Introduction
Type 2 diabetes mellitus (T2DM), a glucose-, lipid-,
protein- and water-electrolyte metabolic disorder, risks micro- and
macrovascular damage (1). T2DM is
associated with various chronic complications, which may result in
high rates of disability and mortality (2). Major features of diabetes are impaired
insulin secretion and insulin resistance (3). Diabetes is developing into a global
health problem that threatens human health. Treatment has improved
over the last decades, but therapeutic effects remain limited
(4,5).
Insulin is used in the clinic to improve utilization
of glucose and accelerate anaerobic glycolysis and aerobic
oxidation of glucose, thereby reducing blood sugar levels (6). However, insulin treatment has side
effects, including hypoglycemic shock, insulin resistance, local
reactions of subcutaneous scleroma and fat atrophy (7). A drug reducing insulin-associated side
effects is needed. The synthetic glucagon-like peptide-1 (GLP-1)
receptor agonist, liraglutide (liragl), shares 97% homology with
the structure of human native GLP-1 (8). Numerous studies have demonstrated that
liragl increases insulin secretion and inhibits glucagon secretion
(9,10). A long circulating half-life with few
side effects make liragl an ideal long-acting antidiabetic drug
(11). Co-administration of insulin
and liragl may describe a novel therapy for T2DM.
Owing to interactions between lipid metabolic
disorder and hyperglycemia, glycolipid metabolic disorder is
becoming a major factor in T2DM and metabolic syndrome (12). In addition, glycolipid metabolic
disorder contributes to diabetes and its associated complications,
including cardiovascular diseases (13,14). A
previous study has indicated that severe chronic vascular disease
(CVD) is a major cause of co-morbidity and mortality in patients
with T2DM (15). Cardiovascular
disease has been identified as a threat to patients with diabetes,
as the association between high blood glucose and cardiovascular
disease has been confirmed, and many trials have tested the
hypothesis that glucose normalization should prevent vascular
injury (16,17). Previous studies have indicated that
patients with diabetes and cardiovascular diseases have increased
mortality rates compared with patients without cardiovascular
diseases (18,19). The main challenge in the successful
management of T2DM is not only to control blood sugar levels, but
also to reduce glycolipid metabolic disorders and cardiac
damage.
The current study investigated whether liragl may
reduce insulin-induced side effects in patients with diabetes,
including glycolipid metabolic disorders and cardiac injury. The
results demonstrated that co-administration of insulin and liragl
may control blood sugar levels, restore the glycolipid metabolic
balance and alleviate cardiac injury.
Materials and methods
Animals and ethics
A total of 40 adult male Sprague Dawley rats (age, 4
weeks; weight 220–250 g) were purchased from the Experimental
Animal Center of Hebei Medical University (Shijiazhuang, China) and
housed in a controlled environment at 25±3°C in 60% humidity, in a
12-h light/dark cycle with free access to food and water. All
experimental protocols were approved by the Committee for
Laboratory Animal Care and Use of the Cangzhou Central Hospital
(Cangzhou, China).
Induction of T2DM
STZ-induced model rats were exposed to high-fat
diets (77% regular diet, 15% lard oil, 5% white sugar, 2%
cholesterol, 0.25% sodium cholate, and 0.75% salt) for 4 weeks
prior to receiving two intraperitoneal streptozotocin (STZ)
injections (60 mg/kg) within 72 h. Rats were fed with the high-fat
diets for a further 2 weeks. Rats with blood glucose level ≥11.1
mmol/l were considered as diabetic and selected for subsequent
experiments. Healthy rats fed a normal diet were assigned as the
control group (n=8) and diabetic rats were randomly divided into
four groups (n=8 per group): STZ, Liragl, Insulin and Insulin +
Liragl. Liragl group was treated with liragl (3 mg/day) by
hypodermic injection in the abdomen; Insulin group was treated with
insulin (50 U/day) by hypodermic injection in the abdomen and
Insulin + Liragl group was treated with liragl (3 mg/day) and
insulin (50 U/day) by hypodermic injection in abdomen. Treatment
continued for 4 weeks. During this period, rats in healthy control
group were fed with normal chow diet and rats with diabetes
continued high-fat diet. All rats were sacrificed by cervical
dislocation for subsequent experiments.
Blood-measured parameters
Rats who were deprived of food overnight for 12 h
were sacrificed by cervical dislocation and blood was collected
from the orbital sinus. Following centrifugation at 3,000 × g for
15 min at 4°C, serum was collected for measurement of total
cholesterol (TC), triglycerides (TG), low-density lipoprotein
cholesterol (LDL-C) and high-density lipoprotein cholesterol
(HDL-C) using a Hitachi 912 photometric chemistry analyzer
(Hitachi, Ltd., Tokyo, Japan). The blood glucose concentration in
the fasting state was measured using a blood glucose measurement
kit (Roche Diagnostics, Basel, Switzerland), utilizing the glucose
dehydrogenase method as previously described (20). Radioimmunoassays (cat. no.
NEX133001KT; PerkinElmer Inc., Krakow, Poland) were performed to
assess fasting insulin and C-peptide as described elsewhere
(21).
