Introduction
Primary hypertension is a common chronic disease of
the circulatory system, with an incidence rate of 25.2% in China
2017 (1), which is also a key factor
in the mortality of patients with cardiovascular and
cerebrovascular diseases. If not properly controlled, primary
hypertension may induce stroke, myocardial infarction, heart
failure and chronic kidney diseases, leading to high mortality
rates and seriously endangering public health (2,3). It has
been demonstrated previously that, due to population aging and
living standard improving in China, the incidence of primary
hypertension continues to rise, and the proportion of young
patients has also been significantly increasing, which has
attracted great attention from Scientists (4). Continued elevation of blood pressure is
a major feature of primary hypertension, and vascular injury is the
underlying mechanism for the dysregulation of blood pressure
(5). It has been identified that
vascular injuries mainly induce the pathological processes
including endothelial cell injury, smooth muscle cell proliferation
and vascular remodeling (2,6). Vascular endothelial cells serve
important roles in the regulation of blood pressure and the
maintenance of blood vessel walls, and vascular endothelial cell
injuries represent the important cause of hypertension (7). Hypertension-induced injuries of
vascular endothelial cells may lead to the imbalance of the
secretion of NO and endothelin, enhance vasoconstriction, increase
the peripheral circulation resistance and promote the development
of hypertension (8). It has
previously been demonstrated that vascular injuries may cause
massive apoptosis of microvascular endothelial cells, induce damage
in the blood parallel pathways and elevate the peripheral
circulation resistance, resulting in elevated blood pressure
(9). Vascular endothelial cell
injuries serve important roles in the development of hypertension,
which is also a focus in medical research (10). However, the associated molecular
mechanism has not yet been fully elucidated.
The transforming growth factor (TGF)-β family of
cytokines was identified in the 1980s and comprises >30 members
(11,12). At present, four subtypes of TGF-β
have been observed in mammals, including TGF-β1, TGF-β2, TGF-β3 and
TGF-β1β2 (13). TGF-β1 is currently
the most well-studied member of the TGF family, which serves
important regulatory roles in various pathophysiological processes,
such as cell proliferation, differentiation, migration and
apoptosis (14,15). Activated TGF-β1 exerts biological
functions through cell surface receptors. The classical TGF-β1
signaling pathway is mainly mediated by the downstream mothers
against decapentaplegic homolog (SMAD) family (16). In addition, TGF-β1 can exert
biological functions through a variety of non-classical pathways,
such as the c-Jun N-terminal kinase, extracellular signal-regulated
kinase, and p38/mitogen-activated protein kinase pathways (17–19). A
previous study has demonstrated that TGF-β1 can regulate the
synthesis of collagen and the expression of extracellular
matrix-related protein, thus promoting myocardial fibrosis
(20,21). Furthermore, in hypertension, TGF-β1
can induce the proliferation of vascular smooth muscle (22). It has also been reported that the
expression of TGF-β1 is positively correlated with disease
development, as detected in the peripheral blood and urine of
patients with myocardial infarction and hypertensive nephropathy
(23). Furthermore, peripheral blood
tests for hypertensive patients have demonstrated that the
concentration of TGF-β1 is significantly increased compared with
the healthy control group (24).
These findings suggest that TGF-β1 may be closely associated with
vascular injuries in hypertensive patients.
VPS34 was initially identified in yeast, which is
analogous tophosphatidylinositol 3-kinase catalytic subunit type 3
(PI3KC3) in mammals (25). PI3KC3
has been demonstrated to serve an important regulatory role in
autophagic processes (26).
Autophagy is an indispensable physiological process through which
normal cells maintain their homeostasis and activities, the main
function of which comprises the recycling of aging organelles and
the degradation of abnormal proteins (27). Abnormal autophagic processes may lead
to mitochondrial damages, thus resulting in cell death (28). Aberrant activity of autophagy has
been demonstrated to serve important roles in vascular injuries. In
diabetic patients, persistent hyperglycemia induces increased
reactive oxygen species in endothelial cells, which in turn
activates the endothelial cell autophagy, resulting in endothelial
cell death and vascular injuries (29).
In recent years, a number of studies have confirmed
that TGF-β1 can regulate the cell autophagy activity, with
differential roles in different disease processes (30,31).
Whether TGF-β1 participates in the vascular injury in hypertensive
patients via autophagy remains unclear. In the present study, the
regulating effect of TGF-β1 on PI3KC3 and its role in vascular
injuries were investigated.
Materials and methods
Study patients
A total of 28 patients with primary hypertension, 17
males and 11 females, with a mean age of 56.3±4.3 years, who were
admitted to the Department of Cardiology at Zaozhuang Municipal
Hospital, (Zaozhuang, China) from January 2016-January 2017, were
included in the present study. According to the Guidelines for
Hypertension Diagnosis and Treatment in China (32), these patients were divided into three
groups as follows: Grade I hypertension (n=10), grade II
hypertension (n=9) and grade III hypertension (n=9). All patients
were first diagnosis cases with no long-term medication history,
combined tumors or other chronic diseases, autoimmune diseases or
family genetic diseases. Furthermore, 20 healthy subjects were
recruited as the control group. From each patient and healthy
subject, 5 ml peripheral blood was collected. Prior written and
informed consent was obtained from all subjects and the present
study protocol was approved by the ethics review board of Zaozhuang
Municipal Hospital (Zaozhuang, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Blood samples were collected and total RNA was
extracted with TRIzol (life Technologies; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) within 30 min. The cDNA template was
obtained via the Ipsogen RT kit (Qiagen GmbH, Hilden, Germany).
qPCR was performed using a Fast Fire qPCR PreMix kit (Tiangen
Biotech Co., Ltd., Beijing, China) with a Step one Plus PCR machine
(Thermo Fisher Scientific, Inc.). Primer sequences were as follows:
PI3KC3, forward 5′-CTCGCTTCGGCAGCACA-3′ and reverse
5′-AACGCTTCACGAATTTGCGT-3′; GAPDH forward,
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse,
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′. The 20-µl PCR reaction mixture
consisted of 10 µl RT-qPCR-Mix, 0.5 µl primereach, 2 µl cDNA and 7
µl ddH2O. The PCR conditions were set as follows: 95°C
for 5 min and 40 cycles of 95°C for 30 sec, 60°C for 45 sec and
72°C for 30 sec; followed by 72°C for 20 sec. The relative
expression levels of the target genes were calculated using the
2−ΔΔCq method (33).
GAPDH was used as the internal control.
Human umbilical vein endothelial cell
(HUVEC) treatment and transfection
HUVECs were purchased from the Cell Bank, Chinese
Academy of Sciences (Shanghai, China). TGF-β1 recombinant protein
was purchased from R&D Systems, Inc. (Minneapolis, MN, USA).
HUVECs were cultured at 37°C in a 5% CO2 incubator, in
RPMI 1640 culture medium (Thermo Fisher Scientific, Inc.)
containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific,
Inc.). For cell treatment (at 37°C in a 5% CO2
incubator), 5 µg/ml TGF-β1 was added into the culture medium, to
incubate the HUVECs at 37°C in a 5% CO2 incubator for 48
h.
For cell transfection, following the treatment with
TGF-β1 for 48 h, HUVECs were seeded in a 24-well plate, at a
density of 1×105 cells/well, and cultured, at 37°C in a
5% CO2 incubator for 12 h, with RPMI-1640 medium
containing 10% FBS. Cells were then divided into the empty vector
(0.5 µg) and PI3KC3 groups. When 60% confluence was reached, 0.5 µg
PI3KC3 plasmid (Shanghai GeneChem Co., Ltd., Shanghai, China) and 2
µl Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.)
were added into 50 µl OptiMem culture medium (Thermo Fisher
Scientific, Inc.), in Eppendorf tubes, at room temperature for 5
min. The solution in the two separate tubes (lipofectamine and
plasmid, respectively) was then mixed together gently, and the
mixture was placed at room temperature for 15 min prior to
incubation at 37°C for 6 h. The incubation medium was then replaced
with RPMI-1640 medium containing 20 ng/ml TGF-β1, followed by
incubation for 48 h at 37°C in a 5% CO2 incubator, prior
to further investigation.
