Introduction
Acute stress disorder, post-traumatic stress
disorder, chronic pain and depression are highly prevalent among
survivors of severe burns (1).
Anesthetic management may modulate this physiological response and
impact post-operative recovery. Maintaining analgesia and
appropriate sedation in patients with burns may be highly
challenging and typically requires high doses of anxiolytics and
analgesics. However, escalating doses of opioids and
benzodiazepines provide little additional benefit and may increase
the incidence and severity of side effects (2).
Dexmedetomidine (DEX) is a selective α-2 adrenergic
agonist that has sympatholytic, analgesic and sedative properties
(3). Various clinical trials have
identified that intra-operative administration of DEX may promote
recovery, enhance the analgesic properties of morphine in
patient-controlled analgesia and reduce morphine consumption along
with its associated adverse effects (4,5). A
recent study indicated that in patients undergoing cervical spine
surgery, the intra-operative and post-operative use of DEX was able
to reduce the post-operative requirement of analgesics and promote
recovery (6). Multiple studies have
indicated that DEX has an active influence on recovery (7–9), even at
a single dose (10). However, few
studies have assessed the effects of DEX on analgesia and general
anesthesia recovery in patients with burns. Of note, DEX provides
sedation and improves the conditions for induction of general
anesthesia, and its use has been reported in patients with burns
during dressing changes or mechanical ventilation during intensive
care unit sedation (11,12). In addition, it has been revealed that
intra-operative DEX infusion reduces the stress response and
therefore improves the quality of recovery following major spinal
surgery (9).
Based on the evidence above, the present randomized,
double-blinded, placebo-controlled study, was performed to test the
hypothesis that the use of DEX improves the analgesic effects of
sufentanil-based patient-controlled intravenous analgesia (PCIA)
and promotes the quality of recovery of patients treated for
moderate-to-severe burns under general anesthesia.
Materials and methods
The present study was approved by the Ethics
Committee of Yantai Yuhuangding Hospital of Qingdao University
(Yantai, China). Written informed consent was obtained from all
participants prior to randomization. A total of 60 consecutive
patients (age, 18–65 years) were enrolled in the present study
between January 2017 and November 2017. Patients exhibited American
Society of Anesthesiologists (ASA) grade II or III burns (13), with burn surface areas that met the
criteria for moderate-to-severe burn. Patients were conscious and
actively cooperated and underwent general anesthesia for tangential
excision skin grafting. The exclusion criteria were as follows:
Known or suspected allergy to α-2 receptor agonists or
non-steroidal anti-inflammatory drugs; sinus bradycardia;
myocardial dysfunction; chronic respiratory insufficiency; impaired
renal or hepatic function; regular use of sedatives or
anti-depressants; additional respiratory tract burns and/or severe
complex injuries. Patients were instructed regarding the use of the
Visual Analogue Scale (VAS) (14),
where 0 indicated no pain and 10 indicated the worst pain
imaginable, and the PCIA pump.
Patients were randomly assigned to two groups using
computer-generated random numbers and the sequentially numbered
opaque sealed envelope technique. The DEX group (Group D) received
an intravenous (i.v.) single-dose bolus injection of 0.5 µg/kg DEX
(Precedex; Aibeinin, Inc., Henrui Pharmaceutical, Lianyungang,
China) >10 min prior to induction of anesthesia. PCIA was
provided to patients at the end of the surgery, which consisted of
100 µg sufentanil plus 200 µg DEX. The control group (Group C)
received volume-matched normal saline prior to the induction of
anesthesia and 100 µg sufentanil infusion in PCIA. To ensure that
the study was blinded, the test drug infusion was prepared by a
different anesthesiologist. In addition, the VAS score was
evaluated by another anesthesiologist.
Routine monitoring, including measurement of the
heart rate (HR), blood pressure (BP), pulse oxygen saturation,
electrocardiograms and depth of sedation according to the
bispectral index (BIS), was performed at 5-min intervals. General
anesthesia was induced with midazolam (0.05 mg/kg), propofol
(1.5–2.0 mg/kg), fentanyl (3.0 µg/kg) and cisatracurium (0.15
mg/kg) as described previously (8,15). After
the administration of cisatracurium, orotracheal intubation was
performed using a 6.5- and 7.5-mm tracheal tube for female and male
patients, respectively. Mechanical ventilation was maintained with
a 6–8 ml/kg tidal volume and a respiratory rate of 12–15 breaths
per min (inspiratory-to-expiratory time ratio, 1:2), which
maintained the end-tidal CO2 at 35–45 mmHg. The i.v.
