Introduction
During pregnancy, changes in the levels of hormones
such as estrogen and human chorionic gonadotropin (HCG) and the
increased excretion of iodine in the kidney result in changes in
thyroid hormones in pregnant women to varying degrees (1). Previous findings showed that the levels
of free thyroxine (FT4) and thyroid stimulating hormone (TSH)
during pregnancy are obviously different from those during
non-pregnancy (2,3). The application of non-pregnancy
reference intervals lead to false detection rates to different
degrees.
In the Guidelines for Diagnosis and Treatment of
Pregnancy and Postpartum Thyroid Diseases stipulated by the
American Thyroid Association (ATA) in 2011 (4) and the Chinese Society of Endocrinology
and Chinese Society of Perinatal Medicine of the Chinese Medical
Association in 2012 (2), it was
proposed that the specific reference interval of thyroid hormones
during pregnancy needs to be established. In recent years, relevant
research has been conducted in many regions in China (5–10), but
no unified standard has been formed, which repeatedly confirms the
necessity for establishing region- and method-specific reference
intervals. Therefore, a statistical analysis was carried out in the
present study for the detection results of thyroid hormones in
healthy pregnant women in Linyi region, so as to establish a
method-specific thyroid hormone reference interval during pregnancy
in this region and provide a basis for accurate clinical diagnosis
and treatment.
Patients and methods
Study objects
A total of 22,235 healthy pregnant women receiving
examinations during pregnancy from October 2016 to October 2017 in
the Women and Children's Health Care Hospital of Linyi (Linyi,
China) were selected, including 11,382 patients in the first
trimester of pregnancy (within 12 weeks of pregnancy), 8,698
patients in the second trimester of pregnancy (13–27 weeks of
