Introduction
COPD is one of the leading causes of death
worldwide. Of note, patients with AECOPD and ventilator support in
the intensive care unit (ICU) have a higher risk of death and
disability (1,2). The duration of mechanical ventilation
support has an impact on healthcare costs as well as quality of
life (3), and directly affect the
prognosis of patients with AECOPD (4,5). It is
important to terminate mechanical ventilation early to prevent
infection, airway pressure injury and ventilator dependence
(6). The standard of management for
acute exacerbation inpatients with moderate-to-severe COPD includes
supplemental oxygen, non-invasive or invasive mechanical
ventilation, short-acting inhaled bronchodilators, systemic
corticosteroids and antibiotics (7).
Non-pharmacologic management includes ventilator support and
administration of supplemental oxygen.
Lentinan is a well-recognized immunomodulator, which
is extracted from shiitake (8).
AECOPD medications include bronchodilator, anti-infective drugs as
required and systemic cortical hormone, but systemic corticosteroid
treatment has obvious adverse effects, including hyperglycemia,
osteoporosis and vertebral compression fractures. Corticosteroid
inhalation therapy is effective and due to its lower dosage, it
causes less adverse reactions, and has almost no direct effects on
target organs and the blood circulation (9). A previous study has indicated that
short-term corticosteroid therapy was as effective as long-term
therapy (10). Budesonide suspension
(Pulmicort Respules) is currently used as inhaled corticosteroids.
Another advantage of atomization inhalation therapy is that
corticosteroid may be mixed with bronchodilator, including β2
agonists and anti-cholinergic drugs, which rapidly relieve
symptoms. In patients with severe COPD inhaled corticosteroids may
reduce the number of exacerbations, the degree of acute
exacerbations and the risk of mortality (10).
Studies reporting on the use of corticosteroids and
lentinanin critically ill patients with AECOPD on ventilator
support are scarce. The present study, the outcome of combined
usage of inhaled immunomodulator and corticosteroid in patients
with AECOPD was compared. In the present study, the clinical
efficacy of lentinan combined with budesonide inhalation in AECOPD
patients receiving ventilation support at the ICU was analyzed, and
the serum concentrations of adiponectin (APN), D-dimer (D-D),
interleukin (IL)-17 and high-sensitivity C-reactive protein
(hs-CRP) were examined. Furthermore, the proportions of
CD3+, CD4+ and CD8+T-cells were
assessed to evaluate the immunity function of the patients.
Materials and methods
Patients
Written informed consent was obtained from all
participants and the present study was approved by the Ethical
Committee of Shanghai Jiao Tong University Affiliated Sixth
People's Hospital (Shanghai, China). The Chinese clinical trial
registry number is ChiCTR1800019088. A total of 72 cases of severe
AECOPD admitted to the ICU between June 2016 and September 2017
with the requirement of ventilator support were enrolled. All of
the patients were divided into two groups by a randomized,
double-blinded method, including 36 cases in each group
(experimental group: 20 males and 16 females; age, 54.69±5.33
years; and healthy control group: 19 males and 17 females; age,
58.54±8.01). The retrospective patients were all without fatal
outcome.
Inclusion and exclusion criteria
All of the patients were diagnosed according to the
diagnostic criteria for AECOPD. The inclusion criteria were set in
accordance with the Global initiative for chronic Obstructive Lung
Disease (GOLD) spirometry definition (11). The exclusion criteria included the
diagnosis of pneumonia or pulmonary embolism, asthma, heart
failure, thromboembolic disease or restrictive respiratory
insufficiency.
Therapies
The experimental group received oral administration
of 0.5 g lentinan twice/day (Jinling Pharmaceutical Co., Ltd.,
Shenzhen, China) and inhalation of 2 ml budesonide (0.5 mg/ml;
Astra Zeneca Pharmaceutical Co., Ltd., Shanghai, China) twice a day
(bid) over 4 days delivered by a breathing machine Y-tube. The
control group only received budesonide atomization inhalation (bid
over 4 days) via a breathing machine Y-tube. All patients received
the same antibiotic therapy (cephalosporins).