infusion was switched to a maintenance syringe pump at a rate of
0.06–0.10 µg/kg/min for remifentanil and a 1.0–2.5 minimal alveolar
concentration of sevoflurane in order to maintain a BIS of 45–60
during the surgery (16,17). Cisatracurium (0.05 mg/kg) was
intermittently used for muscle relaxation. Bradycardia (HR, <50
beats/min) was treated with i.v. atropine (0.5 mg). Tachycardia (HR
>100 beats/min) was treated with i.v. esmolol in 20-mg
increments. Hypotension [systolic BP (SBP) <25% of baseline
level] was treated with i.v. phenylephrine (20 µg). Hypertension
(SBP >25% of baseline level) was treated with i.v. nitroglycerin
(10 µg). Neuromuscular blockade was antagonized by i.v.
administration of 1.0 mg neostigmine and 0.5 mg atropine. Following
extubation, patients were transferred to the post-anesthesia care
unit (PACU) and received nasal O2 supplementation.
All patients were transferred to the ward once they
met the recovery room criteria. A PCIA pump (Disposable Infusion
Pump; WZ-6523C-4; Fornia Medical Device Co., Zhuhai, China) was
connected to the i.v. line and configured to administer sufentanil
and DEX or 1 µg/ml sufentanil diluted in 100 ml normal saline with
a basal infusion of 2 ml/h with a lockout period of 15 min (the
self-controlled doses were set at 0.5 ml each). If the VAS at rest
was ≥4, the PCIA button was pressed. If the pain was not
alleviated, the rescue anesthetic tramadol (0.1 g) was orally
administered.
The primary outcomes were the patient's VAS at rest
and during movement, and the 40-item quality of recovery
questionnaire (QoR-40) score. The secondary outcomes were the
number of PCIA pressings, the cumulative dose of sufentanil and any
opioid-associated side-effects. The VAS at rest and during
movement, the number of times the patient pressed the PCIA pump,
the cumulative dose of sufentanil and any opioid-associated
side-effects were recorded for the first 24 h and then every 4 h
post-surgery. The QoR-40 was used to evaluate the quality of
post-operative recovery at 24 h, as described previously (18,19).
Five categories of recovery were included in the QoR-40: Emotional
state (9 items), physical comfort (12 items), psychological support
(7 items), physical independence (5 items) and pain (7 items). Each
item was graded on a 5-point scale, and global scores ranged from
40 (poor quality of recovery) to 200 (excellent quality of
recovery).
Statistical analysis
The sample size of the study was calculated
according to previous studies, and was based on a pilot study
(6,20). A total of 25 patients in each group
were required to assess their pain using the VAS scoring system;
the statistical power and type I error of the subsequent analysis
was 0.85 and 0.05, respectively. A total of 67 patients scheduled
for tangential excision skin grafting under general anesthesia were
assessed for eligibility. To achieve a statistical power of at
least 85% using repeated-measures analysis of variance (ANOVA) with
a significance level of 0.05 and a dropout rate of 10%, at least 28
subjects were recruited in each group. A total of 60 patients were
therefore enrolled and assigned to Group D (n=30) and Group C
(n=30).
Statistical analyses were performed using SPSS 23.0
software (IBM Corp., Armonk, NY, USA). All quantitative data were
expressed as the mean ± standard deviation. Parameters including
age, weight, body mass index (BMI), duration of surgery, duration
under anesthesia, duration of extubation, duration in the PACU,
number of times the patient pressed the PCIA pump, sufentanil
consumption and recovery scores were compared between the two
groups using an unpaired Student's t-test. HR, mean arterial
pressure (MAP) and VAS score at different time-points (2, 4, 8, 12
and 24 h post-surgery) were compared between the two groups using
two-way ANOVA followed by Bonferroni's post-hoc test. ASA grade,
sex and post-operative adverse effects were evaluated using the
χ2 test or Fisher's exact test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Demographic data and
anesthesia-related information
A total of 67 patients scheduled for tangential
excision skin grafting under general anesthesia were assessed for
eligibility, of which 7 excluded due to ineligibility.
Subsequently, a total of 60 patients were enrolled in the present
clinical trial and randomized into two groups: Group D (n=30) and
Group C (n=30). All patients completed the study (Fig. 1). There were no significant
differences between the two groups in terms of patient
characteristics, type of surgery undergone or anesthesia-associated
variables, including age, sex, weight, BMI, duration of surgery,
duration under anesthesia, duration of extubation and duration of
stay at the PACU (Table I).
 | Table I.Patient characteristics and surgery or
anesthesia-associated information. |
Table I.