pregnancy) and 2,155 patients in the third trimester of pregnancy
(more than 28 weeks of pregnancy). The study patients were of 16–48
years of age and were divided to 6 groups according to their age:
≤20 years group, 21–25 years group, 26–30 years group, 31–35 years
group, 36–40 years group and >40 years group. Exclusion criteria
for the study were: i) patients with a family history and past
history of thyroid-related diseases, ii) patients taking
iodine-containing drugs or drugs affecting thyroid function (except
estrogens), iii) patients with palpable goiters, or iv) patients
with autoimmune diseases. At the same time, 990 healthy
non-pregnant women during the same period undergoing thyroid
hormone detection were selected as the control group. The study was
approved by the Ethics Committee of Women and Children's Health
Care Hospital of Linyi. Patients who participated in this study had
complete clinical data. Signed informed consents were obtained from
the patients or guardians.
Detection methods
Fasting venous blood (3 ml) was collected in the
morning and placed in a yellow vacuum tube containing coagulants.
The two were inverted and mixed 5–8 times. After standing at room
temperature for 30 min, the mixture was centrifuged at 1,680 × g
for 5 min at 25°C to separate the serum for detection on BY-320A
centrifuge (Beijing Baiyang Medical Devices Co., Ltd., Beijing,
China). The levels of FT4, TSH and thyroid peroxidase antibody
(TPOAb) were detected via a Roche E601 automatic
electrochemiluminescence analyzer. All reagents were provided by
Roche.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
19.0 (IBM Corp., Beijing, China) and Excel software were employed
for statistical analysis. Data in each trimester of pregnancy were
expressed as median and 95% bilateral limiting values. Enumeration
data were described by frequency and percentage. Chi-square test
