Introduction
The final common pathological result of chronic
kidney disease (CKD) is renal interstitial fibrosis (1,2), which
is characterized by tubular atrophy and accumulation of
extracellular matrix (3). Apoptosis
of tubular epithelial cells is one of the causes of tubular atrophy
and interstitial fibrosis (4–7).
Hypoxia, oxidative stress, and TGF-β1 treatment are all leading
causes of apoptosis in the model of unilateral ureteral obstruction
(UUO)-induced renal interstitial fibrosis (8,9), which
can promote inflammatory reactions and increase synthesis of
extracellular matrix, eventually leading to renal interstitial
fibrosis. Factors that can reduce apoptosis such as hepatocyte
growth factor (HGF) and bone morphogenetic protein-7 (BMP-7) can
delay renal interstitial fibrosis (6,10,11).
However, the molecular mechanism underlying apoptosis of renal
tubular epithelial cells remains unknown.
MicroRNAs are endogenous non-coding small RNAs that
are widely present in eukaryotic organisms and play an important
role in renal development and renal homeostasis (12–14).
Recent studies have shown positive feedback regulation between
miRNA-34 family (miR-34s) and tumor suppressor protein p53,
suggesting that miRNA-34 may be involved in the regulation of tumor
cell apoptosis (15,16). Mature miR-34s in mammals are divided
into three types, miR-34a, miR-34b, and miR-34c. miR-34a is the
most widely distributed and has the highest expression. miR-34a can
promote cell apoptosis by inhibiting Bcl-2 in hepatoma and
colorectal cancer cells (17,18).
However, the role of miR-34a in kidney diseases has not been widely
studied, so we hypothesized that miR-34a may regulate renal tubular
epithelial cells apoptosis during renal interstitial fibrosis.
miRNAs not only regulate the expression of target
genes directly, but also regulate the expression of target genes in
other cells through the delivery by microvesicles (MVs). In recent
years, MVs have attracted wide attention as the most important link
between cells (19). miRNAs found in
serum derived from tumor cell MVs have been shown to be associated
with tumor metastasis and apoptosis (20–23).
In this study, UUO was used as a renal interstitial
fibrosis model and TGF-β1-treatment was used as an attempt to
investigate the role of MVs containing miR-34a in regulating renal
tubular epithelial cell apoptosis. We found that miR-34a was mainly
distributed in renal tubular interstitial cells and that its
expression was significantly increased in obstructed renal tissues
after obstruction. TGF-β1 treatment upregulated miR-34a expression
in mesenchymal fibroblasts.miR-34a derived from mesenchymal
fibroblast MVs can be transmitted to proximal tubular epithelial
cells through the damaged tubular basement membrane and induced
apoptosis through inhibition of Bcl-2 expression. This study
provides a new molecular mechanism for microRNA-mediated apoptosis
of renal tubular epithelial cells and a new theoretical basis for
the pathogenesis of renal interstitial fibrosis.
Materials and methods
Mice
Male CD-1 mice weighing 18–20 g were purchased from
the Nanjing University Laboratory, and were raised according to the
experimental animal feeding standard. The mice were randomly
divided into 4 groups, which were sham-operated group, UUO groups
for 1, 3, and 7 days (n=6 each group). Mice were sacrificed on 1, 3
and 7 days after operation, respectively and the kidney tissues
were obtained. This study was approved by The Animal Care and Use
Review Committee of Wujiang Hospital Affiliated to Nantong
University (Suzhou, China).
Cell culture and treatment
The rat proximal tubular epithelial cells (NRK-52E)
and the renal interstitial fibroblast cells (NRK-49F) were planted
on a petri dish and cultured at 37°C, 5% CO2
environment. The culture medium was DMEM/F12 (12400–024),
containing 10% fetal bovine serum (FBS; 16000-044) and 1%
penicillin-streptomycin (15140–122) (all from Life Technologies;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). When cells grew
to 80% confluency, the culture medium was replaced with serum-free
medium for 16 h. After 16 h, the serum-free medium was replaced
again, and TGFβ1 (240B; R&D Systems, Inc., Minneapolis, MN,
USA) was added to stimulate the cells. Cells and supernatants were
collected at different times. The control group was collected in
the same way.
