
|---|
| Male/Female | 14/11 | 22/28 | 19/18 | 1.072 | 0.585 |
| Age (years) | 52.6±10.7 | 53.3±11.2 | 53.8±13.2 | 0.562 | 0.649 |
| Course of disease
(months) | 1.8±0.5 | 1.9±0.7 | 1.8±0.6 | 0.253 | 0.864 |
| CRT (µm) | 635±50 | 642±55 | 647±62 | 0.642 | 0.596 |
| BCVA | 0.24±0.08 | 0.25±0.09 | 0.23±0.07 | 0.153 | 0.863 |
| Intraocular pressure
(mmHg) | 20.5±3.6 | 19.6±3.3 | 21.2±3.8 | 0.345 | 0.769 |
| Hypertension [n
(%)] | 6 (24.0) | 10 (20.0) | 7 (18.9) | 0.252 | 0.882 |
| Diabetes [n (%)] | 4 (16.0) | 6
(12.0) | 4 (10.8) | 0.372 | 0.830 |
Research methods
The patients were divided into the following groups:
The trunk occlusion group (12 patients for ranibizumab treatment,
13 patients for ranibizumab+argon ion laser treatment), the branch
occlusion group (25 patients for ranibizumab treatment, 25 patients
for ranibizumab+argon ion laser treatment) and the hemi-occlusion
group (18 patients for ranibizumab treatment, 19 patients for
ranibizumab+argon ion laser treatment). All patients were
intravitreally injected with 0.5 mg ranibizumab (Lucentis; Novartis
International AG, Basel, Switzerland), followed by argon ion laser
photocoagulation after 7 days. The specific steps were as follows:
Prior to operation, levofloxacin hydrochloride eye drops were
dropped into the eyes for 3 days continuously (4 times per day);
following conventional disinfection and topical anesthesia with 50
g/l oxybuprocaine hydrochloride, povidone-iodine eye drops were
used for disinfecting eyeballs, and after 90 sec, normal saline was
used to wash the cornea and bulbar conjunctival sac; 1 ml empty
needle was used to extract 0.05 ml (0.5 mg) ranibizumab and inject
it into the vitreous body at 4 mm behind the inferotemporal corneal
limbus, and change of the intraocular pressure was observed. After
the needle was removed, the sterile wet cotton swab was used to
press the wound for 1–2 min, followed by wound painting using
levofloxacin oculentum and dressing of affected eye. Patients
treated with only 0.5 mg ranibizumab were used as the controls.
The solid laser therapeutic instrument (Iridex,
Mountain View, CA, USA) with the frequency multiplication of 532 nm
was used for grid photocoagulation in the macular region.
Parameters included spot diameter in 100 µm, energy at 100–140 mW,
exposure time at 0.1 msec, spot reaction at I level, distance away
from macula central fovea with 1–2 optic disc diameters, ring
photocoagulation outward and photocoagulation range from the upper,
and lower vascular arcades to the bitamporal junction. The optic
disc maculary fasciculi were retained. According to the FFA results
of patients, if necessary, the retinal non-perfusion area and local
photocoagulation of novel vessels were combined. Photocoagulation
parameters of retinal non-perfusion area: Spot diameter in 200 µm,
energy at 180–260 mW, exposure time at 0.2 msec and spot reaction
at II level. The laser should avoid the retinal hemorrhage or thick
area and the photocoagulation should be 1 optic disc diameter away
from the non-perfusion area or peripheral new vessels.
Observational indexes
BCVA, CRT, macular edema and surgical complications
at 1 month, 3 months and 6 months following operation among all
groups were compared. BCVA was detected using the international
standard visual acuity chart (converted into Log MAR visual
acuity). CRT was measured according to OCT and the decrease of CRT
>100 µm compared with the results detected previously was
regarded as the recession.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
20.0 software (IBM, Corps., Armonk, NY, USA) was used for
statistical analysis. Measurement data were presented as mean ±
standard deviation. One-way analysis of variance (ANOVA) was used
for the comparison among the groups. Least significance
difference-t test was used for pairwise comparison. Repeated
measurement ANOVA was used for value comparisons in different time
of follow-up. Enumeration data were presented as case or percentage
(%) and Chi-square test was used for intergroup comparison.