was used for the comparison between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Comparison of the positive rates of
TPOAb in healthy pregnant women in different pregnancy
trimesters
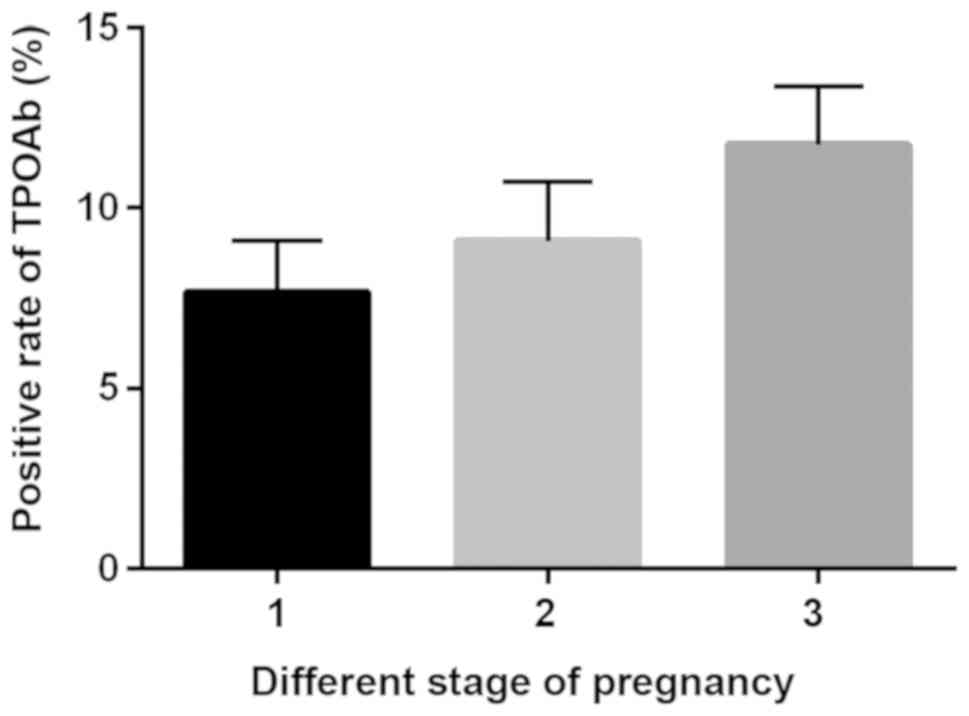
Statistical detection revealed a total of 873
pregnant women with positive TPOAb in the first trimester of
pregnancy, and the positive rate was 7.67%. A total of 791 pregnant
women with positive TPOAb in the second trimester of pregnancy were
detected, with a positive rate of 9.09%. Positive TPOAb was
detected in a total of 253 pregnant women in the third trimester of
pregnancy, with a positive rate of 11.74%. With the prolongation of
pregnancy, the positive rate of TPOAb increased, and the positive
rate in the third trimester was significantly higher than that in
the first and second trimesters of pregnancy (P<0.05). TPOAb ≥34
IU/l set by Roche reagent specifications was considered positive
(Fig. 1).
Analysis of the correlation of FT4 and
TSH detection results in healthy pregnant women according to
age
According to the guideline (2), after patients with positive TPOAb were
excluded, the remaining specimens included 10,509 cases in the
first trimester of pregnancy, 7,907 cases in the second trimester
of pregnancy, and 1,902 cases in the third trimester of pregnancy.
FT4 and TSH detected data were statistically analyzed (Figs. 2–7).
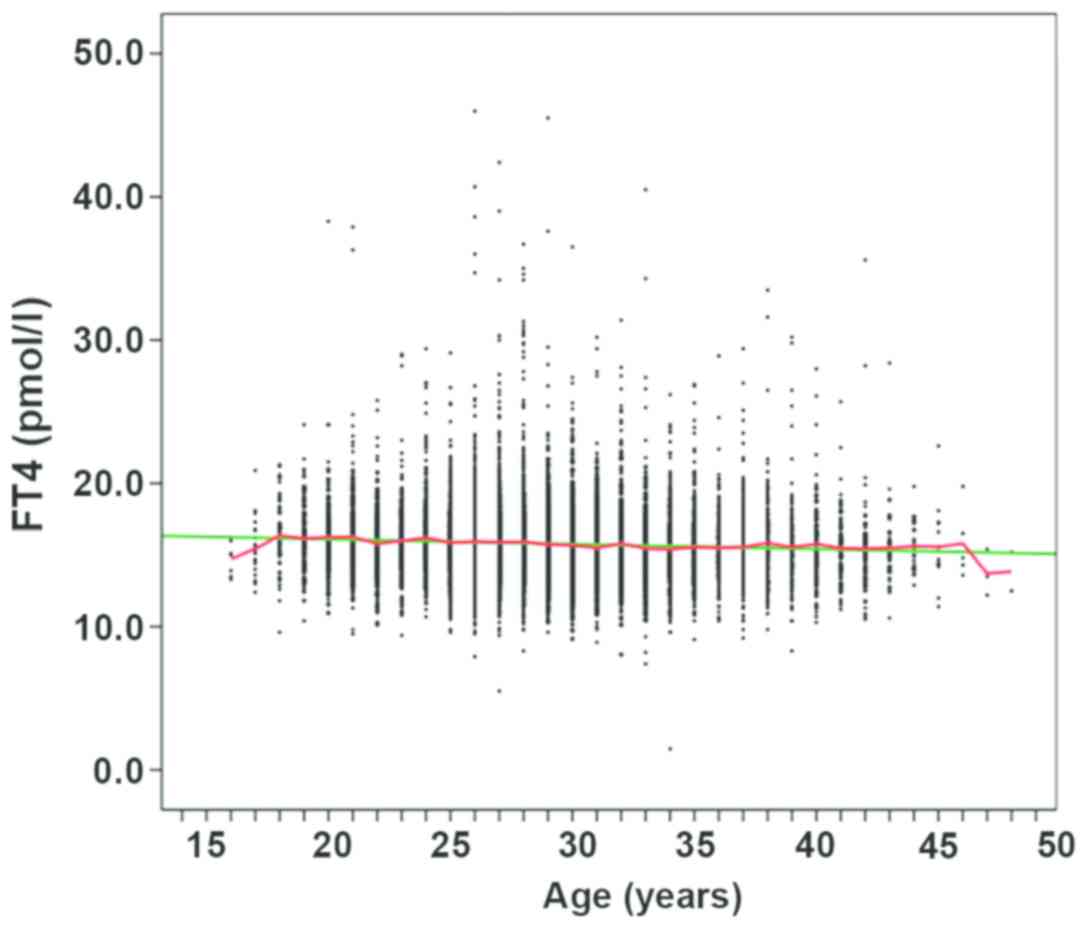
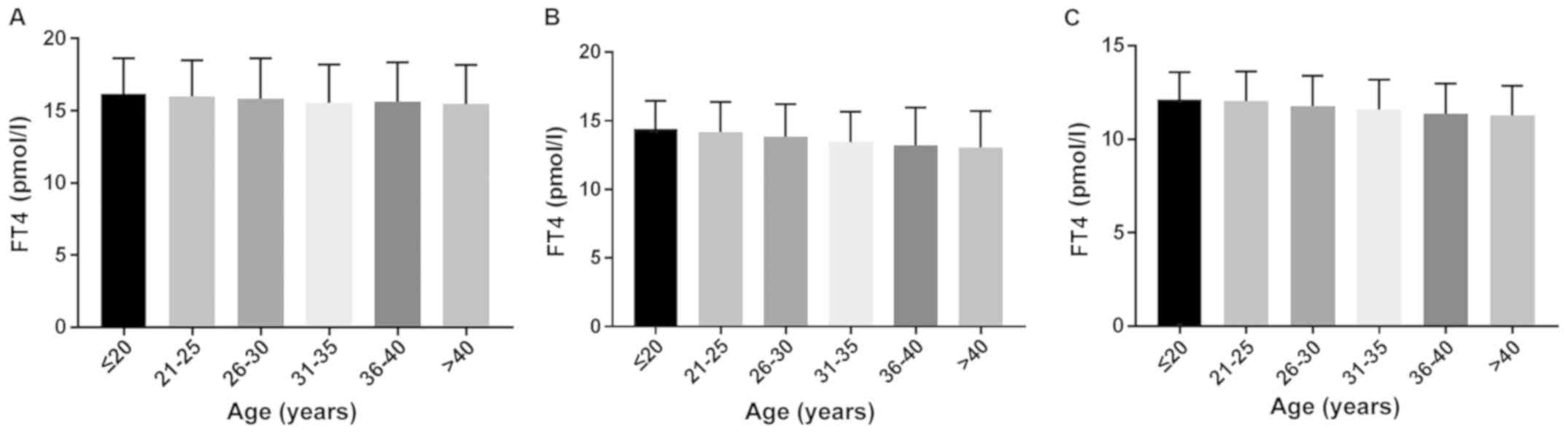
Comparison of the levels of FT4 in
different age groups in the first, second and third trimesters of
pregnancy
The levels of FT4, TSH, and TPOAb were detected
using a Roche E601 automatic electrochemiluminescence analyzer, and
all reagents were provided by Roche. Statistical methods included
SPSS 19.0 and Excel software, which were applied for statistical
analysis. The median FT4 decreased gradually with increase of age
in the second and third trimester of pregnancy (P<0.05)
(Fig. 8).