Microvesicle extraction
We used ultracentrifugation to extract the MVs in
the cell supernatant. Cell supernatants were centrifuged at 300 × g
for 10 min at 4°C, then 1,200 × g for 20 min and 10,000 × g for 30
min. The supernatant was collected, and then centrifuged at 4°C for
110,000 × g for 60 min. Finally, the precipitate was the MVs. The
MVs were resuspended with appropriate phosphate-buffered saline
(PBS). TRIzol reagent (10296-028; Life Technologies; Thermo Fisher
Scientific, Inc.) was used to extract mRNA of MVs.
miRNA detection by reverse
transcriptase-quantitative polymerase chain reaction (RT-qPCR)
mRNA was extracted from tissues or cells using
TRIzol, and the cDNA was synthesized using miScript RT II kit
(15596-026; Life Technologies; Thermo Fisher Scientific, Inc.), and
was stored at −20°C. RT-qPCR was performed using the miScript
SYBR-Green PCR kit (28073; Qiagen, Duesseldorf, Germany) to
investigate the relative gene expressions.
Plasmid transfection
The cells were planted in 6-well plate, and cultured
in DMEM/F12 with 10% FBS. When 90–95% confluency was reached, 1.5
ml of serum-free and antibiotic-free culture medium was replaced
per well. A total of 2.5 µg plasmid (pGPH1/GFP/NEO-BCL-2 shRNA;
Shanghai Yingjun Biotech Company, Shanghai, China) and 5 µl
Liposome 2000 were diluted with 250 µl Opti-MEM (serum reduced
medium) and incubated for 5 min at room temperature, respectively.
The two transfection mixtures were mixed and incubated at room
temperature for 20 min. A total of 500 µl of transfection mixture
was added into each well, and gently mixed with the cell culture
medium. Then the cells were incubated in CO2 incubator.
The medium was changed 6 h later, then the cells were stimulated
with or without TGF-β1. After 48 h, cells were collected.
TUNEL staining
Apoptosis of tissues and cells was detected using
the TACS2 TdT-DAB/Fluor in situ apoptosis detection kit (no.
4812-30-K; Trevigen, Gaithersburg, MD, USA). Briefly, a coverslip
was pre-placed in a 24-well plate, then cells were plated on the
coverslips. The cells were incubated with 50 μl proteinase K
for 30 min at room temperature. After being washed in deionized
water, the cells were immersed in 1X TdT labeling buffer for
another 5 min. Then, the cells were incubated with 50 μl
labeled reaction solution in a wet box at 37°C for 1 h. The stop
solution 1X TdT was dipped in medium at room temperature, for 5
min. After washing twice with deionized water, the cells were
incubated with 50 μl Strep-DAB/Fluor solution for 20 min in
the dark. Finally, the cells were washed with 1X PBS twice. A Nikon
Eclipse 80i (fluorescence) microscope equipped with a DS-Ril (Nikon
Corporation, Tokyo, Japan) digital camera was used to observe and
photograph the cells. A total of 20 random fields of each sample
were selected for capturing images under the microscope at a
magnification of ×200. The number of positive cells was counted and
the average value was calculated to make a histogram of the number
of TUNEL-positive cells.
Immunoblot analysis
Cells were ground or collected with cell scraper to
obtain the total protein of cells by lysate on ice, then
centrifuged at 16,000 × g at 4°C for 30 min. Bicinchoninic acid
(BCA) Protein Assay kit (Beyotime, Shanghai, China) was used to
measure protein concentration. Followed by sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), the
immunoblots were incubated with the antibodies of fibronectin (FN;
cat. no. 3648; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), CD34
(cat. no. sc-15363; Santa Cruz Biotechology, Inc., Santa Cruz, CA,
USA), p-caspase-3 (cat. no. ab59425), caspase-3 (cat. no. ab13847;
both from Abcam, Cambridge, MA, USA), Bcl-2 (cat. no. 2870; Cell
Signaling Technology, Inc., Danvers, MA, USA) and tubulin (cat. no.
ab7291; Abcam).