Western blot assays
Hepatic and myocardial tissues were isolated from
rats and homogenized separately on ice using a 10X RIPA buffer
(Cell Signaling Technology, Inc., Danvers, MA, USA) containing 1%
phenylmethylsulfonyl fluoride. Homogenized samples were washed with
ice-cold PBS and centrifuged at 10,000 × g for 15 min at 4°C. The
supernatant was collected and the protein concentration was
determined using a BCA protein assay kit (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China). Proteins were
separated using 10% SDS-PAGE gels and transferred to polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). Following
blocking with 5% skimmed milk at room temperature for 2 h,
membranes loaded with hepatic proteins were incubated with primary
antibodies at 37°C for 4 h: Rabbit anti-adenosine 5′-monophosphate
kinase-α1 (AMPKα1) (1:1,000; cat. no. ab3759), rabbit
anti-carnitine palmitoyltransferase 1 (CPT-1) (1:5,000; cat. no.
ab198494), rabbit anti-sterol regulatory element-binding protein 1
(SREBP-1c) (1:2,000; cat. no. ab28481) and rabbit anti-GAPDH
(1:1,000, cat. no. ab9485) (all Abcam, Cambridge, UK). Membranes
loaded with myocardial proteins were incubated with rabbit anti-
myoglobin (Mb) (1:2,500; cat. no. ab77232), rabbit anti-creatine
kinase-muscle/brain (CK-MB; 1:1,000; ab31832), rabbit anti-cardiac
troponin I (cTnI; 1:1,000; ab10231) and rabbit anti-GAPDH (1:2,500)
(all Abcam) at 4°C overnight. Membranes were washed with
Tris-buffered saline and Tween-20 three times and incubated with
horseradish peroxidase-conjugated secondary antibody (1:10,000;
cat. no. ab181658; Abcam) for 1 h at room temperature. Proteins
were visualized using enhanced chemiluminescence reagents (Pierce;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Analysis was
performed using ImageJ software (version 1.48; National Institutes
of Health, Bethesda, MD, USA).
Histopathological examination
Formalin fixed and paraffin-embedded heart tissues
were fixed with 10% neutral buffered formalin at 4°C for 4 h. Then
tissues were cut into 4-µm-thick slices. All samples were stained
with hematoxylin and eosin (H&E) at 4°C for 2 h.
Histopathological characteristics of hearts were observed using
light microscopy (magnification, ×400).
Immunohistochemistry
Caspase-3 in heart tissues was measured by
immunohistochemistry. Paraffin sections of heart tissue was fixed
with 10% neutral buffered formalin at 4°C for 4 h. Then the tissues
were deparaffinized in xylene, rehydrated in graded ethanol
solutions and microwaved in sodium citrate buffer. Following
cooling to room temperature, sections were incubated with 3% fresh
H2O2, followed by blocking with 3% bovine
serum albumin for 2 h (Thermo Fisher Scientific, Inc.) at 25°C for
2 h. Sections were incubated with rabbit anti-caspase-3 (cat. no.
#9662; 1:1,000; Cell Signaling Technology, Inc.) at 4°C overnight.
Following washing with Tris buffered saline for 5 min (repeated
three times), all slides were incubated with secondary antibody
(rabbit IgG; cat. no. #A32731; 1:200; Thermo Fisher Scientific,
Inc.) for 30 min at 37°C. Sections were successively stained with
3,3′-diaminobenzidine for 5 min and counter-stained with
hematoxylin for 30 sec at 4°C. Sections were observed using a
digital camera (under magnification, ×400) following dehydrating,
drying and mounting with neutral gum.
Evaluation of oxidative stress in
serum
Malondialdehyde (MDA) in the serum was measured
using an MDA Assay kit (Nanjing Jiancheng Bioengineering Institute,
Nanjing, China). The content of MDA was determined by a
thiobarbituric acid reaction. The absorbance was read at 532 nm by
a spectrophotometer. For superoxide dismutase (SOD) detection, the
cells were lysed using a cell lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) and the lysates were centrifuged at
10,000 g at 4°C for 5 min. The supernatant was collected for SOD
analysis using a SOD kit (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). The absorbance was read at 450 nm by a microplate
reader. The mean value of each group was calculated as the
percentage of the control value.
Statistics analysis
Data were analyzed with SPSS 19.0 (IBM Corp.,
Armonk, NY, USA). Data are presented as the mean ± standard
deviation and a minimum of three repeats were performed for each
experiment. Group statistical comparisons were assessed by one-way
analysis of variance followed by Bonferroni's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Liragl enhances hypoglycemic effect of
insulin in T2DM rats
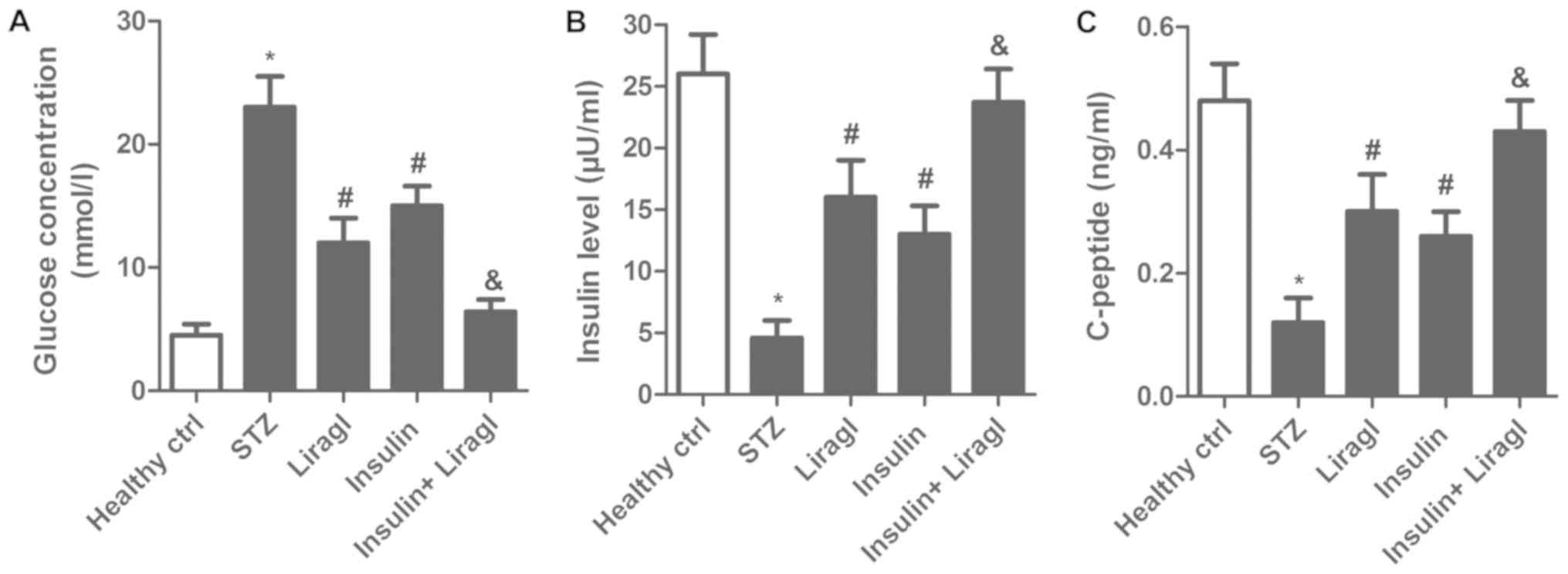
To explore the hypoglycemic effect of combined
liragl and insulin treatment in T2DM rats, fasting blood-glucose,
insulin and c-peptide concentrations were measured. Fig. 1A demonstrated a significant decrease
in the glucose concentration in the Insulin group and the Liragl
group compared with the STZ group, while a decrease was also
detected in the Insulin + Liragl group compared with the Liragl or
Insulin groups. Insulin and c-peptide levels in the Insulin and
Liragl groups were significantly lower compared with the Insulin +
Liragl group (Fig. 1B and C). These
results demonstrated that combined treatment with liragl and
insulin enhanced the hypoglycemic effect.