P<0.05 was considered to indicate a statistically significant
difference.
Results
In the present results revealed that there were 25
cases of trunk occlusion, 50 cases of branch occlusion and 37 cases
of hemi-occlusion. The baseline data of patients in the three
groups presented that there was no difference in sex, age, course
of disease, CRT, BCVA, intraocular pressure, hypertension and
diabetes between three groups (P>0.05, Table I).
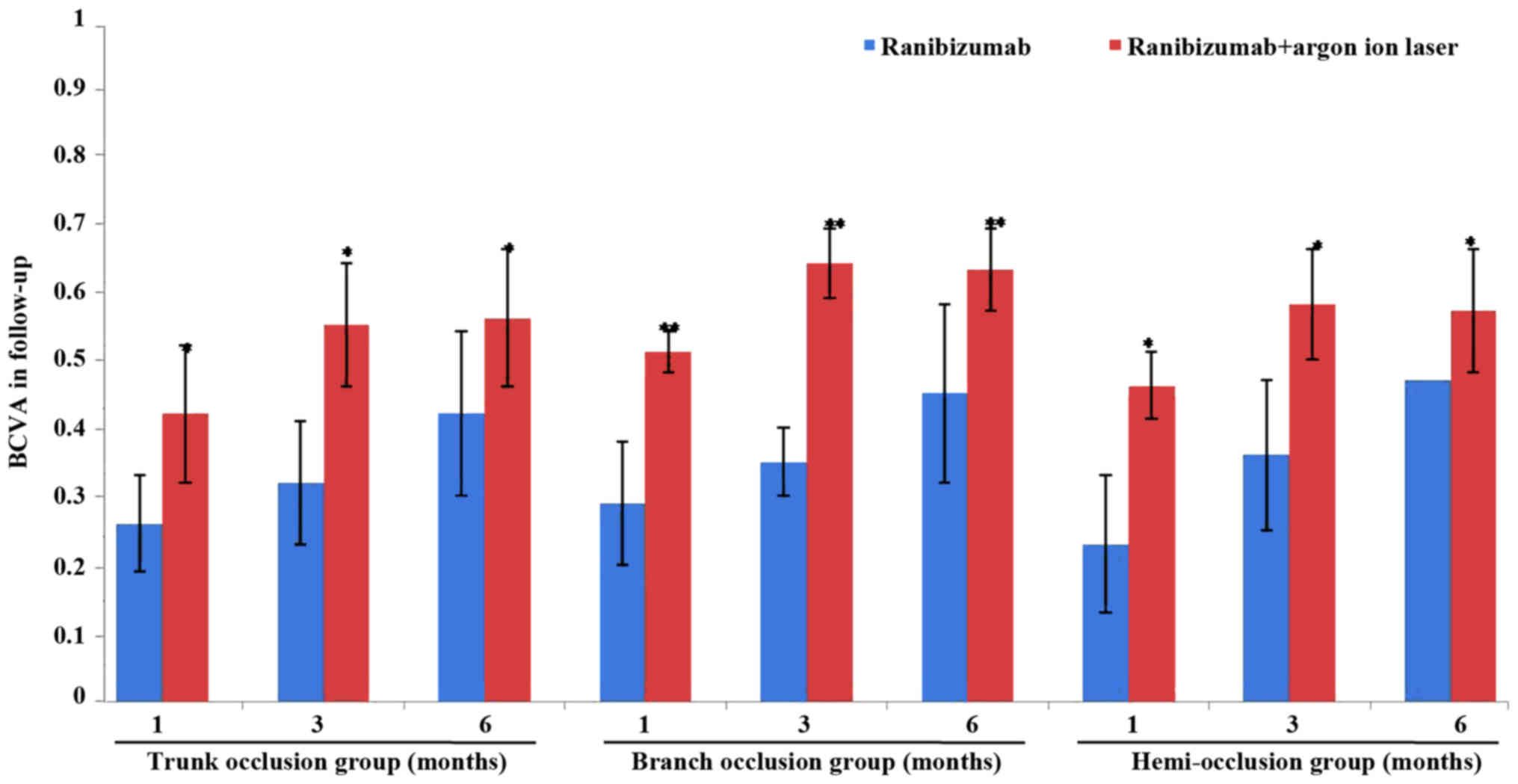
As shown in Fig. 1,
compared with the controls treated with ranibizumab alone, after
patients with the trunk occlusion, the branch occlusion or the
hemi-occlusion were treated with ranibizumab combined with argon
ion laser, the BCVA of all groups improved significantly following
