Introduction
B-cell activating factor (BAFF), also known as
B-lymphocyte stimulator, is a cytokine belonging to the tumor
necrosis factor ligand superfamily, existing either as a type 2
transmembrane protein or in its soluble form (1). BAFF and its homolog A
PRoliferation-Inducing Ligand (APRIL) have an important role in
B-cell maturation, survival, selection and differentiation
(2). The major sources for BAFF
cytokine are several innate immune cell types, including monocytes,
macrophages (3), neutrophils
(4) and dendritic cells in response
to Toll-like receptors, type I and II interferons (IFNs) (5), interleukin-10 (6) and granulocyte colony-stimulating factor
expression (7). Furthermore,
fibroblast-like cells (8) and
astrocytes (9) are also able to
produce BAFF, and so are B cells (10) and T cells (11) in secondary lymphoid tissues,
including the spleen, lymph nodes and tonsils. Increased levels of
BAFF have been associated with autoimmune diseases (12), including systemic lupus erythematosus
(13), Sjögren's syndrome (14) and rheumatoid arthritis (15), as well as with multiple myeloma
(16), non-Hodgkin's lymphoma
(17), B-lineage lymphomas (18) and Hodgkin's lymphoma (19).
A total of 3 BAFF-binding receptors have been
established, namely BAFF receptor (BAFF-R), which is specific for
BAFF, and two others that are shared with the homologue APRIL,
transmembrane activator and cyclophilin ligand interactor (TACI)
and B-cell maturation antigen (BCMA) (2). BAFF-R is essential for B-cell
maturation at early transitional stages, particularly from T1 to T2
B cells, as well as for B-cell survival (20). TACI is important for
T-cell-independent responses of B cells, immunoglobulin (Ig) class
switch and it is considered to be a negative regulator of B-cell
homeostasis (21–23). Whereas, BCMA promotes plasma cells
survival (24). All three BAFF
receptors, BAFF-R, TACI and BCMA, are expressed on B cells; TACI is
additionally expressed on activated T cells, while BAFF-R is also
expressed on follicular helper T cells (TFH) (21,25).
Germinal centers (GCs) are important structures in
secondary lymphoid tissues where T cell-dependent immune responses
occur, and BAFF has been identified to be implicated in their
formation from lymphoid tissues in murine models (26,27), as
well as in tertiary lymphoid structures in autoimmune diseases
(28,29). A study reported that BAFF and APRIL
are associated with artery tertiary lymphoid organs in giant-cell
arteritis, and that BAFF is highly expressed within infiltrating,
vascular and endothelial cells, suggesting their involvement in
ectopic GCs; however, BAFF receptors were not analyzed (28). Likewise, in Hodgkin (30) and non-Hodgkin lymphoma (31), the BAFF/BAFF-R pathway has an
important role and has been proposed as a predictor of lymphoma
development in primary Sjögren's syndrome (32). However, only few studies have
assessed the expression of BAFF receptors in non-neoplastic
lymphoid tissues in humans (30,33,34).
Therefore, the distribution and expression profiles of BAFF and
their receptors, BAFF-R, TACI and BCMA, in secondary follicles from
tonsil tissues were analyzed in the present study.
Materials and methods
Patients
Tonsils were obtained from nine patients submitted
to a routine tonsillectomy performed at the Department of
Otorhinolaryngology of the Mexican Institute of Social Security
(Guadalajara, Mexico). The mean age of the patients was 12 years
(range, 4–41 years), and the cohort comprised 7 females and 2
males. The clinical diagnosis for the majority of the patients was
chronic tonsillitis and grade III or IV tonsillar hypertrophy. All
of the patients, or their guardians in the case of minors, provided
written informed consent in accordance with the Declaration of
Helsinki and the current national guidelines and regulations. The
ethics committee of the University Center for Health Sciences,
University of Guadalajara (Guadalajara, Mexico) approved the study
under the number CI-01215.
Histological technique and
characterization of GCs
Palatine tonsils were fixed in 4% paraformaldehyde
for later paraffin embedding using a tissue processor (TP1020;
Leica Biosystems, Wetzlar, Germany). Tissues were sectioned at 4
microns and mounted on electrocharged slides. After drying, slides
were de-paraffinized in an electric oven at 59°C, re-hydrated in
xylene and a series of graded ethanols followed by distilled water.
Haematoxylin-eosin staining was performed in order to identify GC
morphology in tonsils. A total of four zones in GCs were identified
and considered in the expression analysis: The dark zone (DZ),
where lymphocytes have a large nucleus and a small amount of
cytoplasm; the light zone (LZ), where lymphocytic cells with a
higher amount of cytoplasm may be distinguished due to their
differentiation; the mantle zone (MZ), consisting of a lymphocyte
fringe that surrounds the GC and exhibits an asymmetric
distribution regarding the central axis to the follicle; and the
interfollicular zone (IZ) as the remaining area excluding the GCs.
Finally, the presence of GCs was immunohistochemically confirmed by
CD21 labeling and as described below.
Immunohistochemical staining
Once tissue sections were re-hydrated, antigen
retrieval was accomplished in one step with a water steamer at 96
degrees for 30 min, while slides were submerged in a cup with 10 mM
sodium citrate buffer (pH=6) for later staining with rat monoclonal
antibody to BAFF (cat. no. ab16081; dilution, 1:100), rabbit
monoclonal antibodies to TACI (cat. no. ab79023; dilution, 1:200)
and BCMA (cat. no. ab5972; dilution, 1:400), or 1 mM EDTA buffer
(pH=9) for later staining with mouse monoclonal antibodies to CD21
(cat. no. ab9492; dilution, 1:10) and BAFF-R (cat. no. ab16232;
dilution, 1:500) from Abcam (Cambridge, UK). After cooling, slides
were treated with a peroxidase blocking solution composed of 3%
hydrogen peroxide and 10% methanol solution for 15 min at room
temperature. The slides were then blocked with serum depending on
the secondary antibody source: Horse serum for CD21 and BAFF-R;
rabbit serum for BAFF; and goat serum for TACI and BCMA antibodies
(VECTASTAIN Elite ABC-HRP Kit; Vector Laboratories, Inc.,
Burlingame, CA, USA). The samples were incubated with the primary
antibodies overnight at 4°C. Detection was performed with the
respective provided secondary biotinylated antibody (1:200), for 30
min at room temperature and streptavidin (VECTASTAIN Elite ABC-HRP
Kit; Vector Laboratories, Inc.) using diaminobenzidine (DAB) until
a red-brown color developed. Finally, counterstaining was performed
using Harris' hematoxylin.