Statistical analysis
Statistical analysis was performed using Statistical
Product and Service Solutions (SPSS) 16.1 statistical software
(SPSS, Inc., Chicago, IL, USA). Measurement data were expressed as
mean ± standard error. One-way ANOVA (post-hoc LSD or SNK) was used
for comparison between multiple experimental groups. The
experimental data were compared between the two groups using
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
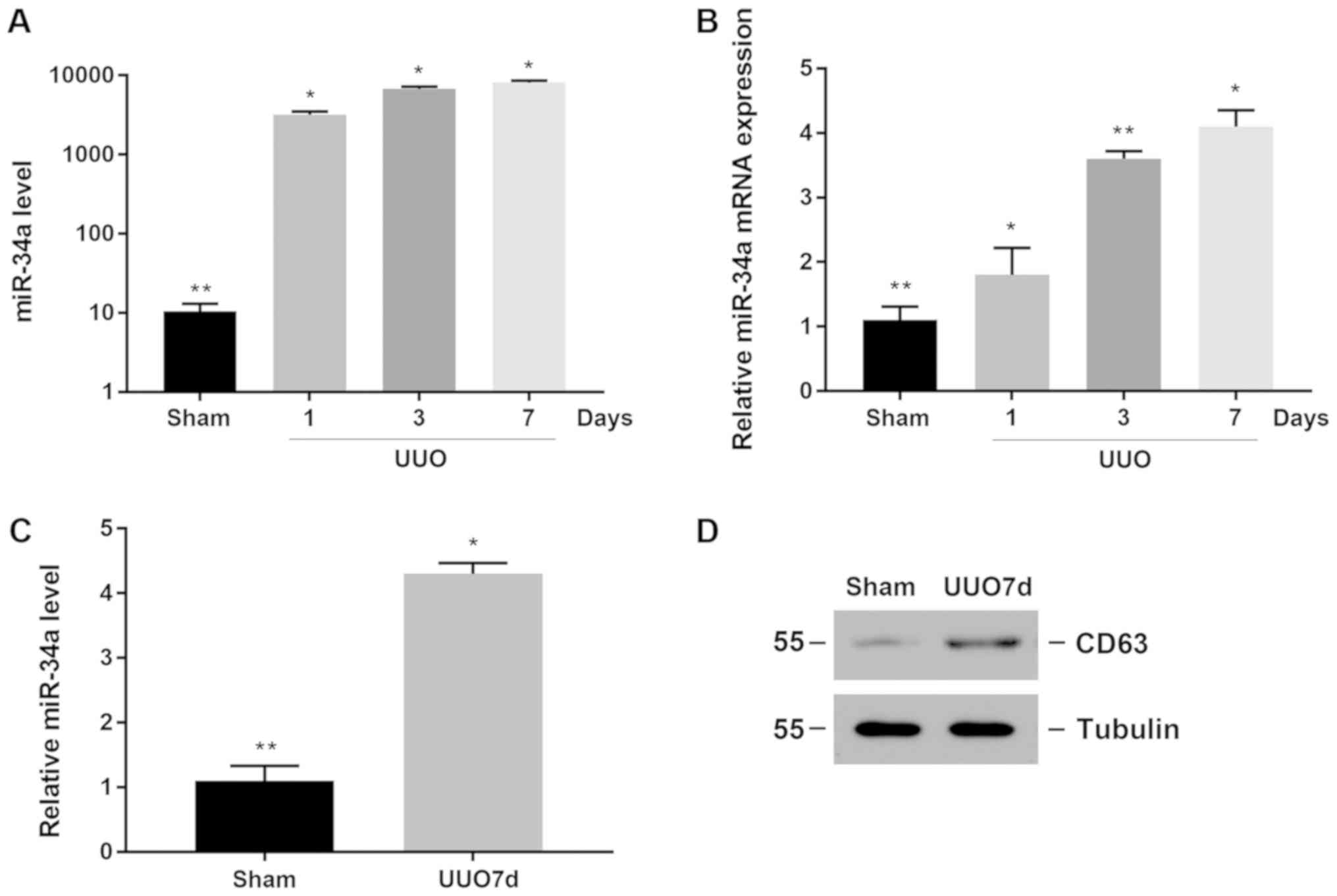
Increased expression of miR-34a in
obstructed renal tissue and MVs
A large amount of cell apoptosis occurred in a
UUO-induced renal interstitial fibrosis model. We first detected
the expression of miR-34a in renal tissue, and found that miR-34a
expression began to increase on the first day after surgery, and
had a time-dependent elevation (Fig.
1A). At the same time, the mRNA expression of miR-34a in renal
tissue was also significantly increased after treatment; it was
also time-dependent (Fig. 1B).
To determine whether the increased expression of
miR-34a in the obstructed kidney was secreted into MVs, we
extracted MVs from the kidney. As shown in Fig. 1C, the expression of miR-34a in the
MVs of the obstructed kidney was significantly increased (Fig. 1C). CD63 is a marker protein of MVs.
CD63 protein expression represents the content of MVs. The results
showed that compared with the sham-operated renal tissue, the
expression of CD63 was increased in obstructed kidney tissue
(Fig. 1D), suggesting that in the
UUO renal interstitial fibrosis model, the secretion of MVs in the
kidney was increased. Thus, these data demonstrated that in the
UUO-induced renal interstitial fibrosis model, MVs containing
microRNA-34a were increased.