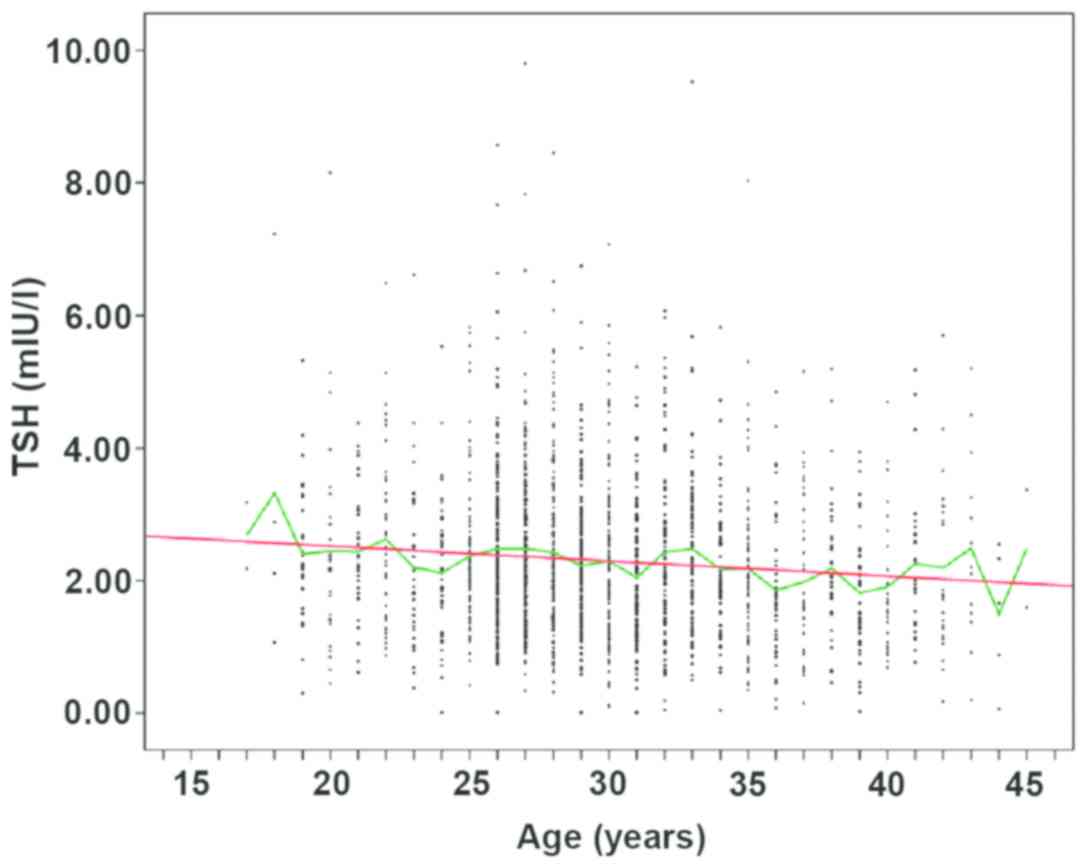
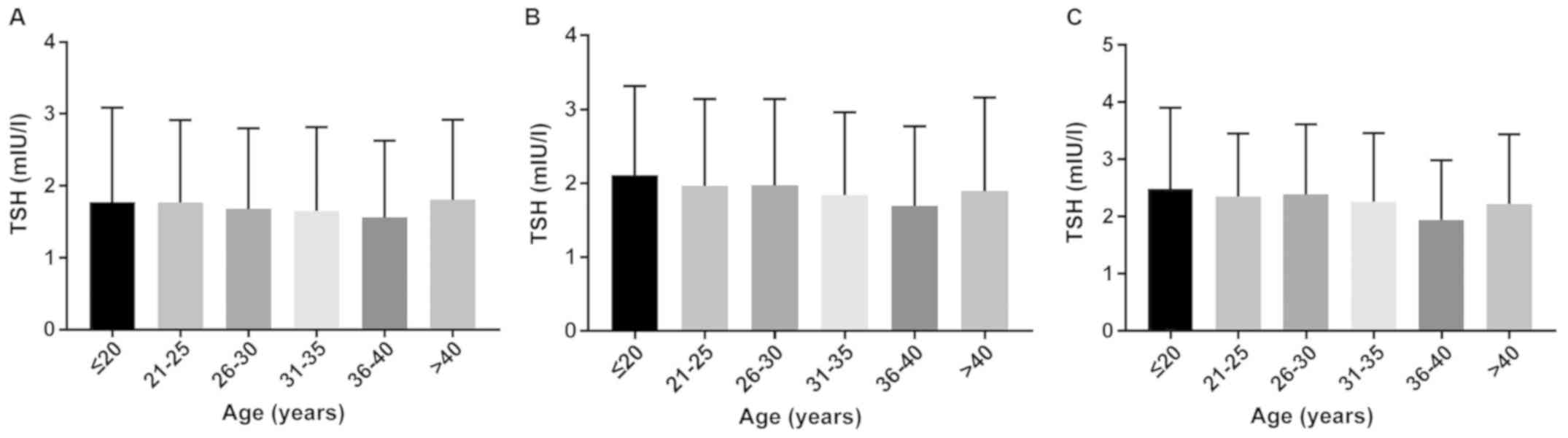
Comparison of the levels of TSH in
different age groups in the first, second and third trimesters of
pregnancy
The levels of TSH in different age groups in the
first, second and third trimesters of pregnancy were compared, and
the Roche E601 automatic electrochemiluminescence analyzer was used
to detect the levels of FT4, TSH and TPOAb. The median value of TSH
decreased in the first and second trimester of pregnancy, but the
median of TSH in the group of >40 years of age was significantly
higher than that in the group of 36–40 years of age (P<0.05)
(Fig. 9).