Immunohistochemistry slide staining for CD3 and CD20
was performed using the automated Ventana Benchmark ULTRA (Roche
Diagnostics, Basel, Switzerland) following the manufacturer's
recommended protocols for paraffin-embedded sections. The primary
antibodies anti-CD3 (2GV6) rabbit monoclonal antibody (cat. no.
790-4341) and anti-CD20 (L26) mouse monoclonal antibody [cat. no.
760-2531; Ventana iVIEW PATHWAY (Roche Diagnostics) were obtained
prediluted by Roche Diagnostics, and were optimized for use on
Ventana staining platforms. Detection was performed with the iVIEW
DAB detection kit (Roche Diagnostics)].
Immunohistochemical image
analysis
After staining, images of the slides were captured
with a digital camera (Axiocam 305; Zeiss AG, Oberkochen, Germany)
attached to an optical microscope (Axio Lab.A1; Zeiss AG) and three
fields of view per slide were tested with a total magnification of
×400 for each case; the same three fields of view were captured for
the analyzed surface area from each sample. AxioVision V4.6
software (Zeiss AG) was used for controlling the light and camera
settings. Image analysis was performed using ImageJ v1.51j8 free
software (National Institutes of Health, Bethesda, MD, USA;
http://imagej.nih.gov/ij/) using the DAB color
deconvolution plugin. In the DAB layer, the analysis of tissue
images was performed considering the pixel intensity values in the
range of 0–255, as previously described by Chatterjee et al
(35). The mean intensity was
identified using ImageJ software using the mean default threshold
feature in the ‘Image’ menu. Finally, the number of positive pixels
in a zone was measured with the measuring tool ‘Analyze’ from the
software menu. The staining quantification was the mean percentage
of stained cells in the field of view.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 6 software (GraphPad Software Inc., La Jolla, CA, USA). Data
were analyzed using the Shapiro-Wilk normality test and 95%
confidence interval. P<0.05 was considered to indicate a
statistically significant difference. For multiple-group
comparisons with parametric data, one-way analysis of variance and
Tukey's post-hoc test was used. Correlation analyses were performed
using Pearson's R2 correlation coefficients.
Results
Identification of GCs in tonsillar
follicles based on CD21 expression
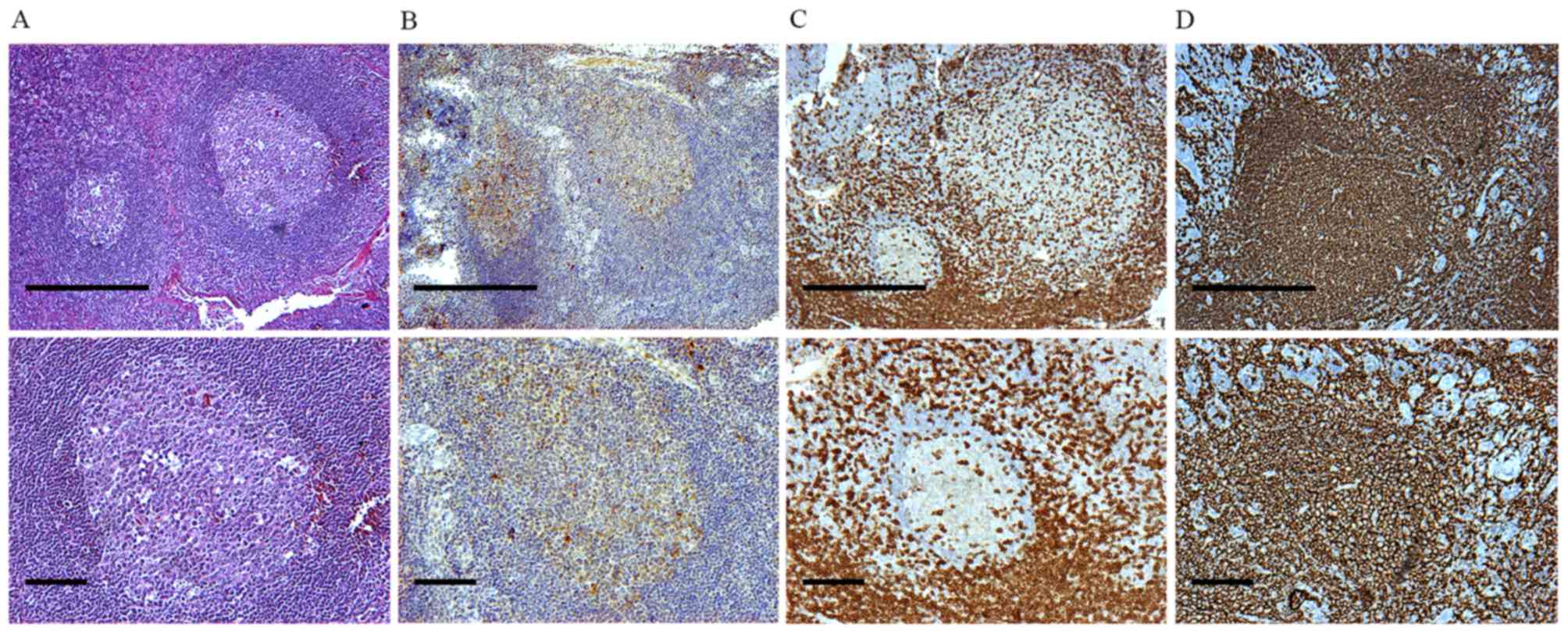
Besides their morphological features and the typical
structure of a secondary lymphoid organ (Fig. 1A and B), immunohistochemical staining
for CD21 was used for locating GCs in tonsillar secondary lymphoid
tissue, in order to identify the presence of follicular dendritic
cell networks. CD21-positive cells were observed mainly in the LZ.
The MZ presented with diffuse staining for CD21 (Fig. 1C and D).
Identification of CD3+ and CD20+ cells
of GCs in tonsillar follicles
The cellular aggregates were further analyzed by
staining with anti-CD3 and anti-CD20 antibodies to detect areas of
T-cell/B-cell aggregation (Fig. 1C and
D). CD3+ cells were located mainly in IZs as well as in the LZ
and DZ. A small amount of CD3+ cells were observed in the MZ
(Fig. 1C). However, CD20+ cells were
the most remarkable cell type in the entire tonsillar follicles
(Fig. 1D).