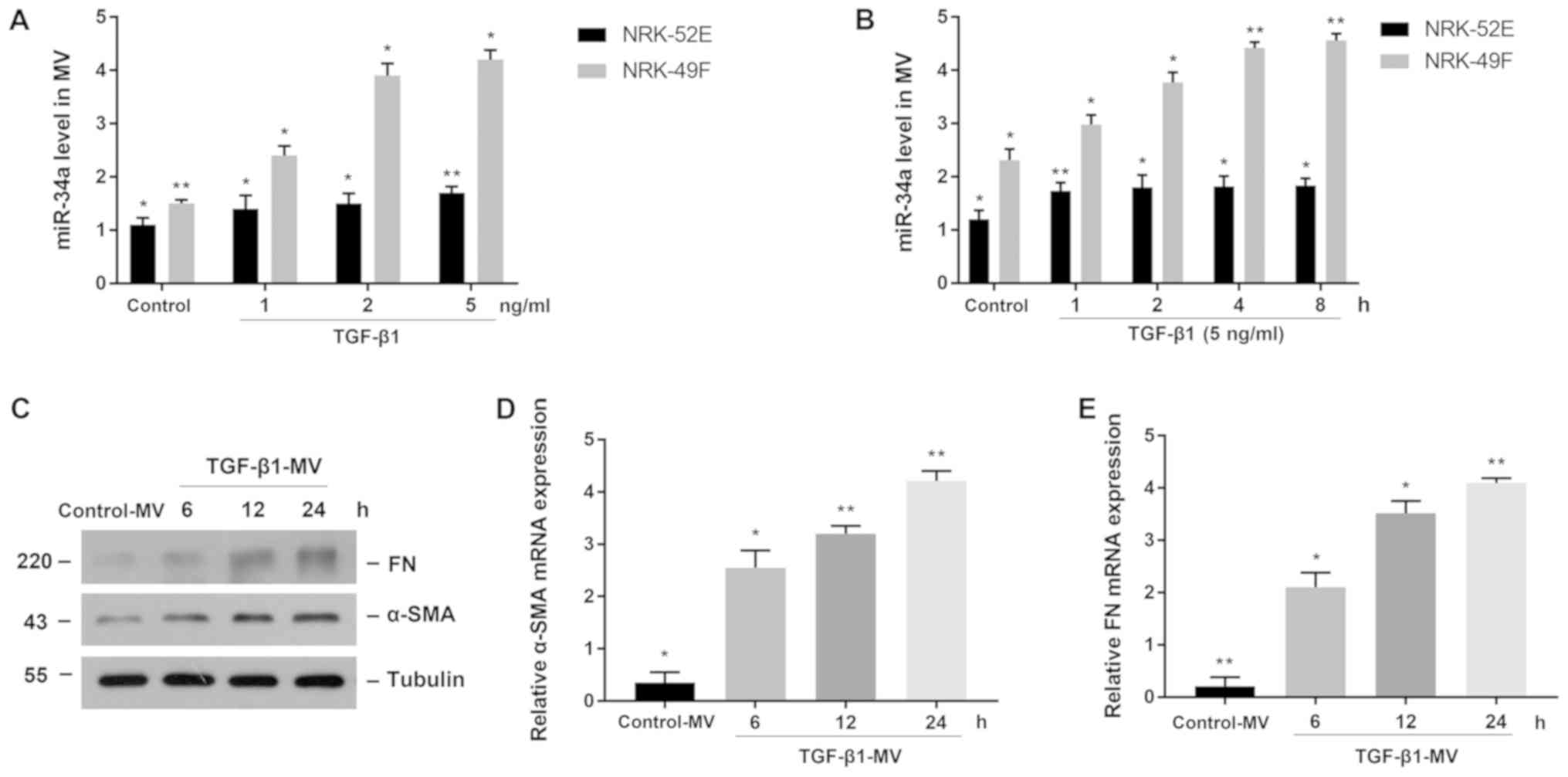
miR-34a is increased in TGF-β1-treated
fibroblasts and aggravated renal interstitial fibrosis
To clarify the expression of miR-34a in the renal
tissue after obstruction, we treated NRK-52E and NRK-49F cells with
TGF-β1 to observe the expression of miR-34a. Firstly, we stimulated
cells with different concentrations of TGF-β1 for 24 h and
extracted MVs from cell culture fluid, then we found that miR-34a
expression was significantly increased in fibroblasts compared to
the control group. The cells treated with TGF-β1 at a concentration
of 5 ng/ml had the highest miR-34a expression. However, miR-34a
expression in tubule epithelial cells did not change significantly
(Fig. 2A). We then treated both cell
lines with 5 ng/ml TGF-β1 for different time-points to detect the
expression of miR-34a in MVs from cell culture fluid. A similar
result was found that the expression of miR-34a was significantly
increased in fibroblasts and was time-dependent, whereas the
expression of miR-34a was slightly increased in tubule epithelial
cells (Fig. 2B). The MVs collected
from the culture fluid of NRK-49F cells were treated with TGF-β1 at
a concentration of 5 ng/ml, then stimulated NRK-52E cells with MVs
at a concentration of 200 nmol/l for different times, then we found
the expression of α-SMA and FN were increased and were
time-dependent (Fig. 2C). Similar
results were found in the mRNA expression of α-SMA and FN which
were increased in experimental groups (Fig. 2D and E).