Inclusion criteria included patients with a
breathing rate <30/min, a normal cardiovascular function, no
abnormal breathing and no airway irritability. The endotracheal
intubation time was limited to 120 h.
Clinicopathological characteristics,
biochemistry and lung function
The full medical history was reviewed for each
patient, and all subjects underwent a full clinical examination and
pulmonary function tests. The airway pressure, mechanical
ventilation running time, length of ICU stay and rate of trachea
incision of patients were recorded. The plasma levels of APN, D-D,
serum IL-17 and hs-CRP were measured for all of the patients prior
to and after treatment (day 4). A total of 5 ml fasting blood as
obtained, and 2 ml plasma was collected in Eppendorf tubes and
centrifuged at 1,509 × g for 10 min at 4°C. The supernatant was put
into a new Eppendorf tube and stored in an −80°C refrigerator. APN
(cat. no. 442308) and IL-17 (cat. no. 433918) quantitative ELISA
kits were purchased from BioLegend, Inc. (San Diego, CA, USA). The
ELISAs for all standards and samples were performed in duplicate.
The levels of APN and IL-17 were detected by reading the absorption
at 450 nm using an ELISA plate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) according to the protocol of the kits. The
concentrations of the analyte proteins were determined through
interpolation from a standard curve. ASYSMEX CA-7000 automatic
blood coagulation analyzer was used to test the level of D-D
through immune turbidimetry (reference value, <0.3 mg/l). A
HITACHI 7600 automatic biochemical analyzer (Hitachi, Ltd., Tokyo,
Japan) was used to determine the level of hs-CRP (reference value,
0–3 mg/l).
Flow cytometric analysis
The antibodies phycoerythrin-CD3 (cat. no.
bs-10498R), fluorescein isothiocyanate-CD4 (cat. no. bs-0647R) and
peridinin chlorophyll/cyanine 5.5-CD8 (cat. no. bs-0648R) were
purchased from BIOSS (Beijing, China). Whole blood cells were
obtained from patients. Cells were fixed with 70% methanol
(overnight at 4°C), permeabilized with 90% ice-cold methanol for 20
min at −20°C, counted and stained with the aforementioned
antibodies at a dilution of 1:1,000 in 106/ml cell
suspension in dark for 30 min at 4°C. Following washing, the
populations of CD3+, CD4+ and
CD8+T-cells were analyzed using a FACSCanto II (BD
Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
Statistical analyses were performed using SPSS
software (version 15.0; SPSS, Inc., Chicago, IL, USA). Measurement
data were expressed as the mean ± standard deviation. An unpaired
t-test was used for comparison between two groups. One-way analysis
of variance followed by Tukey's post hoc test was used for
determining inter-group differences among multiple groups. Count
data were presented as n (%) and analyzed using the χ2
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
A total of 80 consecutive patients with severe
AECPOD were considered for enrollment. Among them, 3 cases were
excluded due to inadequate medical record documentation and 5
patients died during the ICU stay. The baseline characteristics of
evaluable cases included in the present analysis are listed in
Table I. No differences in age, sex,
disease severity, complications and pulmonary function between the
experimental and the control group were noted prior to treatment
(P>0.05).
 | Table I.Baseline characteristics of the
patients. |
Table I.
Baseline characteristics of the
patients.
