Introduction
Pregnancy-induced hypertension syndrome (PIH) is a
common disease in women during pregnancy and is one of the
important causes of maternal bleeding, infection and death.
According to the World Health Organization (WHO), at least one
woman dies of PIH complications every 7 min (1). PIH is divided into four categories:
gestational hypertension, chronic hypertension with preeclampsia,
preeclampsia and eclampsia. According to the clinical
characteristics of the patients, preeclampsia can be divided into
mild and severe preeclampsia (2).
The main cause of maternal mortality is the wrong choice of PIH
treatment methods and untimely treatment (3). PIH may cause a series of complications,
such as chest pain, difficulty breathing, cortical blindness, fetal
growth and development restriction, iatrogenic preterm delivery,
fetal distress in uterus, and even organ failure and fetal death in
severe cases (4).
At present, the pathogenesis of PIH has not been
clarified, and the relationship between cytokines and the
occurrence and development of PIH has attracted increasing
attention. Some scholars believe that due to poor early development
of the placenta, poor immunogenicity may cause extravillous
trophoblasts to invade the parental spiral artery, resulting in
poor remodeling of the blood vessels and reduced maternal blood
supply, which in turn causes hypoxic and ischemic conditions in the
placental tissue. Under this condition, trophoblasts produce
anti-angiogenic factors such as soluble vascular endothelial growth
factor receptor 1 (sFLT-1), and elevated sFlt-1 can cause
endothelial dysfunction and hypoxia (5–7).
Vascular endothelial growth factor (VEGF) is a multifunctional
cytokine produced by vascular endothelial cells and macrophages
(8). Previous studies have shown
that the biological activity of VEGF is regulated by sFLT-1. sFLT-1
is an endogenous inhibitor of VEGF, and excess sFLT-1 can bind VEGF
through high affinity, thereby neutralizing it. sFLT-1 is an
endogenous inhibitor of VEGF, and excess sFLT-1 can bind VEGF to
neutralize it. VEGF and sFLT-1 may play an important role in
endothelial dysfunction (9). VEGF is
mainly expressed on the surface of placental syncytiotrophoblasts
and invasive chorionic trophoblast cells during pregnancy. VEGF is
highly expressed in the blood vessels during early pregnancy and is
abundant in trophoblast cells (10,11). It
has been reported that sFLT-1 increases dramatically during
pregnancy and then rapidly declines after delivery (12).
Previous studies have shown that VEGF and its
receptor sFLT-1 in placenta may play an important role in the
occurrence and development of PIH (13), but the relationship between serum
VEGF and sFLT-1 expression in PIH and inhibited fetal growth and
development is still unknown. The study examined serum VEGF and
sFLT-1 levels in patients with PIH and explored their relationships
with inhibited fetal growth and development.
Patients and methods
Patient information
A retrospective analysis of medical records of 105
PIH pregnant women admitted to The First People's Hospital of
Changzhou (Changzhou, China) from March 2015 to February 2018, aged
21–39 years, with an average age of 26.47±3.15 years, 27–36 weeks
of gestation with an average of 32.41±0.89 weeks, and 1–3
pregnancies with an average of 1.56±0.19 pregnancies was conducted.
According to the severity of disease (14), patients were divided into 3 groups:
group A (n=35) as the hypertension complicating pregnancy, group B
(n=46) the mild preeclampsia and group C (n=24) the severe
preeclampsia. Another 35 healthy pregnant women admitted to The
First People's Hospital of Changzhou during the same period were
selected as the control group. Participants in the control group
were aged 21–35 years, with a mean age of 25.89±2.16 years, 28–35
weeks of gestation, average 33.15±0.79 weeks and 1–2 pregnancies,
average 1.38±0.13 times.
Inclusion and exclusion criteria
Inclusion criteria were: The subjects were diagnosed
in accordance with classification and diagnostic criteria of PIH
(15); aged from 20 to 40, and with
complete clinical data. This study was approved by the Ethics
Committee of The First People's Hospital of Changzhou and all
subjects were informed and agreed to participate in this clinical
study and signed an informed consent. Exclusion criteria were:
Patients with severe liver and kidney disease, diabetes, therioma,
chronic inflammatory disease, hematopoietic dysfunction,
immunological disease, psychosis or family history of mental
illness.