Expression of BAFF in human
tonsils
BAFF expression was examined in GCs from human
tonsils. BAFF was highly expressed in all regions of the GC.
However, the highest percentage of BAFF+ cells, exhibiting a
membrane and intracellular staining pattern, were located in the MZ
corresponding to the naïve B-cell area (P<0.05 vs. LZ, DZ and
IZ; Fig. 2A, D and F).
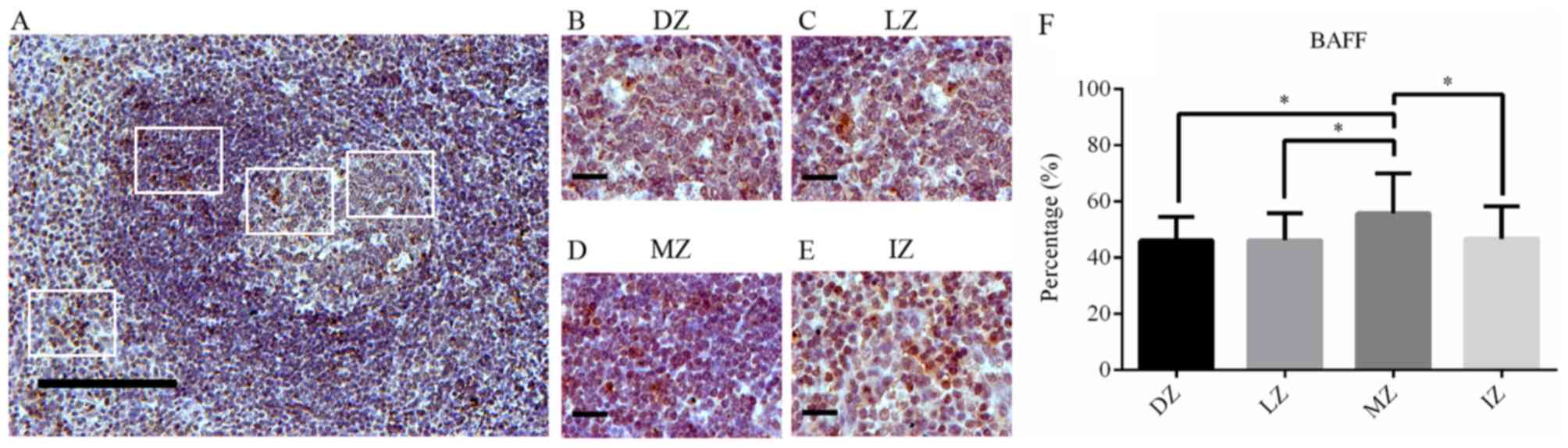 | Figure 2.BAFF expression in germinal centers.
(A) BAFF staining in human tonsils (magnification, ×100; scale bar,
200 µm). (B-E) BAFF staining in (B) the DZ, (C) LZ, (D) MZ and (E)
IZ in germinal centers of human tonsils (magnification, ×400; scale
bar, 50 µm). (F) Percentage of BAFF-positive cells in different
zones of germinal centers in human tonsils. BAFF expression is
showed as brown-orange staining and Harris' hematoxylin was used as
a blue-purple counterstain. Images were quantitatively analyzed
using ImageJ software. Values are expressed as the mean ± standard
deviation. *P<0.05. DZ, dark zone; IZ, interfollicular zone; LZ,
light zone; MZ, mantle zone; BAFF, B-cell activating factor. |
Analysis of BAFF-R expression in human
tonsils
Based on the average staining intensities and
percentages of positively stained cells, a high expression of
BAFF-R was observed in the membrane cells of the MZ (P<0.05 vs.
DZ, LZ and IZ; Fig. 3A-F). The
lowest expression of BAFF-R was identified in the IZ (Fig. 3A, E and F).
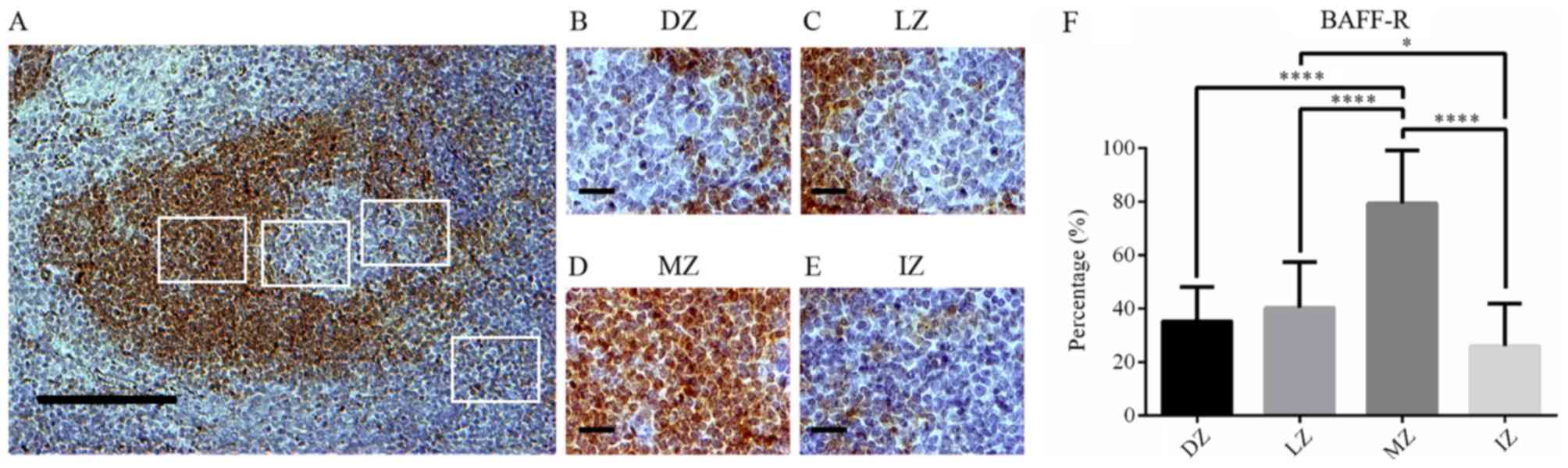 | Figure 3.BAFF-R expression in germinal
centers. (A) BAFF-R staining in human tonsils (magnification, ×100;
scale bar, 200 µm). (B-E) BAFF-R staining in (B) the DZ, (C) LZ,
(D) MZ and (E) IZ in germinal centers of human tonsils
(magnification, ×400; scale bar, 50 µm). (F) Percentage of
BAFF-R-positive cells in different zones of germinal centers in
human tonsils. BAFF-R expression is showed as brown-orange staining
and Harris' hematoxylin was used as a blue-purple counterstain.
