Introduction
As with the visual system, the auditory system can
locate the source of stimuli in space and distinguish between
different stimuli (1). Hearing loss
is one of the most common birth defects (2). Prelingual deafness is a type of hearing
loss and is a profound hearing impairment that is congenital or
occurs in the first few years of life, which is fully acquired
prior to language and speech and can result in brain structure
alterations (3,4). It typically takes place prior to 2
years of age with a high rate of incidence (5). More than 60% of patients with
prelingual deafness are associated with hereditary factors and the
other 40% are associated with environmental or iatrogenic factors
(2,6). Cochlear implantation (CI) serves an
essential role in patients with severe hearing loss of hearing
ability and verbal ability as CI enables patients to have the
stimulation sound to promote auditory center development and
interfere with auditory cortex alienation (7,8). The
assessment of prelingual auditory development is very important in
early identification and for interventions to treat hearing
impairment and deafness (9). The
current predominant methods of evaluating hearing aid effects in
the clinic are sound field audiometry, speech audiometry and
subjective evaluation (10), which
have large limitations.
Auditory cortical evoked potential (ACEP) is a novel
method of evaluating hearing loss in children who do not receive
reliable feedback to all external sounds (11). ACEP has some of the most common
exogenous waves, including P1, N1 and P2, which provide information
about sound information's arrival to the auditory cortex (12). P1 latency is a useful biomarker of
central auditory development and a powerful, positive response that
is easily identified, which occurs following 100–300 msec of
stimulation depending on the age of the child (13,14).
Additionally, N1 is a high negative peak at ~100 msec following the
stimulus initiation, but P2 is a second high positive peak at ~200
msec following stimulus (12). N1
initially emerges in the P1 waveform as a bifurcation, which serves
as a biomarker of higher-order auditory cortical development
(15). The P1-N1-P2 waveform pattern
for people with auditory deficits had been illustrated to result
from the absence of a synchronized neural response to a stimulus
characteristic, whose responses had association with acoustic
changes in speech sounds (16,17).
Therefore, the present study was conducted to evaluate the effect
of the exogenous P1-N1-P2 waves on the ACEP of people with auditory
prosthesis and to evaluate ACEP feasibility in clinical practice
for assessing the application value of auditory effects.
Materials and methods
Ethics statement
All research subjects in the present study met the
inclusion and exclusion criteria. In addition, the present study
was approved by the Ethics Committee of the First People's Hospital
of Kunshan (Kunshan, China), and all patients provided written
informed consent.
Subjects
From January 2012-June 2017, 126 prelingual deaf
patients (aging from 1–18 years old) from The First People's
Hospital of Kunshan (Kunshan, China), were evaluated, including 67
males and 59 females with a mean age of 9.1±2.8 years. There were
69 cases of patients with hearing aids and 57 cases of patients
with CI, in which the minimum age of using auditory prosthesis was
6 months and the maximum age was 18 years. In addition, the
duration of using auditory prosthesis was 1–9 years, and the mean
duration was 8.8±0.8 years. The medical evaluation of 126
prelingual deaf patients was performed for otoscopy canal patency
and tympanic membrane integrity. Additionally, computed tomography
(CT) was used to examine congenital inner ear malformations.
Audiological assessment for pure tone audiometry determined that
phono-sensitive neural hearing was lost (>90 dB) and auditory
brainstem response test had no evoked potential being extracted
(>95 dBnHL); distortion product otoacoustic emissions were not
led out. The acoustic impedance test tympanogram presented with B
type or C type curves, with acoustic reflection that disappeared;
language and language development were hindered by language
barriers; the symptoms were diagnosed as binaural extremely severe
sensorineural deafness (18).
Inclusion criteria of study subjects were as follows: Patients'
details included basic information, the epicophosis history and
other clinical data; patients aged between 1 and 18 years; patients
with binaural extremely severe sensorineural deafness (pure-tone
average >90 dB); the use of auditory prosthesis for >6 months
and the auditory language ability being significantly improved; the
patient and their guardian having a correct understanding of the
present study and voluntary participation in the study. The
presence of non-hearing factors in patients with congenital disease
lead to exclusion. Table I presents
the base characteristics of 126 patients using auditory
prosthesis.
 | Table I.Basic information of 126 patients with
auditory prosthesis. |
Table I.