Sample collection and detection
Venous blood was drawn under fasting condition,
serum was separated by centrifugation at 3,000 × g for 10 min at
4°C and stored at −20°C in a cryogenic refrigerator. ELISA
(16) was used to detect the levels
of VEGF and sFLT-1 in serum. VEGF ELISA kit (Shanghai Kalang
Biotechnology Co., Ltd., Shanghai, China) and sFLT-1 ELISA kit
(Shanghai Jingyang Bioengineering Co., Ltd., Shanghai, China) were
used. All operations were performed in strict accordance with the
manufacturers instructions. No reagent was added into the blank
control well. After adding samples, the plate was covered with film
and incubated at 37°C for 1 h. Then, the plate was washed and 80 µl
affinity chain enzyme was added into each well, followed by
incubation at 37°C for 30 min. Substrate A and B (50 µl) were
added, followed by incubation at 37°C for 10 min. Each well was
added with 50 µl stop solution. An AMR-100 automatic enzyme marker
analyzer (Hangzhou Allsheng Instrument Co., Ltd., Hangzhou, China)
was used to detect the OD value of each well at the wavelength of
450 nm and to calculate the levels of VEGF and sFLT-1.
Observation index
According to the neonatal weight (17), 2.5–4.0 kg was classified as normal
weight newborns, 1.5–2.5 kg were low weight newborns, <1.5 kg
was very low weight newborns, and >4.0 kg is macrosomia. Apgar
scores (18) were used to evaluate
the degree of neonatal asphyxia and respiratory, muscular tension,
heart rate, skin color and laryngeal reflex signs: 8–10 points for
normal newborns, 4–7 points for mild asphyxia and 0–3 points for
severe asphyxia. The lower the score, the more severe asphyxia
was.
Statistical analysis
SPSS 18.0 (Yiyun Information Technology Co., Ltd.,
Shanghai, China) was used for statistical analysis. Measurement
data were presented as mean ± standard deviation (mean ± SD). One
way analysis of variance (ANOVA) was used for multigroup mean
comparisons. Dunnetts t-test was used for pairwise comparison and
Spearmans test for correlation analysis. P<0.05 indicates the