Patient characteristics and surgery or
anesthesia-associated information.
| Parameter | Group D (n=30) | Group C (n=30) | P-value |
|---|
| Age (years) | 37.10±12.17 | 37.57±11.07 | 0.877 |
| Sex (M/F) | 21/9 | 20/10 | 0.781 |
| Weight (kg) | 68.68±6.68 | 70.73±8.33 | 0.298 |
| BMI
(kg/m2) | 22.43±2.30 | 23.07±2.35 | 0.283 |
| Duration of surgery
(min) | 90.00±15.69 | 87.37±20.18 | 0.575 |
| Duration of
anesthesia (min) | 132.73±15.94 | 131.97±18.30 | 0.767 |
| Duration of
extubation (min) | 9.67±3.25 | 11.57±2.70 | 0.772 |
| Duration of stay at
the PACU (min) | 33.73±5.62 | 33.43±5.38 | 0.833 |
MAP and HR at different
time-points
MAP and HR during surgery and at 24 h post-surgery
were determined (Fig. 2). The two
groups were comparable with regard to their baseline HR and MAP. In
addition, the two groups exhibited reductions in HR and MAP upon
induction of anesthesia. Furthermore, Group D and Group C exhibited
increases in HR and MAP in response to intubation. However, Group D
demonstrated a greater hemodynamic stability during the induction
and intubation compared with Group C. Subsequently, the MAP and HR