Co-administration of liragl and
insulin ameliorates disorder of lipid metabolism
To investigate the impact of liragl and insulin on
lipid metabolism, TC, TG, LDL-C and HDL-C levels in serum were
determined. As illustrated in Fig.
2A-C, an increase of TC, TG and LDL-C was observed in STZ rats,
which was significantly decreased by insulin and liragl, with a
further significant decrease observed for the combination
treatment. In addition, an STZ-induced decrease in HDL-C was
significantly reversed by insulin and liragl, with a further
significant increase observed for the combination treatment
(Fig. 2D). These results suggested
that combination of liragl and insulin may better alleviate the
disorder of lipid metabolism more effectively compared with liragl
or insulin alone.
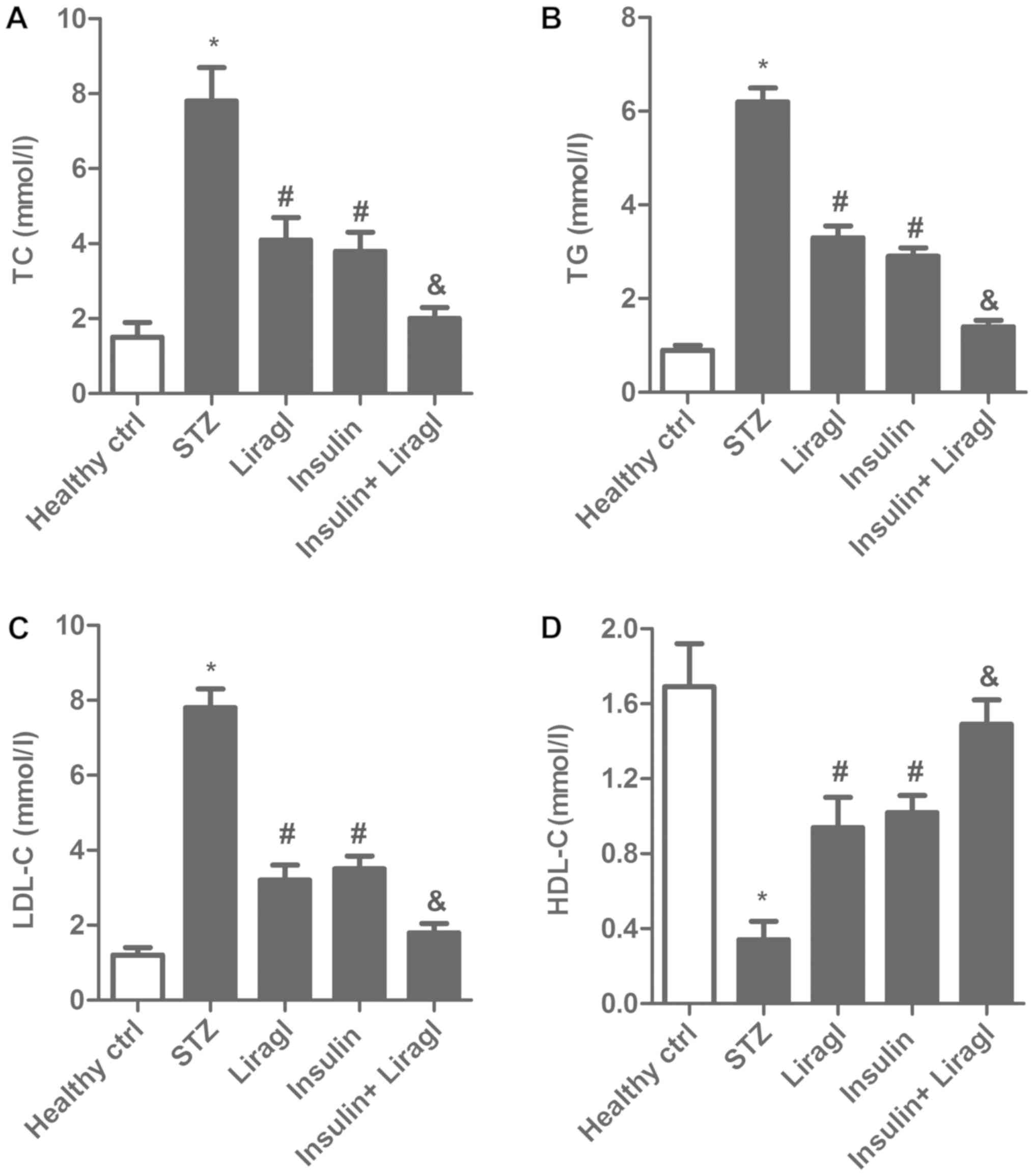 | Figure 2.Co-administration of liragl and
insulin ameliorates the disorder of lipid metabolism. Sprague
Dawley rats were randomly divided into groups: Healthy control
group, healthy rats; STZ group, rats that received STZ injections
to induce type 2 diabetes; Liragl group, diabetic rats treated with
liragl; Insulin group, diabetic rats treated with insulin; Insulin
+ Liragl group, diabetic rats treated with insulin and liragl. (A)
TC, (B) TG, (C) LDL-C and (D) HDL-C levels. *P<0.05 vs. healthy
control group; #P<0.05 vs. STZ group;
&P<0.05 vs. Insulin group. Liragl, liraglutide;
STZ, streptozotocin; TC, total cholesterol; TG, triglycerides;
LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density
lipoprotein cholesterol. |
Combination of liragl and insulin
regulates expression of proteins associated with lipid
metabolism
To further investigate the regulatory role that
liragl and insulin serve in lipid metabolism, AMPKα1, CPT-1 and
SREBP-1c levels were determined using western blot assays. As
presented in Fig. 3, liragl and
insulin suppressed the decrease in AMPKα and CPT-1 levels and the
increase in SREBP-1c induced by STZ. Co-administration of the two
drugs produced greater effects compared with either drug alone.