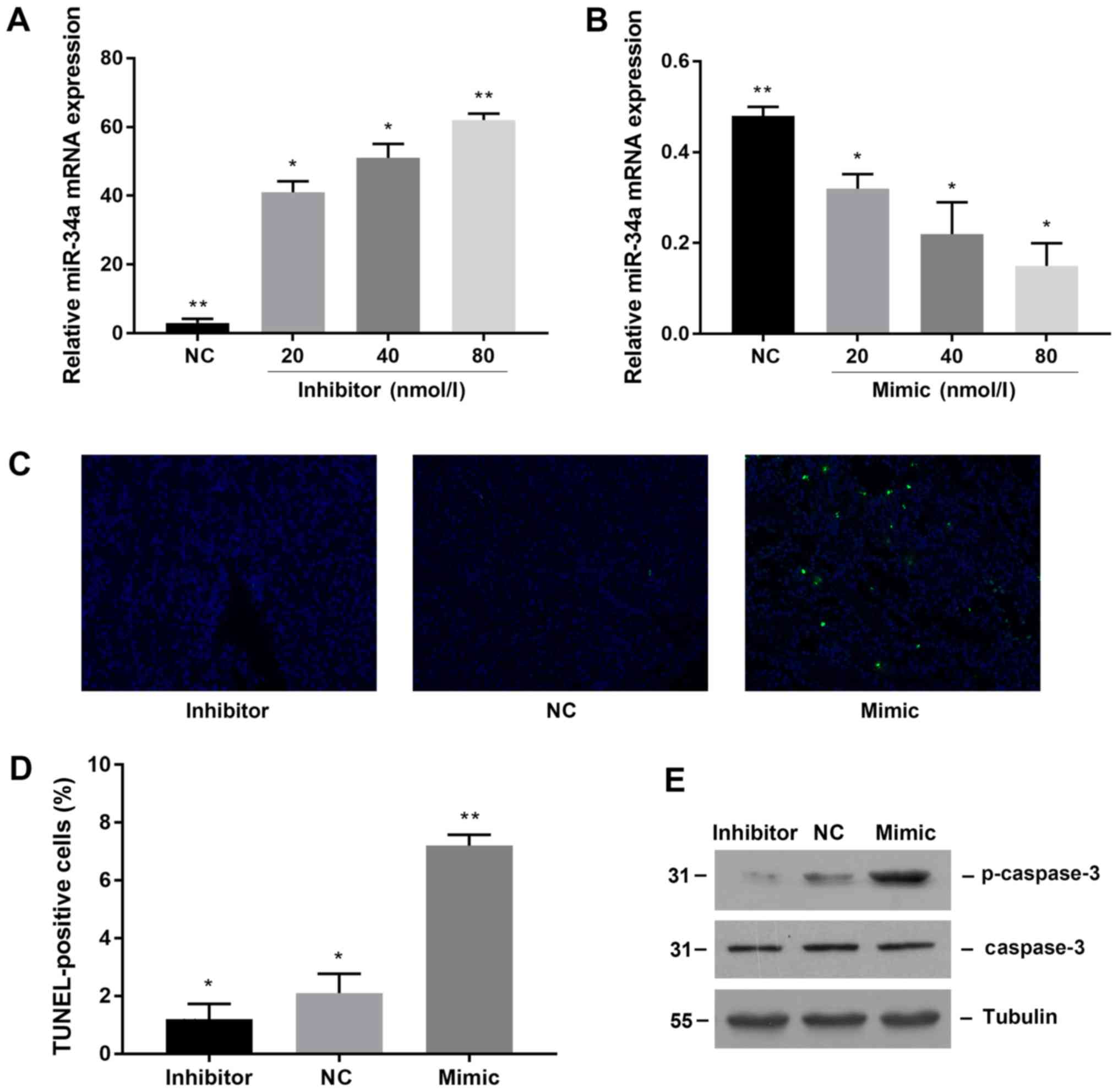
miR-34a induces apoptosis of proximal
tubular epithelial cells
To directly verify whether miR-34a promotes
apoptosis of proximal tubular epithelial cells, we transfected
miR-34a mimic and inhibitor to observe apoptosis to achieve miR-34a
overexpression and knockdown. First, we tested the transfection
efficiency. The results showed that transfection of miR-34a
inhibitor significantly downregulated the expression of miR-34a in
NRK-52E cells (Fig. 3A), while
miR-34a mimic transfection significantly increased the expression
of miR-34a (Fig. 3B). We used TUNEL
staining to compare the apoptosis of proximal tubular epithelial
cells after transfection with 80 nmol/l negative control, miR-34a
mimic and miR-34a inhibitor. The results showed that the number of
apoptotic cells in the mimic group was significantly increased
(Fig. 3C), while the number of
apoptosis in the inhibitor transfected group was decreased. The
same result was obtained by counting the number of cells (Fig. 3D).