difference was statistically significant.
Results
Baseline data
There was no significant difference in age,
gestational age, gravida para, body mass index (BMI), hemoglobin
(HB), red blood cell (RBC) count, or platelet (PLT) count among
groups A, B, C and control group (P>0.05) (Table I).
 | Table I.Baseline data of groups A, B, C and
control group (mean ± SD). |
Table I.
Baseline data of groups A, B, C and
control group (mean ± SD).
| Classification | Group A (n=35) | Group B (n=46) | Group C (n=24) | Control group
(n=35) | F | P-value |
|---|
| Age | 26.12±3.63 | 26.58±3.15 | 25.48±3.47 | 25.89±2.16 | 0.728 | 0.536 |
| Gestational age
(week) | 32.65±0.91 | 32.65±0.84 | 32.87±0.97 | 33.15±0.79 | 1.811 | 0.148 |
| Gravida para | 1.48±0.16 | 1.49±0.25 | 1.45±0.18 | 1.38±0.13 | 2.494 | 0.062 |
| BMI
(kg/m2) | 26.72±2.87 | 26.59±2.87 | 27.32±3.11 | 27.67±3.01 | 1.098 | 0.352 |
| Hb (g/l) | 123.58±9.47 | 128.58±11.37 | 126.19±10.08 | 127.58±10.37 | 1.637 | 0.183 |
| RBC
(×1012/l) | 4.63±0.49 | 4.85±0.52 | 4.59±0.61 | 4.70±0.36 | 2.006 | 0.116 |
| PLT
(×109/l) | 149.36±11.58 | 151.85±9.67 | 155.47±11.09 | 152.26±10.25 | 1.602 | 0.191 |
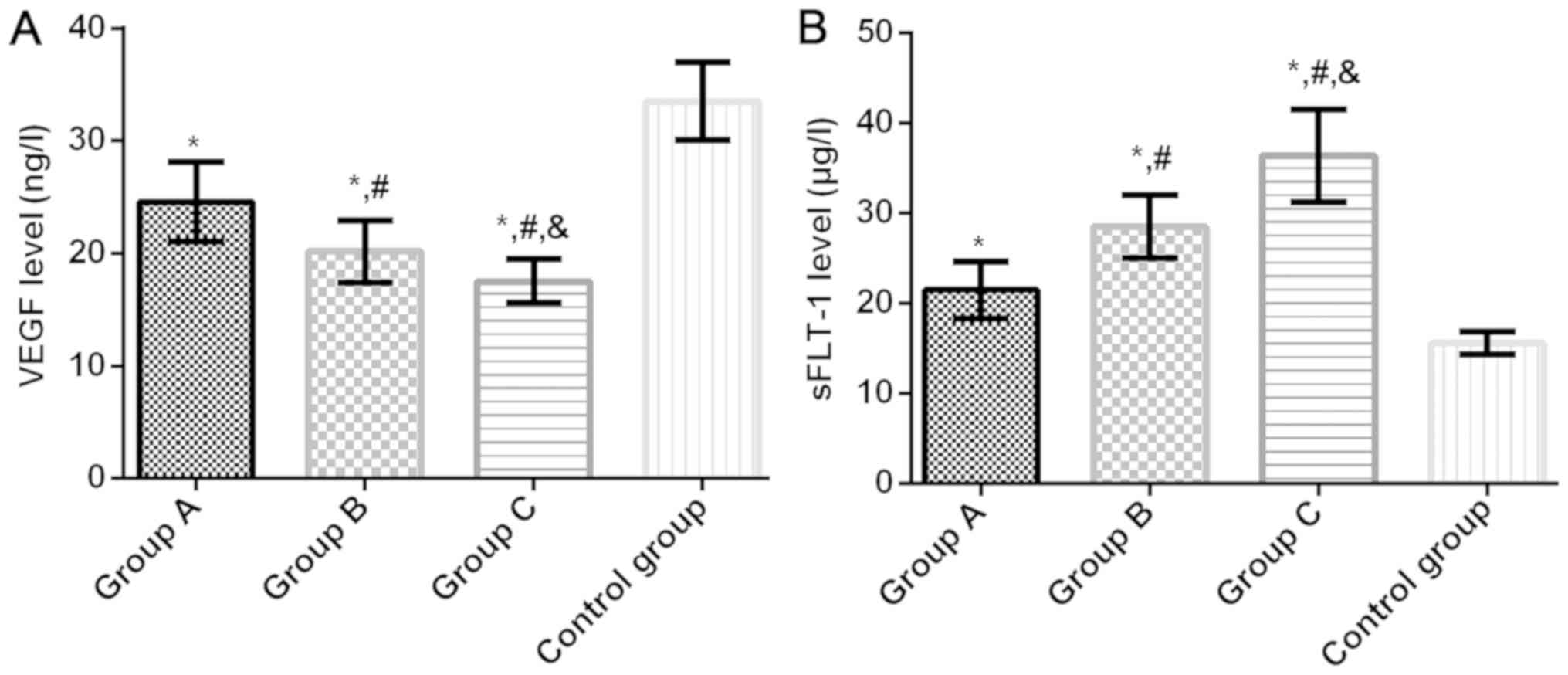
Serum VEGF and sFLT-1 levels in four
groups
Serum VEGF levels in groups A, B, C and control
group were 24.58±3.54, 20.17±2.74, 17.52±1.95 and 33.51±3.47 µg/l,
respectively, and the sFLT-1 levels were 21.49±3.17, 28.52±3.48,
36.41±5.15 and 15.58±1.25 µg/l, respectively. Compared with the
control group, the serum VEGF level in groups A, B and C was
significantly lower (P<0.05). Compared with group A, the level
of serum VEGF in groups B and C was significantly lower
(P<0.05). Compared with group B, the serum VEGF level in group C
decreased significantly (P<0.05). Compared with the control
group, the serum sFLT-1 level in groups A, B and C was
significantly higher (P<0.05). Compared with group A, the serum
sFLT-1 level in groups B and C was significantly higher
(P<0.05). Compared with group B, the serum sFLT-1 level in group
C was significantly higher (P<0.05) (Fig. 1).