Images were quantitatively analyzed using ImageJ software. Values
are expressed as the mean ± standard deviation. *P<0.05;
****P<0.0001. DZ, dark zone; IZ, interfollicular zone; LZ, light
zone; MZ, mantle zone; BAFF-R, B-cell activating factor
receptor. |
Analysis of TACI expression in human
tonsils
TACI-expressing cells were mostly located inside GCs
in the DZ and LZ, and also in the MZ. No significant differences in
TACI+ cells were observed among the different GC areas (Fig. 4A-F).
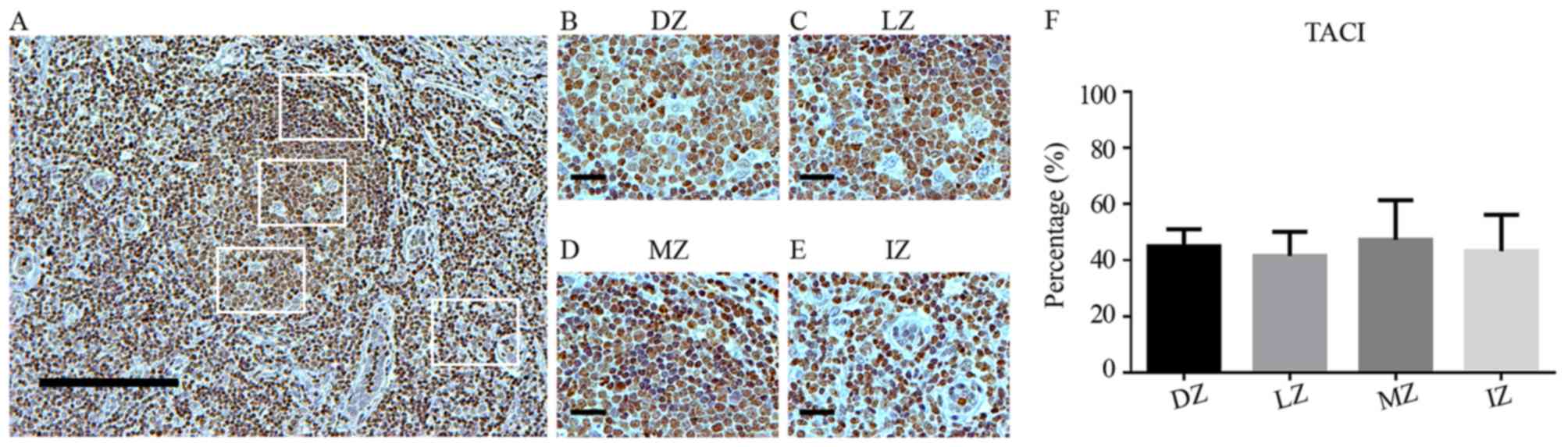 | Figure 4.TACI expression in germinal centers.
(A) TACI staining in human tonsils (magnification, ×100; scale bar,
200 µm). (B-E) TACI staining in (B) the DZ, (C) LZ, (D) MZ and (E)
IZ in germinal centers of human tonsils (magnification, ×400; scale
bar, 50 µm). (F) Percentage of TACI-positive cells in different
zones of germinal centers in human tonsils. TACI expression is
showed as brown-orange staining and Harris' hematoxylin was used as
a blue-purple counterstain. Images were quantitatively analyzed
using ImageJ software. Values are expressed as the mean ± standard
deviation. No statistically significant differences. DZ, dark zone;
IZ, interfollicular zone; LZ, light zone; MZ, mantle zone; TACI,
transmembrane activator and cyclophilin ligand interactor. |
Analysis of BCMA expression in human
tonsils
BCMA exhibited a similar distribution between the
DZ, LZ and MZ (Fig. 5A-F). The
lowest expression of BCMA was observed in the IZ (P<0.05 vs. DZ,
LZ and MZ; Fig. 5A, E and F). In
addition, a small proportion of cells expressing BCMA in the
tonsillar submucosa, particularly in proximity to blood vessels,
were observed (data not shown).
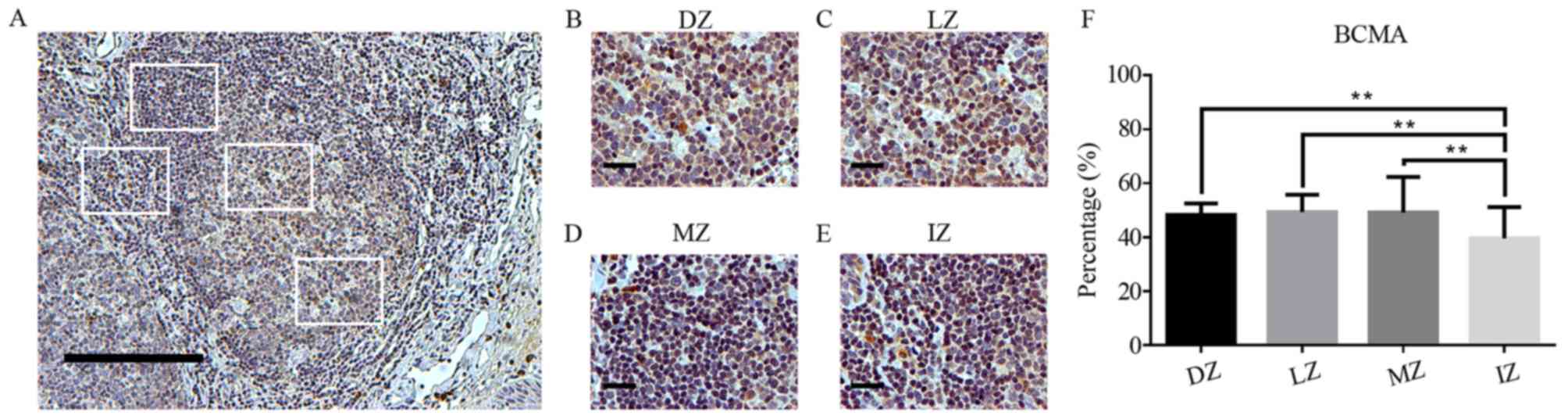 | Figure 5.BCMA expression in germinal centers.
