Introduction
Hypertrophic scar (HS) is characterized by excessive
growth of dense fibrous tissue, which is caused by deep heat or
traumatic injury of the skin (1–3). In
humans, HS is hyperplastic healed fibroplastic diseases, which are
procedural processes that include proliferation, maturation,
inflammation, and remodeling (4).
Presently, there have been several methods for treating HS, such as
surgical resection, injection steroids, radiotherapy, but still no
best regimen, and the clinical behavior of HS is unclear. Some
studies have demonstrated that many different non-coding RNAs and
growth factors are involved in the formation of HS (5,6).
MicroRNAs (miRNAs) are evolutionary conserved
non-coding RNAs of about 19–25 nucleotides, function by regulating
one or more mRNA to regulate gene expression for translation
inhibition or cleavage (7,8). About one-third of the coding genes in
mammalian are regulated by miRNAs (9,10), and
mature miRNAs are not transformed into proteins but are bound to
mRNAs to interfere with the translation process. With regard to
miRNA function, they play a key role in cell proliferation, cell
death and organ development (11,12).
Furthermore, abnormal expression of microRNAs is associated with
several pathological processes, including kidney, lung and heart
metabolism (13). Previous study
have shown that miRNAs contribute to HS or keloid formation, and
the abnormal expressed miRNAs have been identified by genomic
analysis between denatured dermis and normal skin, which means that
multiple signaling pathways participate in wound healing (14). For example, microRNA-98 has been
found can inhibit the cell proliferation of human hypertrophic scar
fibroblasts via targeting Col1A1 (15). MicroRNA-185 plays critical roles in
HS via regulating transforming growth factor-β1 and collagen-1
expression, and may serve as a promising target for HS treatment
(16). miR-21 has been recognized as
a critical regulator for HS formation (17). Recently, the effect of miR-205-5p in
carcinogenesis has been well documented, in which many targets of
miR-205-5p have been defined in cancer cells (18–21), and
the effect of miR-205-5p in HS formation remains unclear.
Increasing evidence have supported that miR-205-5p
may play critical roles in cell proliferation, apoptosis, and
extracellular matrix (ECM) deposition (22,23). And
increased myofibroblasts and excessive ECM accumulation are the
main characteristics of HS formation (24). Thus, we supposed that miR-205-5p may
play critical roles in HS formation. In this study, we revealed the
abnormal expression of miR-205-5p in HS. Meanwhile, miR-205-5p
overexpression prevented HSF cell proliferation and induced
apoptosis. Moreover, we found that Smad2 was a direct target for
miR-205-5p in HSF cells. miR-205-5p might serve as a new potential
therapeutic target for HS.
Materials and methods
Tissue samples
In total, 15 paired Hypertrophic scar (HS) (Age
range: 21–49 years old; sex ratio: 1:1; Location: skin) and normal
skin (NS) (Age range: 23–51 years old; sex ratio: 1:1; Location:
skin) tissues were obtained during biopsy from 15 patients at the
Affiliated Hospital of Nantong University. All tissues were
immediately stored in liquid nitrogen until use. All the protocols
were approved by the Ethics Committee of the Affiliated Hospital of
Nantong University (Nantong, China), and every patient wrote the
informed consent.
Cell culture
The human embryonic skin fibroblasts, CCC-ESF-1
(ESF), and human hypertrophic scar fibroblasts (HSFs) were cultured
in RPMI-1640 medium supplemented with 10% fetal bovine serum and 1%
penicillin/streptomycin. All cells were incubated in a humidified
incubator at 37°C and 5% CO2.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from cells and tissues was extracted by
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and cDNAs were synthesized using miScript
Reverse Transcription kit (Qiagen GmbH, Hilden, Germany) according
to the manufacturer's instructions. The primers for reverse
transcription and amplification of miR-205-5p and U6 were designed
and synthesized by Guangzhou RiboBio Co., Ltd., Guangzhou, China.
Quantitative real-time PCR was conducted to detect the miR-205-5p
and smad2 mRNA using the SYBR Premix Ex Taq™ II (TliRNaseH Plus)
kit (Takara Bio, Inc., Otsu, Japan) with the Bio-Rad machine
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). U6 small nuclear
RNA and GAPDH were used as internal normalized references for miRNA
and mRNA, respectively.
Cell transfection
miR-205-5p mimics, smad2-plasmid and matched
negative control (NC) were obtained from Guangzhou RiboBio Co.,
Ltd. Cell transfections were performed using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instruction. Cells were harvested for further
experiments after 48 h transfection.
Cell proliferation assay
Cell Count Kit-8 assay (CCK-8, Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was used as a qualitative
marker for cell proliferation ability. After 48 h transfection with
miR-205-5p mimics, NC or miR-205-5p mimic+smad2-plamid, HSF cells
were seeded into 96 well plates in triplicate at 5×103
cells per well. At 24 h, 10 µl of CCK-8 solution mixed with 90 µl
of RPMI-1640 was added to each well. After 2 h of incubation, the
absorbance was measured at 450 nm.
Apoptosis assay
HSF cells were transfected with miR-205-5p mimics,
NC or miR-205-5p mimic+smad2-plamid, and 48 h after transfection,
the cells were then subjected to apoptosis assay. Then
106 treated cells were stained with Annexin V/PI using
an apoptosis detection kit (BD Biosciences, Franklin Lakes, NJ
USA). According to the manufacturer's instructions, after
incubating for 15 min in the dark, cell apoptosis was then detected
by flow cytometry.
Luciferase reporter assay
For confirmation of direct target binding, the wild
type (smad2 WT) and mutant (smad2 MUT) 3′UTR of smad2 identified by
TargetScan were cloned into a pmiR-RB-ReportTM dual luciferase
Reporter gene plasmid vector (Guangzhou RiboBio Co., Ltd.). The UTR
region of candidate target gene was inserted downstream of the
sequence of Renilla luciferase, which was designed for reporter
fluorescence (Rluc). For luciferase reporter analysis, luciferase
reporter vectors and mimic control, miR-205-5p mimics, were
transfected into HSF cells using Lipofectamine 2000. After 48 h,
luciferase activity was analyzed by the Dual-Luciferase Assay
System (Promega Corporation, Madison, WI, USA), according to the
manufacturer's protocols.