the operation and the branch occlusion group exhibited the best
results (P<0.05; Table II and
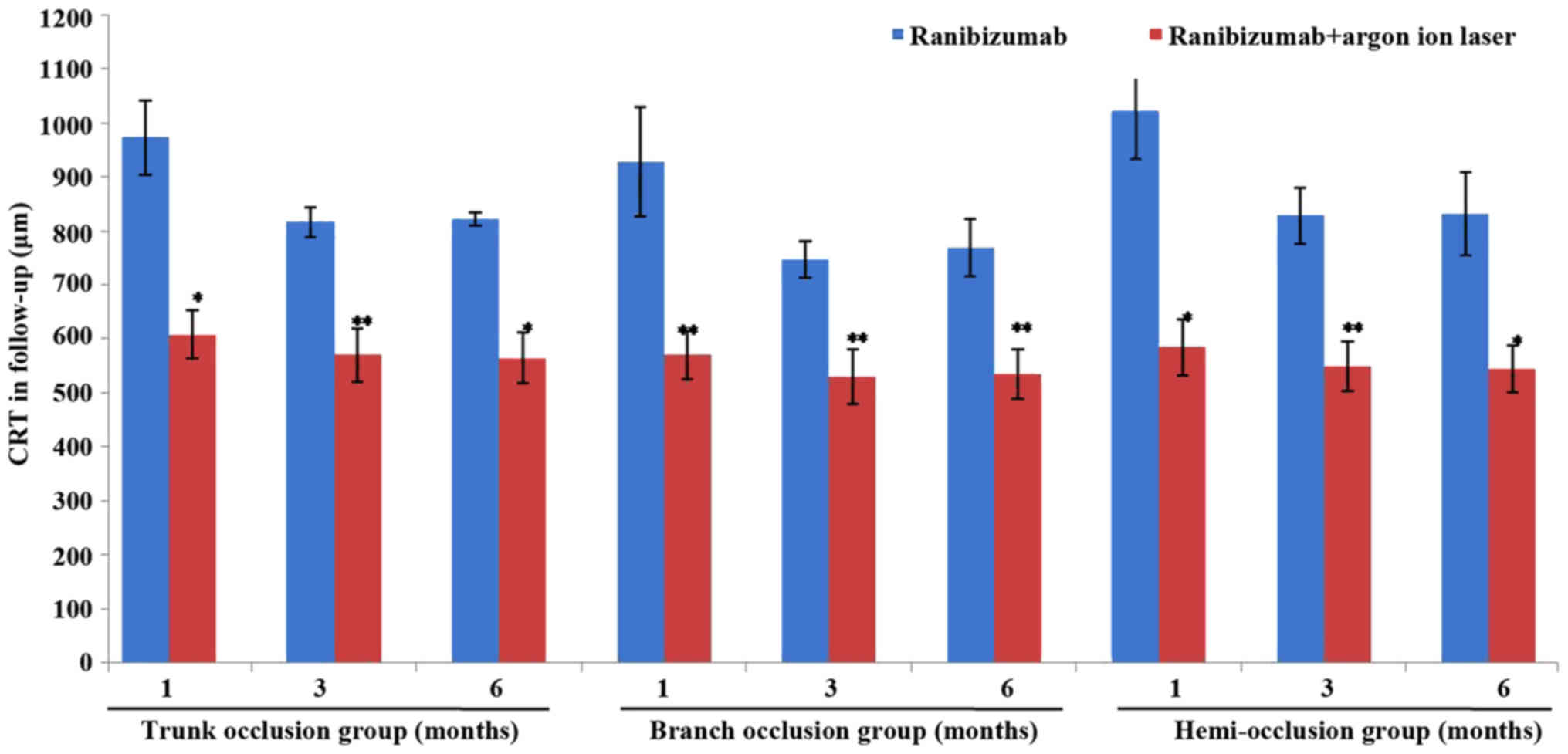
Fig. 1). However, the significant
reduction in the CRT following combination therapy was observed in
all groups and the best effects were noted in the branch occlusion
group (P<0.05; Table III and
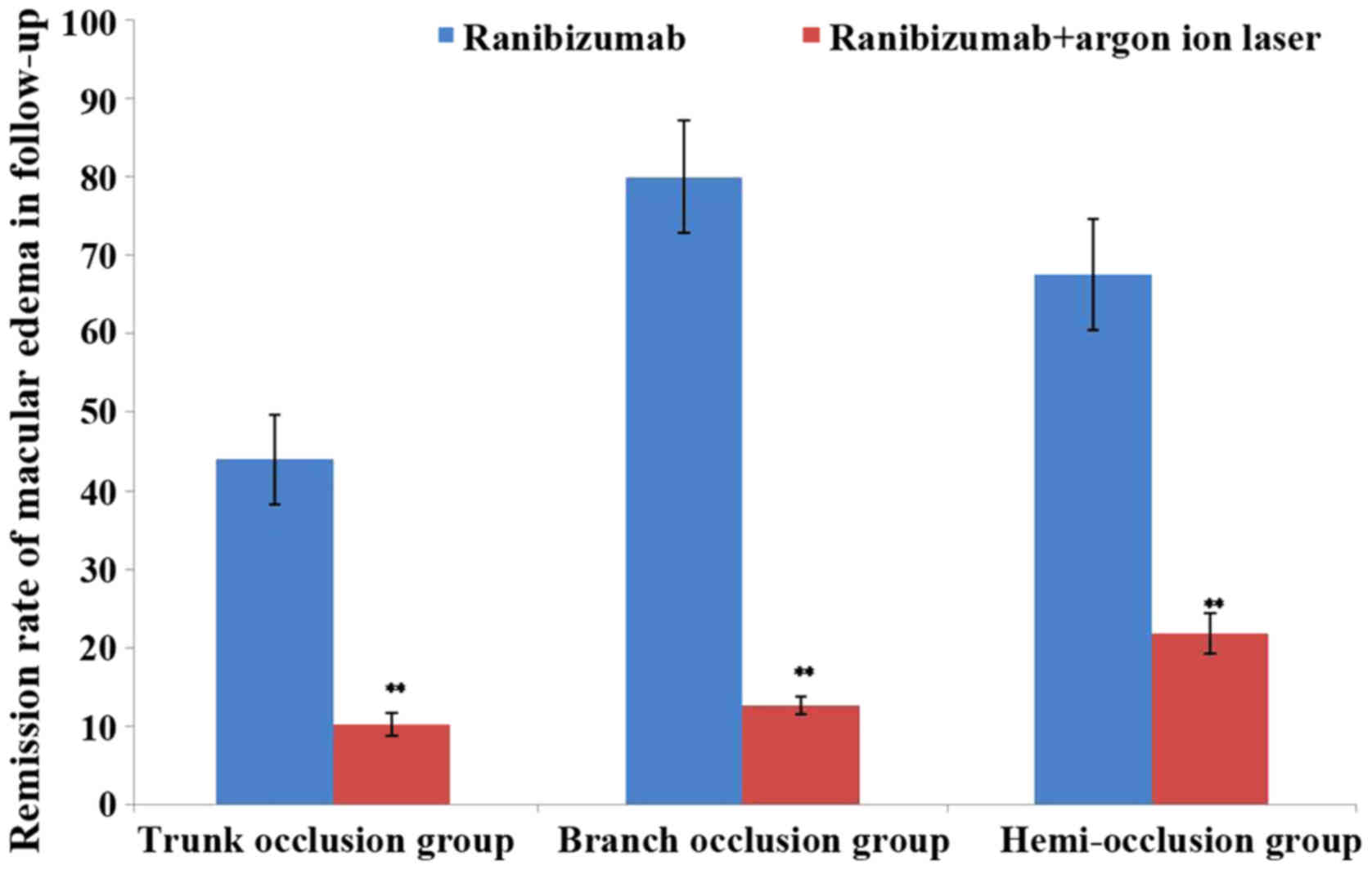
Fig. 2). Furthermore, in the 6-month
follow-up, the remission rate of macular edema of each group
gradually decreased and the effect in branch occlusion group was
the best (Table IV and Fig. 3). In addition, no severe surgical
complications like endophthalmitis, retinal detachment and
intraocular hypertension, were noticed in all three groups (data
not shown).