(A) BCMA staining in human tonsils (magnification, ×100; scale bar,
200 µm). (B-E) BCMA staining in (B) the DZ, (C) LZ, (D) MZ and (E)
IZ in germinal centers of human tonsils (magnification, ×400; scale
bar, 50 µm). (F) Percentage of BCMA-positive cells in different
zones of germinal centers in human tonsils. BCMA expression is
showed as brown-orange staining and Harris' hematoxylin was used as
a blue-purple counterstain. Images were quantitatively analyzed
using ImageJ software. Values are expressed as the mean ± standard
deviation. **P<0.01. DZ, dark zone; IZ, interfollicular zone;
LZ, light zone; MZ, mantle zone; BCMA, B-cell maturation
antigen. |
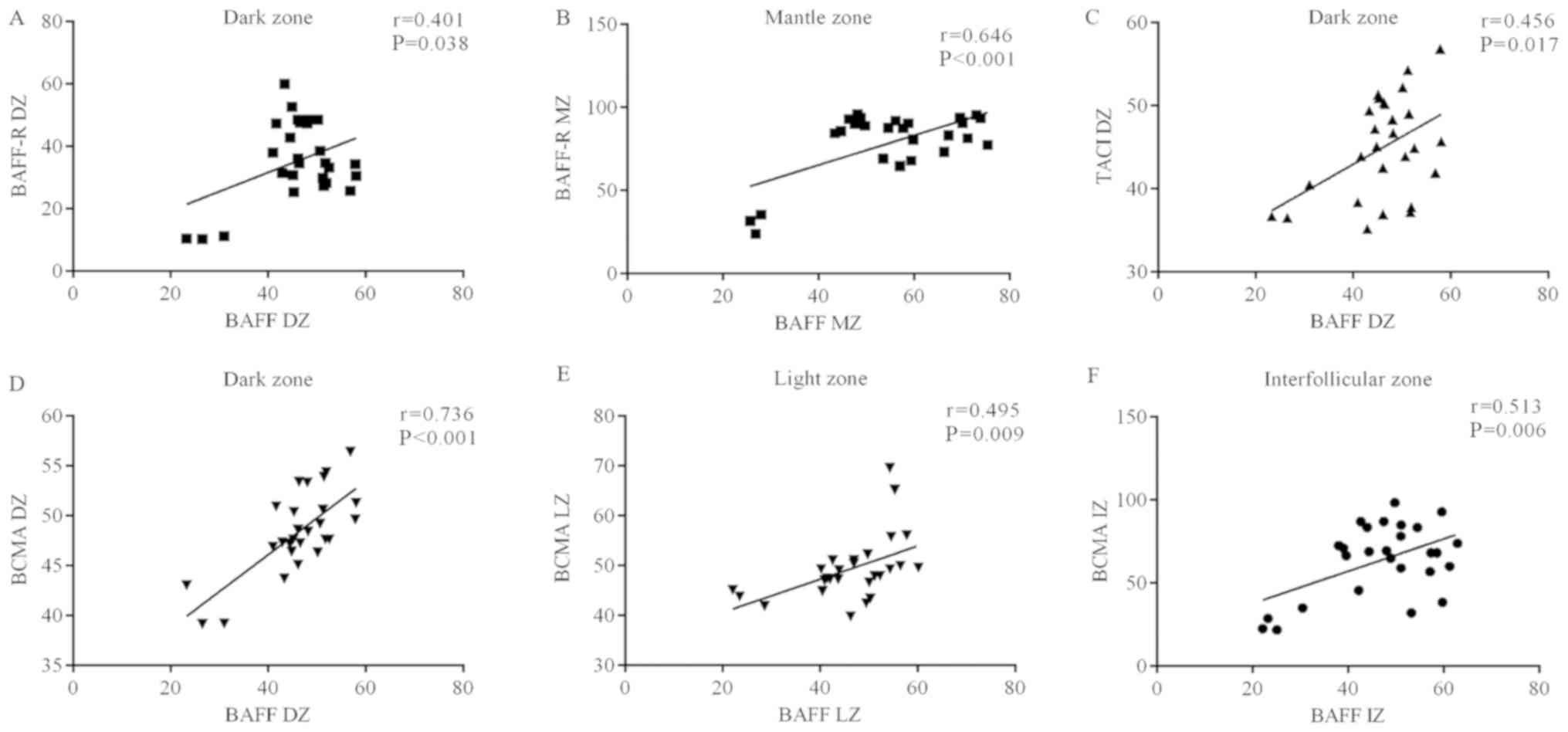
Association between BAFF and its
receptors in GC zones
The association between BAFF and its receptors
regarding their distribution in GC zones was analyzed. A
co-localization of BAFF and BAFF-R according to amount of positive
cells and staining intensity was identified in the DZ (r=0.401,
P=0.038; Fig. 6A) and the MZ
(r=0.646, P<0.001; Fig. 6B).
Regarding the TACI receptor, a positive correlation
with BAFF levels was identified in the DZ (r=0.456, P=0.017;
Fig. 6C).
With regard to BCMA, BAFF+ cells were correlated
with the amount of BCMA+ cells in the DZ (r=0.736, P<0.001;
Fig. 6D), LZ (r=0.495, P=0.009;
Fig. 6E) and IZ (r=0.513, P=0.006;
Fig. 6F).
Discussion
BAFF is a key molecule that mediates B-cell
survival, proliferation and differentiation through its receptors
BAFF-R, TACI and BCMA in different stages of B-cell development
(2). BAFF and BAFF-binding receptors
have been demonstrated to be involved in the formation of GCs in
secondary follicles in murine models and in tertiary lymphoid
structures in autoimmune diseases (28,26,27). GCs
are the major sites for the generation of high-affinity
antibody-secreting plasma cells and memory B cells, through the
processes of proliferation, high-affinity selection and somatic
hypermutation (36,37). GCs are well-organized structures with
defined histological zones referred to as the DZ, LZ and MZ, while
the remaining area is known as the IZ (38). In the present study the expression
profiles of BAFF and their receptors, BAFF-R, TACI and BCMA, were
analyzed in secondary follicles from human tonsillar tissues.
A diffuse expression of BAFF was identified in
extended tonsillar tissue areas, particularly in the MZ, where the
highest percentages of BAFF+ cells were located. The BAFF
expression profiles may be explained by the large abundance of
BAFF-secreting cells in the lymphoid tissue, mainly follicular
dendritic cells (39,40) and TFH cells (41), which are the major sources of BAFF.