gradually returned to baseline levels within 24 h of surgery
(Fig. 2A and B).
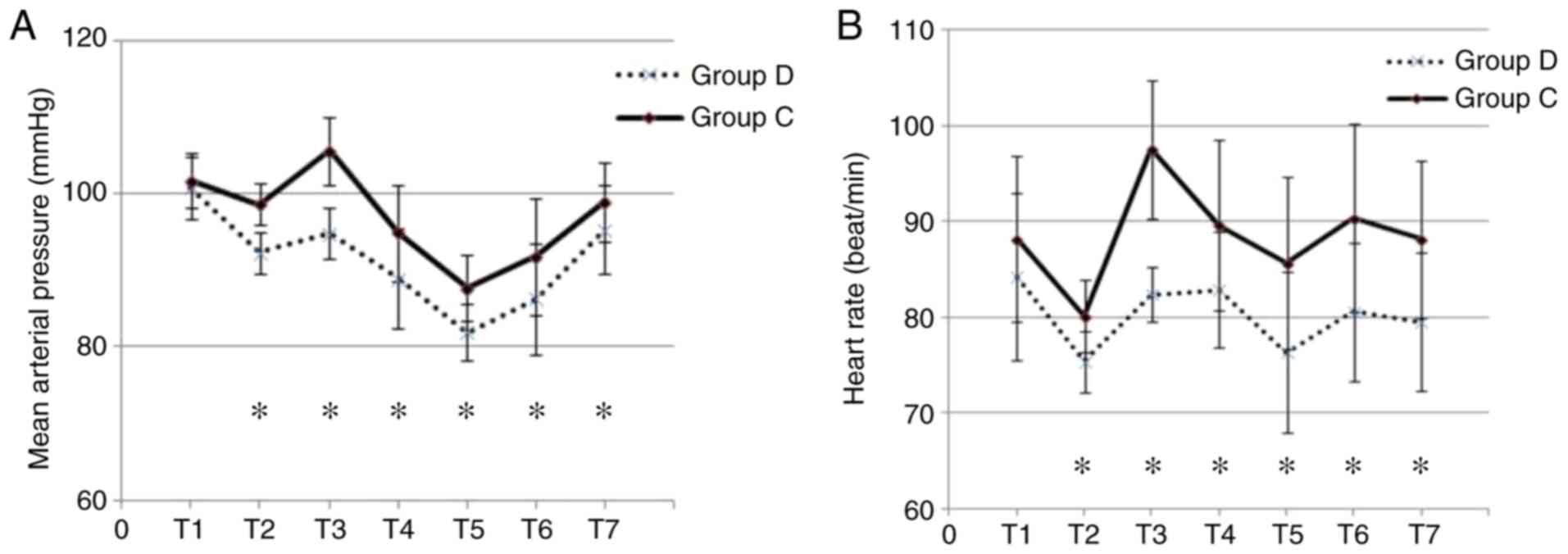 | Figure 2.MAP and HR. (A) MAP and (B) HR at
different time-points. *P<0.05, group D vs. C. T1, baseline; T2,
induction; T3, intubation; T4-T7, 4, 8, 12 and 24 h post-surgery,
respectively; MAP, mean arterial pressure; HR, heart rate; D,
dexmedetomidine; C, control with normal saline. |
Postoperative PCIA evaluation
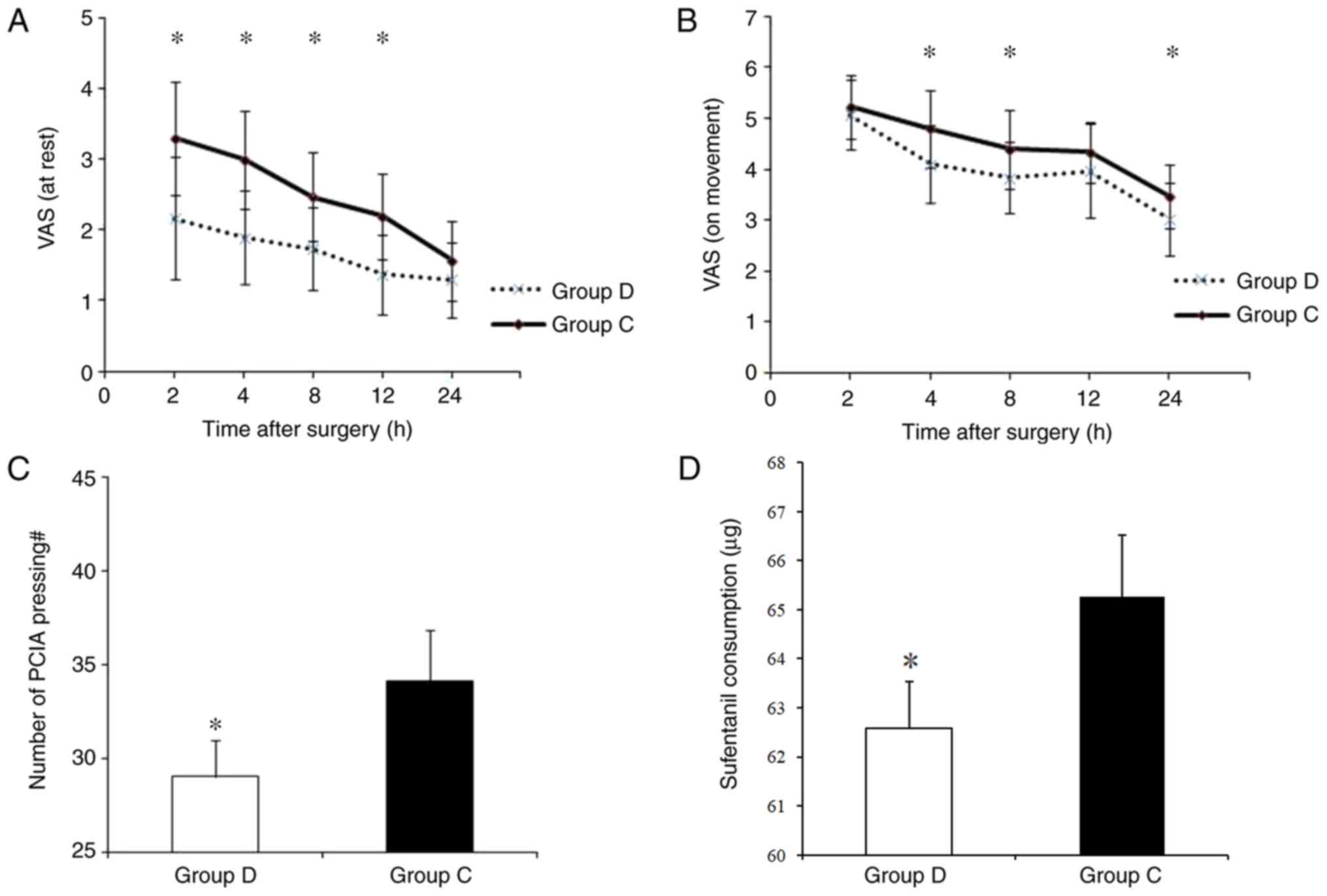
Post-operative pain was assessed using the VAS, and
the number of pain-induced pump presses and sufentanil consumption
was also recorded. During the first 24 h post-surgery, patients in
Group D exhibited a lower VAS score at rest (at 2, 4, 8 and 12 h
post-surgery, P<0.05; Fig. 3A)
and during movement (at 4, 8 and 24 h post-surgery, P<0.05;
Fig. 3B). Compared with that in
Group D, patients in Group C also exhibited a higher number of PCIA
pump presses (29.17±1.91 vs. 34.13±2.73; P<0.05; Fig. 3C) and a higher sufentanil consumption
(62.58±0.96 µg vs. 65.27±1.26 µg; P<0.05; Fig. 3D).