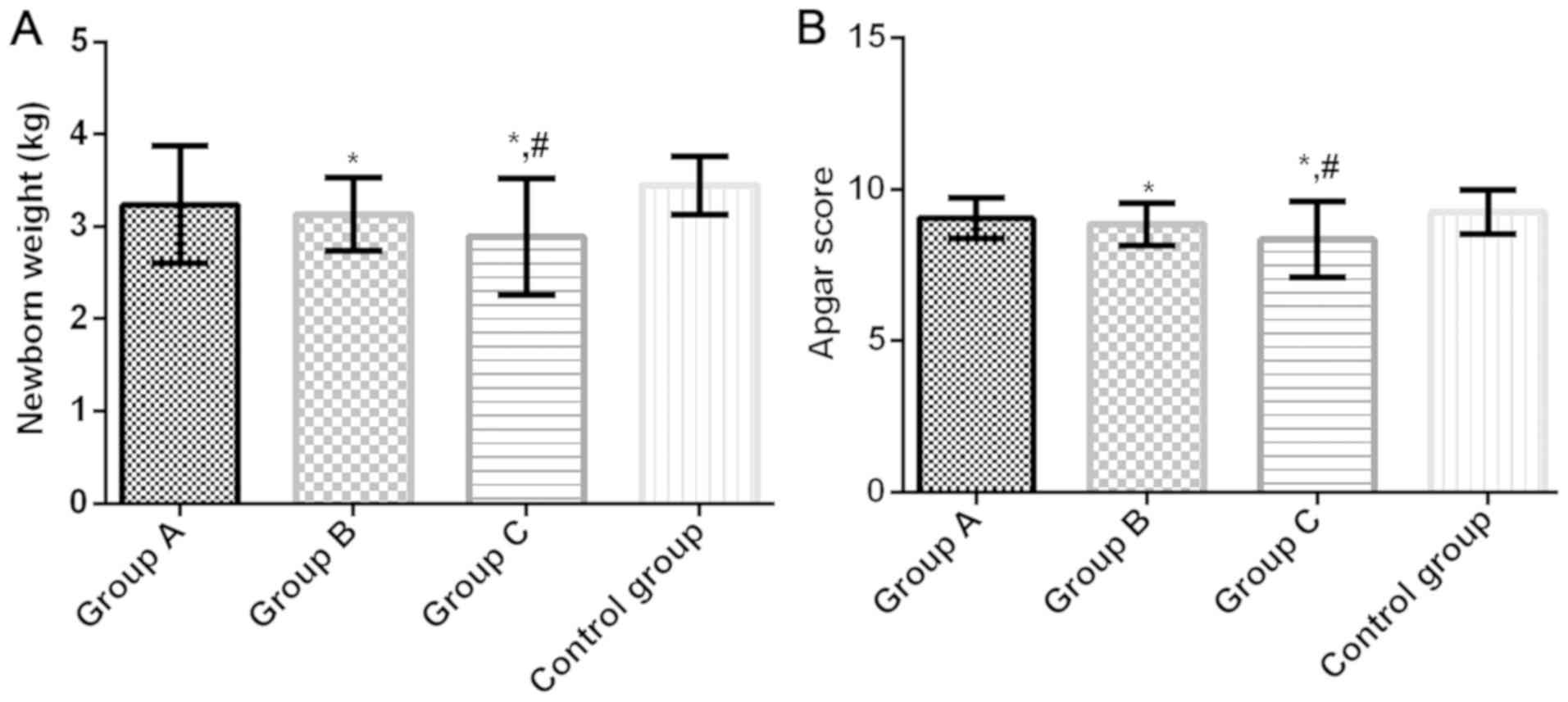
Neonatal weight and Apgar score in
four groups
Neonatal weight of groups A, B, C and control group
were 3.241±0.634, 3.137±0.398, 2.891±0.628 and 3.447±0.312 kg,
respectively, and the Apgar scores were 9.05±0.68, 8.86±0.70,
8.36±1.25 and 9.25±0.73, respectively. Compared with the control
group, neonatal weight and Apgar score in groups B and C were
significantly lower (P<0.05). There was no significant
difference in the neonatal weight and Apgar score between group A
and the control group (P>0.05). Compared with group A, neonatal
weight and Apgar score in group C were significantly lower
(P<0.05). There was no significant difference between groups A
and B (P>0.05) (Fig. 2).