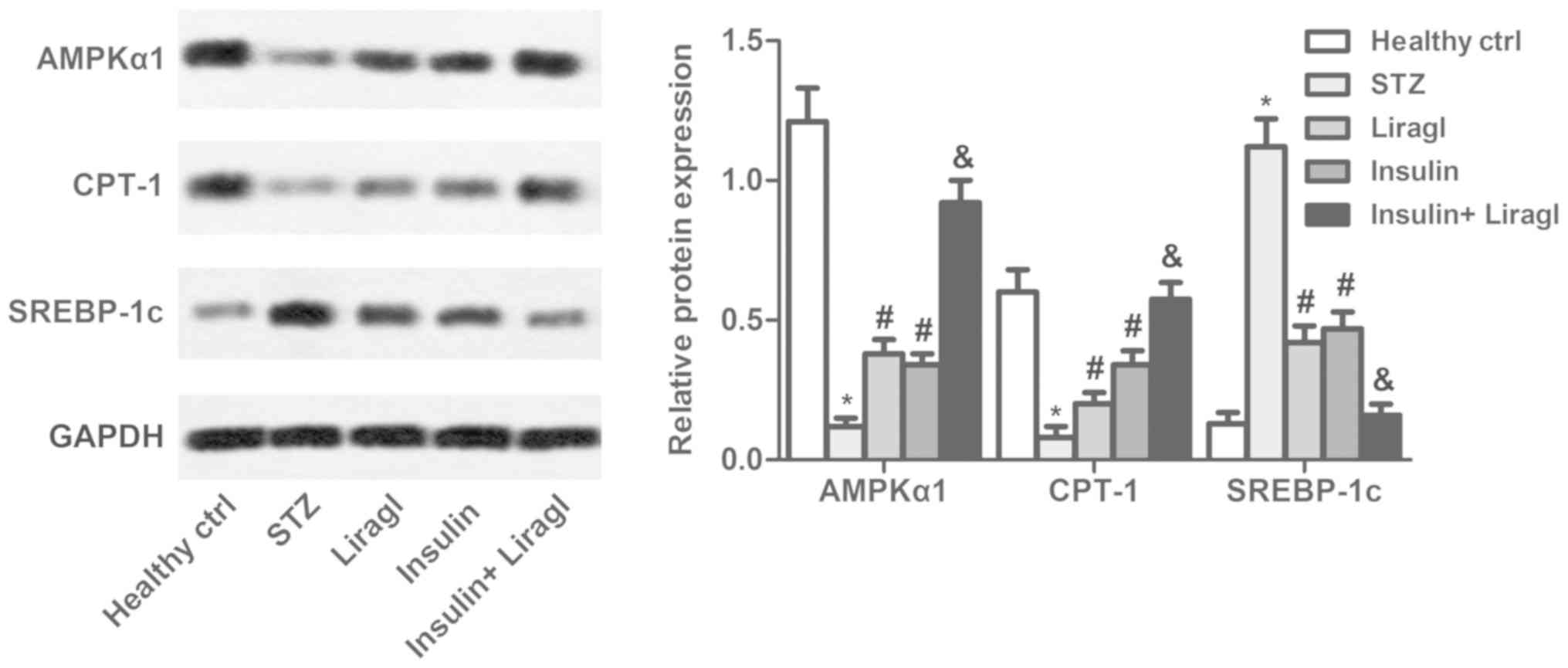 | Figure 3.Combination of liragl and insulin
regulates expressions of lipid metabolism associated proteins.
Sprague Dawley rats were randomly divided into groups: Healthy
control group, healthy rats; STZ group, rats that received STZ
injections to induce type 2 diabetes; Liragl group, diabetic rats
treated with liragl; Insulin group, diabetic rats treated with
insulin; Insulin + Liragl group, diabetic rats treated with insulin
and liragl. AMPKα1, CPT-1 and SREBP-1c expression determined using
western blot analysis. *P<0.05 vs. healthy control group;
#P<0.05 vs. STZ group; &P<0.05 vs.
Insulin group. Liragl, liraglutide; STZ, streptozotocin; AMPKα1,
adenosine 5′-monophosphate kinase-α1; CPT-1, carnitine
palmitoyltransferase 1; SREBP-1c, sterol regulatory element-binding
protein 1. |
Liragl enhances protective effects of
insulin on diabetes-induced myocardial damage
To determine whether combination of liragl and
insulin served a protective role in diabetes-induced myocardial
injury, morphological histological features of heart tissues were
measured using H&E staining. As illustrated in Fig. 4A, serious cardiomyocyte edemas and
intercellular space dilatations were observed in intermuscular
spaces in the STZ group compared with the healthy control group.