| Parameter | Control (n=36) | Experimental group
(n=36) |
|---|
| Age (years) | 54.69±5.33 | 58.54±8.01 |
| Sex
(male/female) | 19 (26.39%)/17
(23.61%) | 20 (27.78%)/16
(22.22%) |
| BMI
(kg/m2) | 30.12±6.87 | 31.81±7.66 |
| FEV1% | 42.88±15.02 | 41.57±16.45 |
|
FEV1/FVC | 40.87±17.98 | 41.71±17.09 |
| GOLD stage |
| 1 | 16 (22.22%) | 12 (16.67%) |
| 2 | 11 (15.28%) | 10 (13.89%) |
| 3 | 6 (8.33%) | 7 (9.72%) |
| 4 | 5 (6.94%) | 5 (6.94%) |
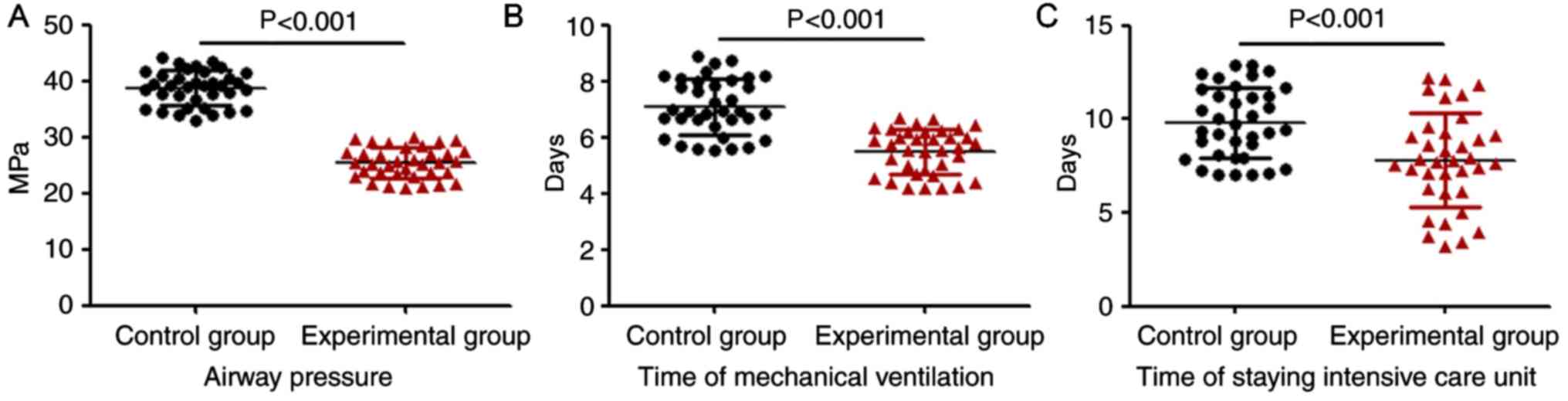
Airway pressure of breathing machine,
mechanical ventilation time and length of ICU stay
A comparison of airway pressure of the breathing
machine, mechanical ventilation time and length of ICU stay between
the experimental and the control group is provided in Fig. 1. The results indicated that the
airway pressure of the breathing machine (P<0.001), the
mechanical ventilation time (P<0.001) and the time of ICU stay
(P<0.001) in the experimental group were significantly decreased
compared with those in the control group.
Serum levels of APN, D-D, IL-17 and
hs-CRP
The laboratory analyses indicated that in each
group, the plasma levels of APN, D-D, IL-17 and hs-CRP after
treatment were obviously lower than those at baseline (P<0.05).
Furthermore, the levels of APN (P<0.001), D-D (P<0.001) and
IL-17 (P<0.001) in the experimental group were significantly
lower than those in the control group, suggesting that
administration of lentinan in addition to budesonide in the
experimental group further reduced the level of APN, D-D and IL-17
(Fig. 2A-C). Regarding the serum
levels of hs-CRPs, the difference between the experimental and the
control group after the treatment was not significant (Fig. 2D). After treatment, as presented in
Table II, the partial oxygen
pressure (PaO2) in two groups was significantly
increased compared with the baseline value (P<0.001), while the
CO2 pressure (PCO2) was significantly
decreased (P<0.001). Furthermore, the PaO2 in the
experimental group was significantly higher than that in the
control group (P<0.001), while the PCO2 was
significantly lower (P<0.05).