Reference intervals of FT4 and TSH in
healthy pregnant women in different trimesters of pregnancy
According to the guideline (2), after patients with positive TPOAb,
there were 10,509 cases in the first trimester of pregnancy, 7,907
cases in the second trimester of pregnancy and 1,902 cases in the
third trimester of pregnancy. Statistical analysis results of FT4
and TSH revealed that all the results presented a skewed
distribution, and the corresponding reference intervals were
represented by median and 95% bilateral limiting values. Specific
data are shown in Table I. Table I also shows that with the
prolongation of pregnancy, the FT4 level tended to be gradually
decreased whereas the TSH level was gradually increased. FT4 levels
in the second and third trimesters of pregnancy were markedly lower
than that in the non-pregnancy, and the differences were
statistically significant. The TSH level in the first trimester of
pregnancy was notably lower than that in the non-pregnancy, with a
statistically significant difference.
 | Table I.Reference intervals of FT4 and TSH in
pregnant women in different pregnancy trimesters in Linyi
region. |
Table I.
Reference intervals of FT4 and TSH in
pregnant women in different pregnancy trimesters in Linyi
region.
| Group | n | FT4 (pmol/l) | TSH (mIU/l) |
|---|
| First-trimester
pregnancy | 10509 | 15.6
(11.5–21.5)b | 1.51
(0.04–4.42)a,b |
| Second-trimester
pregnancy | 7907 | 13.9
(9.9–18.7)a | 1.90 (0.09–4.70) |
| Third-trimester
pregnancy | 1902 | 11.6
(8.8–15.2)a | 2.14 (0.59–5.16) |
| Non-pregnancy | 990 | 15.5 (11.9–20.2) | 1.94 (0.57–4.90) |
Comparison of the detection results of
FT4 and TSH in pregnant women in different age groups
The pregnant women were divided into five groups
according to their age: ≤20-year group, 21–25-year group,
26–30-year group, 31–35-year group, 36–40-year group and
>40-year group. Statistics revealed that the medians of FT4 in
the second and third trimesters of pregnancy showed a gradual
decrease in trend with age, and those of TSH in the first and
second trimesters of pregnancy also exhibited a tendency to decline
with age in the following groups aged below 40 years, but they were
increased again in the >40-year group. In the first, second and
third trimesters of pregnancy, the medians of TSH in the
>40-year group were obviously higher than those in 36- to
40-year group, and the differences were statistically significant
(Table II).
 | Table II.Correlation of FT4 and TSH medians
with the age of pregnant women. |
Table II.
Correlation of FT4 and TSH medians
with the age of pregnant women.
|
| First-trimester
pregnancy | Second-trimester
pregnancy | Third-trimester
pregnancy |
|---|
|
|
|
|
|
|---|
| Group | n | FT4 (pmol/l) | TSH (mIU/l) | n | FT4 (pmol/l) | TSH (mIU/l) | n | FT4 (pmol/l) | TSH (mIU/l) |
|---|
| ≤20 years | 436 | 16.14 | 1.78 | 292 | 14.41 | 2.11 | 65 | 12.13 | 2.49 |
| 21–25 years | 1,895 | 16.00 | 1.77 | 1283 | 14.19 | 1.96 | 260 | 12.03 | 2.35 |
| 26–30 years | 4,823 | 15.85 | 1.68 | 3619 | 13.84 | 1.97 | 847 | 11.76 | 2.39 |
| 31–35 years | 2,164 | 15.54 | 1.65 | 1782 | 13.44 | 1.84 | 458 | 11.58 | 2.26 |
| 36–40 years | 927 | 15.63 | 1.56a | 728 | 13.21 | 1.70a | 199 | 11.35 | 1.94a |
| >40 years | 264 | 15.46 | 1.80 | 203 | 13.05 | 1.90 | 73 | 11.27 | 2.22 |
Comparison of the reference intervals
of FT4 and TSH with those shown in relevant studies in China
The detection results of this study were compared
with those in relevant studies in China (6–10), which
demonstrated that the reference interval of FT4 was not obviously
different from those in the first trimester of pregnancy in Jinan,
in the second trimester of pregnancy in Urumqi and Shenzhen, and in
the third trimester of pregnancy in Jinan, but significantly
different from those in other groups (P<0.05). In addition,
there were no obvious differences in the reference interval of TSH
in comparison with those in the first trimester of pregnancy in
Jinan and in the second trimester of pregnancy in Urumqi, but
significant differences were found in comparison with other groups
(P<0.05). Specific results are shown in Tables III and IV.
 | Table III.Comparison of FT4 detection results in
different regions via different methods. |
Table III.