Caspase-3 is a marker of apoptosis. Therefore, we
observed the effect of miR-34a on apoptosis of renal tubular
epithelial cells by observing the expression of caspase-3. The
results showed that when miR34a was upregulated in NRK-52E cells,
the expression of caspase-3 protein was significantly increased as
well, whereas after downregulating miR-34a, the expression of
caspase-3 protein was decreased (Fig.
3E). The mRNA levels of caspase-3 in each group were found to
be consistent with the protein results. These results suggested
that miR-34a can promote renal tubular epithelial cell
apoptosis.
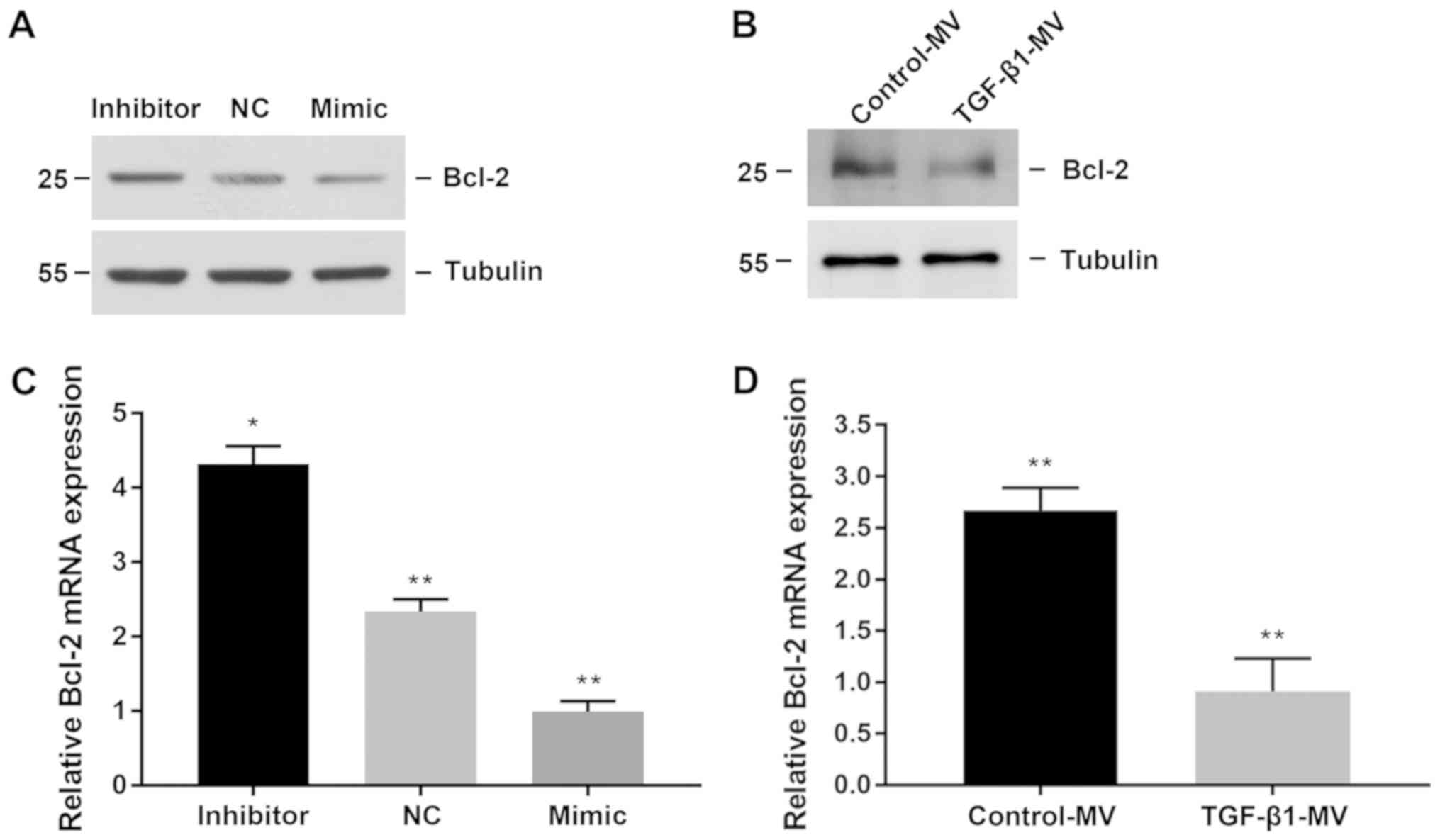
miR-34a participates in renal
interstitial fibrosis by inhibiting apoptosis of target gene
Bcl-2
Bioinformatics studies have shown that Bcl-2 is the
target protein of miR-34a. Previous studies have confirmed that
miR-34a regulates the expression of Bcl-2 in tumor cells (17,18,24). We
then hypothesize whether miR-34a regulates apoptosis through Bcl-2.