Cell Counting Kit (CCK)-8 assay
Proliferation of HUVECs was detected with the CCK-8
assay. Briefly, cells were seeded onto a 96-well plate, at a
density of 2×103 cells/well, and cultured with 200 µl
RPMI-1640 complete medium at 37°C in a 5% CO2 incubator.
According to the manufacturer's protocol, CCK-8 (Beyotime Institute
of Biotechnology, Haimen, China) was added into each well to
incubate the cells for 30 min, and the optical density values at
490 nm were detected at 24, 48 and 72 h. The experiment was
performed in triplicate, and cell proliferation curves were
produced.
Transwell chamber assay
The migration ability of HUVECs was assessed using
the Transwell chamber assay. A total of 2×105 HUVECs
were seeded into the upper chamber of a Transwell insert (8 µm;
Corning Incorporated, Corning, NY, USA) and cultured with 200 µl
serum-free RPMI 1640 culture medium, while 500 µl RPMI 1640 medium
containing 20% FBS was added into the lower chamber. Following 24
h, the cells in the upper chamber were fixed at room temperature
with 4% formaldehyde for 10 min. Giemsa staining was then performed
at room temperature for 2 min, and the cells were observed under a
light microscope (magnification, ×100). Five fields were randomly
selected, and the number of penetrating cells were counted. Cell
migration ability was assessed accordingly.
Flow cytometry
Cellular apoptotic processes were assessed with flow
cytometry. HUVEC apoptosis was induced by hypoxia, as follows:
Following transfection, HUVECs were cultured in RPMI-1640 culture
medium containing TGF-β1 (20 ng/ml) at 37°C for 48 h, and then
cultured in a 1% O2, 5% CO2, 37°C incubator
for 4 h. Following washing, the cells were collected, and the cell
density was adjusted to 1×106 cells/100 µl. Flow
cytometry was performed with the Annexin V/FITC Apoptosis Detection
kit I (BD Biosciences, Franklin Lakes, NJ, USA), according to the
manufacturer's instructions, using a flow cytometer. Annexin V
single-positive cells were undergoing early apoptosis, propidium
iodide (PI; provided by the kit) single-positive cells were
undergoing necrosis, and PI and Annexin V double-positive cells
were undergoing late apoptosis. For the detection of Ki67, cells
were incubated with 20 µl of the PE-labeled Ki67 (BD Biosciences)
in the dark at room temperature for 10 min. Analyses was performed
with the ModFit LT 5.0 software (BD Biosciences).
Ad-GFP-LC3 adenovirus transfection and
detection of autophagosomes
Following treatment with TGF-β1, HUVECs were seeded
onto a culture dish at a density of 1×105 cells/well.
The mRFP-GFP-LC3 adenovirus (Hanbio Biotechnology Co., Ltd.,
Shanghai, China) was used to transfect the cells at multiplicity of
infection=20 for 48 h. The cells were then washed with pre-chilled
PBS twice, and fixed at room temperature with 4% formaldehyde for
15 min. Following washing, the autophagosomes were observed under
laser confocal microscopy (magnification, ×400; SP8; Leica
Microsystems GmbH, Wetzlar, Germany). Five fields were randomly
selected, and the autophagic process was assessed.
Western blot analysis
Cells were lysed with radio immunoprecipitation
assay lysis containing 1% PMSF. Total protein concentration was
determined using the BCA method. Protein samples (20 µg) were
separated by SDS-PAGE, and then electronically transferred onto a
polyvinylidene difluorude membrane. Following blocking with 5%
non-fat milk at room temperature for 1 h, the membrane was
incubated with the rabbit anti-human anti-LC3B primary antibody
(1:1,000; cat. no. AL221; Beyotime Institute of Biotechnology),
rabbit anti-human anti-PI3KC3 primary antibody (1:1,000; cat. no.
ab124905; Abcam, Cambridge, UK), rabbit anti-human anti-p62 primary
antibody (1:1,000; cat. no. ab155686; Abcam) and mouse anti-human
anti-GAPDH primary antibody (1:5,000; cat. no. AF0006; Beyotime
Institute of Biotechnology) at 4°C overnight. Following washing
with TBST, the membrane was incubated with horseradish
peroxidase-conjugated goat anti-mouse (1:4,000; cat. no. A0192;
Beyotime Institute of Biotechnology) or goat anti-rabbit (1:4,000;
cat. no. A0208; Beyotime Institute of Biotechnology) secondary
antibody, at room temperature for 1 h. Following washing with TBST,
protein bands were developed using the P0018 enhanced
chemiluminescence kit (Beyotime Institute of Biotechnology).
Analysis was performed using quantityone software (V4.62; Bio-Rad
Laboratories, Hercules, CA, USA).
ELISA
TGF-β1 levels were detected using the Human TGF-beta
1 Quantikine ELISA kit (cat. no. DB100B; R&D Systems, Inc.),
according to the manufacturer's protocol. Peripheral blood (5 ml)
was collected and incubated at 4°C overnight. Serum was obtained
via centrifugation at 12,000 × g at 4°C for 10 min, to detect
TGF-β1.
lactate dehydrogenase (LDH)
detection
LDH levels were detected using the LDH Cytotoxicity
Assay kit (cat. no. C0016) from Beyotime Institute of
Biotechnology. The culture supernatant was collected and
centrifuged at 12,000 × g at 4°C for 10 min. Detection was
performed according to the manufacturer's protocol.
Statistical analysis
Data were expressed as the mean ± standard
deviation. SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) was
used for statistical analysis. Student's t-test was performed for
comparison between two groups, and one-way analysis of variance was
performed for multiple group comparisons, with a
Student-Newman-Keuls post hoc test. Spearman's rank correlation
analysis was used to measure the correlation between PI3KC3 and
TGF-β1. P<0.05 was considered to indicate a statistically
significant difference.