These histopathological alterations were suppressed by liragl or
insulin used alone and markedly inhibited by their combined
treatment. In addition, the STZ-induced increase in caspase-3
expression could be repressed significantly by liragl or insulin
alone, but a combination produced greater inhibitory effects
(Fig. 4B). SOD, MDA, Mb, CK-MB and
cTnI expression illustrated a similar trend. A decrease in SOD and
an increase in MDA, Mb, CK-MB and cTnI expression as induced by STZ
were significantly reversed by administration of liragl and
insulin, with enhanced results when drugs were administered
together (Fig. 4C and D). These
results demonstrated that combination of liragl and insulin may
significantly alleviate myocardial injury and oxidative stress.
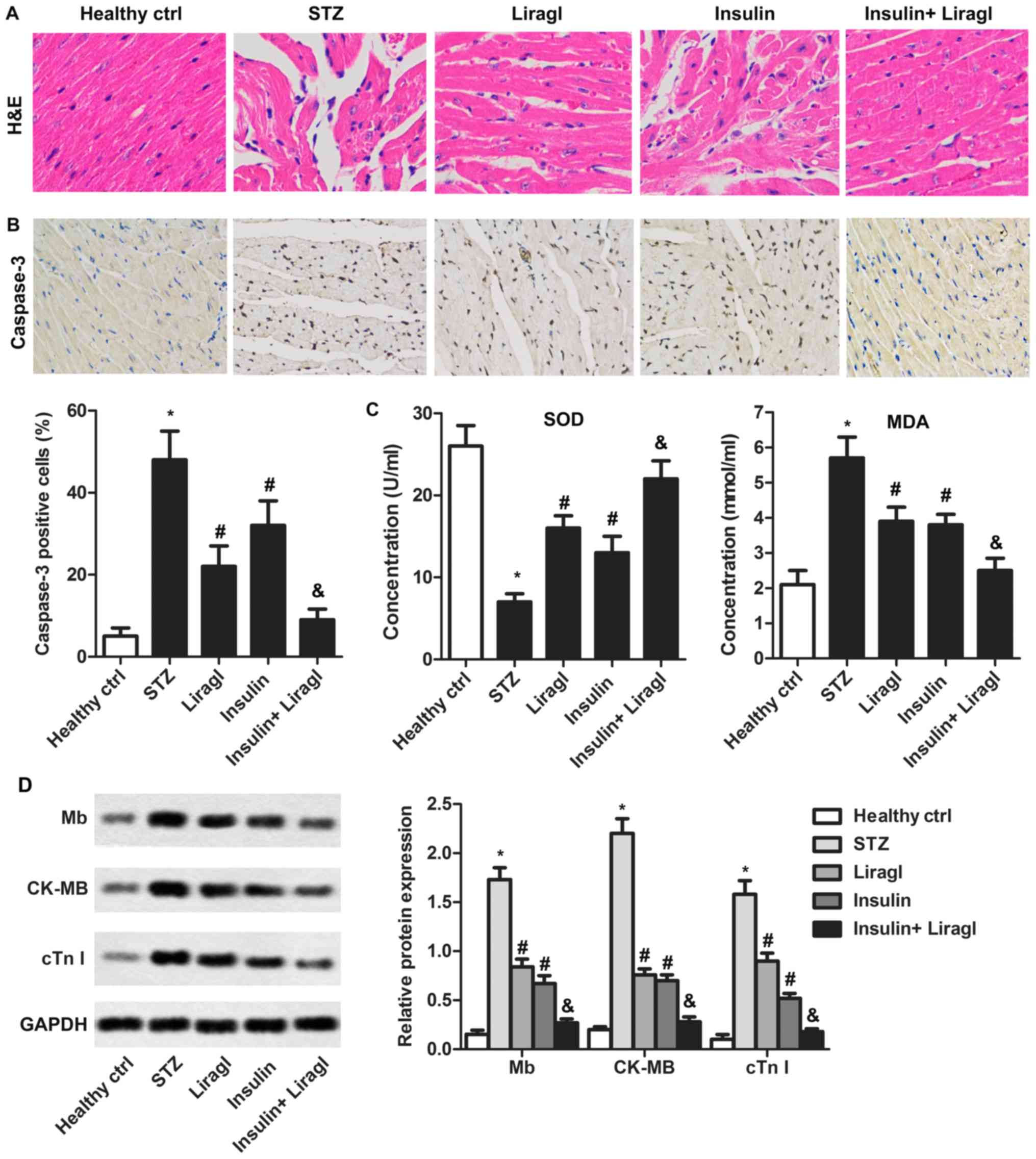 | Figure 4.Liragl enhances protective effect of
insulin on diabetes-induced myocardial damage. Sprague Dawley rats
were randomly divided into groups: Healthy control group, healthy
rats; STZ group, rats that received STZ injections to induce type 2
diabetes; Liragl group, diabetic rats treated with liragl; Insulin
group, diabetic rats treated with insulin; Insulin + Liragl group,
diabetic rats treated with insulin and liragl. (A) Morphological
changes induced by diabetes measured using H&E staining.
Magnification, ×400. (B) The Caspase-3 expression was measured
using immunohistochemistry assays. Magnification, ×400 (C) SOD and
MDA levels measured using commercial detection kits. (D) Mb, CK-MB
and cTnI levels evaluated using western blot assays. *P<0.05 vs.
healthy control group; #P<0.05 vs. STZ group;
&P<0.05 vs. Insulin group. Liragl, liraglutide;
STZ, streptozotocin; SOD, superoxide dismutase; H&E,
hematoxylin and eosin; MDA, malondialdehyde; Mb, myoglobin; CK-MB,
creatine kinase-muscle/brain; cTNI, cardiac troponin I. |
Discussion
Owing to the progressive nature of diabetes, many
patients require multiple therapeutic approaches to control blood
sugar levels (22). One major
treatment is insulin. However, hypoglycemia, which depends on
duration and dose of insulin treatment, limits application of
insulin treatments (23).