However, numerous other types of myeloid origin cell, including
monocytes, macrophages and neutrophils, are important for
BAFF-producing cells (42,43). Furthermore, it has been demonstrated
in experimental models that BAFF is necessary for GC formation,
since BAFF blockade with anti-BAFF-R antibodies decreases the
number of follicles in mouse secondary lymphoid organs (44).
In the present study, expression of the three BAFF
receptors, BAFF-R, TACI and BCMA, was detected in the GCs of the
secondary follicles from human tonsil tissues. However, a different
expression pattern was observed among them. In addition, a
correlation between BAFF and each of the three BAFF-binding
receptors was observed, particularly in the DZ of GCs. This result
may be due to the function of BAFF as a survival factor for
centroblasts, which are highly proliferating cells that are about
to undergo selection, clonal expansion and somatic hypermutation
(45).
BAFF-R expression was observed inside GCs;
furthermore, a high expression was observed in the MZ. In this
region, mainly IgD+ naïve B cells are located (30,46),
which suggests that this receptor is essential at early stages of
B-cell development and promotes their maturation (47). Furthermore, the most evident BAFF and
BAFF-R interaction was identified in the MZ, which was greater than
that in the LZ and DZ. BAFF-R has been previously detected on
B-cells situated in the MZ (33).
Furthermore, an in vitro study that analyzed GC B cell
maturation indicated an initial increment of BAFF-R expression in
early stages, which decreased during plasma cell differentiation,
and was associated with TACI and BCMA expression (34). Recently, it has been proposed that GC
B cells from the DZ, express lower levels of BAFF-R due to the
cleavage process of BAFF-R by A disintegrin and metalloproteinases,
a BAFF- and TACI-dependent mechanism (48).
In addition, T cells in the MZ and inside follicles
were observed in the present study, although at a lower proportion
compared with that of B cells. It has been demonstrated that T
cells are able to express BAFF-R favoring T-cell activation and
their differentiation to TFH (49). In fact, an expansion of
TFH with high IFN-γ production through BAFF-R was
observed in a lupus-prone murine model with BCMA deficiency, which
suggests a potential role of a BCMA-BAFF-R balance in the
maintenance of immune tolerance (25).
However, the expression pattern of TACI identified
in the present study displayed a wide distribution within follicles
from the tonsillar tissue. TACI has been linked to isotype class
switch in T-independent immune responses and it is perceived as a
negative regulator of B cells (21).
These results are supported by a previous experimental study on
TACI gene knockout (KO) mice, which revealed expanding populations
of TFH and GC B cells in their spleens when immunized
with a T-cell-dependent antigen. In addition, TACI KO mice
exhibited decreased plasma cells and antigen-specific antibody
responses (50).
In addition, a correlation between TACI and BAFF was
observed in the DZ, but not in the LZ within the follicle, whereas
a correlation of BCMA and BAFF was observed in the DZ as well as
the LZ. BAFF shares TACI and BCMA receptors with APRIL, which also
co-stimulates B cells and plasma cell survival; however, APRIL
expression was not evaluated in the present study.
It is likely that the BCMA-TACI interaction is a key
mechanism in B-cell differentiation to plasma cells in the LZ. As
demonstrated by a previous study using a Nba2.Yaa spontaneous lupus
mouse model with induced TACI, BCMA or double TACI-BCMA mutations,
the disease activity was increased in BCMA-deficient animals, while
it was decreased in animals with TACI deficiency and TACI-BCMA
double deficiency (51).
BCMA is expressed on B cells, plasmablasts and GC
plasma cells (52). In a murine
lupus model, BCMA deficiency was associated with early lethality.
Exacerbated B cell and plasma cell production and increased
autoantibody levels reveal that BCMA is key in the maintenance of
B-cell homeostasis and self-tolerance control in autoimmunity
responses (53). The presence of
BCMA-expressing cells within tonsillar follicles emphasizes its
possible role in the differentiation process towards
antibody-producing plasma cells, which migrate to regions near
blood vessels or salivary conducts to secrete mucosa-associated
Igs, e.g. IgA. This explains why BCMA was associated with BAFF in
cells located in the IZ.
In conclusion, the high expression and differential
distribution patterns of BAFF, BAFF-R, TACI and BCMA in follicle
zones suggest their involvement in the maintenance of GCs in
tonsillar secondary lymphoid tissue. BAFF-R overexpression in the
MZ associated with BAFF suggests that BAFF, through BAFF-R, is the
principal pathway for maintaining the population of naïve B-cells
in GCs. Furthermore, TACI and BCMA have roles inside GCs, where
processes of B-cell positive selection, proliferation and
differentiation into Ig-secreting plasma cells occur.
Acknowledgements
Not applicable.
Funding
This work was supported by a grant no. 273324 to
EOR, from the National Council of Science and Technology
(CONACYT).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
FJCB: Patient enrollment, immunohistochemistry
assay, immunohistochemical quantification and statistical analysis,
manuscript preparation; EOR: Manuscript preparation, statistical
analysis and study design; RAFT: Histological technique and GC
evaluation; LHGC: Patient enrollment, surgical tissues obtention
and histological technique; FJBR: Histological technique and GC
evaluation; ALPS: Immunohistochemical quantification analysis; AC:
Immunohistochemical quantification analysis and manuscript
preparation; JFMV: Manuscript preparation and analysis and
interpretation of data; CAPS: Patient enrollment, clinical
evaluation, statistical analysis, manuscript preparation and study
design. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
In accordance with the Declaration of Helsinki and
the current national guidelines and regulations, written informed
consent was provided by all of the patients, and in the case of
minors under the age of 16 years, the parents provided written
informed consent. The ethics committee of the University Center for
Health Sciences, University of Guadalajara (Guadalajara, Mexico)
approved the study under the number CI-01215.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests regarding the present study. They have no relationship
with or any financial interests regarding any commercial companies
pertaining to this article.
References
|
1
|
Vincent FB, Saulep-Easton D, Figgett WA,
Fairfax KA and Mackay F: The BAFF/APRIL system: Emerging functions
beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev.
24:203–215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bossen C and Schneider P: BAFF, APRIL and
their receptors: Structure, function and signaling. Semin Immunol.