Comparison of FT4 detection results in
different regions via different methods.
| Region | Instrument | First-trimester
pregnancy | Second-trimester
pregnancy | Third-trimester
pregnancy | Non-pregnancy |
|---|
| Shenzhen | Roche | 17.56
(11.65–22.16)a | 14.16
(9.49–20.14) | 12.38
(10.13–15.88)a | 18.71
(14.35–21.69)a |
| Urumqi | Roche | 14.30
(9.94–20.21)a | 13.52
(9.69–17.77) | 10.92
(7.28–15.99)a | 13.91
(10.09–19.72)a |
| Jinan | Roche | 15.39
(12.60–18.94) | 12.46
(8.74–17.23)a | 11.45
(8.64–17.21) | 14.62
(11.06–18.59)a |
| Zibo | Beckman | 11.10
(8.71–15.86)a | 9.31
(5.98–12.22)a | 8.05
(5.22–10.92)a | 12.12
(7.86–14.41)a |
| Henan | Siemens | 11.07
(9.55–12.59)a | 9.31
(7.89–10.73)a | 8.16
(6.86–9.46)a | 12.81
(11.29–14.33)a |
| Linyi | Roche | 15.6
(11.5–21.5) | 13.9
(9.9–18.7) | 11.6
(8.8–15.2) | 15.5
(11.9–20.2) |
 | Table IV.Comparison of TSH detection results
in different regions via different methods. |
Table IV.
Comparison of TSH detection results
in different regions via different methods.
| Region | Instrument | First-trimester
pregnancy | Second-trimester
pregnancy | Third-trimester
pregnancy | Non-pregnancy |
|---|
| Shenzhen | Roche | 1.10
(0.23–3.58)a | 2.07
(0.13–3.96)a | 2.62
(1.08–3.62)a | 2.63
(1.00–5.14)a |
| Urumqi | Roche | 1.85
(0.06–4.80)a | 1.89
(0.29–5.84) | 2.57
(0.65–7.64)a | 2.09
(0.48–4.56)a |
| Jinan | Roche | 1.52
(0.27–4.27) | 2.39
(0.53–4.75)a | 2.52
(0.76–4.59)a | 2.68
(0.83–4.79)a |
| Zibo | Beckman | 1.78
(0.22–4.53)a | 2.14
(0.42–4.88)a | 2.40
(0.54–5.36)a | 2.67
(0.66–4.60)a |
| Henan | Siemens | 1.68
(0.65–2.71)a | 2.03
(0.96–3.10)a | 2.31
(1.06–3.56)a | 2.35
(1.21–3.49)a |
| Linyi | Roche | 1.51
(0.04–4.42) | 1.90
(0.09–4.70) | 2.14
(0.59–5.16) | 1.94
(0.57–4.90) |
Discussion
During pregnancy, thyroid dysfunction can lead to
miscarriage, thyroid crisis, pregnancy-induced hypertension, fetal
distress, intrauterine growth retardation and a series of maternal
and fetal adverse reactions (1). In
particular, maternal hypothyroidism during pregnancy can cause
fetal developmental disorders in neural intelligence, thus bringing
serious burdens to family and the society. Thus, it is crucial to
maintain maternal normal thyroid function during pregnancy. A
series of compensatory changes occur in maternal thyroid glands due
to hormone and immunophysiological changes during pregnancy, so the
thyroid function indexes during pregnancy are quite different from
those during non-pregnancy. Therefore, the application of reference
intervals of thyroid function indexes during pregnancy may lead to
misdiagnosis and missed diagnosis to a certain degree (3), and it is necessary to establish a
reference interval for specific thyroid function during pregnancy.
In the guideline stipulated in China (2), it is recommended to apply FT4, TSH and
TPOAb as indexes of thyroid screening during pregnancy. The
guideline suggests that the positive diagnostic criteria of TPOAb
is higher than the upper limit of the reference value provided by
the kit. Therefore, statistical analyses were mainly conducted for
reference intervals of the two indexes, FT4 and TSH, in this
study.
TPOAb is produced by the release of thyroid
peroxidase (TPO) from the follicles of thyroid glands into the
blood to stimulate the body's immune system, thereby reducing the
production of thyroid hormones and stimulating TSH secretion.
Positive TPOAb often indicates the presence of thyroid damage and
may increase the risks of miscarriage and premature delivery.
Reports worldwide have demonstrated that the positive rate of TPOAb
during pregnancy is in the range of 9.17–17.58% (11–13).