A recent study indicated that liragl decreased
glycated hemoglobin, enhanced insulin secretion, aided weight loss
and rarely led to hypoglycemia (24). Based on these results, liragl was
selected for combination treatment with insulin in the current
study.
A major characteristic of diabetes is the
disturbance of carbohydrate metabolism, which is caused by damaged
islet β-cells (25). Islet β-cell
injury is associated with increased blood glucose and decreased
insulin and C-peptide levels (26).
Kondo et al (27) described a
retrospective cohort study to demonstrate that β-cell function was
improved in early liragl treatment and increased C-peptide level in
patients with T2DM. A previous study indicated that insulin therapy
enhanced C-peptide expression over a short period (28). In the current study, liragl and
insulin controlled blood sugar levels and increased insulin and
C-peptide serum levels, and the co-administration of liragl and
insulin resulted in more pronounced effects.
Patients with T2DM may exhibit serious damage of
lipid dynamics, manifested as elevated TC, TG, LDL-C, decreased
HDL-C and excessive fat deposition in various tissues (29). According to Liu et al
(30), liragl (1.2 mg/day)
monotherapy exhibited significant lipid-lowering effects in
patients with reduced levels of fasting blood glucose, glycated
hemoglobin, body mass index, TG, TC and LDL-C following 24-week
treatment. However, insulin resistance promoted small dense LDL and
reduced HDL production. In the current study, combining liragl and
insulin significantly elevated HDL-C levels and reduced TC, TG and
LDL-C.
Myocardial damage induced by diabetes is a distinct
entity, which is different from coronary heart disease (31). Increasing numbers of studies have
demonstrated that liragl serves a positive role in cardiac
functional recovery in patients with heart diseases (32,33). It
is reported that primary endpoints of cardiac output, stroke volume
and left ventricular contractile index were remarkably enhanced by
liragl treatment for 7 days in patients with heart failure
(34). For insulin, studies
indicated beneficial effects of insulin on damaged cardiac tissue
(35,36). Xing et al (37) reported activation of protein kinase B
as a result of insulin-induced suppression of PH domain
leucine-rich repeat-containing protein phosphatase 1 serving a
vital role in cardioprotection. In the current study, serious
cardiomyocyte edemas and intercellular space dilatations were
alleviated by combination treatment of liragl and insulin.
Co-administration of liragl and insulin significantly suppressed
Mb, CK-MB and cTnI expression in heart tissue.
Oxidative stress and inflammation result in
cardiomyocyte apoptosis in diabetic hearts, which eventually leads
to cardiac dysfunction (38). Thus,
suppressing apoptosis is extremely important. According to a
published report, liragl treatment suppresses apoptosis of various
cell types (39). Liragl improves
recovery following central nervous system injuries through
inhibiting apoptosis and elevating microtubulin acetylation and
autophagy (40). In addition, in a
previous study, an intramyocardial injection of nanoparticle-liragl
promoted recovery of cardiac functions, alleviated infarct size and
inhibited cardiomyocyte apoptosis at 4 weeks following injection
(41). Insulin was reported to
suppress cardiomyocytes apoptosis in rats with diabetic
cardiomyopathy (42). In the current
study, liragl combined with insulin suppressed apoptosis of
cardiomyocytes via significantly decreased caspase-3
expression.
Increasing evidence indicates that aggregation of
intermediate oxidation products may be a pathogenic factor of
myocardial damage in diabetic rats (43). SOD, an important biological
antioxidant, is involved in eliminating free radicals (44). MDA, a metabolite of lipid
peroxidative damage, evaluates the extent of free radical-induced
damage on cytomembranes (45). A
previous study demonstrated that liragl treatment enhanced SOD and
adiponectin levels in the liver, indicating antioxidative effects
of liragl (46). Ramalingayya et
al (47) observed that insulin
(0.5 U/kg, intraperitoneal) attenuated doxorubicin-induced brain
oxidative stress with an elevation in antioxidant defense systems.
In the current study, combining liragl and insulin significantly
increased SOD and decreased MDA levels in rats with T2DM.
In conclusion, the current study demonstrated that
both liragl and insulin ameliorated diabetes and its complications,
including glucose and lipid metabolism disorder and myocardial
injury. However, combination treatment of insulin and liragl
resulted in increased effects. Therefore, combination treatment of
liragl and insulin may be considered as a potential therapeutic
agent in diabetes treatment in the clinic.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QH analyzed and interpreted the data regarding the
T2DM model and blood-measured parameters. CL was responsible for
designing the study and drafting the manuscript. JRL and LZ
performed the immunohistochemistry. FCH, DW and YJL performed the
western blot and statistical analysis. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The animal experiments in this study were approved
by the Animal Care and Research Committee of Cangzhou Central
Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TC
|
total cholesterol
|
|
TG
|
triglycerides
|
|
LDL-C
|
low-density lipoprotein
cholesterol
|
|
HDL-C
|
high-density lipoprotein
cholesterol
|
|
T2DM
|
type 2 diabetes mellitus
|
|
liragl
|
liraglutide
|
References
|
1
|
Sciatti E, Vizzardi E, Castiello A,
Valentini F, Bonadei I, Gelsomino S, Lorusso R and Metra M: The
role of type 2 diabetes mellitus on hypertensive-related aortic
stiffness. Echocardiography. 35:798–803. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen H, Jiang Y, Yang Z, Hu W, Xiong L,
Wang N, Liu X, Zheng G, Ouyang K and Wang W: Effects of
chimonanthus nitens oliv. Leaf extract on glycolipid metabolism and
antioxidant capacity in diabetic model mice. Oxid Med Cell Longev.