Quality of recovery
The QoR-40 scores at 24 h post-surgery were
determined (Table II). The mean
global QoR-40 score in Group D was 175.97±9.68, which was
significantly higher than the QoR-40 score of 133.53±13.77 in Group
C (P<0.01).
 | Table II.Scores of the 40-item quality of
recovery questionnaire in different sub-categories and global score
at 24 h post-surgery. |
Table II.
Scores of the 40-item quality of
recovery questionnaire in different sub-categories and global score
at 24 h post-surgery.
| Item | Group D (n=30) | Group C (n=30) | P-value |
|---|
| Emotional
state | 39.90±4.61 | 30.03±3.81 | <0.001 |
| Physical
comfort | 54.60±3.18 | 39.93±7.33 | <0.001 |
| Psychological
support | 30.30±2.95 | 24.33±4.56 | <0.001 |
| Physical
independence | 20.63±2.66 | 18.13±4.04 | 0.006 |
| Pain | 30.53±3.05 | 21.13±3.40 | <0.001 |
| Global score | 175.97±9.68 | 133.53±13.77 | <0.001 |
Postoperative adverse effects
At 24 h post-surgery, tramadol (0.1 g) was orally
administered to 2 patients in Group D and 6 patients in Group C,
whose VAS scores were ≥4 at rest (P=0.255). No significant
difference was observed in the post-operative adverse effects
between Group D and Group C during the first 24 h post-surgery
(Table III).
 | Table III.Post-operative side effects. |
Table III.
Post-operative side effects.
| Adverse effect | Group D (n=30) | Group C (n=30) | P-value |
|---|
| Nausea | 2 (6.67) | 3 (10.00) | 0.999 |
| Vomiting | 1 (3.33) | 3 (10.00) | 0.612 |
| Itch | 0 (0.00) | 0 (0.00) | – |
| Respiratory
depression | 0 (0.00) | 0 (0.00) | – |
| Dizziness | 0 (0.00) | 1 (3.33) | 1 |
| Bradycardia | 2 (6.67) | 0 (0.00) | 0.492 |
Discussion
The results of the present prospective,
double-blinded, randomized controlled study indicated that
administration of 0.5 µg/kg DEX pre-operatively followed by 200 µg
DEX and 100 µg sufentanil by post-operative PCIA was effective in
promoting the analgesic efficacy of sufentanil-based PCIA without
any significant side effects. Furthermore, it enhanced the
patient-reported global quality of recovery at 24 h following
tangential excision skin grafting. Patients with moderate-to-severe
burns eligible for tangential excision skin grafting were
recruited. Moderate-to-severe burns are defined as second-degree
burn injuries covering <29% of the body surface area or
third-degree burn injuries covering <10% of the body surface
area. Patients with burns undergo excruciating pain as a result of
their injuries and during the procedures that are associated with
surgery and wound care (21).
Perception of the pain depends on the degree of the burn and the
sensory input into the burn area, and also displays an
inter-patient variability. The aim of the present study was to
explore whether DEX infusion provides any beneficial effects as an
effective multi-modal analgesic regimen with PCIA sufentanil and
improve the recovery after surgery.
Opioids are the fundamental drugs used for
post-operative analgesia. Sufentanil is specifically suitable for
post-operative PCIA due to its fast response and excellent
analgesic effects. However, with increasing doses, sufentanil
produces dose-dependent adverse reactions, and studies have been
performed to identify novel drugs, consolidate currently available
drugs and reduce opioid use in order to avoid side effects,
including nausea, vomiting and itching (22). DEX is an α-2 adrenergic agonist that
was developed in the 1990s and was first used as a short-term
sedative in intensive care units (23). Clinical trials have confirmed that
combining DEX with sufentanil for PCIA allows for a significant
reduction in the amount of sufentanil administered and improves
analgesia (24,25). Furthermore, similar results were
obtained in a previous study for thoracic surgery where an infusion
dose of 0.04 µg/kg/h DEX in combination with 0.02 µg/kg/h
sufentanil reduced post-operative pain during the initial 72 h
post-surgery (26). In the present
study, DEX was administered at an infusion dose of ~0.03 µg/kg/h
DEX, which is an approach that has been used for patient-controlled
analgesia in Asian patients (7). The
present results indicated that the combined use of DEX with
sufentanil had an improved capacity to relieve post-operative acute
pain at rest and during movement compared with sufentanil alone.
Specifically, pre-operative administration of 0.5 µg/kg DEX
followed by post-operative DEX and sufentanil by PCIA improved the
QoR-40 pain scores in patients that underwent tangential excision
skin grafting at 24 h post-surgery. In addition, the number of PCIA
pump presses and the sufentanil consumption were reduced. However,
there was no significant difference between Groups C and D with
regard to the supplemental requirement for tramadol. Of note, the
analgesic and opioid dose-reducing/sparing effects of DEX have been
well documented in previous studies in adult and pediatric patients
(7,8,27).