We found that transfection with miR-34a mimic inhibited Bcl-2
protein expression, whereas transfection with miR-34a inhibitor
increased Bcl-2 protein expression (Fig.
4A). Similarly, at the gene level, Bcl-2 mRNA levels decreased
when miR-34a was upregulated, whereas Bcl-2 mRNA levels increased
when miR-34a was downregulated (Fig.
4B). Later, we treated NRK-49F and NRK-52E cells with TGF-β1
and found that Bcl-2 protein levels and mRNA levels were all
reduced (Fig. 4C and D).
Downregulated Bcl-2 induces apoptosis
of proximal tubular epithelial cells
Previously, we found that upregulation of miR-34a or
TGF-β1 treatment of NRK-49F micro-vessels can inhibit the
expression of Bcl-2 in proximal tubular epithelial cells and induce
apoptosis. Then we transfected Bcl-2-specific plasmids to
downregulate the expression of Bcl-2 in NRK-52E cells to observe
whether apoptosis and fibrosis were further aggravated. We first
observed the transfection efficiency of different concentrations of
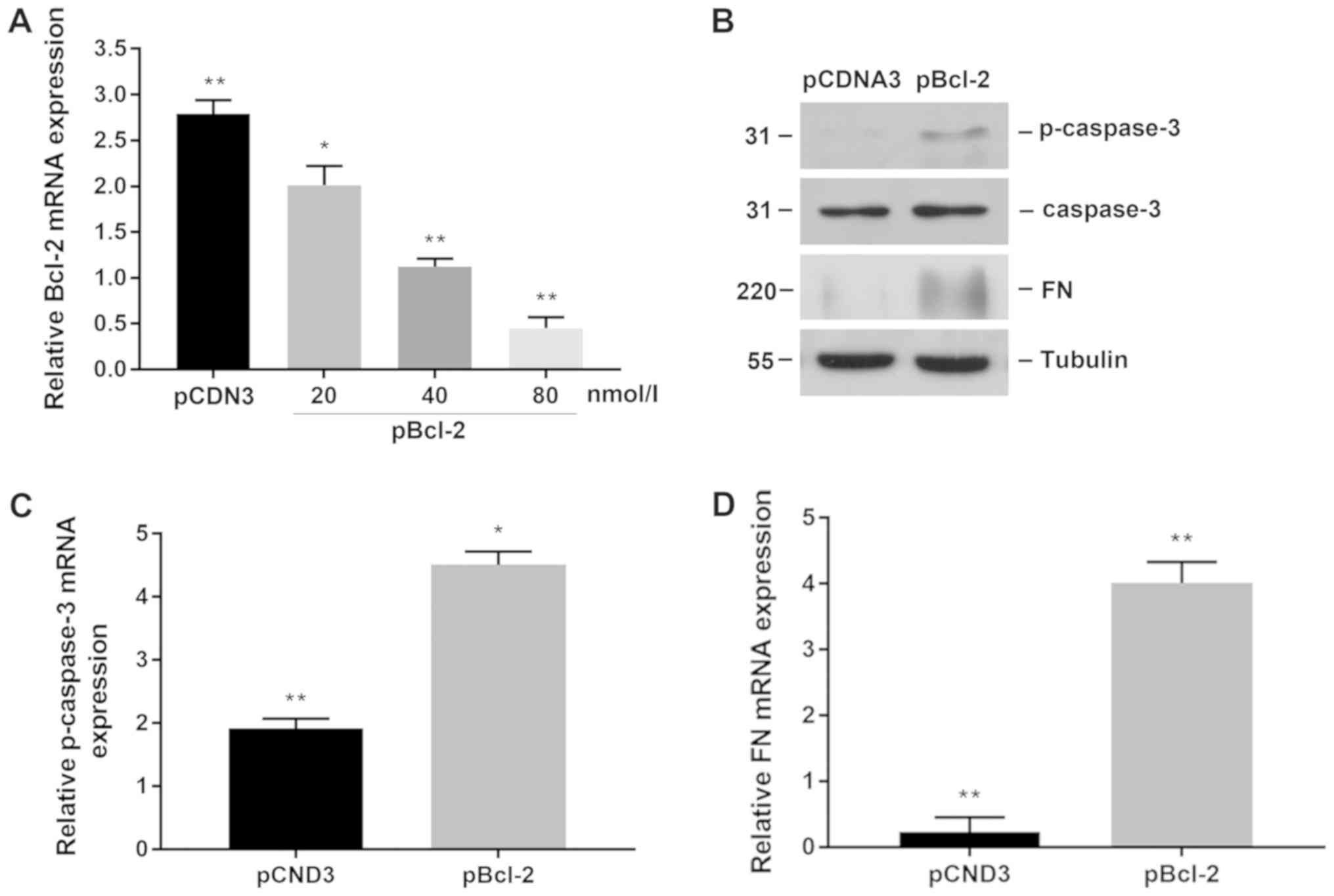
plasmids. We selected a concentration of 80 nmol/l. After treatment
of NRK-52E 24 h, we found that downregulation of Bcl-2 increased
the expression of p-caspase-3 and promoted interstitial fibrosis
(Fig. 5B). Similar results were
found in mRNA level analysis (Fig. 5C
and D). The data show that the downregulation of Bcl-2 can
promote apoptosis of proximal tubular epithelial cells and
aggravate renal interstitial fibrosis.