Western blotting
HSF cells were transfected with miR-205-5p mimics,
NC or miR-205-5p mimic+smad2-plamid for 48 h, then cells were
collected and total proteins were extracted in 40 mM Tris-HCl (pH
7.4) containing 150 mM NaCl and 1% (v/v) Triton X-100, supplemented
with protease inhibitors. Protein concentration was determined
using the bicinchoninic acid protein assay (Pierce; Thermo Fisher
Scientific, Inc.). Equal amounts of proteins were resolved on 10%
SDS-PAGE gels, and then transferred to a PVDF membrane (EMD
Millipore, Billerica, MA, USA). After blocking with 5% skimmed milk
in TBST, then probed with antibodies against smad2, Col I, Col III,
β-actin (All buy from Cell Signaling Technology, Inc., Danvers, MA,
USA). After three times' washing, blots were then incubated with
horseradish peroxidase (HRP) conjugated secondary antibodies.
Immunoreactive bands were visualized using the enhanced
chemiluminescence detection system. The protein levels of the
stripes were normalized based on the gray value of β-actin.
Statistical analysis
SPSS17.0 software was used to analyze the data.
Values were expressed as mean ± SD of experiments performed in
triplicate. Data were analyzed by one-way ANOVA or Student's
t-test. Statistical significance was defined as P<0.05.
Results
miR-205-5p is downregulated in HS
tissues and HSFs
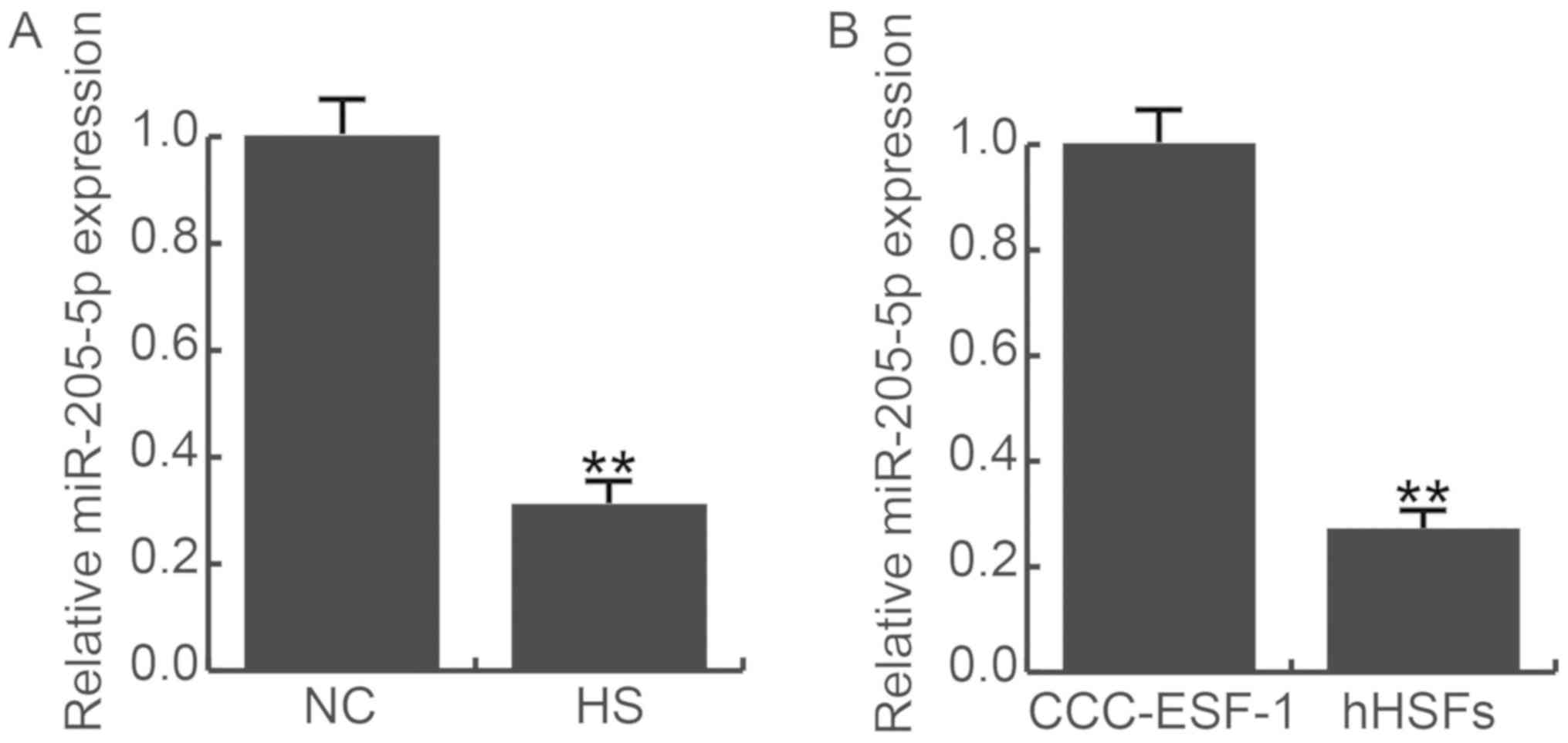
Fifteen HS and 15 paired normal skin (NS) tissues
were recruited in this study. Compared to the normal skin, the
expression of miR-205-5p were significantly decreased in HS tissues
(Fig. 1A). Meanwhile we detected the
expression of miR-205-5p in human embryonic skin fibroblasts
(CCC-ESF-1) and human hypertrophic scar fibroblasts (HSFs), the
result was consistent with tissues, HSFs have lower expression of
miR-205-5p (Fig. 1B). Thus, we
choose HSF cells for the further study.