Basic information of 126 patients with
auditory prosthesis.
| Characteristics | Patients (n) |
|---|
| Age (years) |
|
| ≤10 | 78 |
|
>10 | 48 |
| Duration of deafness
(years) |
|
| ≤10 | 85 |
|
>10 | 41 |
| Age at initial use of
auditory prosthesis (years) |
|
| ≤8 | 71 |
|
>8 | 55 |
| Location of an
auditory prosthesis |
|
| Auris
sinistra | 65 |
| Auris
dextra | 61 |
ACEP test
ACEP of all enrolled subjects was tested using the
HEARLab™ system (Frye Electronics, Inc., Beaverton, OR, USA). The
tests were conducted in a sound insulation room in the
Ear-Nose-Throat Department of the First People's Hospital of
Kunshan in which the indoor temperature was 25°C, the relative
humidity was 30–50% and the background noise was <30 dB. In the
testing process, the patients were permitted to sit in a more
comfortable position and were instructed to watch a silent
animation in order to stay alert and quiet, to minimize physical
activity, and to rest when not in a good condition. All of these
were performed to ensure that the test results were true and
reliable.
An ethanol solution with a volume fraction of 95%
was used to wipe the body part of the receiver's electrode and
remove pollutants of grease and dandruff and other pollutants prior
to testing. The recording electrode, grounding electrode, and
reference electrode were placed in the middle of the calvarium, the
middle of forehead, ear and mastoid synapse without being on the
side of the auditory prosthesis in which electrode impedance should
be <5 kΩ.
Once the HEARLab™ system was opened and logged under
the normal working conditions of the auditory prosthesis, the basic
information of the subjects was entered, ACA was chosen as the test
pattern, and test conditions were as those for an auditory
prosthesis. Then, the calibrated sound field was pressed. The
speaker distance from subjects was 1.5 m, both being placed at the
angle of 90°, in which the speaker and receiver test ear were at
the same level. The initial test strength was calibrated as 60 dB
sound pressure level. /m/, /g/ and /t/ were used as the stimulus
sound, which represented the stimulation of low frequency (0.2–0.5
kHz), intermediate frequency (0.8–1.6 kHz), and high frequency (2–8
kHz), and the duration time was 30, 30, and 20 msec, respectively,
with 1,125 msec as a repeat circle. Additionally, the waveform
window time included 200 msec prior to stimulation sound, 600 msec
following stimulation sound, and the artifact rejection range was
±150 mv, in which superposition times were 2 times/sec. The
waveform was extracted by judging the P-value according to system
automatic statistical analysis and was extracted when
P<0.05. Each stimulus was tested 2 times, recording
subjects' amplitude and incubation of P2, N1 and P1 under 3 types
of stimulation sounds.
Gauge score records
In the present study, speech intelligibility rating
(SIR) and categories of auditory performance (CAP) established by
the University of Nottingham (19)
were used to conduct grading evaluation of speech production and
auditory perception in all patients with auditory prosthesis. SIR
(Table II) and CAP (Table III) were divided into 1–5 and 1–8
levels, respectively, according to the extent to which patients'
self-speaking with auditory prosthesis was understood and the
hearing level in daily life. Both scores were obtained through
face-to-face investigation or telephone follow-up of the patients
themselves and relatives who had close contact with the patients in
their daily lives.
 | Table II.Speech intelligibility rating. |
Table II.
Speech intelligibility rating.
| Grade | Judgment
standards |
|---|
| 5 | Coherent speech could
be understood by all people, and children's language is easy to
understand in daily contexts |
| 4 | Coherent speech could
be understood by a person who had not spoken to the deaf |
| 3 | Coherent speech could
be understood with listener's concentration combined with
lipreading |
| 2 | Coherent speech could
not be understood by listener, but a few words could be understood
based on condition of context and lipreading |
| 1 | Daily speech could
not be understood by anyone, and the main method of communication
is gesture |
 | Table III.Categories of auditory performance
(28). |
Table III.
Categories of auditory performance
(28).
| Grade | Judgment
standards |
|---|
| 8 | Using telephone to
chat with familiar people |
| 7 | Chatting with others
without lipreading |
| 6 | Understanding common
statements without lipreading |
| 5 | Differentiating
speech sound without lipreading |
| 4 | Distinguishing
environment sound |
| 3 | Having response to
speech (example: Walking) |
| 2 | Being aware of the
sound in the environment |
| 1 | Being unaware of the
sound in the environment |
Statistical analysis
All data were processed using SPSS 20.0 statistical
software (IBM Corp. Armonk, N.Y., USA), and the measurement data
were expressed as the mean ± standard deviation. Data with normal
distribution and homogenous variance were analyzed with an
independent sample t-test; whereas data that did not conform to
normal distribution or homogeneous variance were analyzed with the
Wilcoxon rank-sum test. Two groups of different acoustic
stimulations were analyzed by repeated measures of one-way analysis
of variance. Post hoc test was performed using the Bonferroni test.
Categorical data were expressed as percentage or rate and analyzed
using χ2 test. Correlation analysis was conducted via
Pearson correlation analysis. All tests were two-tailed and
P<0.05 was considered to indicate a statistically significant
difference.