Results
PI3KC3 is negatively correlated with
TGF-β1 in peripheral blood of hypertensive patients
Previous studies have demonstrated that the TGF-β1
expression is significantly elevated in the peripheral blood of the
hypertensive patients (34), which
has diagnostic values for the hypertension-induced heart or kidney
injuries. Therefore, the mRNA expression levels of PI3KC3 in the
peripheral blood samples of the patients with hypertension were
initially investigated via RT-qPCR. The results demonstrated that,
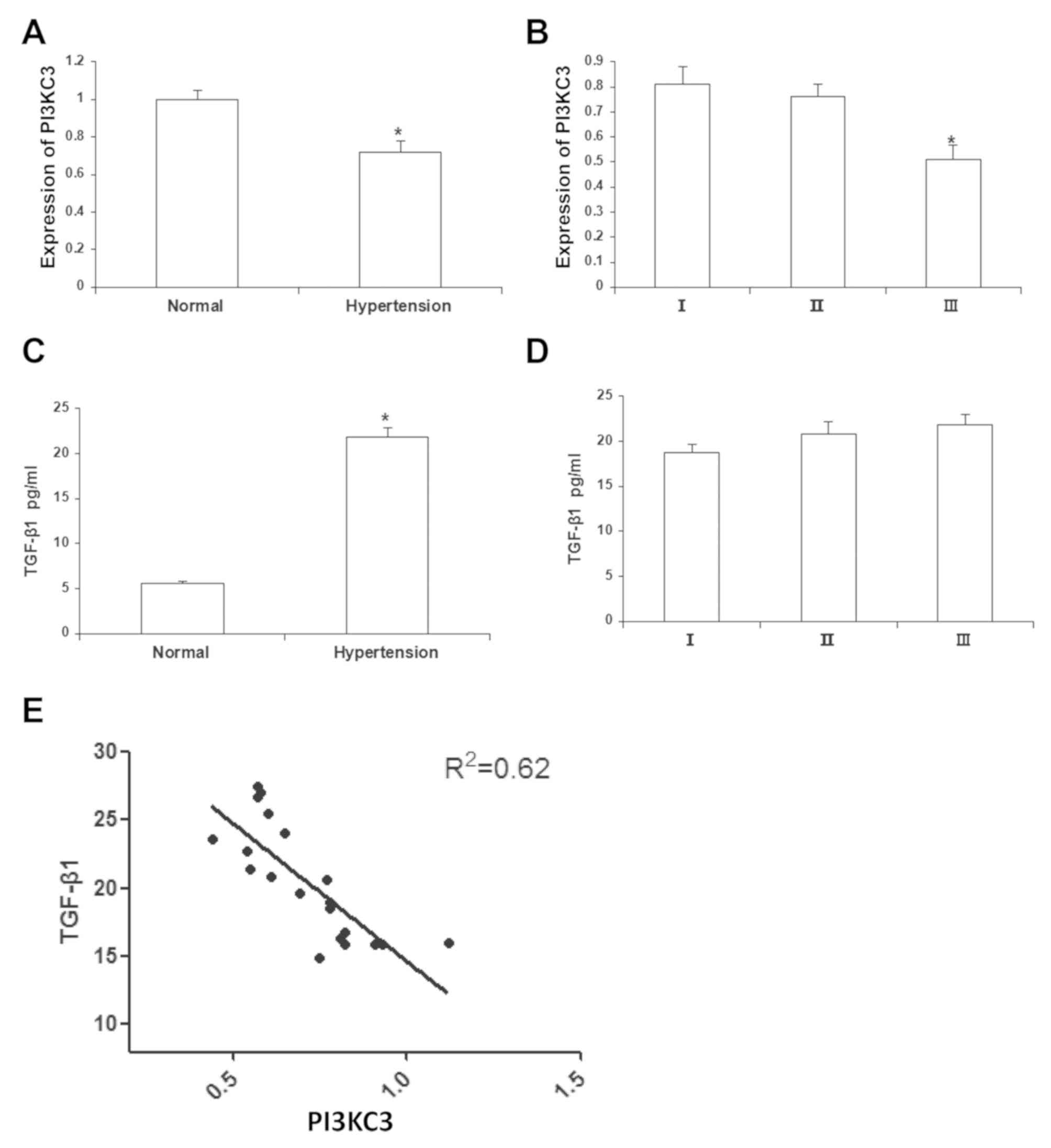
compared with the normal control subjects, the mRNA expression
levels of PI3KC3 in the peripheral blood of the hypertensive
patients were significantly declined (0.72±0.06; P<0.05;
Fig. 1A). For the sub-group
analysis, the results demonstrated that the mRNA expression levels
of PI3KC3 in the grades I and II hypertensive patients were
0.81±0.07 and 0.76±0.05, respectively, which were both
significantly higher than the grade III hypertensive patients
(0.51±0.08; P<0.05; Fig. 1B).
Furthermore, the TGF-β1 contents in the peripheral blood of these
patients were detected with ELISA. The results demonstrated that,
the TGF-β1 contents in the peripheral blood of the hypertensive
patients were significantly elevated (21.70±0.34 pg/ml) compared
with normal controls (P<0.05; Fig.
1C), whereas no significant differences were observed in the
peripheral TGF-β1 content between patients with different grades of
hypertension (Fig. 1D). The
association between TGF-β1 content and PI3KC3 mRNA expression in
the peripheral blood was analyzed. The results demonstrated that
there was a negative correlation between the TGF-β1 content and
PI3KC3 mRNA expression (R2=0.62; P<0.05; Fig. 1E). These results suggest that TGF-β1
and PI3KC3 are negatively correlated in the peripheral blood of the
patients with hypertension.
TGF-β1 reduces PI3KC3 expression and
autophagic activities in HUVECs
Continuously increased peripheral blood pressure in
patients with hypertension represents a stress factor on
endothelial cells, and autophagy, as a physiological process to
maintain intracellular stability, is of great importance for the
repair of endothelial cell injuries. PI3KC3 is a key regulator for
the autophagic process (35).
Therefore, the effects of TGF-β1 on the regulation of PI3KC3 and
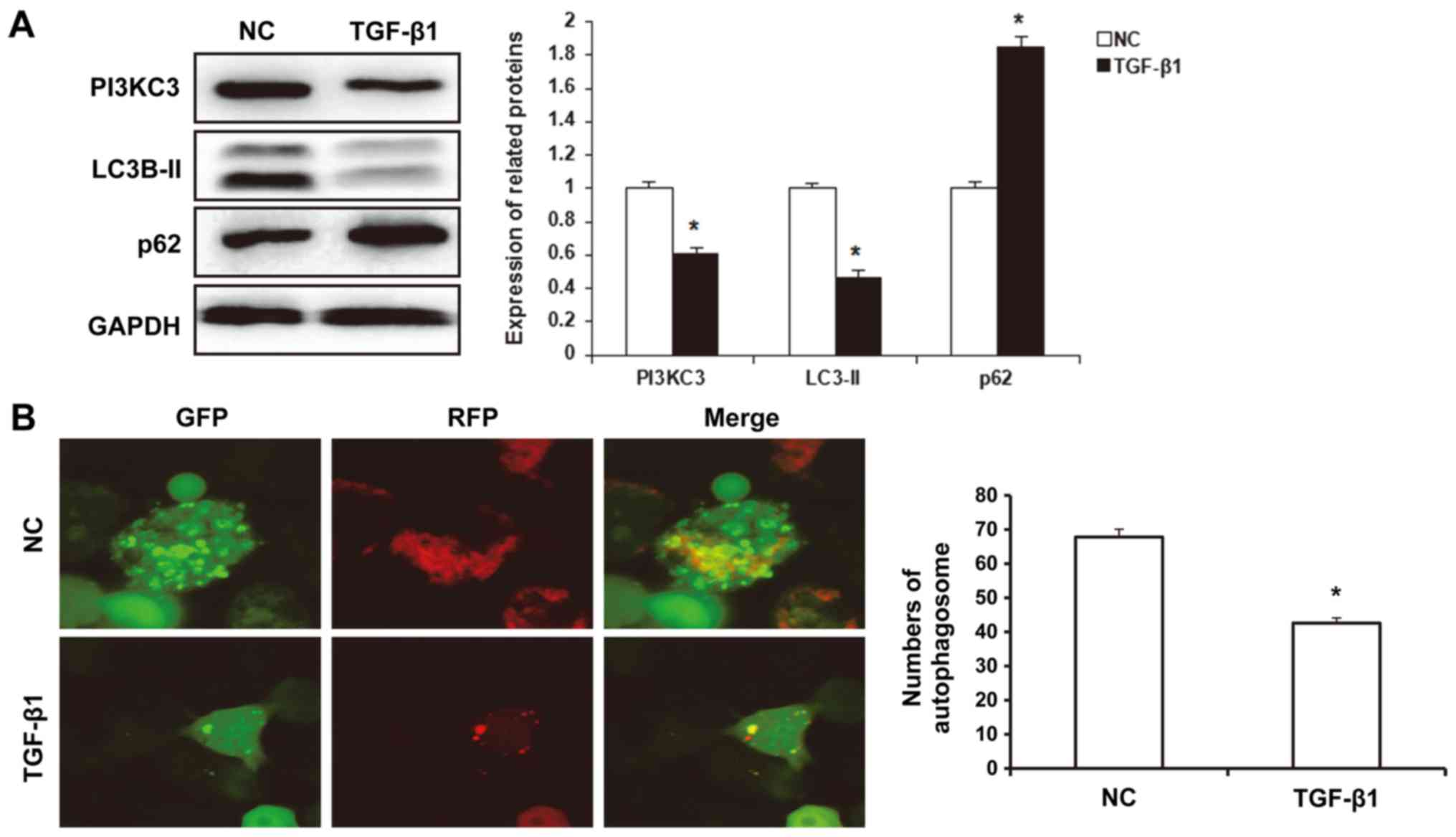
the autophagic activities were investigated. The results from the
western blot analysis demonstrated that, under hypoxia conditions,
the expression levels of LC3B-II protein in the TGF-β1-treated
HUVECs (0.46±0.05) were significantly reduced compared with
controls (P<0.05), whereas the p62 protein expression levels
(1.85±0.06) were significantly elevated compared with the control
group (P<0.05; Fig. 2A). Laser
scanning confocal microscopy demonstrated that, under hypoxia
conditions, the number of autophagosomes in the TGF-β1-treated
HUVECs (42.6±1.5) was significantly declined, compared with the
control group (67.8±2.3; P<0.05; Fig.