miR-205-5p directly targets smad2
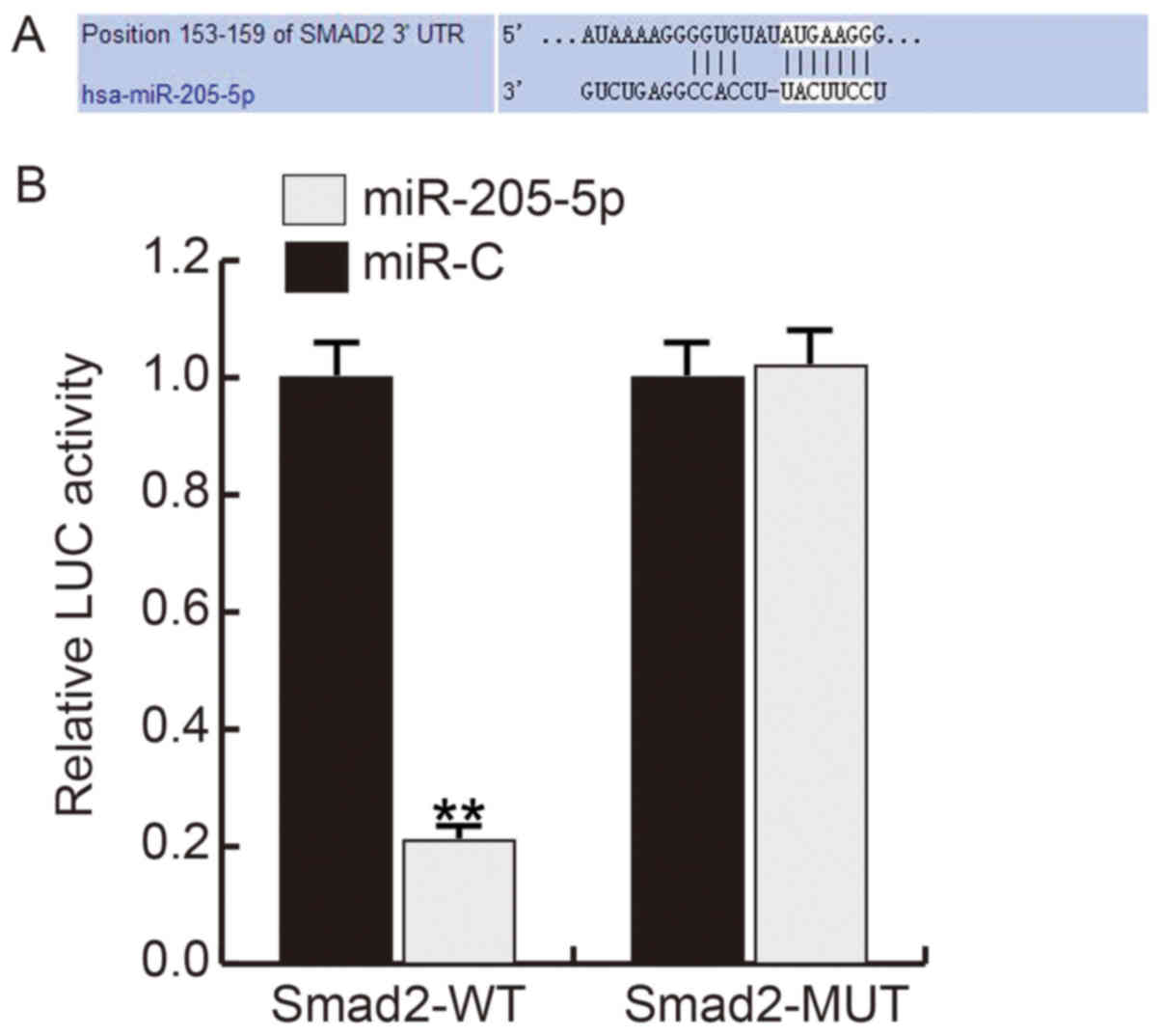
Firstly, we use TargetScan to predict the potential
targets of miR-205-5p, and about 600 genes were found as the
potential target genes of miR-205-5p, including smad2 (Fig. 2A). Smad2, one of the important
members of the transforming growth factor b (TGF-β) pathway family
which involved in cell growth regulation, plays critical roles in
ECM synthesis and degradation. Thus, we choose smad2 for further
analysis. And the luciferase reporter assay showed that the
relative luciferase activity in miR-205-5p mimics and smad2 WT
3′UTR reporter co-transfected hHSFs significantly decreased
compared with cells co-transfected with mimic control and and smad2
WT 3′UTR reporter (Fig. 2B).