2017:76485052017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang M, Zhou J, Liu Y, Sun X, Luo X, Han
C, Zhang L, Wang B, Ren Y, Zhao Y, et al: Risk of type 2 diabetes
mellitus associated with plasma lipid levels: The rural Chinese
cohort study. Diabetes Res Clin Pract. 135:150–157. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Q, Zhang X, Fang L, Guan Q, Guan L
and Li Q: Prevalence, awareness, treatment and control of diabetes
mellitus among middle-aged and elderly people in a rural Chinese
population: A cross-sectional study. PLoS One. 13:e01983432018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Riemenschneider H, Saha S, van den Broucke
S, Maindal HT, Doyle G, Levin-Zamir D, Muller I, Ganahl K, Sørensen
K, Chang P, et al: State of diabetes self-management education in
the european union member states and Non-EU Countries: The diabetes
literacy project. PLoS One. 2018:14671712018.
|
|
6
|
Davies ML, Pham DQ and Drab SR: GLP1-RA
Add-on therapy in patients with type 2 diabetes currently on a
bolus containing insulin regimen. Pharmacotherapy. 36:893–905.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiménez-Osorio AS, Monroy A and Alavez S:
Curcumin and insulin resistance-Molecular targets and clinical
evidences. Biofactors. 42:561–580. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Neumiller JJ: Differential chemistry
(structure), mechanism of action, and pharmacology of GLP-1
receptor agonists and DPP-4 inhibitors. J Am Pharm Assoc (2003). 49
Suppl 1:S16–S29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Andreozzi F, Raciti GA, Nigro C, Mannino
GC, Procopio T, Davalli AM, Beguinot F, Sesti G, Miele C and Folli
F: The GLP-1 receptor agonists exenatide and liraglutide activate
Glucose transport by an AMPK-dependent mechanism. J Transl Med.
14:2292016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kramer CK, Zinman B, Choi H, Connelly PW
and Retnakaran R: The impact of chronic liraglutide therapy on
glucagon secretion in type 2 diabetes: Insight from the libra
trial. J Clin Endocrinol Metab. 100:3702–3709. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nauck MA: Incretin-based therapies for
type 2 diabetes mellitus: Properties, functions, and clinical
implications. Am J Med. 124 (1 Suppl):S3–S18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Perry RJ, Samuel VT, Petersen KF and
Shulman GI: The role of hepatic lipids in hepatic insulin
resistance and type 2 diabetes. Nature. 510:84–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lan YL, Huang SP, Heng XP, Chen L, Li PH,
Wu J, Yang LQ, Pan XD, Lin T, Cheng XL, et al: Dan-gua fang
improves glycolipid metabolic disorders by promoting hepatic
adenosine 5′-monophosphate activated protein kinase expression in
diabetic Goto-Kakizaki rats. Chin J Integr Med. 21:188–195. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yki-Järvinen H: Management of type 2
diabetes mellitus and cardiovascular risk: Lessons from
intervention trials. Drugs. 60:975–983. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stadler S, Jalili S, Schreib A, Jung B,
Zeman F5, Böger CA, Heid IM and Arzt M; DIACORE study group, :
Association of sleep-disordered breathing with severe chronic
vascular disease in patients with type 2 diabetes. Sleep Med.
48:53–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Szuszkiewicz-Garcia MM and Davidson JA:
Cardiovascular disease in diabetes mellitus: Risk factors and
medical therapy. Endocrinol Metab Clin North Am. 43:25–40. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rydén L, Shahim B and Mellbin L: Clinical
implications of cardiovascular outcome trials in type 2 diabetes:
From DCCT to EMPA-REG. Clin Ther. 38:1279–1287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao Q, Yang Y, Chen Z, Yu H and Xu H:
Changes in characteristics, risk factors, and in-hospital mortality
among patients with acute myocardial infarction in the capital of
China over 40 years. Int J Cardiol. 265:30–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vilbergsson S, Sigurdsson G, Sigvaldason H
and Sigfusson N: Coronary heart disease mortality amongst
non-insulin-dependent diabetic subjects in Iceland: The independent
effect of diabetes. the reykjavik study 17-year follow up. J Intern
Med. 244:309–316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maguire GA and Price CP: Kinetic glucose
dehydrogenase method for glucose measurement with a discrete
kinetic analyzer overcomes interference by ascorbate. Clin Chem.
30:157–158. 1984.PubMed/NCBI
|
|
21
|
Zheng H, Fan X, Li X, Zhang Y, Fan Y,
Zhang N, Song Y, Ren F, Shen C, Shen J and Yang J: The association
between single nucleotide polymorphisms of the Apelin gene and
diabetes mellitus in a Chinese population. J Pediatr Endocrinol
Metab. 29:1397–1402. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bally L, Thabit H and Hovorka R:
Glucose-responsive insulin delivery for type 1 diabetes: The
artificial pancreas story. Int J Pharm. 544:309–318. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de Galan BE: Prevention of insulin-induced
hypoglycaemia in the elderly. Ned Tijdschr Geneeskd.