Results
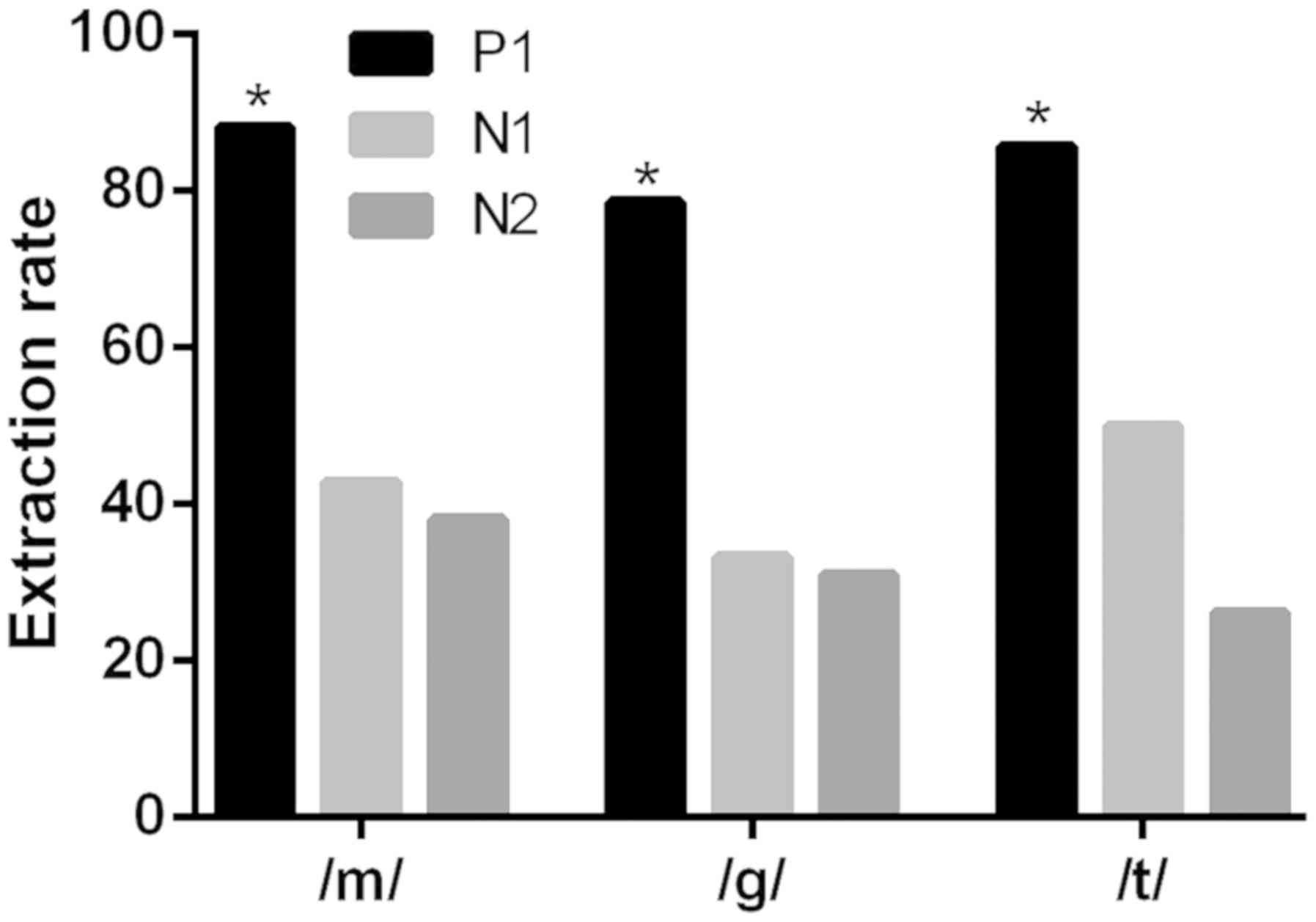
Extraction rates of P1 and N1, P1 and
P2 waves of patients using auditory prosthesis under different
acoustic stimulations are different
Initially, the extraction rates of different waves
were compared under different stimuli in patients with hearing
aids. Table IV and Fig. 1 present the results of extraction
rates of P1, N1 and P2 waves of patients using auditory prosthesis
under different acoustic stimulations. With different acoustic
stimulations of /m/, /g/ and /t/, extraction rates of P1 and N1
wave were significantly different (χ2=57.040;
χ2=52.310; χ2=66.030; all P<0.05); there
was also a significant difference between the extraction rates of
P1 and P2 waves (χ2=67.640; χ2=57.670;
χ2=90.570; all P<0.05), however, no significant
difference was observed between the extraction rates of P2 and N1
waves (χ2=0.593; χ2=0.164;
χ2=2.674; all P>0.05).
 | Table IV.Extraction rates of P1, N1 and P2 wave
under different acoustic stimulations. |
Table IV.