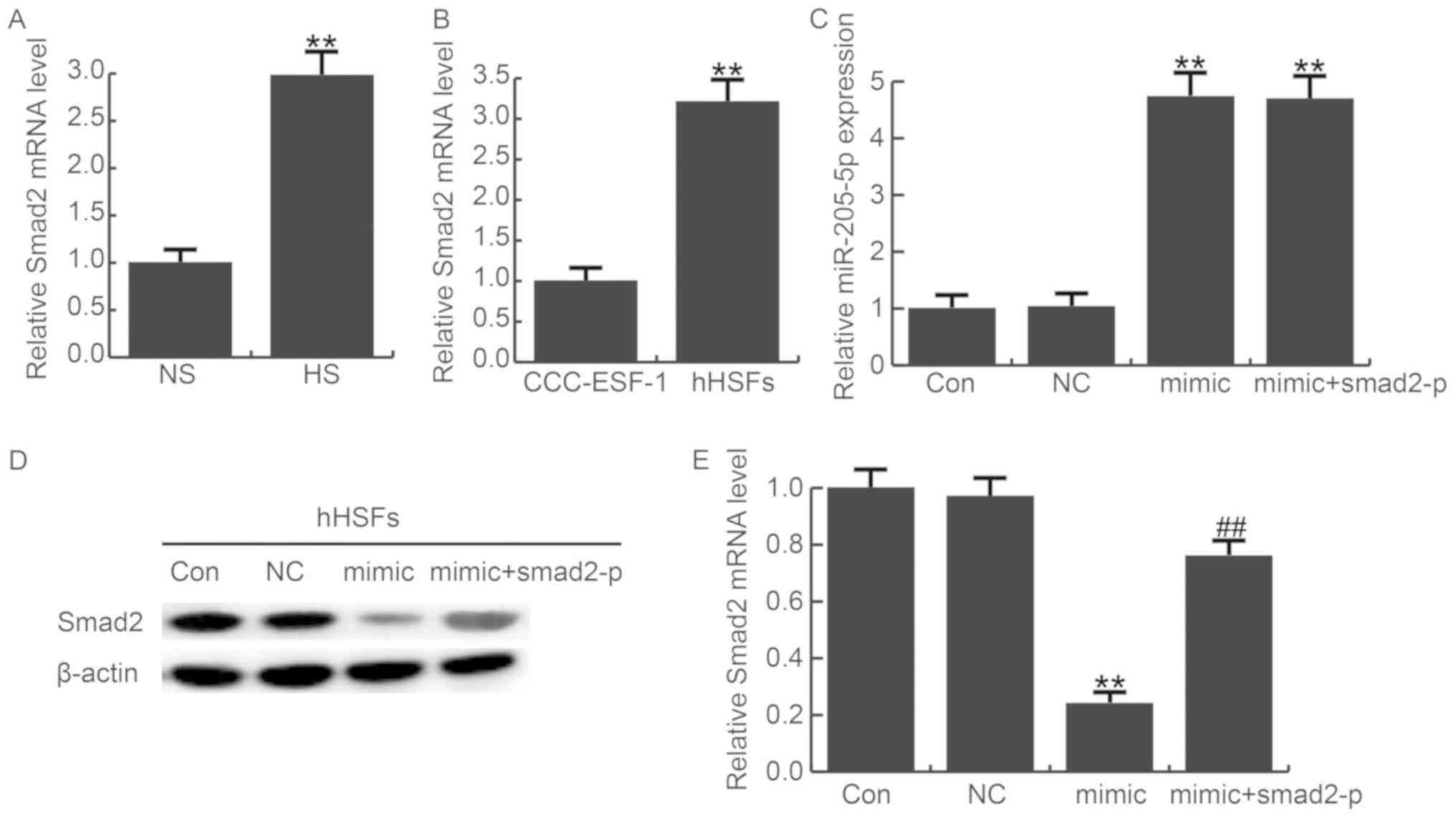
In addition, the expression of smad2 in HS tissues
and HSF cells were detected by qRT-PCR, the data showed that smad2
was significantly upregulated in HS tissues and HSF cells compared
with normal tissues or cells (Fig. 3A
and B). Compared with miR-205-5p, the approximate reverse
pattern of smad2 expression was found.
Furthermore, we transfected HSF cells with
miR-205-5p mimics, miR-205-5p mimic+smad2-plasmid or NC. The
transfection efficiency was examined by qRT-PCR (Fig. 3C). Overexpression of miR-205-5p
markedly decreased the protein level of smad2, and this decrease
can be reversed by smad2-plasmid (Fig.
3D). Moreover, we detected the mRNA level of smad2 after
transfecting miR-205-5p mimics, miR-205-5p mimic+smad2-plasmid or
NC in HSF cells. As expected, overexpression of miR-205-5p
downregulated the mRNA level of smad2 in HSFs. However, the effect
was eliminated when co-transfected the miR-205-5p mimics with
smad2-plasmid (Fig. 3E). These
results suggest that miR-205-5p directly targets smad2.
miR-205-5p suppresses HSF cell
proliferation
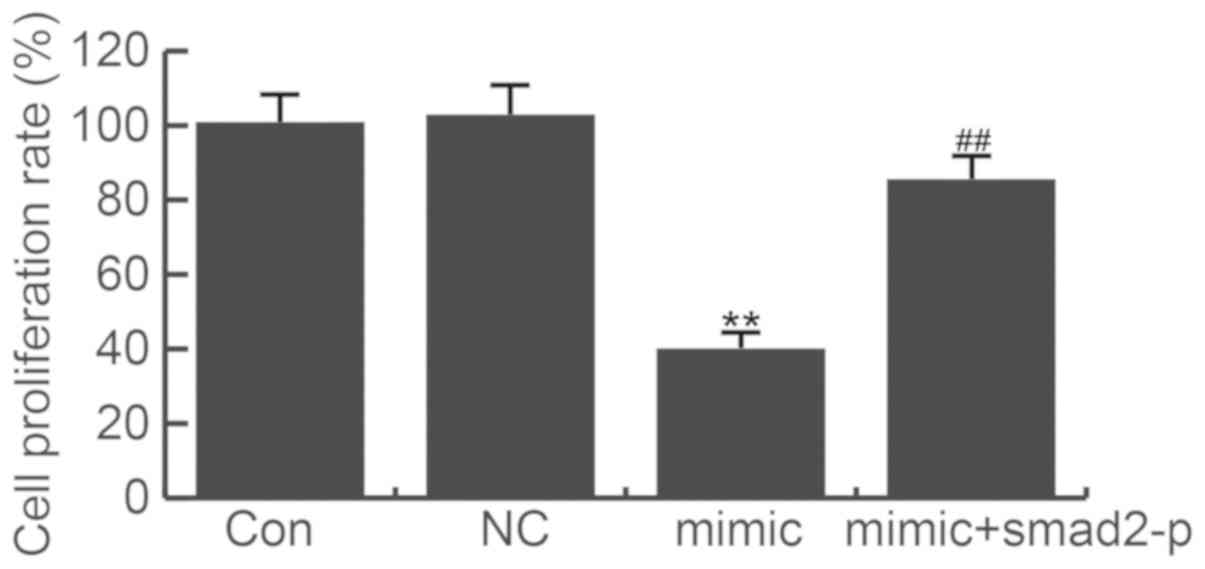
Given our limited understanding of the role played
by miR-205-5p in HSF cells, we examined the effects of miR-205-5p
gain-of-function on HS formation. After transfection for 48 h, cell
proliferation assay was applied. The CCK-8 results showed that
miR-205-5p mimics significantly suppressed HSF cell proliferation
(Fig. 4). Meanwhile, co-transfected
smad2-plasmid with miR-205-5p mimics did not significantly
decreased the cell proliferation ability. The data indicate that
miR-205-5p suppresses HSF cell proliferation through targeting
smad2.