Together with these results, the present study demonstrated that
pre-operative administration of 0.5 µg/kg DEX and the
administration of DEX in combination with sufentanil PCIA provided
effective analgesia following tangential excision skin
grafting.
DEX has been reported to cause common
treatment-associated adverse effects, including hemodynamic
changes, including hypotension (30%), hypertension (12%) and
bradycardia (9%) where mean infusion dose was 7.1 µg/kg (the
percentages represent the frequencies at which they occurred in
subjects) (23). For general
anesthesia, DEX may be applied in three primary ways, namely
continuous infusion, a single injection and a loading dose
injection followed by continuous infusion (15). A loading dose followed by a
continuous injection is most likely to cause adverse events. A
recent study indicated that a single pre-operative dose of DEX for
central venous catheter insertion reduced procedural pain and
provided clinically acceptable sedation (28). In the present study, a single-dose
bolus injection of DEX was administered to patients prior to
induction of anesthesia. In contrast to previous studies, where DEX
in a dose range of 1–2 µg/kg was used (29,30), it
was decided that 0.5 µg/kg DEX would be used in the present study;
DEX has a moderate analgesic effect and achieves a significant
capping effect at 0.5 µg/kg. However, in a preliminary experiment
that used a dose of 1 µg/kg, significant bradycardia and
hypotension was observed in the majority of patients, who therefore
required pharmacological intervention. In the present study, in
order to avoid hypertension, hypotension and bradycardia, a single
dose of 0.5 µg/kg DEX was safely applied >10 min prior to
induction of anesthesia, as described previously (31). In certain clinical studies,
intravenous administration of lower doses of DEX (0.5–0.6 µg/kg)
was reported to attenuate pressor responses (32,33). In
Group D of the present study, where a single-dose bolus injection
of DEX was applied, a significant difference in HR and MAP from
that in Group C was observed, and the profiles suggested that
administration of DEX provided more stable hemodynamics. Of note,
DEX attenuated the increase in HR and MAP following intubation. A
significant reduction in HR and MAP following induction of
anesthesia was also observed; however, values remained within the
normal clinical range during the surgery. These results demonstrate
the safety of using a low-dose (0.5 µg/kg) DEX infusion.
The QoR-40 is a useful objective measure of the
quality of recovery following anesthesia and surgery. It is a
valid, reliable and clinically acceptable method to assess the
recovery score, which may be comparable to the VAS score and a
nine-item questionnaire (19,34). In
the present study, the global QoR-40 scores were significantly
higher in Group D compared with those in Group C, which is
consistent with the results of a recent study by Lee et al
(35). In the present study, it was
observed that the QoR-40 scores referring to all categories were
improved among patients in Group D compared with those in Group C.
Population-based studies have suggested an association between the
efficacy of intra-operative DEX infusion and patient-perceived
quality of recovery as a initial evaluation method (4,5,7,36). In
previous studies, the QoR-40 scores were different between the DEX
and control groups following abdominal colectomy and radical
mastectomy (5,37). Together with these previous results,
the present study indicated that pre- and post-operative
administration of DEX promotes recovery, alleviates pain and
reduces opioid-induced adverse effects, including nausea and
vomiting, in the early post-operative period.
Of note, the present study had certain limitations.
First, evaluation using the QoR-40 questionnaire was not performed
pre-operatively or on days 2 and 3 following surgery. Furthermore,
even though an anesthesiologist who was not involved in the present
study prepared the test drug infusion, administration of DEX may
cause significant hemodynamic changes compared with the placebo,
which may have been identified during surgery; this may have caused
a certain bias to observers. Overall, based on the relatively
restricted sample sizes, the present study provided reliable and
valid results.
In conclusion, pre-operative administration of 0.5
µg/kg DEX followed by post-operative PCIA of 200 µg DEX and 100 µg
sufentanil improved the quality of analgesia and recovery compared
with that of 100 µg sufentanil by PCIA only at 24 h following
tangential excision skin grafting in patients with
moderate-to-severe burns.
Acknowledgements
Not applicable.
Funding
The study was funded by the Nature and Science Fund
of Shandong Province, China (grant no. ZR2014HL109) and the Science
and Technology Program Foundation of Yantai, China (grant no.
2014WS009).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
MJ and QS performed the study and were also major
contributors in writing the manuscript. QS and GL analyzed data. JM
and HQ designed the study and revised manuscript. JM revised
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Institutional Human
Investigations Committee of Yantai Yuhuangding Hospital of Qingdao
University and IRB. All patients provided their written informed
consent.