18:263–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Craxton A, Magaletti D, Ryan EJ and Clark
EA: Macrophage- and dendritic cell-dependent regulation of human
B-cell proliferation requires the TNF family ligand BAFF. Blood.
101:4464–4471. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scapini P, Bazzoni F and Cassatella MA:
Regulation of B-cell-activating factor (BAFF)/B lymphocyte
stimulator (BLyS) expression in human neutrophils. Immunol Lett.
116:1–6. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
MacLennan I and Vinuesa C: Dendritic
cells, BAFF, and APRIL: Innate players in adaptive antibody
responses. Immunity. 17:235–238. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ogden CA, Pound JD, Batth BK, Owens S,
Johannessen I, Wood K and Gregory CD: Enhanced apoptotic cell
clearance capacity and B cell survival factor production by
IL-10-activated macrophages: Implications for burkitt's lymphoma. J
Immunol. 174:3015–3023. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scapini P, Nardelli B, Nadali G, Calzetti
F, Pizzolo G, Montecucco C and Cassatella MA: G-CSF-stimulated
neutrophils are a prominent source of functional blys. J Exp Med.
197:297–302. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohata J, Zvaifler NJ, Nishio M, Boyle DL,
Kalled SL, Carson DA and Kipps TJ: Fibroblast-like synoviocytes of
mesenchymal origin express functional B cell-activating factor of
the TNF family in response to proinflammatory cytokines. J Immunol.
174:864–870. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krumbholz M, Theil D, Derfuss T, Rosenwald
A, Schrader F, Monoranu CM, Kalled SL, Hess DM, Serafini B, Aloisi
F, et al: BAFF is produced by astrocytes and up-regulated in
multiple sclerosis lesions and primary central nervous system
lymphoma. J Exp Med. 201:195–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chu VT, Enghard P, Riemekasten G and Berek
C: In vitro and in vivo activation induces BAFF and APRIL
expression in B cells. J Immunol. 179:5947–5957. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suzuki K, Setoyama Y, Yoshimoto K, Tsuzaka
K, Abe T and Takeuchi T: Effect of interleukin-2 on synthesis of B
cell activating factor belonging to the tumor necrosis factor
family (BAFF) in human peripheral blood mononuclear cells.
Cytokine. 44:44–48. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pers JO, Daridon C, Devauchelle V, Jousse
S, Saraux A, Jamin C and Youinou P: BAFF overexpression is
associated with autoantibody production in autoimmune diseases. Ann
N Y Acad Sci. 1050:34–39. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vincent FB, Morand EF and Mackay F: BAFF
and innate immunity: New therapeutic targets for systemic lupus
erythematosus. Immunol Cell Biol. 90:293–303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Daridon C, Devauchelle V, Hutin P, Le
Berre R, Martins-Carvalho C, Bendaoud B, Dueymes M, Saraux A,
Youinou P and Pers JO: Aberrant expression of BAFF by B lymphocytes
infiltrating the salivary glands of patients with primary Sjögren's
syndrome. Arthritis Rheum. 56:1134–1144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bosello S, Pers JO, Rochas C, Devauchelle
V, De Santis M, Daridon C, Saraux A, Ferraccioli GF and Youinou P:
BAFF and rheumatic autoimmune disorders: Implications for disease
management and therapy. Int J Immunopathol Pharmacol. 20:1–8. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pan J, Sun Y, Zhang N, Li J, Ta F, Wei W,
Yu S and Ai L: Characteristics of BAFF and APRIL factor expression
in multiple myeloma and clinical significance. Oncol Lett.
14:2657–2662. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Novak AJ, Grote DM, Stenson M, Ziesmer SC,
Witzig TE, Habermann TM, Harder B, Ristow KM, Bram RJ, Jelinek DF,
et al: Expression of BLyS and its receptors in B-cell non-Hodgkin
lymphoma: Correlation with disease activity and patient outcome.
Blood. 104:2247–2253. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vaskua BJ, Bienerta P, Kodytkovab D,
Zlamala F, Tomandld J, Tomandlovad M, Vaskua A and Sterbab J: BAFF
levels are elevated in paediatric patients with acute lymphoblastic
leukaemia compared to other B-lineage neoplasms. J Hematol.
1:20–22. 2012.
|
|
19
|
Oki Y, Georgakis GV, Migone TS, Kwak LW
and Younes A: Prognostic significance of serum B-lymphocyte
stimulator in hodgkin's lymphoma. Haematologica. 92:269–270. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rowland SL, Leahy KF, Halverson R, Torres
RM and Pelanda R: BAFF receptor signaling aids the differentiation
of immature B cells into transitional B cells following tonic BCR
signaling. J Immunol. 185:4570–4581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mackay F and Schneider P: TACI, an
enigmatic BAFF/APRIL receptor, with new unappreciated biochemical
and biological properties. Cytokine Growth Factor Rev. 19:263–276.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
von B, ülow GU, van Deursen JM and Bram
RJ: Regulation of the T-independent humoral response by TACI.
Immunity. 14:573–582. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan M, Wang H, Chan B, Roose-Girma M,
Erickson S, Baker T, Tumas D, Grewal IS and Dixit VM: Activation
and accumulation of B cells in TACI-deficient mice. Nat Immunol.
2:638–643. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
O'Connor BP, Raman VS, Erickson LD, Cook
WJ, Weaver LK, Ahonen C, Lin LL, Mantchev GT, Bram RJ and Noelle
RJ: BCMA is essential for the survival of long-lived bone marrow
plasma cells. J Exp Med. 199:91–98. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coquery CM, Loo WM, Wade NS, Bederman AG,
Tung KS, Lewis JE, Hess H and Erickson LD: BAFF regulates
follicular helper T cells and affects their accumulation and
interferon-γ production in autoimmunity: BAFF regulates follicular
helper T cells. Arthritis Rheumatol. 67:773–784. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rahman ZS, Rao SP, Kalled SL and Manser T:
Normal induction but attenuated progression of germinal center
responses in BAFF and BAFF-R signaling-deficient mice. J Exp Med.