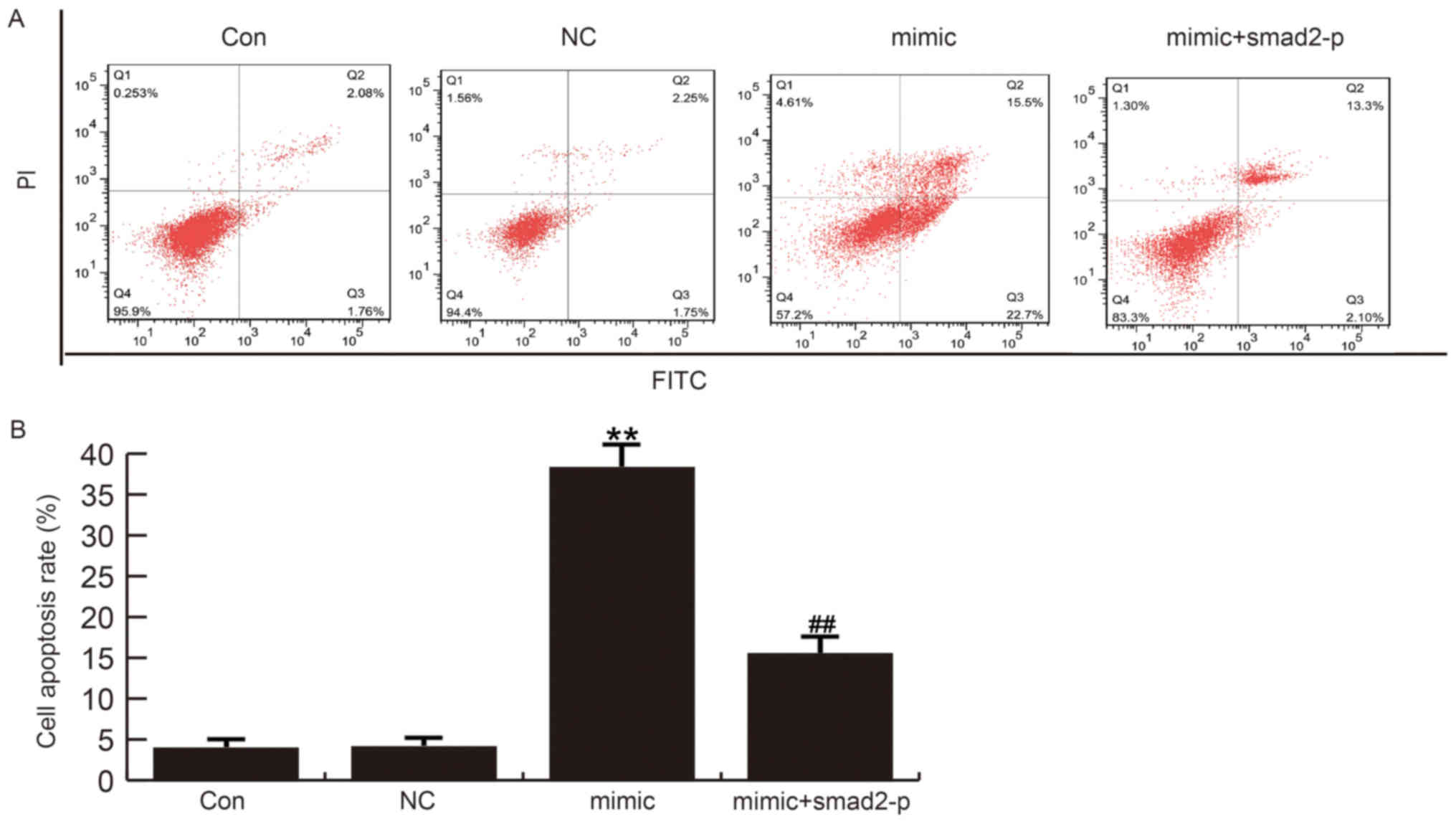
miR-205-5p induces HSF cell apoptosis
in vitro
HSF cell apoptosis was detected 48 h after
transfected with miR-205-5p mimics, miR-205-5p mimic+smad2-plasmid
or NC. Flow cytometry analysis demonstrated that cell apoptosis was
increased in miR-205-5p-mimics-transfected HSFs compared to control
groups. Moreover, co-transfected smad2-plasmid with miR-205-5p
mimics did not notably induced cell apoptosis rate (Fig. 5). Together, these data indicate that
miR-205-5p inhibits cell grows and promotes apoptosis of HSF
cells.