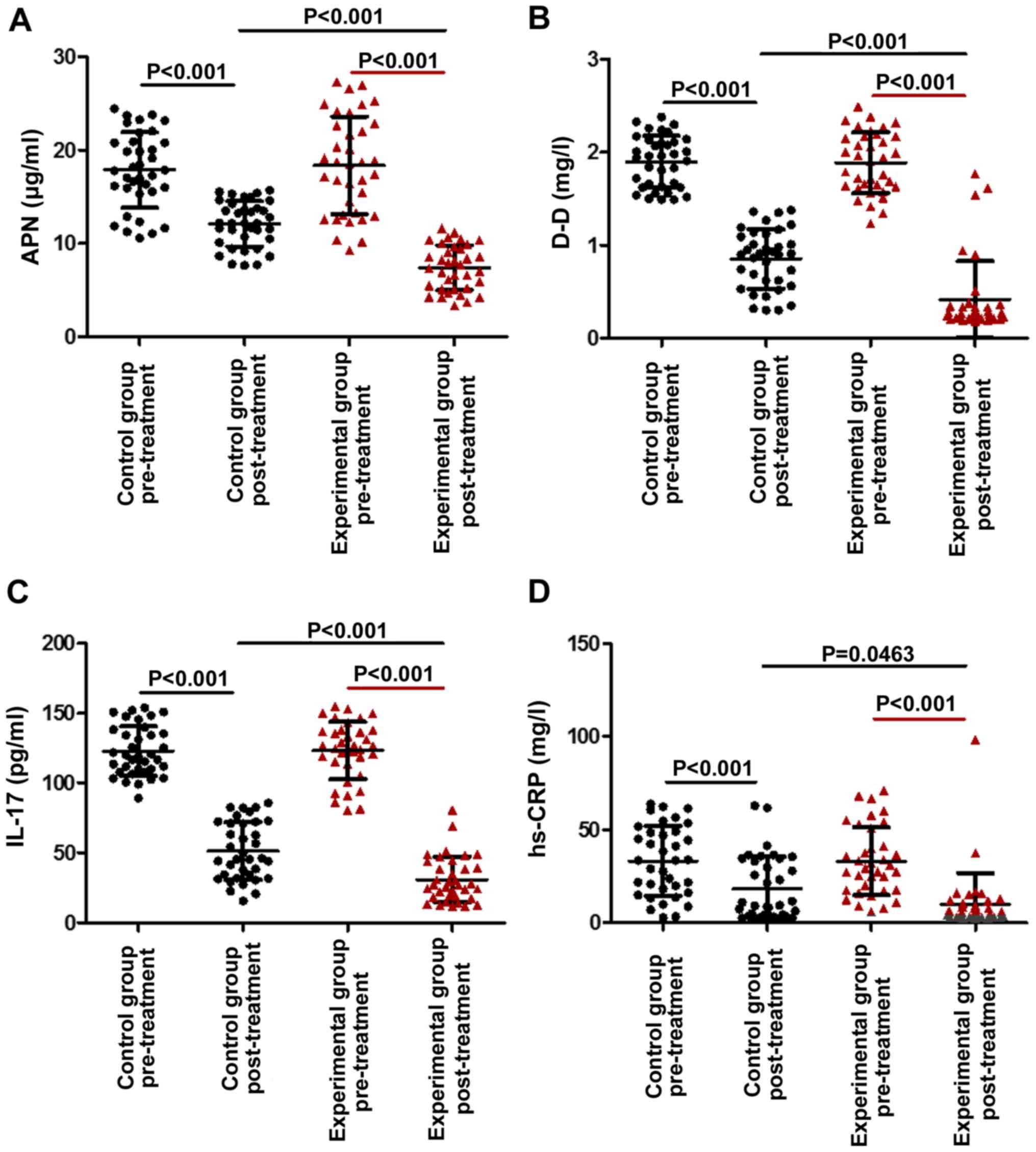 | Figure 2.Comparison of the levels of APN, D-D,
IL-17 and hs-CRP between AECOPD patients treated with lentinan and
budesonide, and those who received budesonide only, under
mechanical ventilation. (A) APN, (B) D-D, (C) IL-17 and (D) hs-CRP
levels in the two groups prior to and post-treatment are provided.
Values are expressed as the mean ± standard deviation. AECOPD,
acute exacerbation of chronic obstructive pulmonary disease. D-D,
D-dimer; IL, interleukin; APN, adiponectin; hs-CRP,
high-sensitivity C-reactive protein. |
 | Table II.Comparison of blood gas pressure
parameters between the two groups. |
Table II.