2B). Furthermore, the results from the western blot analysis
demonstrated that, the expression levels of PI3KC3 in the
TGF-β1-treated HUVECs (0.61±0.03) were significantly declined
compared with controls (P<0.05; Fig.
2A). These results suggest that TGF-β1 reduced the expression
levels of PI3KC3 and the autophagic activities in the HUVECs.
TGF-β1 promotes HUVEC injuries via
inhibiting PI3KC3-mediated regulation of autophagy
Autophagy helps regulate cellular stress, and
factors that inhibit autophagic activities may lead to cellular
damages (36). To further
investigate whether TGF-β1 regulates cell viability through
PI3KC3-mediated autophagy regulation, the cellular autophagic
activities were detected. The results demonstrated that, compared
with the TGF-β1 group, the expression level of LC3B-II protein was
markedly elevated in the TGF-β1+PI3KC3 over-expression group
compared with the TGF-β1 group, which indicated enhanced autophagic
activities, and LCSM revealed that the number of autophagosomes in
the TGF-β1-treatment group was significantly lower than the control
group, while the number of autophagosomes was significantly higher
in the TGF-β1+PI3KC3 overexpression group compared with the TGF-β1
group. These results indicate that PI3KC3 restored TGF-β1-induced
autophagy inhibition (Fig. 3A and
B). To assess the tolerance of HUVECs against hypoxia-induced
stress, the cellular apoptosis rates were detected by flow
cytometry. The results demonstrated that for the NC group, the
HUVEC apoptosis rate was 18.5±1.1%; for the TGF-β1 group, the
apoptosis rate was 33.8±2.7%; and for the TGF-β1+PI3KC3
over-expression group, the apoptosis rate was 19.7±0.9% (Fig. 3C). The LDH release in the cell
culture supernatant was also investigated. The results demonstrated
that, compared with the control group, the relative LDH content in
the culture supernatant in the TGF-β1 group was 3.5±0.54-fold
greater, and for the TGF-β1+PI3KC3 over-expression group, the
relative LDH content was 1.97±0.36-fold greater, which was
significantly decreased compared with the TGF-β1 group (Fig. 3D). These results suggest that TGF-β1
could promote HUVEC injuries via inhibiting PI3KC3-mediated
regulation of autophagy.
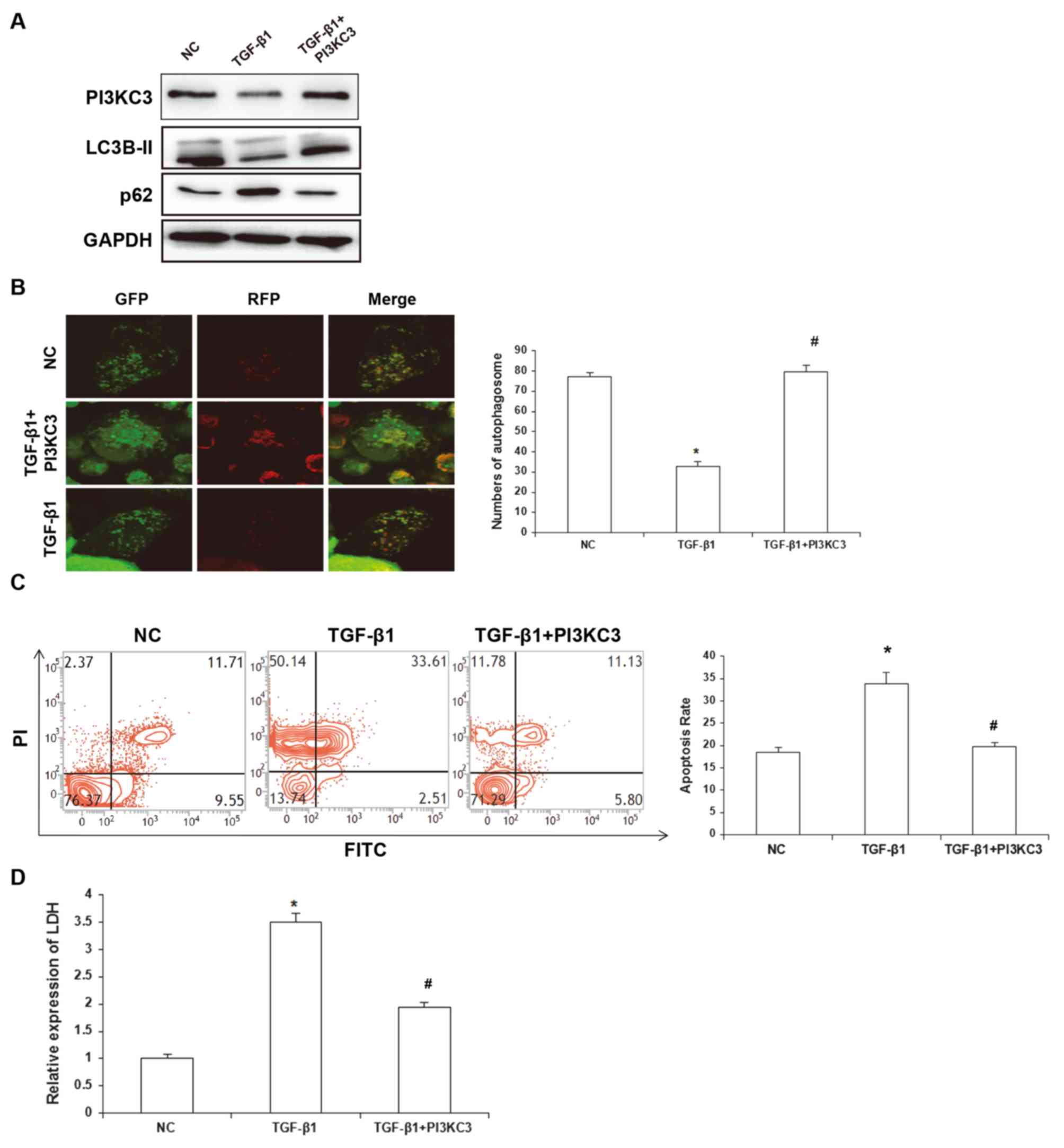 | Figure 3.Effects of TGF-β1 and PI3KC3
overexpression on autophagic activity and apoptotic process of
HUVECs. (A) Following the treatment of TGF-β1, the HUVECs were
overexpressed with PI3KC3 and the expression levels of PI3KC3,
LC3B-II and p62 were detected via western blot analysis. (B)
Following the treatment of TGF-β1, the HUVECs were overexpressed
with PI3KC3 and the autophagosomes were detected with laser
scanning confocal microscopy. (C) Following the treatment of TGF-β1
and overexpression of PI3KC3, under hypoxia conditions the
apoptosis of HUVECs was detected with flow cytometry. (D) Following
the treatment of TGF-β1 and overexpression of PI3KC3, under hypoxia
conditions, the LDH release in the HUVEC culture supernatant was
determined. Magnification, ×400. *P<0.05 vs. NC;
#P<0.05 vs. TGF-β1. TGF, transforming growth factor;
PI3KC3, phosphatidylinositol 3-kinase catalytic subunit type 3;
HUVECs, human umbilical vein endothelial cells; LDH, lactate
dehydrogenase; NC, negative control; PI, propidium iodide; FITC,
fluorescein isothiocyanate. |
TGF-β1 inhibits HUVEC proliferation
via downregulating PI3KC3 expression
Following vascular endothelial injury, endothelial
cells may enter a proliferative cycle, which is a key factors for
the vascular repair process (37).