Patient consent for publication
All patients provided written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
de Castro RJ, Leal PC and Sakata RK: Pain
management in burn patients. Braz J Anesthesiol. 63:149–153. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Asmussen S, Maybauer DM, Fraser JF,
Jennings K, George S and Maybauer MO: A meta-analysis of analgesic
and sedative effects of dexmedetomidine in burn patients. Burns.
39:625–631. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gerlach AT and Dasta JF: Dexmedetomidine:
An updated review. Ann Pharmacother. 41:245–252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ge DJ, Qi B, Tang G and Li JY:
Intraoperative dexmedetomidine promotes postoperative analgesia and
recovery in patients after abdominal hysterectomy: A double-blind,
randomized clinical trial. Sci Rep. 6:215142016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ge DJ, Qi B, Tang G and Li JY:
Intraoperative dexmedetomidine promotes postoperative analgesia and
recovery in patients after abdominal colectomy: A
CONSORT-prospective, randomized, controlled clinical trial.
Medicine (Baltimore). 94:e17272015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gandhi KA, Panda NB, Vellaichamy A, Mathew
PJ, Sahni N and Batra YK: Intraoperative and postoperative
administration of dexmedetomidine reduces anesthetic and
postoperative analgesic requirements in patients undergoing
cervical spine surgeries. J Neurosurg Anesthesiol. 29:258–263.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xin J, Zhang Y, Zhou L, Liu F, Zhou X, Liu
B and Li Q: Effect of dexmedetomidine infusion for intravenous
patient-controlled analgesia on the quality of recovery after
laparotomy surgery. Oncotarget. 8:100371–100383. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheung CW, Qiu Q, Ying AC, Choi SW, Law WL
and Irwin MG: The effects of intra-operative dexmedetomidine on
postoperative pain, side-effects and recovery in colorectal
surgery. Anaesthesia. 69:1214–1221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bekker A, Haile M, Kline R, Didehvar S,
Babu R, Martiniuk F and Urban M: The effect of intraoperative
infusion of dexmedetomidine on the quality of recovery after major
spinal surgery. J Neurosurg Anesthesiol. 25:16–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim SH, Oh YJ, Park BW, Sim J and Choi YS:
Effects of single-dose dexmedetomidine on the quality of recovery
after modified radical mastectomy: A randomised controlled trial.
Minerva Anestesiol. 79:1248–1258. 2013.PubMed/NCBI
|
|
11
|
Gündüz M, Sakalli S, Güneş Y, Kesiktaş E,
Ozcengiz D and Işik G: Comparison of effects of ketamine,
ketamine-dexmedetomidine and ketamine-midazolam on dressing changes
of burn patients. J Anaesthesiol Clin Pharmacol. 27:220–224. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin H, Faraklas I, Sampson C, Saffle JR
and Cochran A: Use of dexmedetomidine for sedation in critically
ill mechanically ventilated pediatric burn patients. J Burn Care
Res. 32:98–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hackett NJ, De Oliveira GS, Jain UK and
Kim JY: ASA class is a reliable independent predictor of medical
complications and mortality following surgery. Int J Surg.
18:184–190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu J, Chen Y and Luo Y: Evaluation of the
visual analog score (VAS) to assess acute mountain sickness (AMS)
in a hypobaric chamber. PLoS One. 9:e1133762014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim SY, Kim JM, Lee JH, Song BM and Koo
BN: Efficacy of intraoperative dexmedetomidine infusion on
emergence agitation and quality of recovery after nasal surgery. Br
J Anaesth. 111:222–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bi SS, Deng CH, Zhou TY, Guan Z, Li L, Li
HQ, Zhang LP, Yang L and Lu W: Remifentanil-sevoflurane interaction
models of circulatory response to laryngoscopy and circulatory
depression. Br J Anaesth. 110:729–740. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heyse B, Proost JH, Schumacher PM,
Bouillon TW, Vereecke HE, Eleveld DJ, Luginbühl M and Struys MM:
Sevoflurane remifentanil interaction: Comparison of different
response surface models. Anesthesiology. 116:311–323. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gornall BF, Myles PS, Smith CL, Burke JA,
Leslie K, Pereira MJ, Bost JE, Kluivers KB, Nilsson UG, Tanaka Y
and Forbes A: Measurement of quality of recovery using the QoR-40:
A quantitative systematic review. Br J Anaesth. 111:161–169. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Myles PS, Weitkamp B, Jones K, Melick J
and Hensen S: Validity and reliability of a postoperative quality
of recovery score: The QoR-40. Br J Anaesth. 84:11–15. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Charan J and Biswas T: How to calculate
sample size for different study designs in medical research? Indian
J Psychol Med. 35:121–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gamst-Jensen H, Vedel PN, Lindberg-Larsen
VO and Egerod I: Acute pain management in burn patients: Appraisal
and thematic analysis of four clinical guidelines. Burns.