Comparison of blood gas pressure
parameters between the two groups.
|
| Experimental group
(n=36) | Control (n=36) |
|---|
|
|
|
|
|---|
| Parameter | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment |
|---|
| PaO2
(mmHg) | 50.58±7.35 |
78.82±8.57a,b | 51.39±5.76 |
67.39±11.25a |
| PCO2
(mmHg) | 69.91±9.46 |
46.71±9.28a,c | 68.39±10.42 |
53.15±9.72a |
Proportion of CD3+,
CD4+ and CD8+ T-cells
The effect of lentinan combined with budesonide on
the proportions of CD3+, CD4+ and
CD8+ T-cells was then examined. As presented in Fig. 3, the percentages of CD3+
and CD4+ T-cells were significantly increased after
treatment, compared with those at baseline and in the control group
(P<0.01). In addition, the percentage of CD8+ T-cells
in the experimental group was decreased compared with that at
baseline and in the control group (P<0.001).
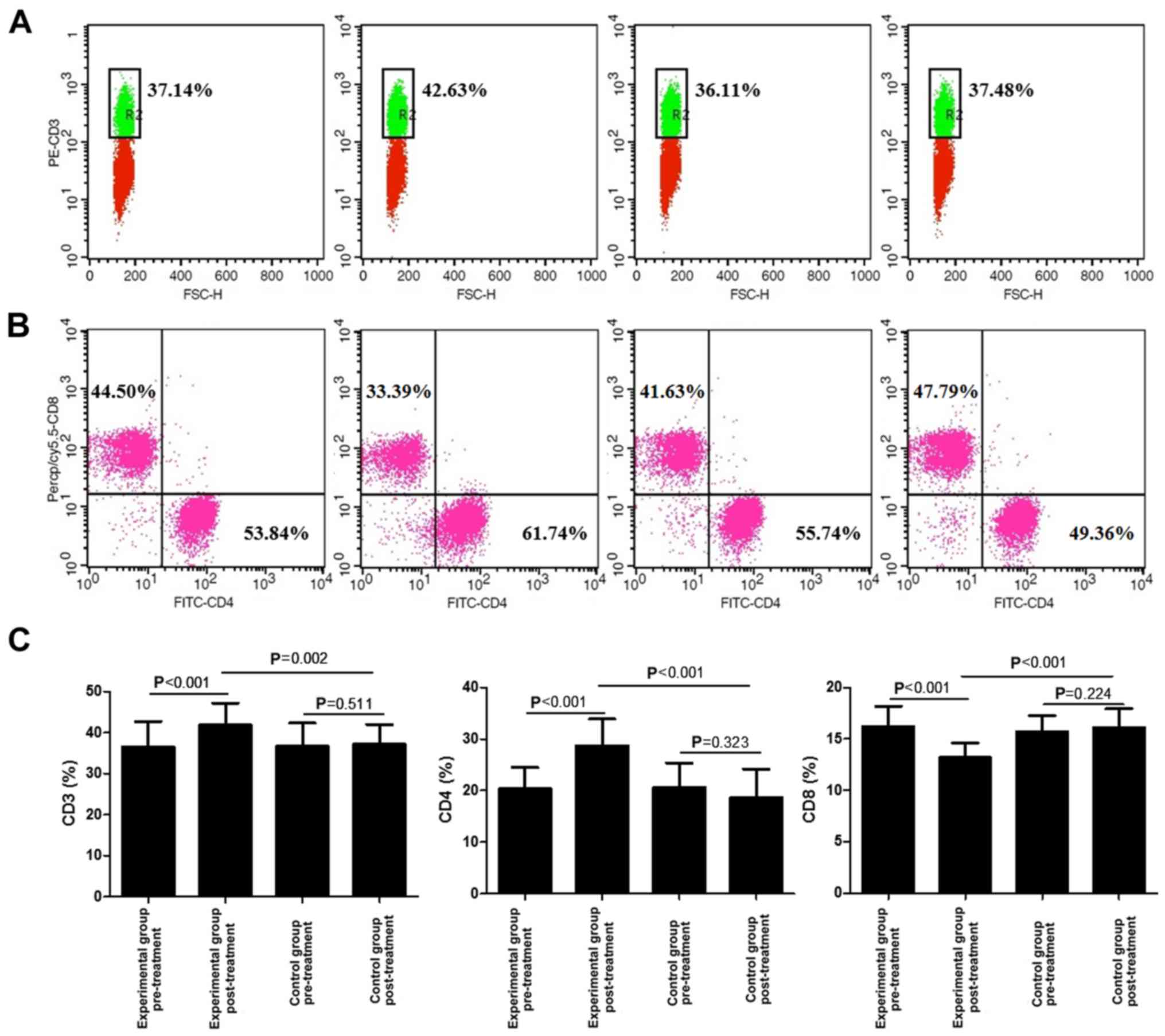 | Figure 3.Analysis of CD3+,
CD4+ and CD8+ T cells in the blood of acute
exacerbation of chronic obstructive pulmonary disease patients
treated with lentinan and budesonide, and those who received
budesonide only, under mechanical ventilation. (A) FACS plots of
CD3+ T cells; (B) FACS plots of CD4+ and
CD8+ T cells; (C) Average percentages of
CD3+, CD4+ and CD8+ T cells,
calculated from the FACS analysis, prior to and post-treatment are
provided. Values are expressed as the mean ± standard deviation.
FACS, fluorescence-assisted cell sorting; FITC, fluorescein
isothiocyanate; Percp, peridinin chlorophyll; cy, cyanine; FSC,
forward scatter; PE, phycoerythrin. |
Discussion
COPD has become an important public health problem
due to the high mortality, as well as the social and economic
burden associated with it. At present, COPD is the fourth most
common cause of death worldwide. The World Bank and the World
Health Organization have announced that COPD will be the world's
fifth economic burden in 2020 (12).
AECOPD in combination with respiratory failure is the most
important reason for readmission of COPD patients to the hospital.
Thus, it is of great significance to improve the means of