In order to study the effect of TGF-β1-induced downregulation of
PI3KC3 expression on the proliferation of HUVECs, a CCK-8 assay was
performed. The results demonstrated that, compared with the NC
group, HUVEC proliferation ability was significantly declined in
the TGF-β1 group (P<0.05) at 24–72 h. Compared with the TGF-β1
group, the OD490 values at 24–72 h in the TGF-β1+PI3KC3
over-expression group were significantly increased (P<0.05;
Fig. 4A). The Ki67 positive cell
numbers were further assessed by flow cytometry. The results
demonstrated that, the number of Ki67 positive HUVECs in the TGF-β1
group was significantly lower than the control group and the
TGF-β1+PI3KC3 over-expression group (P<0.05), indicating
decreased cell proliferation ability (Fig. 4B). These results suggest that TGF-β1
may inhibit the HUVEC proliferation ability via downregulation of
PI3KC3, and the decreased cell proliferation ability may inhibit
the vascular endothelial injury repair process.
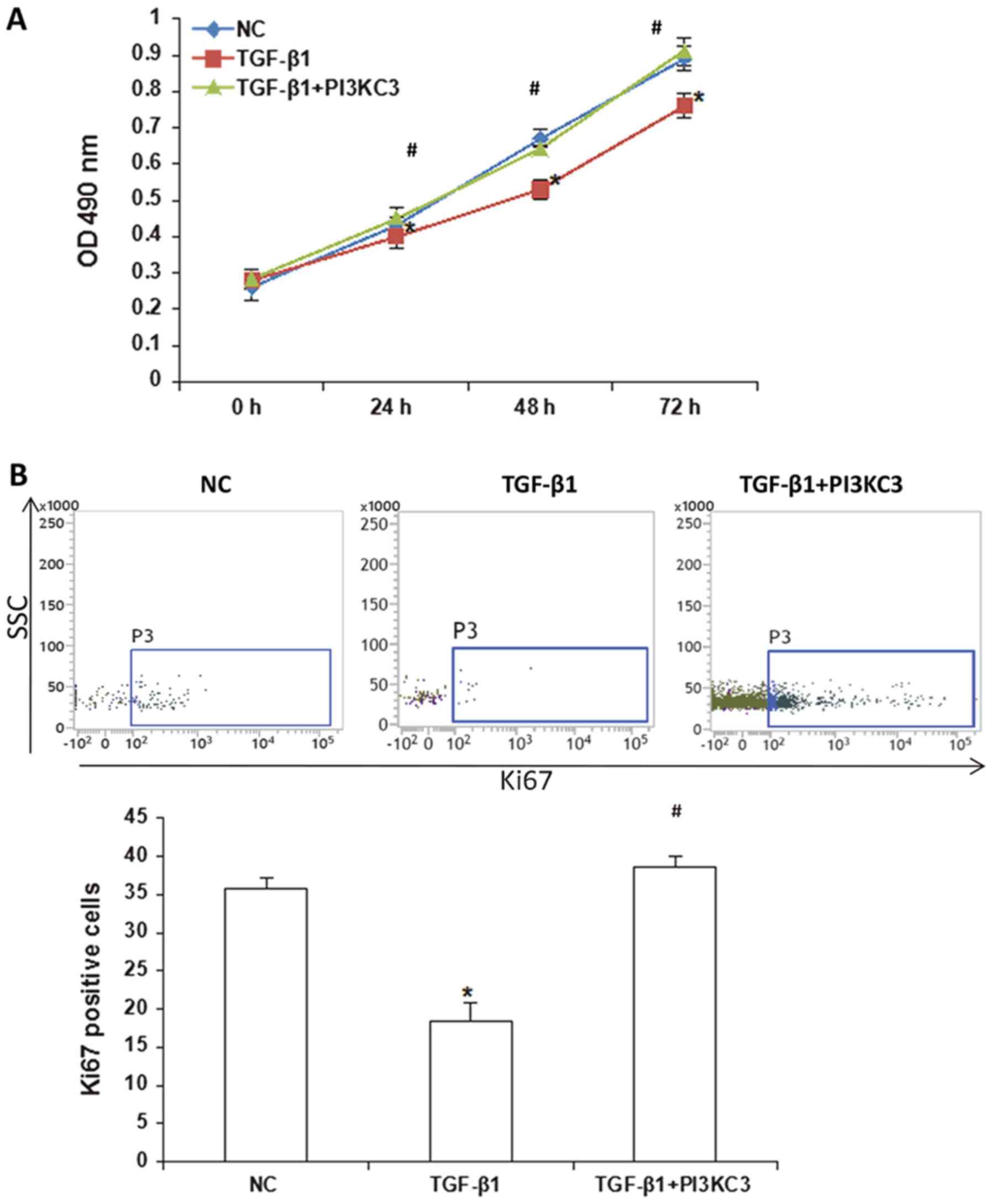 | Figure 4.Effects of TGF-β1 and PI3KC3
overexpression on proliferation of HUVECs. (A) Following the
treatment of TGF-β1, the HUVECs were overexpressed with PI3KC3, and
the cell proliferation was detected via a cell counting kit-8
assay. (B) Following the treatment of TGF-β1, the HUVECs were
overexpressed with PI3KC3, and the Ki67 positive-level was detected
with flow cytometry. *P<0.05 vs. NC; #P<0.05 vs.