Extraction rates of P1, N1 and P2 wave
under different acoustic stimulations.
|
| /m/ | /g/ | /t/ |
|---|
|
|
|
|
|
|---|
| Wave | Extracted (%) | Not extracted
(%) | Extracted (%) | Not extracted
(%) | Extracted (%) | Not extracted
(%) |
|---|
| P1 | 111 (88.1) | 15 (11.9) | 99 (78.6) | 27 (21.4) | 108 (85.7) | 18 (14.3) |
| N1 | 54 (42.9) | 72 (57.1) | 42 (33.3) | 84 (66.7) | 45 (35.7) | 81 (64.3) |
| P2 | 48 (38.1) | 78 (61.9) | 39 (31.0) | 87 (69.0) | 33 (26.2) | 93 (73.8) |
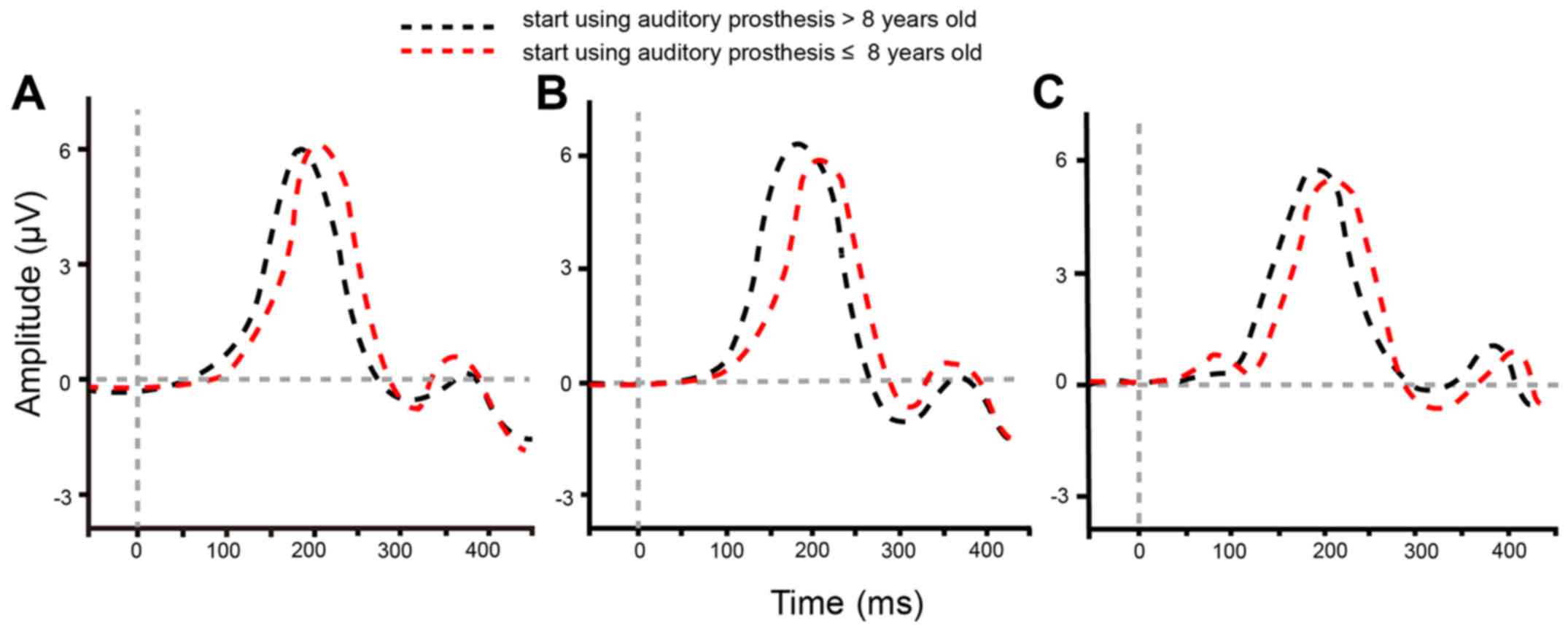
P1 wave growth of auditory prosthesis
users is affected by initial age of using the device
The influence of age on P1-N1-P2 waveforms and P1
latency was then investigated. Under different acoustic
stimulations, P1-N1-P2 waveforms were relatively typical for 126
patients who began using auditory prosthesis at the age of ≤8 or
>8 years old (Fig. 2). Under the
acoustic stimulations of /m/, /g/ and /t/, the target subjects of
two groups (≤8 or >8) presented a significant difference in P1
latency (all P<0.017) following correction via the Bonferroni
method (Table V), which suggested
that the P1 wave growth of auditory prosthesis users was affected
by their initial age of using the device; that earlier initial use
was better for auditory pathway remolding.
 | Table V.P1 latency of auditory prosthesis
users who initially used the device at ≤8 and >8 years of
age. |
Table V.