prevention of AECOPD, and in particular, to improve the success
rate of treating AECOPD combined with respiratory failure.
Significant progress has been made regarding the clinical
application of mechanical ventilation in the treatment of AECOPD,
which has greatly improved the quality of life of affected patients
(13).
COPD is a type of chronic bronchitis with
characteristics of airflow obstruction and/or emphysema and
progressive development, which is associated with airway
hyper-responsiveness. The long-term use of a breathing machine
leads to an increased incidence of ventilator-associated pneumonia
(VAP) (14). For AECOPD patients
with a VAP resistance phenomenon, generic drug-resistant
boydii Acinetobacter strains, fungal infection and drug
resistance have been observed (15),
and adverse effects including an increase of airway damage were
also observed (16). However,
atomization therapy with budesonide may reduce airway resistance,
breathing machine plenum pressure drop and airway pressure damage
(17). The effect of standard doses
of lentinan combined with budesonide for AECOPD has been rarely
reported.
APN, secreted by fat cells, is an endogenous
anti-inflammatory factor in airway epithelial cells, is released by
autocrine and paracrine systems, and is a novel marker of airway
inflammation with a high predictive value for AECOPD. It was
reported that the severity of COPD and the expression levels of APN
were positively correlated (18,19).
COPD patients with chronic hypoxia, infection and accumulated
inflammatory cytokines may develop endothelial damage, which
eventually promotes thrombosis (20). D-D reflects the high coagulation
state in the body and secondary fibrinolytic hyperfunction. IL-17
promotes the release of other associated inflammatory cytokines,
causing neutrophil aggregation in the lung and damage to the
structure of the airways (21).
AECOPD is mostly caused by infection, with bacterial infection
accounting for 40–50% of AECOPD incidence (22). To the best of our knowledge, hs-CRP
is a sensitive indicator that reflects bacterial infections.