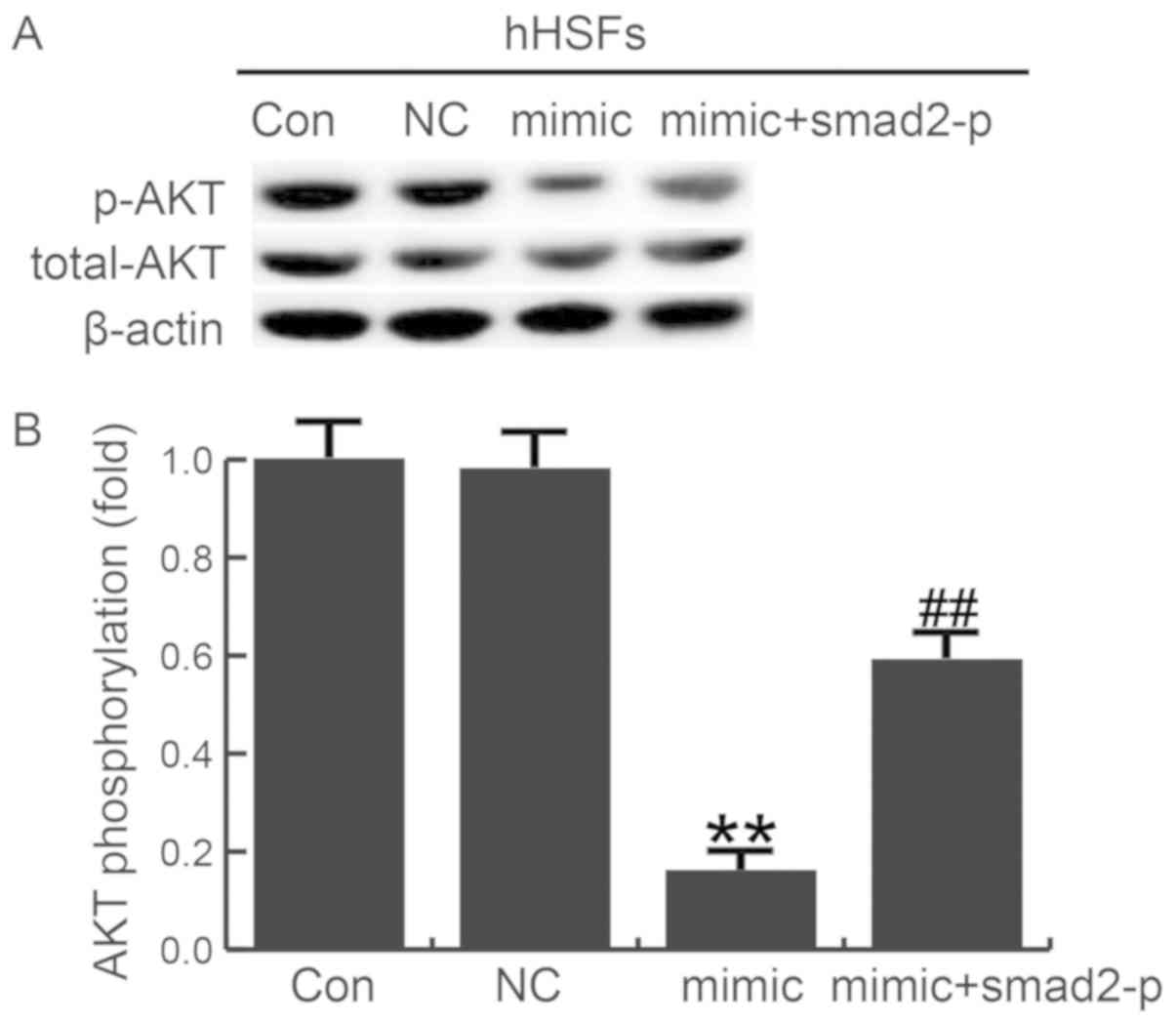
miR-205-5p mediated suppressive effect
on ECM production associated proteins by preventing AKT
phosphorylation
To further investigate the molecular mechanism of
the miR-205-5p effects, we evaluated the role of AKT signaling in
miR-205-5p-mediated smad2 in HSF. The phosphorylation level of AKT
was examined in HSFs transfected with miR-205-5p mimics, miR-205-5p
mimic+smad2-plasmid or NC. Overexpression of miR-205-5p decreased
AKT phosphorylation. However, co-transfected smad2-plasmid with
miR-205-5p mimics could eliminate the changes caused by miR-205-5p
mimics (Fig. 6). Thus, the
miR-205-5p inhibited AKT signaling pathway was activated by smad2
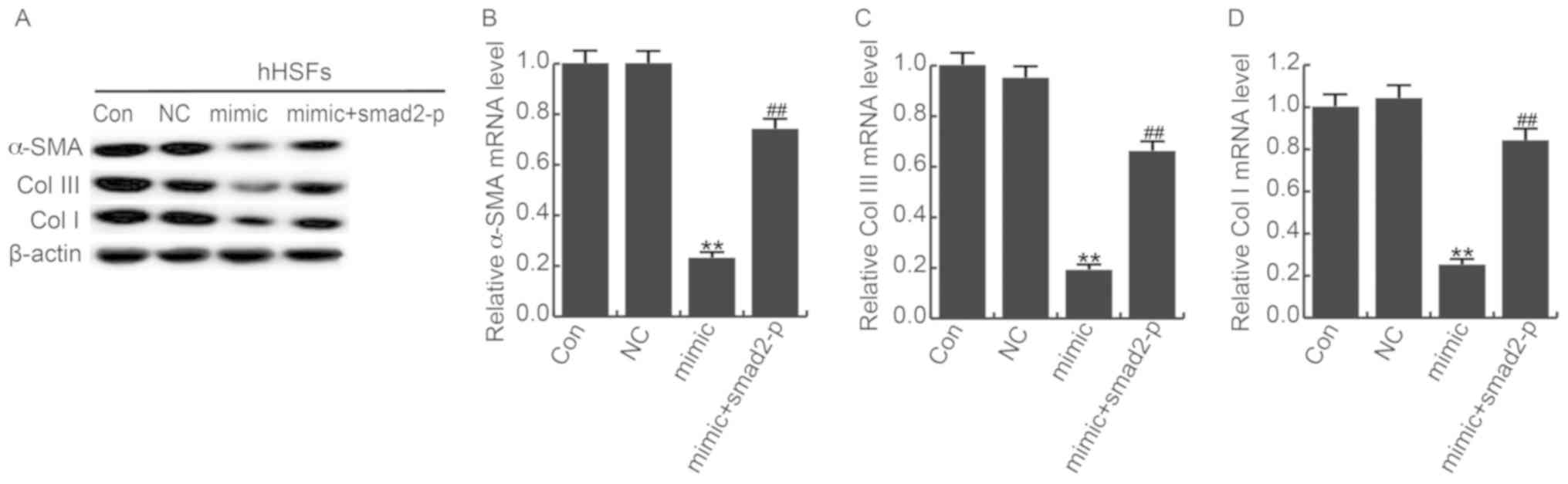
overexpression. In addition, mRNA and protein expression of α-SMA,
Col I and Col III were downregulated in miR-205-5p mimics
transfected HSFs, and the smad2-plasmid significantly counteracted
this effect (Fig. 7). Taken
together, these data indicate that miR-205-5p suppresses AKT
phosphorylation, downregulates Col I, Col III, and α-SMA in HSFs
through smad2 mediator.