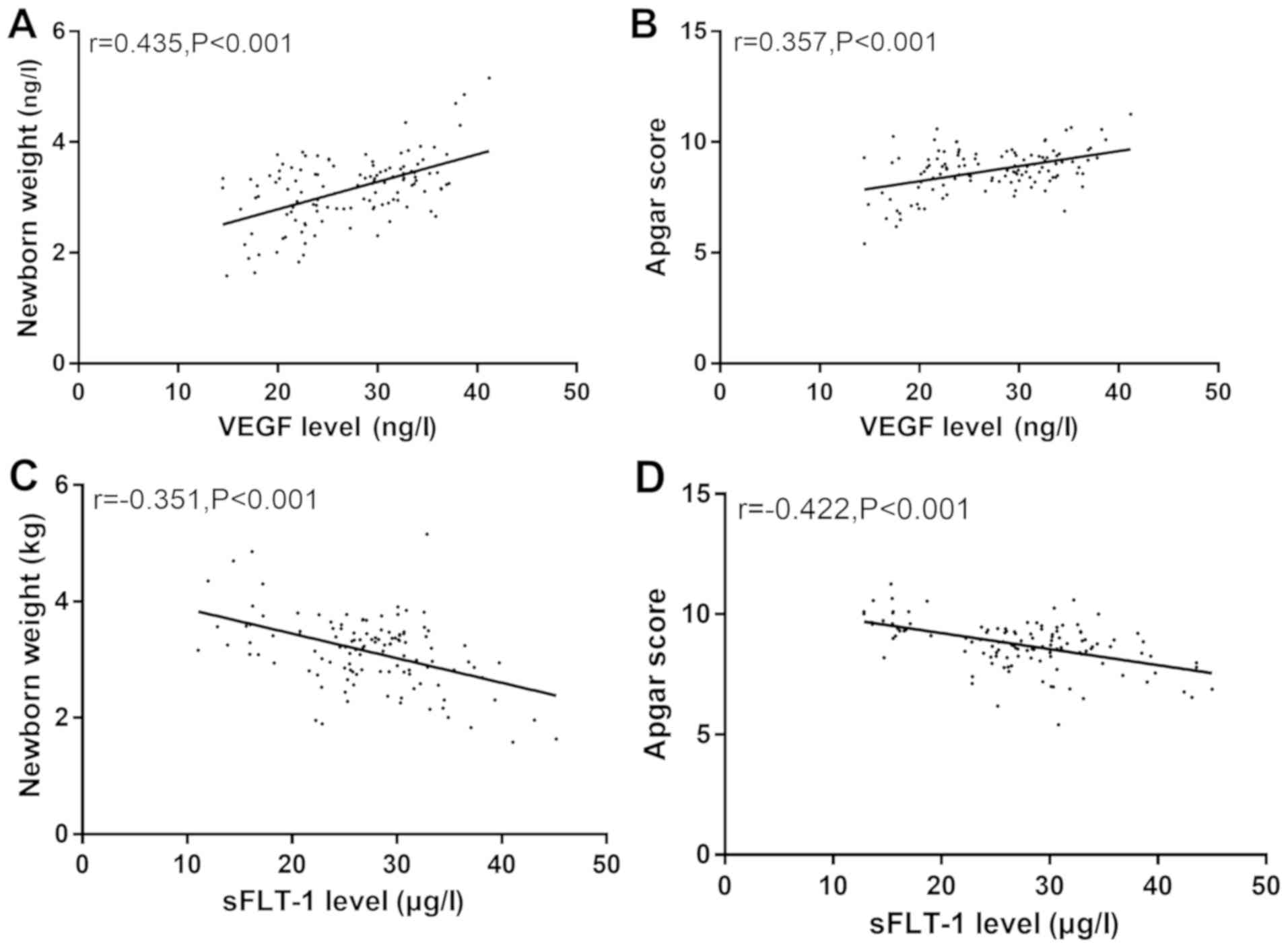
Correlation between serum VEGF, sFLT-1
level and postpartum neonatal weight and Apgar score
Spearmans test showed that there was a positive
correlation between the serum VEGF level and neonatal weight and
Apgar score (r=0.435, P<0.001; r=0.357, P<0.001) and a
negative correlation between serum sFLT-1 level and neonatal weight
and Apgar score (r=−0.351, P<0.001; r=−0.422, P<0.001)
(Fig. 3).
Discussion
Pregnancy-induced hypertension syndrome (PIH) is a
disease in pregnant women and one of the main causes of maternal
and perinatal mortality. At present, the specific physiological and
pathological mechanism of PIH is not clear (19). Pathophysiological studies suggest
that PIH is caused by insufficient trophoblastic infiltration due
to reduced placental perfusion (20). Abnormal placenta is one of the main
causes of preeclampsia. Due to abnormal placenta implantation,
uterine and placental perfusion can cause oxidative stress in the
body, eventually causing hypoxia and the release of anti-angiogenic
factors. These anti-angiogenic factors can cause a wide range of
endothelial dysfunction, which in turn leads to maternal
hypertension and vasoconstriction (21).