The present study indicated that the airway
pressure, usage time of breathing machine, length of stay at the
ICU in the experimental group was significantly lower than that in
the control group. The levels of APN, D-D, IL-17 and hs-CRP of
AECOPD patients at baseline were higher than those after treatment,
indicating the presence of a certain extent of infection, a high
coagulation state and fibrinolytic hyperfunction in AECOPD
patients. The levels of APN, D-D, IL-17 and hs-CRP after treatment
were significantly lower than those prior to treatment in each
group, suggesting that the infection, high coagulation state and
fibrinolytic state were relieved. The levels of APN, D-D, IL-17 and
hs-CRP in the experimental group were significantly lower than
those in the control group, suggesting that the combined use of
lentinan with budesonide is better than monotreatment with
budesonide, which may therefore be worthy of implementation in the
clinic. In comparison with those in the control group, the
PaO2 was significantly increased and the PCO2
was significantly increased, suggesting that combination treatment
of lentinan with budesonide provided a better outcome. Flow
cytometric analysis suggested that combination of lentinan with
budesonide greatly improved the immunity function of patients with
AECOPD, which may contribute to the improved clinical outcome.
In the present study, only single dose of lentinan
was administered to the experimental group, which indicated that
lentinan could improve the immunity function and clinical outcome
of patients with AECOPD. However, the optimal dose of lentinan were
not determined in the current study. This represents a limitation
and more studies are required to explore the clinical outcome of
multiple dosages of lentinan and to analyze the optimal dose in
AECOPD patients.
In summary, a significant benefit for AECOPD
patients receiving mechanical ventilation was observed by
combination treatment with lentinan and budesonide, with a shorter
duration of mechanical ventilation, improvement of the serum levels
of APN, D-D, IL-17 and hs-CRP, as well as the PaO2 and
PCO2, as compared with the outcomes of treatment with
budesonide alone. The present study provided a novel strategy for
the combined administration of these medications.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GZ conceived and designed the study, wrote the
manuscript and was involved in its critical revision. GZ and JS
retrieved the samples and performed the experiments, they
collected, processed and analysed the data and interpreted the
results.
Ethical approval and consent to
participate
Written informed consent was obtained from all
participants and the present study was approved by the Ethical
Committee of Shanghai Jiao Tong University Affiliated Sixth
People's Hospital (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests regarding this study.
References
|
1
|
Spyratos D and Sichletidis L: Umeclidinium
bromide/vilanterol combination in the treatment of chronic
obstructive pulmonary disease: A review. Ther Clin Risk Manag.
11:481–487. 2015.PubMed/NCBI
|
|
2
|
Zhong J and Roth M: Clinical potential of
aclidinium bromide in chronic obstructive pulmonary disease. Ther
Clin Risk Manag. 10:449–453. 2014.PubMed/NCBI
|
|
3
|
Vitacca M, Bianchi L, Bazza A and Clini
EM: Advanced COPD patients under home mechanical ventilation and/or
long term oxygen therapy: Italian healthcare costs. Monaldi Arch
Chest Dis. 75:207–214. 2011.PubMed/NCBI
|
|
4
|
Faenza S and Petrini F: Mechanical
ventilation in the emergency room. Minerva Anestesiol. 66:883–887.
2000.(In Italian). PubMed/NCBI
|
|
5
|
Brijker F, van den Elshout FJ, de Rijk A,
Folgering HT and Bosch FH: Use of noninvasive mechanical
ventilation to avoid intubation during acute respiratory
insufficiency. Ned Tijdschr Geneeskd. 143:1819–1823. 1999.(In
Dutch). PubMed/NCBI
|
|
6
|
Hashemian SM, Mortaz E, Jamaati H, Bagheri
L, Mohajerani SA, Garssen J, Movassaghi M, Barnes PJ, Hill NS and
Adcock IM: Budesonide facilitates weaning from mechanical
ventilation in difficult-to-wean very severe COPD patients:
Association with inflammatory mediators and cells. J Crit Care.
44:161–167. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dixit D, Bridgeman MB, Andrews LB,
Narayanan N, Radbel J, Parikh A and Sunderram J: Acute
exacerbations of chronic obstructive pulmonary disease: Diagnosis,
management, and prevention in critically ill patients.