 | Table II.Comparison of best corrected visual
acuity in follow-up. |
Table II.
Comparison of best corrected visual
acuity in follow-up.
|
| Trunk occlusion
group | Branch occlusion
group | Hemi-occlusion
group |
|---|
|
|
|
|
|
|---|
| Items | Ranibizumab
(n=17) | Ranibizumab + argon
ion laser (n=18) | Ranibizumab
(n=25) | Ranibizumab + argon
ion laser (n=25) | Ranibizumab
(n=18) | Ranibizumab + argon
ion laser (n=19) |
|---|
| 1 month | 0.26±0.07 |
0.42±0.16a | 0.29±0.09 |
0.51±0.23b | 0.23±0.10 |
0.46±0.15a |
| 3 months | 0.32±0.09 |
0.55±0.19a | 0.35±0.15 |
0.64±0.25b | 0.36±0.11 |
0.58±0.18a |
| 6 months | 0.42±0.12 |
0.56±0.21a | 0.45±0.13 |
0.63±0.26b | 0.47±0.06 |
0.57±0.19a |
 | Table III.Comparison of central retinal
thickness in follow-up (µm). |
Table III.
Comparison of central retinal
thickness in follow-up (µm).
|
| Trunk occlusion
group | Branch occlusion
group | Hemi-occlusion
group |
|---|
|
|
|
|
|
|---|
| Items | Ranibizumab
(n=17) | Ranibizumab + argon
ion laser (n=18) | Ranibizumab
(n=25) | Ranibizumab + argon
ion laser (n=25) | Ranibizumab
(n=18) | Ranibizumab + argon
ion laser (n=19) |
|---|
| 1 month | 973±68 | 608±45a | 928±102 | 570±45b | 1021±89 | 585±52a |
| 3 months | 816±27 | 570±50b | 747±34 | 530±50b | 828±51 | 550±46b |
| 6 months | 823±12 | 565±48a | 769±52 | 535±46b | 832±77 | 545±43a |
 | Table IV.Comparison of remission rate of
macular edema in follow-up (%). |
Table IV.
Comparison of remission rate of
macular edema in follow-up (%).