P1 latency of auditory prosthesis
users who initially used the device at ≤8 and >8 years of
age.
|
| P1 latency
(msec) |
|
|---|
|
|
|
|
|---|
| Acoustic
stimulation | >8 | ≤8 | P-value |
|---|
| /m/ | 129.42±8.09 | 135.97±18.26 | 0.015 |
| /g/ | 124.59±15.50 | 133.07±13.90 | 0.002 |
| /t/ | 122.64±10.89 | 134.19±12.87 | <0.001 |
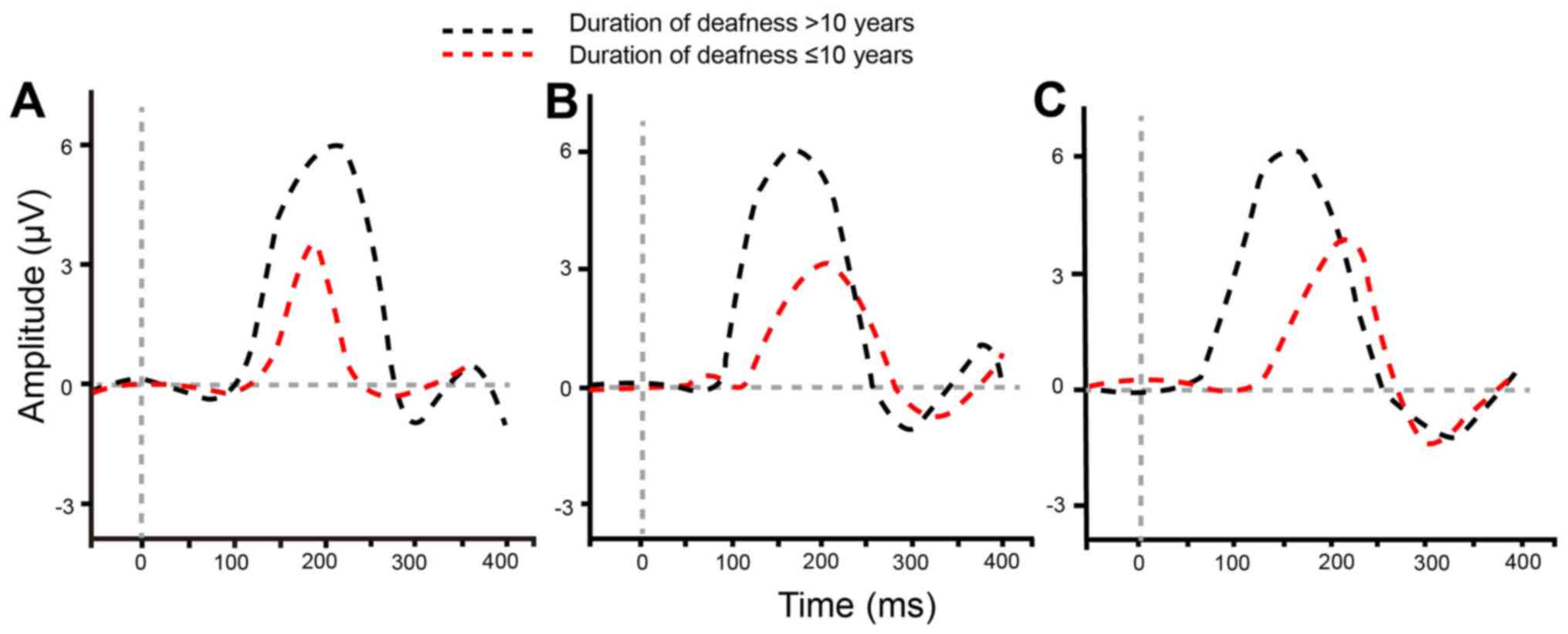
Deafness duration affects waveforms of
P1-N1-P2 and P1 latency
The effect of deafness duration on P1-N1-P2 waveform
and P1 latency was also explored. Under different acoustic
stimulations, the different P1-N1-P2 waveforms were observed
between patients who had ≤10 years of deafness and those who had
>10 years of deafness prior to using an auditory prosthesis
(Fig. 3). Compared with the patients
who had ≤10 years of deafness, the waveforms were not typical for
those with >10 years of deafness. Under the acoustic
stimulations of /m/, /g/ and /t/, P1 latency of patients in the two
groups was increased significantly (P<0.017; Table VI).
 | Table VI.P1 latency of auditory prosthesis
users who had ≤10-year and >10-year deafness duration prior to
using auditory prosthesis. |
Table VI.