VEGF is an important angiogenesis regulatory factor
and a highly conserved glycoprotein in the human body. VEGF can
accelerate angiogenesis and promote endothelial cell division,
playing an important role in physiological or pathological
angiogenesis (22). sFLT-1 can act
as an effective anti-angiogenic molecule by binding to free VEGF in
the blood. sFLT-1 can affect the infiltration of trophoblast cells
by inhibiting the synthesis of trophoblast cells, which in turn
leads to shallow implantation of placental villi and the
development of preeclampsia (23).
Results of this study showed that compared with the control group,
the serum VEGF levels in groups A, B and C were significantly
decreased, while sFLT-1 levels were significantly increased. VEGF
levels were significantly decreased, while sFLT-1 levels were
significantly increased with increasing severity of disease. These
data suggest that VEGF and sFLT-1 may be involved in the occurrence
and development of PIH. Consistently, Kleinrouweler et al
(24) showed that VEGF levels are
reduced and sFLT-1 levels are elevated in patients with
preeclampsia during pregnancy. Maynard et al (25) suggested that excessive sFlt-1
production is the result of abnormal placental hypoxia. Gilbert
et al (26) showed that
hypertension caused by decreased intrauterine perfusion in pregnant
rats is closely related to increased sFLT-1 levels. Pathological
pregnancy may cause local tissue hypoxia-ischemia, and placenta
will release toxic cytokines into the mothers blood circulation,
resulting in endothelial cell damage and trophoblast cell
proliferation and differentiation. Therefore, infiltration ability
of trophoblast cells is reduced, causing a decrease in VEGF
secretion and an increase in sFLT-1 levels.
Normal function of the placenta is an important
factor in fetal growth and maintenance of the pregnancy. Placenta
can provide oxygen and nutrients to the fetus, and can eliminate
the metabolic waste generated by the fetus, so as to ensure the
healthy growth of fetus (27).
Normal pregnancy requires trophoblastic physiological invasion into
the maternal uterine iris tissue, which promotes the exchange of
blood circulation between the placenta and fetus. If this process
fails, preeclampsia and fetal growth restriction in pregnancy will
occur (28).
The basic lesions of PIH are hemodynamic changes and
small arterial spasm, which can cause maternal placental
thrombosis, atherosclerosis of the placental artery and poor blood
supply and circulation. So the reserve capacity of the placenta is
reduced, and the supply of nutrients to fetus from mother is
hindered (25). Results of this
study showed that compared with the control group, weight and Apgar
scores of the newborns in groups B and C were significantly lower.
Compared with group A, weight and Apgar scores of newborns in group
C were significantly lower. Serum VEGF levels were positively
correlated with neonatal weight and Apgar scores, and serum sFLT-1
levels were negatively correlated with neonatal weight and Apgar
scores. It has been reported that (29) the expression of sFlt-1 mRNA and
protein in placenta of severe intrauterine growth restriction was
significantly upregulated compared to that in normal gestational
age placenta, which is similar to the results of this study. Klein
et al (30) considered that
the risk of maternal and fetal adverse outcomes associated with
preeclampsia increases with increasing sFlt-1/PlGF ratio and is the
highest among women with sFlt-1/PlGF ratios of 85 and above. In the
study of Zeisler et al (31),
the sFlt-1: PlGF ratio is 38 or lower, which can be used to predict
women with short-term suspected preeclampsia and PlGF and VEGF
function similarly. Although serum levels of vascular endothelial
growth factor were positively correlated with neonatal weight and
Apgar score, and sFlt-1 level was negatively correlated with
neonatal weight and Apgar score, this study did not investigate the
correlation between VEGF and sFLT-1 levels and maternal and fetal
adverse outcomes, so these data do not necessarily mean that VEGF
and sFlt-1 will affect pregnancy outcomes, which is a limitation in
our design. Further studies are required on this aspect.