40:1463–1469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu J, Sang N, Ren L and Huang Y: Effect of
different sufentanil concentration regimens on postoperative pain
control and its adverse events in intravenous patient-controlled
analgesia. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 39:406–410.
2017.PubMed/NCBI
|
|
23
|
Bhana N, Goa KL and McClellan KJ:
Dexmedetomidine. Drugs. 59:263–270. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qin M, Chen K, Liu T and Shen X:
Dexmedetomidine in combination with sufentanil for postoperative
analgesia after partial laryngectomy. BMC Anesthesiol. 17:662017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nie Y, Liu Y, Luo Q and Huang S: Effect of
dexmedetomidine combined with sufentanil for post-caesarean section
intravenous analgesia: A randomised, placebo-controlled study. Eur
J Anaesthesiol. 31:197–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong CS, Zhang J, Lu Q, Sun P, Yu JM, Wu C
and Sun H: Effect of dexmedetomidine combined with sufentanil for
post-thoracotomy intravenous analgesia: A randomized, controlled
clinical study. BMC Anesthesiol. 17:332017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bloor BC, Ward DS, Belleville JP and Maze
M: Effects of intravenous dexmedetomidine in humans. II.
Hemodynamic changes. Anesthesiology. 77:1134–1142. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Samantaray A, Hanumantha Rao M and Sahu
CR: Additional analgesia for central venous catheter insertion: A
placebo controlled randomized trial of dexmedetomidine and
fentanyl. Crit Care Res Pract. 2016:90626582016.PubMed/NCBI
|
|
29
|
Reddy SV, Balaji D and Ahmed SN:
Dexmedetomidine versus esmolol to attenuate the hemodynamic
response to laryngoscopy and tracheal intubation: A randomized
double-blind clinical study. Int J Appl Basic Med Res. 4:95–100.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gogus N, Akan B, Serger N and Baydar M:
The comparison of the effects of dexmedetomidine, fentanyl and
esmolol on prevention of hemodynamic response to intubation. Rev
Bras Anestesiol. 64:314–319. 2014.(In Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kumari K, Gombar S, Kapoor D and Sandhu
HS: Clinical study to evaluate the role of preoperative
dexmedetomidine in attenuation of hemodynamic response to direct
laryngoscopy and tracheal intubation. Acta Anaesthesiol Taiwan.
53:123–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sulaiman S, Karthekeyan RB, Vakamudi M,
Sundar AS, Ravullapalli H and Gandham R: The effects of
dexmedetomidine on attenuation of stress response to endotracheal
intubation in patients undergoing elective off-pump coronary artery
bypass grafting. Ann Card Anaesth. 15:39–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Basar H, Akpinar S, Doganci N, Buyukkocak
U, Kaymak C, Sert O and Apan A: The effects of preanesthetic,
single-dose dexmedetomidine on induction, hemodynamic, and
cardiovascular parameters. J Clin Anesth. 20:431–436. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guimarães-Pereira L, Costa M, Sousa G and
Abelha F: Quality of recovery after anaesthesia measured with
QoR-40: A prospective observational study. Braz J Anesthesiol.
66:369–375. 2016.PubMed/NCBI
|
|
35
|
Lee SH, Lee CY, Lee JG, Kim N, Lee HM and
Oh YJ: Intraoperative dexmedetomidine improves the quality of
recovery and postoperative pulmonary function in patients
undergoing video-assisted thoracoscopic surgery: A
CONSORT-prospective, randomized, controlled trial. Medicine
(Baltimore). 95:e28542016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aouad MT, Zeeni C, Al Nawwar R,
Siddik-Sayyid SM, Barakat HB, Elias S and Yazbeck Karam VG:
Dexmedetomidine for improved quality of emergence from general
anesthesia: A dose-finding study = Anesth Analg. 29–Dec;2017.[Epub
ahead of print]. View Article : Google Scholar
|
|
37
|
Duncan JA, Bond JS, Mason T, Ludlow A,
Cridland P, O'Kane S and Ferguson MW: Visual analogue scale scoring
and ranking: A suitable and sensitive method for assessing scar
quality? Plast Reconstr Surg. 118:909–918. 2006. View Article : Google Scholar : PubMed/NCBI
|