P1 latency of auditory prosthesis
users who had ≤10-year and >10-year deafness duration prior to
using auditory prosthesis.
|
| P1 latency
(msec) |
|
|---|
|
|
|
|
|---|
| Acoustic
stimulation | >10-year
deafness duration | ≤10-year deafness
duration | P-value |
|---|
| /m/ | 128.68±9.27 | 135.25±16.76 | 0.021 |
| /g/ | 125.24±14.56 | 131.36±15.12 | 0.033 |
| /t/ | 123.23±11.02 | 132.00±13.42 | <0.001 |
Latency and amplitude of the P1 wave
were negatively associated with usage time of auditory
prosthesis
Correlation between latency and amplitude of P1 wave
and time of using hearing aids under different stimuli was
analyzed. Under the acoustic stimulations of /m/, /g/ and /t/, the
P1 latency and amplitude of patients are presented in Table VII. According to the Pearson
correlation analysis concerning latency and amplitude of P1 wave
and usage time of auditory prosthesis, under the acoustic
stimulation of /m/, latency and amplitude of P1 wave were both
negatively associated with the usage time of auditory prosthesis
(P<0.05), with respective correlation coefficients of
−0.222 and −0.774; provided acoustic stimulations were /g/ and /t/,
no significant correlation existed between latency and amplitude of
the P1 wave and usage time of auditory prosthesis (all P>0.05;
Table VIII).
 | Table VII.PI latency and amplitude under
different acoustic stimulations. |
Table VII.
PI latency and amplitude under
different acoustic stimulations.
| Acoustic
stimulation | Latency (msec) | Amplitude (µV) |
|---|
| /m/ | 133.11±15.02 | 6.52±1.51 |
| /g/ | 129.37±15.16 | 6.46±1.86 |
| /t/ | 129.15±13.30 | 5.54±1.46 |
 | Table VIII.Correlation of latency and amplitude
of P1 wave and usage time of auditory prosthesis under different
acoustic stimulations. |
Table VIII.
Correlation of latency and amplitude
of P1 wave and usage time of auditory prosthesis under different
acoustic stimulations.
|
| P1 latency and
usage time of auditory prosthesis | P1 amplitude and
usage time of auditory prosthesis |
|---|
|
|
|
|
|---|
| Acoustic
stimulation | r | P-value | r | P-value |
|---|
| /m/ | −0.222 | 0.013 | −0.774 | <0.001 |
| /g/ | 0.153 | 0.088 | −0.058 | 0.522 |
| /t/ | −0.030 | 0.741 | −0.037 | 0.679 |
SIR and CAP are associated with
initial age of using auditory prosthesis and deafness duration
In terms of the initial age of use of auditory
prosthesis, deafness duration and usage time of auditory
prosthesis, 126 patients were divided into two groups. The rank-sum
test was used to statistically analyze SIR and CAP of the two
groups under the same influencing factors (Table IX). The results demonstrated that
SIR and CAP were significantly associated with the initial age of
use of auditory prosthesis and deafness duration (both P<0.05)
but not with the usage time of auditory prosthesis (P>0.05).
 | Table IX.Influence of different parameters on
SIR and CAP. |
Table IX.
Influence of different parameters on
SIR and CAP.
| Parameter | Cases (n) | SIR | P-value | CAP | P-value |
|---|
| Initial age of
using auditory prosthesis (years) |
|
| 0.024 |
| <0.001 |
| ≤8 | 71 | 3.23±1.01 |
| 6.76±1.52 |
|
|
>8 | 55 | 2.43±0.75 |
| 5.67±1.72 |
|
| Deafness duration
(years) |
|
| <0.001 |
| 0.011 |
|
≤10 | 85 | 3.08±1.05 |
| 6.55±1.70 |
|
|
>10 | 41 | 2.46±0.68 |
| 5.74±1.56 |
|
| Using time of
auditory prosthesis (years) |
|
| 0.190 |
| 0.153 |
| ≤8 | 113 | 2.84±0.98 |
| 6.23±1.71 |
|
|
>8 | 13 | 3.22±1.02 |
| 6.74±1.53 |
|
Discussion
ACEPs are an emerging hearing aid evaluation tool
for young children who fail to provide reliable behavioral
feedback. It is effective in determining the association between
the sensitivity of ACEPs and the sensation level of speech sounds,
which is the ratio between the sum of detections and non-detections
and the number of detections (11).
P1, N1, and P2 are obligatory ACEP components, which are generated
with input from the primary auditory cortex, auditory
thalamocortical, cortico-cortical pathways and various association
cortices (19). In the present
study, via analysis of the P1-N1-P2 waves of the ACEP, the changes
of the P1 wave were evaluated for auditory prosthesis users in
order to investigate the feasibility of ACEP in clinical auditory
effect assessment.