TGF-β1. TGF, transforming growth factor; PI3KC3,
phosphatidylinositol 3-kinase catalytic subunit type 3; HUVECs,
human umbilical vein endothelial cells; NC, negative control; OD,
optical density; SSC, side-scattering. |
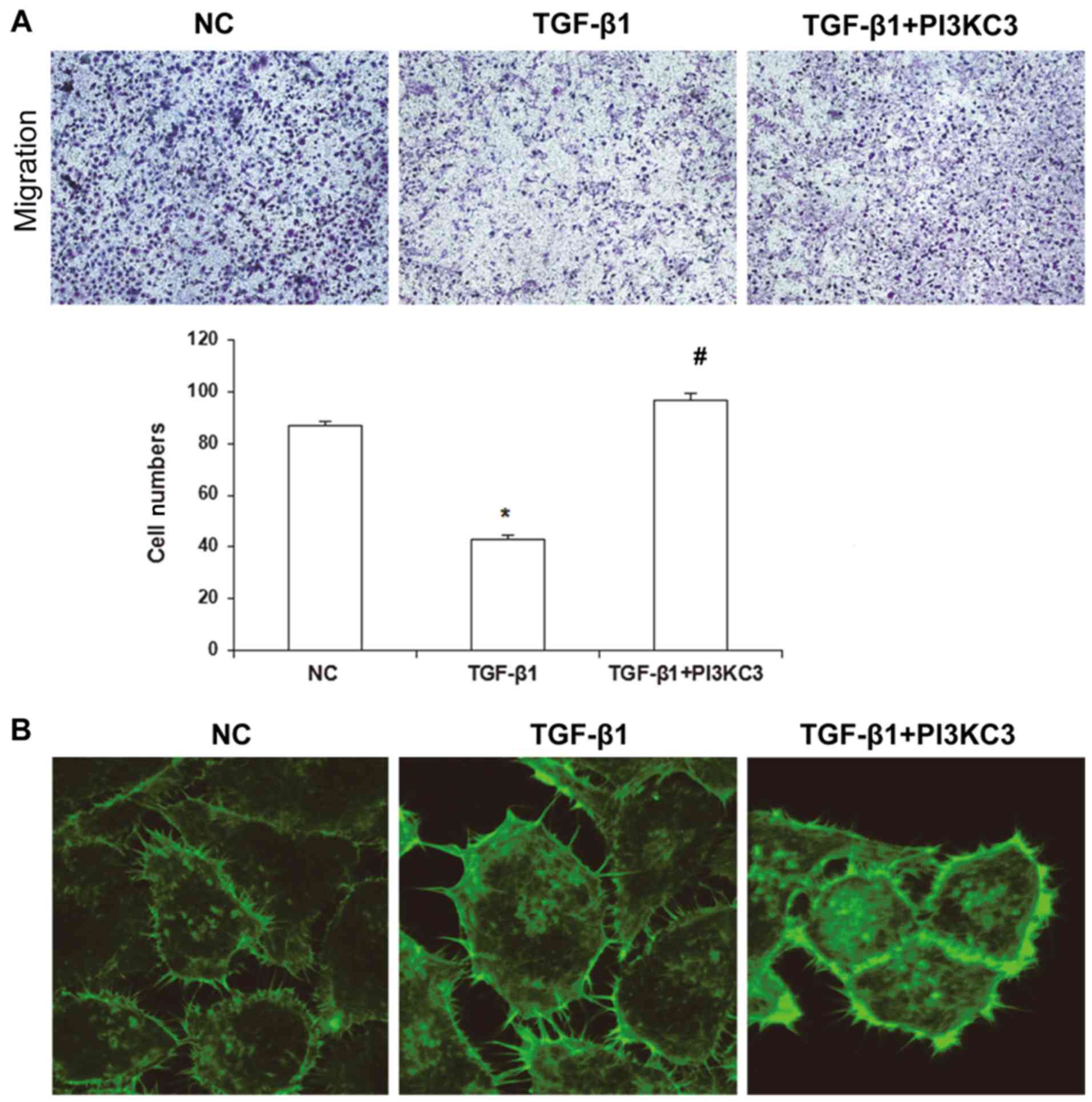
TGF-β1 inhibits HUVEC migration
ability via downregulating PI3KC3 expression
It has been demonstrated that endothelial cells have
the ability to migrate. Following vascular injury, the adjacent
vascular endothelial cells may begin to proliferate or migrate to
the lesion sites under the direction of local chemotactic factors,
repairing the vascular wall (38).
To investigate the migration ability of HUVECs, a Transwell chamber
assay was performed. The results demonstrated that, compared with
the NC group, the HUVEC migration ability in the TGF-β1 group was
significantly decreased, as indicated by the decreased penetrating
cells (P<0.05), whereas the penetrating cells were significantly
increased in the TGF-β1+PI3KC3 over-expression group compared with
the TGF-β1 group (85.6±3.5; P<0.05; Fig. 5A). The cytoskeletal changes in these
cells were further observed by laser confocal microscopy. The
results demonstrated that, compared with the NC and TGF-β1+PI3KC3
groups, the synapses on the HUVEC membrane were significantly
decreased in the TGF-β1 group, as indicated by the declined
fluorescence intensity and quantity, which also suggests decreased
migration ability (Fig. 5B). These
results suggest that TGF-β1 may inhibit the migration ability of
HUVECs via downregulating PI3KC3 expression.
Discussion
Vascular endothelial injury is one of the most
commonly reported pathological changes in patients with
hypertension, which has a marked impact on the occurrence and
development of hypertension and associated complications (29). TGF-β1 is associated with various
physiological and pathological biological processes. Due to its
diverse functions and complex mechanism of action, TGF-β1 has
notable clinical value in various diseases, including tumor
metastasis and tissue fibrosis (13,39), and
has thus become a focus in medical research. Previous studies have
demonstrated that TGF-β1 serves an important role in the
development of hypertension and its associated complications,
particularly in the regulation of various pathological processes
(40,41). In the present study, the results
demonstrated that the expression of TGF-β1 in the peripheral blood
was significantly elevated in patients with hypertension, which was
negatively correlated with the autophagy regulator PI3KC3. The
in vitro results demonstrated that TGF-β1 could downregulate
the expression of PI3KC3 to inhibit the proliferation, migration
and autophagy of HUVECs, while promoting cellular apoptosis and
aggregating the vascular endothelial injuries in patients with
hypertension.
TGF-β1, as a multi-functional cytokine, serves an
important role in the development of hypertension. A number of
studies have demonstrated that the contents of TGF-β1 in the
peripheral blood are significantly increased in patients with
hypertension (42,43). In accordance with this, the present
results demonstrated that, compared with the healthy control group,
the TGF-β1 contents in the peripheral blood were significantly
elevated in hypertensive patients, which was also associated with
hypertension grading. In addition, TGF-β1 is associated with
various hypertension-induced complications. Wei et al
(44) have recently demonstrated
that the TGF-β1/SMAD3 pathway mediates hypertension-induced
myocardial fibrosis (45). The
TGF-β1/p53 signaling pathway serves an important role in
hypertension-induced renal fibrosis (46). For vascular injuries, it has been
reported that, TGF-β1 can promote the proliferation of vascular
smooth muscle, leading to thickened vascular muscle layer and
enhanced contraction, further increasing the peripheral circulation
resistance and promoting hypertension development (47). For vascular endothelial injuries, the
role of TGF-β1 remains unclear. Li et al (48) have recently demonstrated that
miRNA-146a-mediated TGF-β1 silencing can promote angiogenesis. In
the present study, the results demonstrated that following
treatment with TGF-β1, the HUVEC proliferation and migration
ability were significantly decreased, and under hypoxic conditions,
the cellular apoptosis rates were significantly elevated. In
addition, the release of LDH in the cell culture supernatant was
also investigated. The results demonstrated that TGF-β1 could
promote the release of LDH, indicating the occurrence of
endothelial cell injuries. These results suggest that TGF-β1 may
promote the development of vascular endothelial injuries.
Autophagy is an important biological process in
vascular injury, and PI3KC3 is a key gene regulating autophagic
activity. It has been demonstrated that TGF-β1 is associated with
the regulation of autophagy (49,50). In
the present study, the expression levels of PI3KC3 mRNA in the
peripheral blood and its correlation with TGF-β1 were analyzed. The
results demonstrated that the PI3KC3 mRNA expression levels were
significantly decreased in patients with hypertension, which was
also associated with the disease grading. Correlation analysis
demonstrated that the TGF-β1 and PI3KC3 mRNA expression
demonstrated moderate negative correlation. In vitro results
demonstrated that, TGF-β1 could inhibit the PI3KC3 expression and
the autophagic activity in the HUVECs. In order to further verify
whether TGF-β1 functioned through PI3KC3, following treatment with
PI3KC3, the HUVECs were overexpressed with PI3KC3. The
overexpression of PI3KC3 significantly ameliorated the
proliferation, migration and hypoxia tolerance of the HUVECs. These
results suggest that TGF-β1 was able to inhibit autophagic activity
through PI3KC3 and promote the injury of vascular endothelial
cells. Conversely, it has previously been reported that TGF-β1 can
promote the EMT of vascular endothelial cells and enhance the
migration ability (51). The present
authors speculate that this disparity may be attributed to the
different downstream pathways of TGF-β1 and the different models
used. Further in-depth studies are required to elucidate the
detailed mechanism.