In summary, VEGF and sFLT-1 may be involved in the
occurrence and development of PIH. The decrease of serum VEGF level
and the increase of sFLT-1 level may be related to fetal growth and
development inhibition.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
YT analyzed the general data of patients. WY and XL
helped with the sample collection and detection. YL and CY recorded
and analyzed the Apgar score. JW was responsible for the
statistical analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First People's Hospital of Changzhou (Changzhou,
China). Patients who participated in this research, signed an
informed consent and had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Susan ZS, Bulbul S, Ferdows JA and Nayeem
A: Perinatal findings in pregnancy induced hypertension: A study in
a Tertiary Teaching Hospital in Dhaka City. J Natl Inst Neurosci
Bangladesh. 2:10–13. 2017. View Article : Google Scholar
|
|
2
|
Kintiraki E, Papakatsika S, Kotronis G,
Goulis DG and Kotsis V: Pregnancy-induced hypertension. Hormones
(Athens). 14:211–223. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu FM, Zhao M, Wang M, Yang HL and Li L:
Effect of regular oral intake of aspirin during pregnancy on
pregnancy outcome of high-risk pregnancy-induced hypertension
syndrome patients. Eur Rev Med Pharmacol Sci. 20:5013–5016.
2016.PubMed/NCBI
|
|
4
|
Magee LA, Pels A, Helewa M, Rey E, von
Dadelszen P, Magee LA, Audibert F, Bujold E, Côté A-M, Douglas MJ,
et al Canadian Hypertensive Disorders of Pregnancy Working Group, :
Diagnosis, evaluation, and management of the hypertensive disorders
of pregnancy: Executive summary. J Obstet Gynaecol Can. 36:416–441.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tateishi A, Ohira S, Yamamoto Y and Kanno
H: Histopathological findings of pregnancy-induced hypertension:
Histopathology of early-onset type reflects two-stage disorder
theory. Virchows Arch. 472:635–642. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Steegers EAP, von Dadelszen P, Duvekot JJ
and Pijnenborg R: Pre-eclampsia. Lancet. 376:631–644. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roberts JM and Hubel CA: The two stage
model of preeclampsia: Variations on the theme. Placenta. 30 Suppl
A:S32–S37. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ren Y, Wang H, Qin H, Yang J, Wang Y,
Jiang S and Pan Y: Vascular endothelial growth factor expression in
peripheral blood of patients with pregnancy induced hypertension
syndrome and its clinical significance. Pak J Med Sci. 30:634–637.
2014.PubMed/NCBI
|
|
9
|
Pang L, Wei Z, Li O, Huang R, Qin J, Chen
H, Fan X and Chen ZJ: An increase in vascular endothelial growth
factor (VEGF) and VEGF soluble receptor-1 (sFlt-1) are associated
with early recurrent spontaneous abortion. PLoS One. 8:e757592013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andraweera PH, Dekker GA, Laurence JA and
Roberts CT: Placental expression of VEGF family mRNA in adverse
pregnancy outcomes. Placenta. 33:467–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Demir R: Expression of VEGF receptors
VEFGR-1 and VEGFR-2, angiopoietin receptors Tie-1 and Tie-2 in
chorionic villi tree during early pregnancy. Folia Histochem
Cytobiol. 47:435–445. 2009.PubMed/NCBI
|
|
12
|
Clark DE, Smith SK, He Y, Day KA, Licence
DR, Corps AN, Lammoglia R and Charnock-Jones DS: A vascular
endothelial growth factor antagonist is produced by the human
placenta and released into the maternal circulation. Biol Reprod.
59:1540–1548. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nadar SK, Karalis I, Al Yemeni E, Blann AD
and Lip GY: Plasma markers of angiogenesis in pregnancy induced
hypertension. Thromb Haemost. 94:1071–1076. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Myatt L and Roberts JM: Preeclampsia:
Syndrome or disease? Curr Hypertens Rep. 17:832015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suzuki Y, Yamamoto T, Watanabe K,
Yoshimatsu J, Matsubara K, Mimura K, Tanaka K, Nishizawa H, Makino
S, Nohira T, et al: Home blood pressure measurement (HBPM) for the
early detection of hypertensive disorders of pregnancy (HDP) in
Japanese women: A multicenter prospective study. Hypertens Res
Pregnancy. 5:36–38. 2017. View Article : Google Scholar
|
|
16
|
Prince HE, Lapé-Nixon M, Givens TS,
Bradshaw T and Nowicki MJ: Elimination of falsely reactive results
in a commercially-available West Nile virus IgM capture
enzyme-linked immunosorbent assay by heterophilic antibody blocking
reagents. J Immunol Methods. 444:24–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Simmons MA and Laughon MM: 50 years ago in
the Journal of Pediatrics: A practical classification of newborn
infants by weight and gestational age. J Pediatr. 187:332017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cnattingius S, Norman M, Granath F,
Petersson G, Stephansson O and Frisell T: Apgar score components at
5 minutes: Risks and prediction of neonatal mortality. Paediatr
Perinat Epidemiol. 31:328–337. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shirasuna K, Karasawa T, Usui F, Kobayashi
M, Komada T, Kimura H, Kawashima A, Ohkuchi A, Taniguchi S and
Takahashi M: NLRP3 deficiency improves angiotensin II-induced
hypertension but not fetal growth restriction during pregnancy.