|
| Trunk occlusion
group | Branch occlusion
group | Hemi-occlusion
group |
|---|
|
|
|
|
|
|---|
| Items | Ranibizumab
(n=17) | Ranibizumab + argon
ion laser (n=18) | Ranibizumab
(n=25) | Ranibizumab + argon
ion laser (n=25) | Ranibizumab
(n=18) | Ranibizumab + argon
ion laser (n=19) |
|---|
| 6 months | 44.0±5.7 |
10.2±1.5b | 80.0±7.22 |
12.6±1.2b | 67.6±7.10 |
21.8±2.52b |
Discussion
Therapeutic options for CRVO have been demonstrated
during the last 5 years, which include intravitreally delivered
corticosteroids and intravitreal injections of agents against
vascular endothelial growth factor (VEGF) (10,11). It
was demonstrated in an animal model that obstructed cerebral venous
drainage can lead to increased fundus arteriovenous pressure,
capillary non-perfusion, tissue ischemia, hypoxia and inflammatory
response, and promote the increased release of VEGF and induce
neovascularization. Furthermore, the structure and function of the
novel vessels is imperfect, especially with respect to the
increased permeability, easy bleeding, leakage and edema,
aggravating the retinal and macular pathological changes (12,13). In
the present study, to avoid bias of results, all the patients were
randomly chosen and their situations were different. If the group
size was made the same on purpose it may have lead to a much bigger
bias of results. A literature search was performed and there are
also a number of studies including patient groups of different
sizes. Furthermore, the patients were divided into the following
groups: The trunk occlusion group (12 patients for ranibizumab
treatment and 13 patients for ranibizumab+argon ion laser
treatment), the branch occlusion group (25 patients for ranibizumab
treatment and 25 patients for ranibizumab+argon ion laser
treatment) and the hemi-occlusion group (18 patients for
ranibizumab treatment and 19 patients for ranibizumab+argon ion
laser treatment). Then, the effects of intravitreal injection of
ranibizumab in combination with argon ion laser therapy were
evaluated in the treatment of CRVO in different degrees and present
the safety, and efficacy of this treatment approaches for CRVO in
different degrees.
Ranibizumab is a novel anti-VEGF agent with
humanized monoclonal antibody fragment targeting all isoforms of
VEGF, which is frequently applied in eyes diseases. For instance,
it has been reported that ranibizumab used in the specific
resistance to VEGF can inhibit the production of novel blood
vessels to a greater extent, thereby reducing the retinal exudation
and bleeding (14,15). Studies have confirmed that macular
edema is caused by the damaged blood retinal barrier (internal
barrier) and pigment epithelial barrier (external barrier). CRVO
can lead to internal and external barrier dysfunction at the same
time, and release a variety of endogenous cytokines, among which
VEGF is the most studied (16,17). At
present, the clinical treatment of CRVO-associated persistent
non-ischemic macular edema with laser photocoagulation is still the
standard method (18), which reduces
edema to a certain extent, but the efficacy on complex edema is
poor with severe side effects (19)
and limited vision improvement (20,21).
Previous findings demonstrated that intravitreal injection of
ranibizumab or conbercept combined with laser therapy is an
effective therapeutic option in Coats' disease (22). Another research team confirmed that
ranibizumab 0.5 mg can treat patients with BRVO. Addition of laser
treatment did not lead to better functional outcomes or a reduced
treatment requirement (23). In the
present study, intravitreal injection of ranibizumab combined with
argon ion laser therapy is demonstrated to be a good method for
CRVO treatment.
The results of the present study suggest that BCVA
of the three groups is increased, CRT is reduced, macular edema is
improved and the best status can be obtained at 3 months following
ranibizumab combined with argon ion laser therapy. No obvious
complications occur and the effects in branch occlusion group are
the best. Therefore, it is hypothesized that the intravitreal
injection of ranibizumab combined with argon ion laser
photocoagulation has better safety and effectiveness in the
treatment of different degrees of CRVO. The impairment of vision
from trunk occlusion or hemi-occlusion is more serious than in
branch occlusion and CRT is increased significantly, accompanied by
severe local edema, bleeding, and leakage (24,25);
trunk occlusion or hemi-occlusion frequently involves the distal
branch vessels; although there may be more target vessels in branch
occlusion, pathological changes, including ischemia and
inflammation, are mild, and it has a better response to the
ranibizumab combined with laser photocoagulation therapy (26,27).