Discussion
The regulation of wound healing and its disorders in
HS are complex and incomplete (25,26).
Histologically, HS is characterized by increased myofibroblasts and
mast cells, hypervascularity, excessive extracellular matrix (ECM)
(24). Unfortunately, the current
treatment for HS has limited efficacy (27). Abnormal expression of miRNAs may
contribute significantly to the progression of skin fibrosis
(28). miR-205-5p, a functional
miRNA, has received much attention from researchers in recent
years. The above studies suggested that miR-205-5p acts either as
an oncogene or tumor suppressor gene, depending on the cellular
environment. Our preliminary data show that miR-205-5p is
significantly reduced in HS tissues and HSF cells compared to
normal tissues and cells. Also, we found that the expression of
smad2 was negatively correlated with miR-205-5p. Therefore, we
hypothesis smad2 gene was associated with miR-205-5p.
In order to confirm this hypothesis, firstly we use
TargetScan to predict the potential targets of miR-205-5p. Smad2
was identified as a potential miR-205-5p target gene. In addition,
luciferase reporter assay verified miR-205-5p directly binding to
the 3′-UTR of smad2. Moreover, the expression of smad2 in HS
tissues and HSF cells were significantly upregulated, smad2
expression was the opposite pattern compared to miR-205-5p.
Overexpression of miR-205-5p could downregulate smad2 expression.
These results suggest that miR-205-5p directly targets smad2.
Smad2 is a tumor suppressor that belongs to the
receptor-activated SMAD family (29). Previous studies have revealed that
upregulation of smad2 suppressed TGF-β induced EMT and cell
motility and invasion. In this study, hHSFs transfected with
miR-205-5p mimics showed obvious suppression effect in cell
proliferation, and cell apoptosis increased significantly.
Co-transfected smad2-plasmid with miR-205-5p mimics could eliminate
the effect of miR-205-5p mimics. Further confirmed that miR-205-5p
played a role through regulating smad2.
Collagen is the most important extracellular matrix
structural protein, and 28 different types of collagen has been
identified. Among these types, type 1, 2 and 3 are the most
abundant collagen (30). A large
number of collagen synthesis and changes are considered to be the
main features of HS formation. In previous studies, the expression
of Col I and Col III in HSF cells and HS tissues was significantly
higher than that in normal cells and healthy tissues (31). Fibroblasts, especially Col I and Col
III overdose are responsible for keloid and hypertrophic scar
formation. α-smooth muscle actin (α-SMA) has been shown to be a key
regulator of extracellular matrix metabolism in many tissues
(32). In our study, the protein
expression of Col I, Col III was significantly reduced in
miR-205-5p mimics transfected HSFs by targeting smad2, suggesting
that miR-205-5p works as an anti-fibrotic factor in HSFs.
PI3K/AKT is mediated by multifunctional signaling
pathways in cell proliferation, motility, differentiation, fibrosis
and lipid metabolism. Previous studies have shown that AKT
signaling plays a key role in the development of fibrosis
associated with diabetic nephropathy (33). Our results indicated that the AKT
signaling pathway was inhibited by miR-205-5p mimics; however, the
smad2-plasmid significantly counteracted the effects of miR-205-5p
mimics. In addition, the downregulated expression of ColI, Col III,
and α-SMA caused by miR-205-5p mimics was also eliminate by
smad2-plasmid. Taken together, these results indicate that
miR-205-5p mediates HS formation, partly due to AKT inhibition by
targeting smad2.
In conclusion, our study shows that miR-205-5p
inhibits HSF cell proliferation and promotes apoptosis and reduces
the expression of ECM-related proteins through inhibiting AKT
pathway by directly targeting smad2, thus affecting HS formation.
Thus, this study provided evidence to determine that miR-205-5p may
be a useful target for the treatment of HS. However, as age and
tissue location are important factors when assessing the degree of
healing of the scar tissue, our research may have some limitations,
thus, further researches are needed to prove our conclusion. We
will study the relationship between miR-205-5p expression and the
age of patient or tissue location, and further explore the role of
miR-205-5p in HS formation in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors’ contributions
JQ and YW designed the study. JQ, YL, KH, YZ and XZ
were responsible for data access and analysis. All authors
collaborated to interpret the results and develop the
manuscript.
Ethics approval and consent to
participate
All the protocols were approved by the Ethics
Committee of the Affiliated Hospital of Nantong University
(Nantong, China), and every patient provided informed consent.
Patient consent for publication
All patients provided informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Aarabi S, Bhatt KA, Shi Y, Paterno J,
Chang EI, Loh SA, Holmes JW, Longaker MT, Yee H and Gurtner GC:
Mechanical load initiates hypertrophic scar formation through
decreased cellular apoptosis. FASEB J. 21:3250–3261. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van der Veer WM, Bloemen MC, Ulrich MM,
Molema G, van Zuijlen PP, Middelkoop E and Niessen FB: Potential
cellular and molecular causes of hypertrophic scar formation.
Burns. 35:15–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Younai S, Nichter LS, Wellisz T, Reinisch
J, Nimni ME and Tuan TL: Modulation of collagen synthesis by
transforming growth factor-beta in keloid and hypertrophic scar
fibroblasts. Ann Plast Surg. 33:148–154. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi-Wen X, Leask A and Abraham D:
Regulation and function of connective tissue growth factor/CCN2 in
tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev.