In conclusion, the present results demonstrated that
TGF-β1 may inhibit the autophagic activity of vascular endothelial
cells via the PI3KC3 pathway, promoting the vascular endothelial
injuries. The present study primarily investigated the mechanism
through which TGF-β1 regulated the autophagy, and the results
revealed the regulating correlation between TGF-β1 and PI3KC3.
These findings may contribute to the understanding of the role of
TGF-β1 in hypertension-induced vascular injuries.
Acknowledgements
The authors wish to thank Professor Yuxiang Dai
(Department of Cardiology, Zhongshan Hospital Affiliated to Fudan
University, Shanghai, China) for assisting with data collection,
statistical analysis, and manuscript preparation.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QZ, HL and JY designed the current study, performed
the experiments, collected the data, performed statistical analysis
and prepared the manuscript.
Ethics approval and consent to
participate
Prior written informed consent was obtained from all
subjects and the present study protocol was approved by the ethics
review board of Zaozhuang Municipal Hospital (Zaozhuang,
China).
Patient consent for publication
Prior written informed consent was obtained from all
subjects.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang Y, Zhang E, Zhang J, Chen S, Yu G,
Liu X, Peng C, Lavin MF, Du Z and Shao H: Relationship between
occupational noise exposure and the risk factors of cardiovascular
disease in China: A meta-analysis. Medicine (Baltimore).
97:e117202018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang X, Cai X, Wang W, Jin Y, Chen M,
Huang X, Zhu X and Wang L: Effect of asiaticoside on endothelial
cells in hypoxiainduced pulmonary hypertension. Mol Med Rep.
17:2893–2900. 2018.PubMed/NCBI
|
|
3
|
Kuang ZM, Wang Y, Feng SJ, Jiang L and
Cheng WL: Association between plasma homocysteine and
microalbuminuria in untreated patients with essential hypertension:
A case-control study. Kidney Blood Press Res. 42:1303–1311. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johns CS, Wild JM, Rajaram S, Swift AJ and
Kiely DG: Current and emerging imaging techniques in the diagnosis
and assessment of pulmonary hypertension. Expert Rev Respir Med.
12:145–160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yeates K, Campbell N, Maar MA, Perkins N,
Liu P, Sleeth J, Smith C, McAllister C, Hua-Stewart D, Wells G and
Tobe SW: The effectiveness of text messaging for detection and
management of hypertension in indigenous people in Canada: Protocol
for a randomized controlled trial. JMIR Res Protoc. 6:e2442017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roy C, Tabiasco J, Caillon A, Delneste Y,
Merot J, Favre J, Guihot AL, Martin L, Nascimento DC, Ryffel B, et
al: Loss of vascular expression of nucleoside triphosphate
diphosphohydrolase-1/CD39 in hypertension. Purinergic Signal.
14:73–82. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rocha HNM, Garcia VP, Batista GMS, Silva
GM, Mattos JD, Campos MO, Nóbrega ACL, Fernandes IA and Rocha NG:
Disturbed blood flow induces endothelial apoptosis without
mobilizing repair mechanisms in hypertension. Life Sci.
209:103–110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bakrania BA, Spradley FT, Satchell SC,
Stec DE, Rimoldi JM, Gadepalli RSV and Granger JP: Heme oxygenase-1
is a potent inhibitor of placental ischemia-mediated endothelin-1
production in cultured human glomerular endothelial cells. Am J
Physiol Regul Integr Comp Physiol. 314:R427–R432. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Navia-Pelaez JM, Campos GP, Araujo-Souza
JC, Stergiopulos N and Capettini LSA: Modulation of
nNOSser852 phosphorylation and translocation by PKA/PP1
pathway in endothelial cells. Nitric Oxide. 72:52–58. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rol N, de Raaf MA, Sun X, Kuiper VP, da
Silva Gonçalves, Bos D, Happé C, Kurakula K, Dickhoff C, Thuillet
R, Tu L, et al: Nintedanib improves cardiac fibrosis but leaves
pulmonary vascular remodeling unaltered in experimental pulmonary
hypertension. Cardiovasc Res. Jul 18–2018.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ungefroren H, Witte D, Fiedler C, Gädeken
T, Kaufmann R, Lehnert H, Gieseler F and Rauch BH: The role of PAR2
in TGF-β1-induced ERK activation and cell motility. Int J Mol Sci.
18:E27762017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liakouli V, Elies J, El-Sherbiny YM,
Scarcia M, Grant G, Abignano G, Derrett-Smith EC, Esteves F,
Cipriani P, Emery P, et al: Scleroderma fibroblasts suppress
angiogenesis via TGF-β/caveolin-1 dependent secretion of pigment
epithelium-derived factor. Ann Rheum Dis. 77:431–440. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin X, Aimaiti Y, Chen Z, Wang W and Li D:
Hepatic stellate cells promote angiogenesis via the
TGF-β1-Jagged1/VEGFA axis. Exp Cell Res. 15:34–43. 2018. View Article : Google Scholar
|
|
14
|
Wang Z, Wang K, Wang R and Liu X:
SUMOylation regulates TGF-β1/Smad4 signalling in-resistant glioma
cells. Anticancer Drugs. Dec 18–2017.(Epub ahead of print).
View Article : Google Scholar
|
|
15
|
Huang CH, Chang YH, Lin CY, Wang WH, Kuan
HC, Hsieh YJ, Wang YW, Yang CH, Chiu JY, Tsai SF, et al: Shared IgG
infection signatures vs. hemorrhage-restricted IgA clusters in
human dengue: A phenotype of differential class-switch via TGFβ1.
Front Immunol. 8:17262017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng W, Song J, Zhang Y, Chen S, Ruan H
and Fan C: Metformin prevents peritendinous fibrosis by inhibiting
transforming growth factor-β signaling. Oncotarget.
8:101784–101794. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ohmaru-Nakanishi T, Asanoma K, Fujikawa M,
Fujita Y, Yagi H, Onoyama I, Hidaka N, Sonoda K and Kato K:
Fibrosis in preeclamptic placentas is associated with stromal
fibroblasts activated by the transforming growth factor-β1
signaling pathway. Am J Pathol. 188:683–695. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qu Y, Zhang H, Sun W, Han Y, Li S, Qu Y,
Ying G and Ba Y: MiR-155 promotes gastric cancer growth and
invasion by negatively regulating transforming growth factor β
receptor 2. Cancer Sci. 109:618–628. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Niu J, Yue W, Le-Le Z, Bin L and Hu X:
Mesenchymal stem cells inhibit T cell activation by releasing
TGF-β1 from TGF-β1/GARP complex. Oncotarget. 8:99784–99800. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qiong W, Xiaofeng G and Junfang W:
Transforming growth factor-β1 (TGF-β1) induces mouse
precartilaginous stem cell differentiation through
TGFRII-CK1ε-β-catenin signalling. Int J Exp Pathol. 99:113–120.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang P, Luo L, Shen Q, Shi G, Mohammed A,
Ni S and Wu X: Rosuvastatin improves myocardial hypertrophy after
hemodynamic pressure overload via regulating the crosstalk of
Nrf2/ARE and TGF-β/smads pathways in rat heart. Eur J Pharmacol.