In conclusion, the present study suggests that the
clinical application of intravitreal injection of ranibizumab
combined with argon ion laser photocoagulation is suitable for the
treatment of CRVO in different degrees, providing an important
reference for the early screening of population with the optimal
efficacy. This information can be used to help patients achieving
good visual recovery. However, the specific mechanism remains to be
further studied. The shortcomings in this study are the small
sample size, lack of treatment randomization and disunited criteria
of different occlusion degrees.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
DW designed the study and wrote the manuscript. XW
and KW collected the clinical data, JW and GX conducted the
statistical analysis. As the corresponding author, ZC contributed
to the conception and design of the study, approved the final
manuscript as submitted, and revised the manuscript. All authors
approved this edited version of article to be published.
Ethics approval and consent to
participate
Written informed consent was provided by the
patients. The study was approved by the Ethics Committee of Huizhou
Municipal Central Hospital (Huizhou, China).
Patient consent for publication
Not applicable.
Competing interests
All authors declared no competing interests.
References
|
1
|
Wittström E: Central retinal vein
occlusion in younger Swedish adults: Case reports and review of the
literature. Open Ophthalmol J. 11:89–102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yau JW, Lee P, Wong TY, Best J and Jenkins
A: Retinal vein occlusion: An approach to diagnosis, systemic risk
factors and management. Intern Med J. 38:904–910. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nroev VV: Modern aspects of diabetic
retinopathy and diabetic macularoedema treatment. Vestn Ross Akad
Med Nauk. 1:61–65. 2012.
|
|
4
|
Kolar P: Risk factors for central and
branch retinal vein occlusion: A meta-analysis of published
clinical data. J Ophthalmol. 2014:7247802014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuo JZ, Lai CC, Ong FS, Shih CP, Yeung L,
Chen TL, Chen KJ and Wu WC: Central retinal vein occlusion in a
young Chinese population: Risk factors and associated morbidity and
mortality. Retina. 30:479–484. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Priluck IA, Robertson DM and Hollenhorst
RW: Long-term follow-up of occlusion of the central retinal vein in
young adults. Am J Ophthalmol. 90:190–202. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spaide RF, Chang LK, Klancnik JM, Yannuzzi
LA, Sorenson J, Slakter JS, Freund KB and Klein R: Prospective
study of intravitreal ranibizumab as a treatment for decreased
visual acuity secondary to central retinal vein occlusion. Am J
Ophthalmol. 147:298–306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Keshav BR, Zacharia G, Bhat VK, Joseph MK
and Ideculla T: Laser therapy in diabetic macular edema. Oman Med
J. 23:28–31. 2008.
|
|
9
|
Ashraf M, Souka AA and Singh RP: Central
retinal vein occlusion: Modifying current treatment protocols. Eye
(Lond). 30:505–514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ip MS, Scott IU, VanVeldhuisen PC, Oden
NL, Blodi BA, Fisher M, Singerman LJ, Tolentino M, Chan CK and
Gonzalez VH: SCORE Study Research Group: A randomized trial
comparing the efficacy and safety of intravitreal triamcinolone
with observation to treat vision loss associated with macular edema
secondary to central retinal vein occlusion: The Standard Care vs
Corticosteroid for Retinal Vein Occlusion (SCORE) Study Report 5.