Pharmacotherapy. 35:631–648. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu YH, Ma SD, Fu QJ, Zhao LY, Li Y, Wang
HQ and Li MC: Effect of lentinan on membrane-bound protein
expression in splenic lymphocytes under chronic low-dose radiation.
Int Immunopharmacol. 22:505–514. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maltais F, Ostinelli J, Bourbeau J, Tonnel
AB, Jacquemet N, Haddon J, Rouleau M, Boukhana M, Martinot JB and
Duroux P: Comparison of nebulized budesonide and oral prednisolone
with placebo in the treatment of acute exacerbations of chronic
obstructive pulmonary disease: A randomized controlled trial. Am J
Respir Crit Care Med. 165:698–703. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma Z and Zhang W: Short-term versus longer
duration of glucocorticoid therapy for exacerbations of chronic
obstructive pulmonary disease. Pulm Pharmacol Ther. 40:84–90. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vogelmeier CF, Criner GJ, Martínez FJ,
Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M,
Fabbri LM, et al: Global strategy for the diagnosis, management,
and prevention of chronic obstructive lung disease 2017 report:
GOLD executive summary. Arch Bronconeumol. 53:128–149. 2017.(In
English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rudan I, Campbell H, Marušić A, Sridhar D,
Nair H, Adeloye D, Theodoratou E and Chan KY: Assembling GHERG:
Could ‘academic crowd-sourcing’ address gaps in global health
estimates? J Glob Health. 5:0101012015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uren NG, Davies SW, Jordan SL and Lipkin
DP: Inhaled bronchodilators increase maximum oxygen consumption in
chronic left ventricular failure. Eur Heart J. 14:744–750. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yalçınsoy M, Salturk C, Takır HB, Kutlu
SB, Oguz A, Aksoy E, Balcı M, Kargın F, Mocin OY, Adıguzel N, et
al: Case fatality rate related to nosocomial and
ventilator-associated pneumonia in an ICU: A single-centre
retrospective cohort study. Wien Klin Wochenschr. 128:95–101. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boixeda R, Bacca S, Elias L, Capdevila JA,
Vilà X, Mauri M and Almirall J: Pneumonia as comorbidity in chronic
obstructive pulmonary disease (COPD). Differences between acute
exacerbation of COPD and pneumonia in patients with COPD. Arch
Bronconeumol. 50:514–520. 2014.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McCurdy BR: Noninvasive positive pressure
ventilation for acute respiratory failure patients with chronic
obstructive pulmonary disease (COPD): An evidence-based analysis.
Ont Health Technol Assess Ser. 12:1–102. 2012.
|
|
17
|
Xiong G, Xu L, Wei L and Li X: Atomization
inhalation of terbutaline and budesonide efficiently improved
immunity and lung function of AECOPD patients. Cell Mol Immunol.
5:287–291. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leivo-Korpela S, Lehtimäki L, Vuolteenaho
K, Nieminen R, Kööbi L, Järvenpää R, Kankaanranta H, Saarelainen S
and Moilanen E: Adiponectin is associated with dynamic
hyperinflation and a favourable response to inhaled glucocorticoids
in patients with COPD. Respir Med. 108:122–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kirdar S, Serter M, Ceylan E, Sener AG,
Kavak T and Karadağ F: Adiponectin as a biomarker of systemic
inflammatory response in smoker patients with stable and
exacerbation phases of chronic obstructive pulmonary disease. Scand
J Clin Lab Invest. 69:219–224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang M, Zhang J, Zhang Q, Yang X, Shan H,
Ming Z, Chen H, Liu Y, Yin J and Li Y: D-dimer as a potential
biomarker for the progression of COPD. Clin Chim Acta. 455:55–59.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beringer A, Noack M and Miossec P: IL-17
in Chronic Inflammation: From Discovery to Targeting. Trends Mol
Med. 22:230–241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai BQ, Cai SX, Chen RC, et al: Expert
consensus on acute exacerbation of chronic obstructive pulmonary
disease in the People's Republic of China. Int J Chron Obstruct
Pulmon Dis. 9:381–95. 2014.PubMed/NCBI
|