Endocrinology. 156:4281–4292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aggarwal PK, Chandel N, Jain V and Jha V:
The relationship between circulating endothelin-1, soluble fms-like
tyrosine kinase-1 and soluble endoglin in preeclampsia. J Hum
Hypertens. 26:236–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McDonald SD, Han Z, Walsh MW, Gerstein HC
and Devereaux PJ: Kidney disease after preeclampsia: A systematic
review and meta-analysis. Am J Kidney Dis. 55:1026–1039. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ogunleye O, Campo B, Herrera D, Post
Uiterweer ED and Conrad KP: Relaxin confers cytotrophoblast
protection from hypoxia-reoxygenation injury through the
phosphatidylinositol 3-kinase-Akt/protein kinase B cell survival
pathway. Am J Physiol Regul Integr Comp Physiol. 312:R559–R568.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Burton GJ: Intrauterine devices, the
endometrium and the risk of pre-eclampsia. BJOG. 123:796. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kleinrouweler CE, Wiegerinck MMJ,
Ris-Stalpers C, Bossuyt PM, van der Post JA, von Dadelszen P, Mol
BW and Pajkrt E; EBM CONNECT Collaboration, : Accuracy of
circulating placental growth factor, vascular endothelial growth
factor, soluble fms-like tyrosine kinase 1 and soluble endoglin in
the prediction of pre-eclampsia: A systematic review and
meta-analysis. BJOG. 119:778–787. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maynard SE, Min JY, Merchan J, Lim KH, Li
J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et
al: Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may
contribute to endothelial dysfunction, hypertension, and
proteinuria in preeclampsia. J Clin Invest. 111:649–658. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gilbert JS, Babcock SA and Granger JP:
Hypertension produced by reduced uterine perfusion in pregnant rats
is associated with increased soluble fms-like tyrosine kinase-1
expression. Hypertension. 50:1142–1147. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ganguly A, Tamblyn JA, Finn-Sell S, Chan
SY, Westwood M, Gupta J, Kilby MD, Gross SR and Hewison M: Vitamin
D, the placenta and early pregnancy: Effects on trophoblast
function. J Endocrinol. 236:R93–R103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carreras-Badosa G, Prats-Puig A, Puig T,
Vázquez-Ruíz M, Bruel M, Mendoza E, de Zegher F, Ibáñez L,
López-Bermejo A and Bassols J: Circulating fatty acid synthase in
pregnant women: Relationship to blood pressure, maternal metabolism
and newborn parameters. Sci Rep. 6:241672016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nevo O, Many A, Xu J, Kingdom J, Piccoli
E, Zamudio S, Post M, Bocking A, Todros T and Caniggia I: Placental
expression of soluble fms-like tyrosine kinase 1 is increased in
singletons and twin pregnancies with intrauterine growth
restriction. J Clin Endocrinol Metab. 93:285–292. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Klein E, Schlembach D, Ramoni A, Langer E,
Bahlmann F, Grill S, Schaffenrath H, van der Does R, Messinger D,
Verhagen-Kamerbeek WD, et al: Influence of the sFlt-1/PlGF ratio on
clinical decision-making in women with suspected preeclampsia. PLoS
One. 11:e01560132016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeisler H, Llurba E, Chantraine F, Vatish
M, Staff AC, Sennström M, Olovsson M, Brennecke SP, Stepan H,
Allegranza D, et al: Predictive value of the sFlt-1:PlGF ratio in
women with suspected preeclampsia. N Engl J Med. 374:13–22. 2016.
View Article : Google Scholar : PubMed/NCBI
|