198:1157–1169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vora KA, Wang LC, Rao SP, Liu ZY, Majeau
GR, Cutler AH, Hochman PS, Scott ML and Kalled SL: Cutting edge:
Germinal centers formed in the absence of B cell-activating factor
belonging to the TNF family exhibit impaired maturation and
function. J Immunol. 171:547–551. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ciccia F, Rizzo A, Maugeri R, Alessandro
R, Croci S, Guggino G, Cavazza A, Raimondo S, Cannizzaro A,
Iacopino DG, et al: Ectopic expression of CXCL13, BAFF, APRIL and
LT-β is associated with artery tertiary lymphoid organs in giant
cell arteritis. Ann Rheum Dis. 76:235–243. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kang S, Fedoriw Y, Brenneman EK, Truong
YK, Kikly K and Vilen BJ: BAFF induces tertiary lymphoid structures
and positions T cells within the glomeruli during lupus nephritis.
J Immunol. 198:2602–2611. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chiu A, Xu W, He B, Dillon SR, Gross JA,
Sievers E, Qiao X, Santini P, Hyjek E, Lee JW, et al: Hodgkin
lymphoma cells express TACI and BCMA receptors and generate
survival and proliferation signals in response to BAFF and APRIL.
Blood. 109:729–739. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang S, Li JY and Xu W: Role of
BAFF/BAFF-R axis in B-cell non-hodgkin lymphoma. Crit Rev Oncol
Hematol. 91:113–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nocturne G and Mariette X: Sjögren
syndrome-associated lymphomas: An update on pathogenesis and
management. Br J Haematol. 168:317–327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakamura N, Hase H, Sakurai D, Yoshida S,
Abe M, Tsukada N, Takizawa J, Aoki S, Kojima M, Nakamura S and
Kobata T: Expression of BAFF-R (BR3) in normal and neoplastic
lymphoid tissues characterized with a newly developed monoclonal
antibody. Virchows Arch. 447:53–60. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang X, Park CS, Yoon SO, Li L, Hsu YM,
Ambrose C and Choi YS: BAFF supports human B cell differentiation
in the lymphoid follicles through distinct receptors. Int Immunol.
17:779–788. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chatterjee S, Malhotra R, Varghese F,
Bukhari AB, Patil A, Budrukkar A, Parmar V, Gupta S and De A:
Quantitative immunohistochemical analysis reveals association
between sodium iodide symporter and estrogen receptor expression in
breast cancer. PLoS One. 8:e540552013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Allen CD, Okada T and Cyster JG:
Germinal-center organization and cellular dynamics. Immunity.
27:190–202. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Corcoran LM and Tarlinton DM: Regulation
of germinal center responses, memory B cells and plasma cell
formation-an update. Curr Opin Immunol. 39:59–67. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Victora GD and Nussenzweig MC: Germinal
centers. Annu Rev Immunol. 30:429–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hase H, Kanno Y, Kojima M, Hasegawa K,
Sakurai D, Kojima H, Tsuchiya N, Tokunaga K, Masawa N, Azuma M, et
al: BAFF/BLyS can potentiate B-cell selection with the B-cell
coreceptor complex. Blood. 103:2257–2265. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Suzuki K, Maruya M, Kawamoto S, Sitnik K,
Kitamura H, Agace WW and Fagarasan S: The sensing of environmental
stimuli by follicular dendritic cells promotes immunoglobulin a
generation in the gut. Immunity. 33:71–83. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Treml LS, Carlesso G, Hoek KL, Stadanlick
JE, Kambayashi T, Bram RJ, Cancro MP and Khan WN: TLR stimulation
modifies BLyS receptor expression in follicular and marginal zone B
cells. J Immunol. 178:7531–7539. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schneider P: The role of APRIL and BAFF in
lymphocyte activation. Curr Opin Immunol. 17:282–289. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Thien M, Phan TG, Gardam S, Amesbury M,
Basten A, Mackay F and Brink R: Excess BAFF rescues self-reactive B
cells from peripheral deletion and allows them to enter forbidden
follicular and marginal zone niches. Immunity. 20:785–798. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sharma A, Kiripolsky J, Klimatcheva E,
Howell A, Fereidouni F, Levenson R, Rothstein TL and Kramer JM:
Early BAFF receptor blockade mitigates murine Sjögren's syndrome:
Concomitant targeting of CXCL13 and the BAFF receptor prevents
salivary hypofunction. Clin Immunol. 164:85–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bräuninger A, Yang W, Wacker HH, Rajewsky
K, Küppers R and Hansmann ML: B-cell development in progressively
transformed germinal centers: Similarities and differences compared
with classical germinal centers and lymphocyte-predominant hodgkin
disease. Blood. 97:714–719. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Le Pottier L, Devauchelle V, Fautrel A,
Daridon C, Saraux A, Youinou P and Pers JO: Ectopic germinal
centers are rare in sjogren's syndrome salivary glands and do not
exclude autoreactive B cells. J Immunol. 182:3540–3547. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tussiwand R, Rauch M, Flück LA and Rolink
AG: BAFF-R expression correlates with positive selection of
immature B cells: Leukocyte signaling. Eur J Immunol. 42:206–216.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Smulski CR, Kury P, Seidel LM, Staiger HS,
Edinger AK, Willen L, Seidl M, Hess H, Salzer U, Rolink AG, et al:
BAFF- and TACI-dependent processing of BAFFR by ADAM proteases
regulates the survival of B cells. Cell Rep. 18:2189–2202. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ng LG, Sutherland AP, Newton R, Qian F,
Cachero TG, Scott ML, Thompson JS, Wheway J, Chtanova T, Groom J,
et al: B cell-activating factor belonging to the TNF family
(BAFF)-R is the principal BAFF receptor facilitating BAFF
costimulation of circulating T and B cells. J Immunol. 173:807–817.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ou X, Xu S and Lam KP: Deficiency in
TNFRSF13B (TACI) expands T-follicular helper and germinal center B
cells via increased ICOS-ligand expression but impairs plasma cell
survival. Proc Natl Acad Sci USA. 109:15401–15406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tran NL, Schneider P and Santiago-Raber
ML: TACI-dependent APRIL signaling maintains autoreactive B cells
in a mouse model of systemic lupus erythematosus. Eur J Immunol.
47:713–723. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Novak AJ, Darce JR, Arendt BK, Harder B,
Henderson K, Kindsvogel W, Gross JA, Greipp PR and Jelinek DF:
Expression of BCMA, TACI, and BAFF-R in multiple myeloma: A
mechanism for growth and survival. Blood. 103:689–694. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jiang C, Loo WM, Greenley EJ, Tung KS and
Erickson LD: B cell maturation antigen deficiency exacerbates
lymphoproliferation and autoimmunity in murine lupus. J Immunol.
186:6136–6147. 2011. View Article : Google Scholar : PubMed/NCBI
|