19:133–144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kashiyama K, Mitsutake N, Matsuse M, Ogi
T, Saenko VA, Ujifuku K, Utani A, Hirano A and Yamashita S:
miR-196a downregulation increases the expression of type I and III
collagens in keloid fibroblasts. J Invest Dermatol. 132:1597–1604.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li P, He QY and Luo CQ: Overexpression of
miR-200b inhibits the cell proliferation and promotes apoptosis of
human hypertrophic scar fibroblasts in vitro. J Dermatol.
41:903–911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hammond SM: An overview of microRNAs. Adv
Drug Deliv Rev. 87:3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilson RC and Doudna JA: Molecular
mechanisms of RNA interference. Annu Rev Biophys. 42:217–239. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun W, Julie Li YS, Huang HD, Shyy JY and
Chien S: microRNA: A master regulator of cellular processes for
bioengineering systems. Annu Rev Biomed Eng. 12:1–27. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Flynt AS and Lai EC: Biological principles
of microRNA-mediated regulation: Shared themes amid diversity. Nat
Rev Genet. 9:831–842. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miska EA: How microRNAs control cell
division, differentiation and death. Curr Opin Genet Dev.
15:563–568. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bushati N and Cohen SM: microRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang P, Lv C, Jiang B, Long X, Zhang P,
Zhang M, Xie T and Huang X: MicroRNA profiling in denatured dermis
of deep burn patients. Burns. 38:534–540. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bi S, Chai L, Yuan X, Cao C and Li S:
MicroRNA-98 inhibits the cell proliferation of human hypertrophic
scar fibroblasts via targeting Col1A1. Biol Res. 50:222017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao K, Luo X, Wang X and Gao Z:
MicroRNA-185 regulates transforming growth factor-β1 and collagen-1
in hypertrophic scar fibroblasts. Mol Med Rep. 15:1489–1496. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li G, Zhou R, Zhang Q, Jiang B, Wu Q and
Wang C: Fibroproliferative effect of microRNA-21 in hypertrophic
scar derived fibroblasts. Exp Cell Res. 345:93–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng Y, Zhu J, Shen D, Qin H, Lei Z, Li W,
Liu Z and Huang JA: MicroRNA-205 targets SMAD4 in non-small cell
lung cancer and promotes lung cancer cell growth in vitro and in
vivo. Oncotarget. 8:30817–30829. 2017.PubMed/NCBI
|
|
19
|
Nguyen-Vu T, Wang J, Mesmar F,
Mukhopadhyay S, Saxena A, McCollum CW, Gustafsson JÅ, Bondesson M
and Williams C: Estrogen receptor beta reduces colon cancer
metastasis through a novel miR-205-PROX1 mechanism. Oncotarget.
7:42159–42171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tucci P, Agostini M, Grespi F, Markert EK,
Terrinoni A, Vousden KH, Muller PA, Dötsch V, Kehrloesser S, Sayan
BS, et al: Loss of p63 and its microRNA-205 target results in
enhanced cell migration and metastasis in prostate cancer. Proc
Natl Acad Sci USA. 109:15312–15317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan F, Mao H, Bu F, Tong X, Li J, Zhang S,
Liu X, Wang L, Wu L, Chen R, et al: Sp1-mediated transcriptional
activation of miR-205 promotes radioresistance in esophageal
squamous cell carcinoma. Oncotarget. 8:5735–5752. 2017.PubMed/NCBI
|
|
22
|
Jiang M, Zhong T, Zhang W, Xiao Z, Hu G,
Zhou H and Kuang H: Reduced expression of miR-205-5p promotes
apoptosis and inhibits proliferation and invasion in lung cancer
A549 cells by upregulation of ZEB2 and downregulation of erbB3. Mol
Med Rep. 15:3231–3238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jang SJ, Choi IS, Park G, Park G, Moon DS,
Choi JS, Nam MH, Yoon SY, Choi CH and Kang SH: MicroRNA-205-5p is
upregulated in myelodysplastic syndromes and induces cell
proliferation via PTEN suppression. Leuk Res. 47:172–177. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tredget EE, Nedelec B, Scott PG and
Ghahary A: Hypertrophic scars, keloids, and contractures. The
cellular and molecular basis for therapy. Surg Clin North Am.
77:701–730. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Armour A, Scott PG and Tredget EE:
Cellular and molecular pathology of HTS: Basis for treatment. Wound
Repair Regen. 15 Suppl 1:S6–S17. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schäfer M and Werner S: Transcriptional
control of wound repair. Annu Rev Cell Dev Biol. 23:69–92. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kwan P, Hori K, Ding J and Tredget EE:
Scar and contracture: Biological principles. Hand Clin. 25:511–528.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Babalola O, Mamalis A, Lev-Tov H and
Jagdeo J: The role of microRNAs in skin fibrosis. Arch Dermatol
Res. 305:763–776. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Syed V: TGF-β signaling in cancer. J Cell
Biochem. 117:1279–1287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Volk SW, Wang Y, Mauldin EA, Liechty KW
and Adams SL: Diminished type III collagen promotes myofibroblast
differentiation and increases scar deposition in cutaneous wound
healing. Cells Tissues Organs. 194:25–37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou R, Zhang Q, Zhang Y, Fu S and Wang C:
Aberrant miR-21 and miR-200b expression and its pro-fibrotic
potential in hypertrophic scars. Exp Cell Res. 339:360–366. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Satish L, Gallo PH, Baratz ME, Johnson S
and Kathju S: Reversal of TGF-β1 stimulation of α-smooth muscle
actin and extracellular matrix components by cyclic AMP in
Dupuytren's-derived fibroblasts. BMC Musculoskelet Disord.
12:1132011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu Q, Zuo WZ, Ji XJ, Zhou YX, Liu YQ, Yao
XQ, Zhou XY, Liu YW, Zhang F and Yin XX: Ethanolic Ginkgo biloba
leaf extract prevents renal fibrosis through Akt/mTOR signaling in
diabetic nephropathy. Phytomedicine. 22:1071–1078. 2015. View Article : Google Scholar : PubMed/NCBI
|