820:173–182. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang N, Dong M, Luo Y, Zhao F and Li Y:
Danshensu prevents hypoxic pulmonary hypertension in rats by
inhibiting the proliferation of pulmonary artery smooth muscle
cells via TGF-β-smad3-associated pathway. Eur J Pharmacol. 820:1–7.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beaudoin J, Dal-Bianco JP, Aikawa E,
Bischoff J, Guerrero JL, Sullivan S, Bartko PE, Handschumacher MD,
Kim DH, Wylie-Sears J, et al: Mitral leaflet changes following
myocardial infarction: Clinical evidence for maladaptive valvular
remodeling. Circ Cardiovasc Imaging. 10:e0065122017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Tan H, Chen M and Zhou S:
Transforming growth factor beta 1 related gene polymorphisms in
gestational hypertension and preeclampsia: A case-control candidate
gene association study. Pregnancy Hypertens. 12:155–160. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stjepanovic G, Baskaran S, Lin MG and
Hurley JH: Unveiling the role of VPS34 kinase domain dynamics in
regulation of the autophagic PI3K complex. Mol Cell Oncol.
4:e13678732017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thongchot S, Yongvanit P, Loilome W,
Seubwai W, Phunicom K, Tassaneeyakul W, Pairojkul C, Promkotra W,
Techasen A and Namwat N: High expression of HIF-1α, BNIP3 and
PI3KC3: Hypoxia-induced autophagy predicts cholangiocarcinoma
survival and metastasis. Asian Pac J Cancer Prev. 15:5873–5878.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Q, Wang D, Zhang H, Wang M, Li P,
Fang X and Cai X: Detection of autophagy processes during the
development of nonarticulated laticifers in Euphorbia kansui Liou.
Planta. 247:845–861. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang B, Sun J, Yuan Y and Sun Z: Effects
of atorvastatin on autophagy in skeletal muscles of diabetic rats.
J Diabetes Investig. 9:753–761. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Holmberg J, Bhattachariya A, Alajbegovic
A, Rippe C, Ekman M, Dahan D, Hien TT, Boettger T, Braun T, Swärd
K, et al: Loss of vascular myogenic tone in miR-143/145 knockout
mice is associated with hypertension-induced vascular lesions in
small mesenteric arteries. Arterioscler Thromb Vasc Biol.
38:414–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao P, Wu W, Ye J, Lu YW, Adam AP, Singer
HA and Long X: Transforming growth factor β1 suppresses
proinflammatory gene program independent of its regulation on
vascular smooth muscle differentiation and autophagy. Cell Signal.
50:160–170. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng JZ, Chen JJ, Wang ZG and Yu D:
MicroRNA-185 inhibits cell proliferation while promoting apoptosis
and autophagy through negative regulation of TGF-β1/mTOR axis and
HOXC6 in nasopharyngeal carcinoma. Cancer Biomark. 23:107–123.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Khera R, Lu Y, Lu J, Saxena A, Nasir K,
Jiang L and Krumholz HM: Impact of 2017 ACC/AHA guidelines on
prevalence of hypertension and eligibility for antihypertensive
treatment in United States and China: Nationally representative
cross sectional study. BMJ. 362:k23572018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun M, Ishii R, Okumura K, Krauszman A,
Breitling S, Gomez O, Hinek A, Boo S, Hinz B, Connelly KA, et al:
Experimental right ventricular hypertension induces regional
β1-integrin-mediated transduction of hypertrophic and profibrotic
right and left ventricular signaling. J Am Heart Assoc.
7:e0079282018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li L, Huang C, He Y, Sang Z, Liu G and Dai
H: Knockdown of long non-coding RNA GAS5 increases miR-23a by
targeting ATG3 involved in autophagy and cell viability. Cell
Physiol Biochem. 48:1723–1734. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen J, Yan L, Wang H, Zhang Z, Yu D, Xing
C, Li J, Li H, Li J and Cai Y: ZBTB38, a novel regulator of
autophagy initiation targeted by RB1CC1/FIP200 in spinal cord
injury. Gene. 678:8–16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McDonald AI, Shirali AS, Aragón R, Ma F,
Hernandez G, Vaughn DA, Mack JJ, Lim TY, Sunshine H, Zhao P, et al:
Endothelial regeneration of large vessels is a biphasic process
driven by local cells with distinct proliferative capacities. Cell
Stem Cell. 23:210–225.e6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rong X, Ge D, Shen D, Chen X, Wang X,
Zhang L, Jia C, Zeng J, He Y, Qiu H, et al: miR-27b suppresses
endothelial cell proliferation and migration by targeting Smad7 in
Kawasaki disease. Cell Physiol Biochem. 48:1804–1814. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Grampp S and Goppelt-Struebe M:
Receptor-independent modulation of TGF-β-induced pro-fibrotic
pathways by relaxin-2 in human primary tubular epithelial cells.
Cell Tissue Res. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen K and Sun Z: Activation of DNA
demethylases attenuates aging-associated arterial stiffening and
hypertension. Aging Cell. Apr 16–2018.(Epub ahead of print).
View Article : Google Scholar
|
|
41
|
Fu S, Li YL, Wu YT, Yue Y, Qian ZQ and
Yang DL: Icariside II attenuates myocardial fibrosis by inhibiting
nuclear factor-κB and the TGF-β1/Smad2 signalling pathway in
spontaneously hypertensive rats. Biomed Pharmacother. 100:64–71.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bellaye PS, Yanagihara T, Granton E, Sato
S, Shimbori C, Upagupta C, Imani J, Hambly N, Ask K, Gauldie J, et
al: Macitentan reduces progression of TGF-β1-induced pulmonary
fibrosis and pulmonary hypertension. Eur Respir J. 52:17018572018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cai Z, Li J, Zhuang Q, Zhang X, Yuan A,
Shen L, Kang K, Qu B, Tang Y, Pu J, et al: MiR-125a-5p ameliorates
monocrotaline-induced pulmonary arterial hypertension by targeting
the TGF-β1 and IL-6/STAT3 signaling pathways. Exp Mol Med.
50:452018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wei W, Rao F, Liu F, Xue Y, Deng C, Wang
Z, Zhu J, Yang H, Li X, Zhang M, et al: Involvement of Smad3
pathway in atrial fibrosis induced by elevated hydrostatic
pressure. J Cell Physiol. 233:4981–4989. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Higgins SP, Tang Y, Higgins CE, Mian B,
Zhang W, Czekay RP, Samarakoon R, Conti DJ and Higgins PJ:
TGF-β1/p53 signaling in renal fibrogenesis. Cell Signal. 43:1–10.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu Z, Huang XR and Lan HY: Smad3 mediates
ANG II-induced hypertensive kidney disease in mice. Am J Physiol
Renal Physiol. 302:F986–F997. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shang P, Liu T, Liu W, Li Y, Dou F, Zhang
Y, Sun L, Zhang T, Zhu Z, Mu F, et al: Telmisartan improves
vascular remodeling through ameliorating prooxidant and profibrotic
mechanisms in hypertension via the involvement of transforming
growth factor-β1. Mol Med Rep. 16:4537–4544. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li Y, Zhu H, Wei X, Li H, Yu Z, Zhang H
and Liu W: LPS induces HUVEC angiogenesis in vitro through
miR-146a-mediated TGF-β1 inhibition. Am J Transl Res. 9:591–600.
2017.PubMed/NCBI
|
|
49
|
Ding Y and Choi ME: Regulation of
autophagy by TGF-β: Emerging role in kidney fibrosis. Semin
Nephrol. 34:62–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang H, Zhang Y, Wu Q, Wang YB and Wang W:
miR-16 mimics inhibit TGF-β1-induced epithelial-to-mesenchymal
transition via activation of autophagy in non-small cell lung
carcinoma cells. Oncol Rep. 39:247–254. 2018.PubMed/NCBI
|
|
51
|
Wang Z, Fei S, Suo C, Han Z, Tao J, Xu Z,
Zhao C, Tan R and Gu M: Antifibrotic effects of hepatocyte growth
factor on endothelial-to-mesenchymal transition via transforming
growth factor-beta1 (TGF-β1)/Smad and Akt/mTOR/P70S6K signaling
pathways. Ann Transplant. 23:1–10. 2018. View Article : Google Scholar : PubMed/NCBI
|