Arch Ophthalmol. 127:1101–1114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Haller JA, Bandello F, Belfort R Jr,
Blumenkranz MS, Gillies M, Heier J, Loewenstein A, Yoon YH, Jiao J,
Li XY, et al: Ozurdex GENEVA Study Group: Dexamethasone
intravitreal implant in patients with macular edema related to
branch or central retinal vein occlusion twelve-month study
results. Ophthalmology. 118:2453–2460. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sellam A, Glacet-Bernard A, Coscas F and
Souied EH: Abnormal retinal artery perfusion and optical coherence
tomography angiography. J Fr Ophtalmol. 40:353–362. 2017.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vogl WD, Waldstein SM, Gerendas BS,
Schmidt-Erfurth U and Langs G: Predicting macular edema recurrence
from spatio-temporal signatures in optical coherence tomography
images. IEEE Trans Med Imaging. 36:1773–1783. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Puche N, Glacet A, Mimoun G, Zourdani A,
Coscas G and Soubrane G: Intravitreal ranibizumab for macular
oedema secondary to retinal vein occlusion: A retrospective study
of 34 eyes. Acta Ophthalmol. 90:357–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mete A, Saygili O, Mete A, Gungor K,
Bayram M and Bekir N: Does ranibizumab (Lucentis®)
change retrobulbar blood flow in patients with neovascular
age-related macular degeneration? Ophthalmic Res. 47:141–145. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lucatto LFA, Magalhães-Junior O, Prazeres
JMB, Ferreira AM, Oliveira RA, Moraes NS, Hirai FE and Maia M:
Incidence of anterior segment neovascularization during
intravitreal treatment for macular edema secondary to central
retinal vein occlusion. Arq Bras Oftalmol. 80:97–103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghasemi Falavarjani K, Iafe NA, Hubschman
JP, Tsui I, Sadda SR and Sarraf D: Optical coherence tomography
angiography analysis of the foveal avascular zone and macular
vessel density after anti-VEGF therapy in eyes with diabetic
macular edema and retinal vein occlusion. Invest Ophthalmol Vis
Sci. 58:30–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bitirgen G, Belviranli S, Malik RA,
Kerimoglu H and Ozkagnici A: Effects of panretinal laser
photocoagulation on the corneal nerve plexus and retinal nerve
fiber layer in retinal vein occlusion. Eur J Ophthalmol.
27:591–595. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pikkel YY, Sharabi-Nov A, Beiran I and
Pikkel J: Comparison of anti-vascular endothelial growth factors,
laser treatments and a combination of the both for treatment of
central retinal vein occlusion. Int J Ophthalmol. 9:431–433.
2016.PubMed/NCBI
|
|
20
|
Patel A, Nguyen C and Lu S: Central
retinal vein occlusion: A review of current evidence-based
treatment options. Middle East Afr J Ophthalmol. 23:44–48. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yeh S, Kim SJ, Ho AC, Schoenberger SD,
Bakri SJ, Ehlers JP and Thorne JE: Therapies for macular edema
associated with central retinal vein occlusion: A report by the
American Academy of Ophthalmology. Ophthalmology. 122:769–778.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang L, Ke Y, Wang W, Shi X, Hei K and Li
X: The efficacy of conbercept or ranibizumab intravitreal injection
combined with laser therapy for Coats' disease. Graefes Arch Clin
Exp Ophthalmol. 256:1339–1346. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tadayoni R, Waldstein SM, Boscia F,
Gerding H, Gekkieva M, Barnes E, Das Gupta A, Wenzel A and Pearce
I: BRIGHTER Study Group: Sustained benefits of ranibizumab with or
without laser in branch retinal vein occlusion: 24-month results of
the BRIGHTER Study. Ophthalmology. 124:1778–1787. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bertelmann T, Frank HU, Fuchs HA and
Feltgen N: Branch retinal vein occlusion, macular ischemia, and
intravitreal anti-VEGF therapy. Case Rep Ophthalmol. 8:271–278.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ehlers JP, Kim SJ, Yeh S, Thorne JE,
Mruthyunjaya P, Schoenberger SD and Bakri SJ: Therapies for macular
edema associated with branch retinal vein occlusion: A report by
the american academy of ophthalmology. Ophthalmology.
124:1412–1423. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sakanishi Y, Usui-Ouchi A, Tamaki K,
Mashimo K, Ito R and Ebihara N: Short-term outcomes in patients
with branch retinal vein occlusion who received intravitreal
aflibercept with or without intravitreal ranibizumab. Clin
Ophthalmol. 11:829–834. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chung CY and Li KKW: Optical coherence
tomography angiography wide-field montage in branch retinal vein
occlusion before and after anti-vascular endothelial-derived growth
factor injection. Int Ophthalmol. 38:1305–1307. 2018. View Article : Google Scholar : PubMed/NCBI
|