However, in this study, the positive rates of TPOAb in the first,
second and third trimesters of pregnancy were 7.67, 9.09 and
11.74%, respectively, with the overall positive rate of 8.62%,
which was slightly lower than results in other reports. This may be
related to the detection method, the iodine status of the region,
and the number of the detected individuals. This study showed that
the TPOAb positive rate in the third trimester of pregnancy is
considerably higher than that in the first trimester of pregnancy,
indicating a statistically significant difference. For pregnant
women in the third trimester of pregnancy, monitoring of TPOAb
should be strengthened. It was also found in this study that the
FT4 level in the third trimester is remarkably lower than that in
the first trimester pregnancy, while the TSH level in the third
trimester of pregnancy was notably higher than that in the first
trimester of pregnancy, suggesting that the probability of
hypothyroidism in pregnant women in the third trimester of
pregnancy is greater, which needs particular attention.
The present study revealed that with the
prolongation of pregnancy, the FT4 level exhibited a gradually
decreasing trend while the TSH level had a gradually increasing
trend, which is consistent with the research findings of other
scholars (6–11). The FT4 level in the third trimester
of pregnancy was significantly lower than that in the first
trimester. The underlying cause may be that the increased excretion
rate of iodine in the kidney of pregnant women in the third
trimester of pregnancy and the increased demand for iodine in the
fetus result in a relative lack of maternal iodine (14), thus leading to the decreased
production of thyroid hormones. TSH level in the first trimester of
pregnancy was obviously lower than that in the third trimester of
pregnancy. This is mainly because HCG peaks at 8–10 weeks of
pregnancy and then continues to decline, the alpha subunits of HCG
and TSH are similar, and the negative feedback regulation leads to
a marked decrease in the TSH level in the first trimester of
pregnancy, which is then gradually increased. In the second and
third trimesters of pregnancy, the FT4 level tended to decrease
with the increase of the age of pregnant woman, which is similar to
the results of Kuo et al (15) and Ademuyiwa et al (16). This may be related to the
hyposecretion of hormones with the increase of age (16), but the specific reason has yet to be
determined. The changed trend in TSH in this study is contrary to
that in the study of Ademuyiwa et al (16). In this study, it was found that TSH
exhibited a tendency to decline with the increase of age in women
aged <40 years, and it only tended to be increased in the
>40-year group, which might be due to the different research
groups and the number of included patients. In the 40-year group,
the FT4 level was the lowest among all age groups, while the TSH
level was markedly higher than that in 35- to 40-year group,
suggesting that the occurrence probability of hypothyroidism is
higher in the 40-year group.
Recent studies worldwide have proposed that region-
and method-specific reference intervals for thyroid function during
pregnancy need to be established for self-serving populations in
various prenatal screening centers (5,17).
Comparisons of study results of related institutions in China
(6–10) also verify this view. Using the same
detection method, significant differences were found in FT4 and TSH
levels in groups in Linyi except FT4 levels in the first and third
trimesters of pregnancy and TSH level in the first trimester of
pregnancy compared with those in Jinan. Compared with those in
Urumqi, significant differences were detected in FT4 and TSH levels
in all groups except FT4 and TSH levels in the second trimester of
pregnancy. Compared with those in Shenzhen, there were notable
differences in FT4 and TSH levels in all the groups except FT4
level in the second trimester of pregnancy (P<0.05). Detection
results were notably different among different detection methods
regardless of the region (P<0.05). Therefore, it is imperative
to establish region- and method-specific thyroid hormone reference
intervals during pregnancy.
In summary, thyroid hormones during pregnancy vary
with pregnancy, region, and detection methods. Each region or
medical institution should establish its own specific thyroid
hormone reference intervals during pregnancy to provide accurate
diagnostic criteria for the region or medical institution. Thyroid
hormones during pregnancy change with age and pregnancy. For
pregnant women aged over 40 years in the third trimester of
pregnancy, thyroid hormone levels need intensive monitoring.
Acknowledgements
Not applicable.
Funding
This study was supported by the Science and
Technology Development and Innovation Project in Linyi (contract
no. 201717050).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ and YZ collected the data of patents and
extracted venous blood. JZ and XY recorded and interpreted FT4
level. YH and HL analyzed TSH levels. QZ, YZ and CM were
responsible for the statistical analysis. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Women and Children's Health Care Hospital of Linyi (Linyi, China).
Patients who participated in this study had complete clinical data.
Signed informed consents were obtained from the patients or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Teng WP and Shan ZY: Confusion and thought
of the diagnosis and treatment for thyroid diseases in pregnancy.