In the present study, under different acoustic
stimulations, auditory prosthesis users had significantly higher
extraction rates of the P1 wave than N1 and P2 waves; the auditory
prosthesis users' shorter deafness duration prior to device usage
and younger initial usage meant more marked P1-N1-P2 waveforms and
longer P1 latency. A previous study noted that the P1 peak occurs
at a latency of ~300 msec in infants with normal hearing; this
latency decreases gradually until the end of the second decade of
life, and at that time, P1 is observed at ~60 msec (13). The morphology and amplitude of the
CAEP waveform are reported to vary with age, and the P1 of
amplitude significantly decreases by adolescence (20). Additionally, in the present study,
the amplification and latency of the P1 wave were both negatively
associated with the usage time of auditory prosthesis under the
acoustic stimulation of /m/. According to Alcántara et al,
adults with severe deafness usually suffer hearing loss at high
frequencies, leading to reduced audibility of speech signals of
high frequency; therefore, once an auditory prosthesis is worn, the
high-frequency acoustic stimulation cannot be recorded in auditory
cortices with extended low-frequency response due to long-term
stimulation by background noise (21).
In addition, SIR and CAP are associated with the
initial age of use of auditory prosthesis and deafness duration.
The SIR was used to provide a general outcome to measure speech
production in various communicative contexts of real-life
situations (22). CAP is designed to
approximately describe how a child responds to sound from the
cochlear implant; the lowest category represents no awareness of
environmental sounds, whereas the highest category describes the
ability to talk with a known speaker on a telephone in a nonlinear
hierarchical scale (23). Long
latency auditory evoked potentials (P1, N1, and P2) of exogenous
cortical responses generated from primary or secondary auditory
cortices (24). It has been verified
that P1 latency changes with age and that P1 latency can be used as
a biomarker for maturation of central auditory development in
children (25). ACEP can reflect the
neural detection of acoustic cues that are essential for speech
perception (26). Additionally, as
the P1 latency varies with different frequency stimulations, it
could effectively reflect a range of acoustic frequencies, making
it necessary for speech recognition (25). A previous study further confirmed the
result that children who have normal P1 latencies (normal-hearing
or age-matched children) exhibit better speech perception in the
multi-syllabic lexical neighborhood test than those with abnormal
P1 latencies (27). Therefore, P1
latency may be used as a biomarker for central auditory development
in hearing-impaired children, determining the effectiveness of
intervention strategies for hearing-impaired children, as speech
production and auditory perception are associated with the initial
age of auditory prosthesis and deafness duration.
In conclusion, ACEP P1-N1-P2 waveforms and the
development of the P1 wave were studied to evaluate their
feasibility in assessing the effectiveness of an auditory
prosthesis, which may be a theoretical foundation for clinical use.
However, there were a limited number of samples, thus there was no
completely representative result, and there was no separate
discussion for patients who were wearing an auditory prosthesis and
CI. For future studies, the authors will continue to collect cases
and to study the application value of ACEP for hearing aids and CI
children, which may provide more detailed references for the
clinical application of ACEP and the evaluation of hearing recovery
effects in clinically deaf children.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JDe, JDu and XM designed the present study. JDe and
JDu performed the experiments. XM and PZ collected and analyzed the
data, contributed to sample collection and provided intellectual
input. JDe and JDu drafted and the manuscript. XM and PZ assisted
with experimental design, interpreted the results and critically
revised the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First People's Hospital of Kunshan (Kunshan,
China), and all patients provided written informed consent.
Patient consent for publication
All patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Richardson GP, de Monvel JB and Petit C:
How the genetics of deafness illuminates auditory physiology. Annu
Rev Physiol. 73:311–334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vivero RJ, Fan K, Angeli S, Balkany TJ and
Liu XZ: Cochlear implantation in common forms of genetic deafness.
Int J Pediatr Otorhinolaryngol. 74:1107–1112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miao W, Li J, Tang M, Xian J, Li W, Liu Z,
Liu S, Sabel BA, Wang Z and He H: Altered white matter integrity in
adolescents with prelingual deafness: A high-resolution tract-based
spatial statistics imaging study. AJNR Am J Neuroradiol.
34:1264–1270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaplan DM and Puterman M: Pediatric
cochlear implants in prelingual deafness: Medium and long-term
outcomes. Isr Med Assoc J. 12:107–109. 2010.PubMed/NCBI
|
|
5
|
Kral A and O'Donoghue GM: Profound
deafness in childhood. N Engl J Med. 363:1438–1450. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fu S, Dong J, Wang C and Chen G: Parental
attitudes toward genetic testing for prelingual deafness in China.