158:A77222014.(In Dutch). PubMed/NCBI
|
|
24
|
Singh S, Wright EE Jr, Kwan AY, Thompson
JC, Syed IA, Korol EE, Waser NA, Yu MB and Juneja R: Glucagon-like
peptide-1 receptor agonists compared with basal insulins for the
treatment of type 2 diabetes mellitus: A systematic review and
meta-analysis. Diabetes Obes Metab. 19:228–238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen LN, Lyu J, Yang XF, Ji WJ, Yuan BX,
Chen MX, Ma X and Wang B: Liraglutide ameliorates glycometabolism
and insulin resistance through the upregulation of GLUT4 in
diabetic KKAy mice. Int J Mol Med. 32:892–900. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kalinowska A, Orlińska B, Panasiuk M,
Jamiołkowska M, Zasim A, Florys B, Wojtkielewicz K, Łuczyński W,
Głowińska-Olszewska B and Bossowski A: Assessment of preservation
of beta-cell function in children with long-standing type 1
diabetes with ‘ultrasensitive c-peptide’ method. Pediatr Endocrinol
Diabetes Metab. 23:130–138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kondo Y, Satoh S, Osada UN and Terauchi Y:
Early liraglutide treatment improves β-cell function in patients
with type 2 diabetes: A retrospective cohort study. Endocr J.
62:971–980. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Davis TME, Davis WA and Jeffrey G:
Successful withdrawal of insulin therapy after post-treatment
clearance of hepatitis C virus in a man with type 2 diabetes. Am J
Case Rep. 18:414–417. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schalch DS and Kipnis DM: Abnormalities in
carbohydrate tolerance associated with elevated plasma
nonesterified fatty acids. J Clin Invest. 44:2010–2020. 1965.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Y, Jiang X and Chen X: Liraglutide and
Metformin alone or combined therapy for type 2 diabetes patients
complicated with coronary artery disease. Lipids Health Dis.
16:2272017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Latha R, Shanthi P and Sachdanandam P:
Kalpaamruthaa modulates oxidative stress in cardiovascular
complication associated with type 2 diabetes mellitus through
PKC-β/Akt signaling. Can J Physiol Pharmacol. 91:901–912. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kumarathurai P, Anholm C, Nielsen OW,
Kristiansen OP, Mølvig J, Madsbad S, Haugaard SB and Sajadieh A:
Effects of the glucagon-like peptide-1 receptor agonist liraglutide
on systolic function in patients with coronary artery disease and
type 2 diabetes: A randomized double-blind placebo-controlled
crossover study. Cardiovasc Diabetol. 15:1052016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Okerson T and Chilton RJ: The
cardiovascular effects of GLP-1 receptor agonists. Cardiovasc Ther.
30:e146–e155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang JY, Wang XY and Wang X: Effects of
liraglutide on hemodynamic parameters in patients with heart
failure. Oncotarget. 8:62693–62702. 2017.PubMed/NCBI
|
|
35
|
Chen T, Ding G, Jin Z, Wagner MB and Yuan
Z: Insulin ameliorates miR-1-induced injury in H9c2 cells under
oxidative stress via Akt activation. Mol Cell Biochem. 369:167–174.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li J, Lin J, Song Y, Xiang L and Wu Z:
Effects of insulin-like growth factor-1 on the myocardium in
diabetic rats. Zhonghua Yi Xue Za Zhi. 94:3329–3333. 2014.(In
Chinese). PubMed/NCBI
|
|
37
|
Xing Y, Sun W, Wang Y, Gao F and Ma H:
Mutual inhibition of insulin signaling and PHLPP-1 determines
cardioprotective efficiency of Akt in aged heart. Aging (Albany
NY). 8:873–888. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cai L, Li W, Wang G, Guo L, Jiang Y and
Kang YJ: Hyperglycemia-induced apoptosis in mouse myocardium:
Mitochondrial cytochrome C-mediated caspase-3 activation pathway.
Diabetes. 51:1938–1948. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
De L, eón DD, Crutchlow MF, Ham JY and
Stoffers DA: Role of glucagon-like peptide-1 in the pathogenesis
and treatment of diabetes mellitus. Int J Biochem Cell Biol.
38:845–859. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen J, Wang Z, Mao Y, Zheng Z, Chen Y,
Khor S, Shi K, He Z, Li J, Gong F, et al: Liraglutide activates
autophagy via GLP-1R to improve functional recovery after spinal
cord injury. Oncotarget. 8:85949–85968. 2017.PubMed/NCBI
|
|
41
|
Qi Q, Lu L, Li H, Yuan Z, Chen G, Lin M,
Ruan Z, Ye X, Xiao Z and Zhao Q: Spatiotemporal delivery of
nanoformulated liraglutide for cardiac regeneration after
myocardial infarction. Int J Nanomedicine. 12:4835–4848. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu T, Liu Y, Deng Y, Meng J, Li P, Xu X
and Zeng J: Insulin combined with selenium inhibit p38MAPK/CBP
pathway and suppresses cardiomyocyte apoptosis in rats with
diabetic cardiomyopathy. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
32:926–930. 2016.(In Chinese). PubMed/NCBI
|
|
43
|
Min Q, Bai Y, Zhang Y, Yu W, Zhang M, Liu
D, Diao T and Lv W: Hawthorn leaf flavonoids protect against
diabetes-induced cardiomyopathy in rats via PKC-α signaling
pathway. Evid Based Complement Alternat Med. 2017:20719522017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bresciani G, da Cruz IB and
González-Gallego J: Manganese superoxide dismutase and oxidative
stress modulation. Adv Clin Chem. 68:87–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ayala A, Muñoz MF and Argüelles S: Lipid
peroxidation: Production, metabolism, and signaling mechanisms of
malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev.
2014:3604382014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gao H, Zeng Z, Zhang H, Zhou X, Guan L,
Deng W and Xu L: The glucagon-like peptide-1 analogue liraglutide
inhibits oxidative stress and inflammatory response in the liver of
rats with diet-induced non-alcoholic fatty liver disease. Biol
Pharm Bull. 38:694–702. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ramalingayya GV, Sonawane V, Cheruku SP,
Kishore A, Nayak PG, Kumar N, VShenoy RS and Nandakumar K: Insulin
protects against brain oxidative stress with an apparent effect on
episodic memory in doxorubicin-induced cognitive dysfunction in
wistar rats. J Environ Pathol Toxicol Oncol. 36:121–130. 2017.
View Article : Google Scholar : PubMed/NCBI
|