Zhonghua Nei Ke Za Zhi. 51:1–4. 2012.(In Chinese). PubMed/NCBI
|
|
2
|
Alexander EK, Pearce EN, Brent GA, Brown
RS, Chen H, Dosiou C, Grobman WA, Laurberg P, Lazarus JH, Mandel
SJ, et al: 2017 Guidelines of the American Thyroid Association for
the diagnosis and management of thyroid disease during pregnancy
and the postpartum. Thyroid. 27:315–389. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stricker R, Echenard M, Eberhart R,
Chevailler MC, Perez V, Quinn FA and Stricker R: Evaluation of
maternal thyroid function during pregnancy: The importance of using
gestational age-specific reference intervals. Eur J Endocrinol.
157:509–514. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stagnaro-Green A, Abalovich M, Alexander
E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP,
Sullivan S, et al: American Thyroid Association Taskforce on
Thyroid Disease During Pregnancy and Postpartum: Guidelines of the
American Thyroid Association for the diagnosis and management of
thyroid disease during pregnancy and postpartum. Thyroid.
21:1081–1125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang QW, Yu B, Huang RP, Cao F, Zhu ZQ,
Sun DC and Zhou H: Assessment of thyroid function during pregnancy:
The advantage of self-sequential longitudinal reference intervals.
Arch Med Sci. 7:679–684. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu B, Wang QW, Huang RP, Cao F, Zhu ZQ,
Sun DC, Zhou H and Zhang YM: Establishment of self-sequential
longitudinal reference intervals of maternal thyroid function
during pregnancy. Exp Biol Med (Maywood). 235:1212–1215. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duan Y, Peng L, Cui Y and Jiang Y:
Reference intervals for thyroid function and the negative
correlation between FT4 and HbA1c in pregnant women of West China.
Clin Lab. 61:777–783. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Yao B, Li C, Mao J, Wang W, Xie
X, Teng X, Han C, Zhou W, Li C, et al: Reference intervals of
thyroid function during pregnancy: Self-sequential longitudinal
study versus cross-sectional study. Thyroid. 26:1786–1793. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang J, Li W, Chen QB, Liu LY, Zhang W,
Liu MY, Wang YT, Li WY and Zeng LZ: Establishment of
trimester-specific thyroid stimulating hormone and free thyroxine
reference interval in pregnant Chinese women using the Beckman
Coulter UniCel™ DxI 600. Clin Chem Lab Med. 53:1409–1414. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang D, Li D, Guo X, Yu S, Qiu L, Cheng X,
Xu T, Li H and Liu H: Effects of sex, age, sampling time, and
season on thyroid-stimulating hormone concentrations: A
retrospective study. Biochem Biophys Res Commun. 506:450–454. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao X, Li Y, Li J, Liu A, Sun W, Teng W
and Shan Z: Gestational TSH and FT4 reference intervals in Chinese
women: A systematic review and meta-analysis. Front Endocrinol
(Lausanne). 9:4322018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sarkhail P, Mehran L, Askari S,
Tahmasebinejad Z, Tohidi M and Azizi F: Maternal thyroid function
and autoimmunity in 3 trimesters of pregnancy and their offspring's
thyroid function. Horm Metab Res. 48:20–26. 2016.PubMed/NCBI
|
|
13
|
Thangaratinam S, Tan A, Knox E, Kilby MD,
Franklyn J and Coomarasamy A: Association between thyroid
autoantibodies and miscarriage and preterm birth: Meta-analysis of
evidence. BMJ. 342:d26162011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shan Z, Chen L, Lian X, Liu C, Shi B, Shi
L, Tong N, Wang S, Weng J, Zhao J, et al: Iodine status and
prevalence of thyroid disorders after introduction of mandatory
universal salt iodization for 16 years in China: A cross-sectional
study in 10 cities. Thyroid. 26:1125–1130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuo FC, Su SW, Wu CF, Huang MC, Shiea J,
Chen BH, Chen YL and Wu MT: Relationship of urinary phthalate
metabolites with serum thyroid hormones in pregnant women and their
newborns: A prospective birth cohort in Taiwan. PLoS One.
10:e01238842015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ademuyiwa O, Odusoga OL, Adebawo OO and
Ugbaja R: Endogenous antioxidant defences in plasma and
erythrocytes of pregnant women during different trimesters of
pregnancy. Acta Obstet Gynecol Scand. 86:1175–1182. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Veltri F, Belhomme J, Kleynen P, Grabczan
L, Rozenberg S, Pepersack T and Poppe K: Maternal thyroid
parameters in pregnant women with different ethnic backgrounds: Do
ethnicity-specific reference ranges improve the diagnosis of
subclinical hypothyroidism? Clin Endocrinol (Oxf). 86:830–836.
2017. View Article : Google Scholar : PubMed/NCBI
|