Int J Pediatr Otorhinolaryngol. 74:1122–1125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vlastarakos PV, Proikas K,
Papacharalampous G, Exadaktylou I, Mochloulis G and Nikolopoulos
TP: Cochlear implantation under the first year of age-the outcomes.
A critical systematic review and meta-analysis. Int J Pediatr
Otorhinolaryngol. 74:119–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Teoh SW, Pisoni DB and Miyamoto RT:
Cochlear implantation in adults with prelingual deafness. Part II.
Underlying constraints that affect audiological outcomes.
Laryngoscope. 114:1714–1719. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng Y, Soli SD, Wang K, Meng J, Meng Z,
Xu K and Tao Y: A normative study of early prelingual auditory
development. Audiol Neurootol. 14:214–222. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jalilvand H, Pourbakht A and Jalaee S: The
relationship between hearing aid frequency response and acceptable
noise level in patients with sensorineural hearing loss. Adv Biomed
Res. 4:2562015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van Dun B, Carter L and Dillon H:
Sensitivity of cortical auditory evoked potential detection for
hearing-impaired infants in response to short speech sounds. Audiol
Res. 2:e132012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Durante AS, Wieselberg MB, Carvalho S,
Costa N, Pucci B, Gudayol N and Almeida KD: Cortical auditory
evoked potential: Evaluation of speech detection in adult hearing
aid users. Codas. 26:367–373. 2014.(In English, Portuguese).
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Campbell JD, Cardon G and Sharma A:
Clinical application of the P1 cortical auditory evoked potential
biomarker in children with sensorineural hearing loss and auditory
neuropathy spectrum disorder. Semin Hear. 32:147–155. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thabet MT and Said NM: Cortical auditory
evoked potential (P1): A potential objective indicator for auditory
rehabilitation outcome. Int J Pediatr Otorhinolaryngol.
76:1712–1718. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharma A, Campbell J and Cardon G:
Developmental and cross-modal plasticity in deafness: Evidence from
the P1 and N1 event related potentials in cochlear implanted
children. Int J Psychophysiol. 95:135–144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wagner M, Roychoudhury A, Campanelli L,
Shafer VL, Martin B and Steinschneider M: Representation of
spectro-temporal features of spoken words within the P1-N1-P2 and
T-complex of the auditory evoked potentials (AEP). Neurosci Lett.
614:119–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elangovan S and Stuart A: A
cross-linguistic examination of cortical auditory evoked potentials
for a categorical voicing contrast. Neurosci Lett. 490:140–144.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lina-Granade G, Truy E, Porot M, Collet L
and Disant F: Hearing impairment in children: Early diagnosis is
essential. Arch Pediatr. 7:991–1000. 2000.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo S, Li H, Chen B and Dai C: Study of
categories of auditory performance and speech intelligibility
rating of post-lingual cochlear implantes. Lin Chung Er Bi Yan Hou
Tou Jing Wai Ke Za Zhi. 28:955–957, 960. 2014.(In Chinese).
PubMed/NCBI
|
|
20
|
Gilley PM, Sharma A, Dorman M and Martin
K: Developmental changes in refractoriness of the cortical auditory
evoked potential. Clin Neurophysiol. 116:648–657. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alcantara JL, Moore BC, Kühnel V and
Launer S: Evaluation of the noise reduction system in a commercial
digital hearing aid. Int J Audiol. 42:34–42. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang VW, Ching TY, Van Buynder P, Hou S,
Flynn C, Burns L, McGhie K and Wong AO: Aided cortical response,
speech intelligibility, consonant perception and functional
performance of young children using conventional amplification or
nonlinear frequency compression. Int J Pediatr Otorhinolaryngol.
78:1692–1700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lonka E, Hasan M and Komulainen E: Spoken
language skills and educational placement in Finnish children with
cochlear implants. Folia Phoniatr Logop. 63:296–304. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Russo N, Zecker S, Trommer B, Chen J and
Kraus N: Effects of background noise on cortical encoding of speech
in autism spectrum disorders. J Autism Dev Disord. 39:1185–1196.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sharma A, Martin K, Roland P, Bauer P,
Sweeney MH, Gilley P and Dorman M: P1 latency as a biomarker for
central auditory development in children with hearing impairment. J
Am Acad Audiol. 16:564–573. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tremblay KL, Billings CJ, Friesen LM and
Souza PE: Neural representation of amplified speech sounds. Ear
Hear. 27:93–103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sharma A and Dorman MF: Central auditory
development in children with cochlear implants: Clinical
implications. Adv Otorhinolaryngol. 64:66–88. 2006.PubMed/NCBI
|
|
28
|
Zhou H, Chen Z, Shi H, Wu Y and Yin S:
Categories of auditory performance and speech intelligibility
ratings of early-implanted children without speech training. PLoS
One. 8:e538522013. View Article : Google Scholar : PubMed/NCBI
|