Discussion
Renal interstitial fibrosis is a common pathological
result of CKD, in which apoptosis of renal tubular epithelial cells
plays an important role. However, the molecular mechanism is still
not clear. Apoptosis, a programmed cell death, is a common form of
cell death (25). Many studies have
shown that there is abundant apoptosis of cells in various fibrotic
organs, suggesting that apoptosis is likely to induce and promote
the occurrence and development of organ tissue fibrosis (26). Renal interstitial fibrosis is
characterized by tubular atrophy and extracellular accumulation of
tubulointerstitial cells (3).
Apoptosis of renal tubular epithelial cells is one of the causes of
renal tubular atrophy and tubulointerstitial fibrosis (5–7).
Previous studies have found evidence of apoptosis in fibrotic
kidney tissue by detecting changes in cellular DNA, mitochondria,
cell membranes, and cell morphology.
Recent studies have found that miR-34s can regulate
tumor cell apoptosis. Among them, the transcriptional expression of
miR-34a alone is most widely distributed in various tissues. Bcl-2
is an important anti-apoptosis gene in caspase cell apoptosis
pathway. Studies have shown that Bcl-2 expression was inhibited
after miR-34s transfection into colon cancer cells. After silencing
of miR-34 expression, the expression of Bcl-2 was increased and the
anti-apoptotic ability of cells was enhanced (18). Therefore, we hypothesize that miR-34a
may also have a role in apoptosis of renal tubular epithelial
cells.
To investigate whether miR-34a is involved in the
regulation of renal tubular epithelial cell apoptosis, we first
examined the expression of miR-34a in the UUO renal interstitial
fibrosis model. As a result, we found that the expression of
miR-34a as well as the MVs was significantly increased in the renal
tissue. Afterwards, we found that MVs containing miR-34a also
increased significantly. Subsequently, we used in vitro
experiments to detect the distribution of miR-34a. We used TGF-β1
to treat NRK-49F and NRK-52E cells to alter their phenotypes,
producing extracellular matrix and promoting fibrosis. We found
that miR-34a was only significantly increased in interstitial
fibroblasts, suggesting that miR-34a is mainly distributed in
interstitial fibroblasts but not in tubule epithelial cells.
Studies have confirmed that TGF-β1 can be transmitted from injured
epithelial cells to fibroblasts and induce renal interstitial
fibrosis (27). Therefore, we
hypothesize that in the obstruction model, the tubule basement
membrane was broken and lost its integrity, allowing miR-34a to
pass from the mesenchymal fibroblasts to the renal tubular
epithelial cells, inducing apoptosis of the renal tubular
epithelial cells and promoting renal interstitial fibrosis.
In conclusion, this study shows that in the process
of renal interstitial fibrosis, interstitial fibroblasts can
secrete miR-34a-containing MVs and transmit signals to renal
tubular epithelial cells through the ruptured basement
membrane.miR-34a can inhibit the target protein Bcl-2, activate
caspase apoptosis pathway, induce apoptosis of renal tubular
epithelial cells, and promote renal interstitial fibrosis. MVs
containing miRNAs serve as an important molecular platform for
mediating cells, helping to further understand the molecular
mechanisms of renal tubular epithelial cell apoptosis and
tubulointerstitial fibrosis. Our identification of miR-34a in renal
interstitial fibrosis suggests miR-34a mimics or inhibitors as
potential therapeutics for treating renal interstitial
fibrosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HL and KL designed the study and performed the
experiments, YuX, QZ and HX established the animal models, HX and
YaX collected the data, HL and YuX analyzed the data, HL and KL
prepared the manuscript. All authors read and approved the final
study.
Ethics approval and consent to
participate
This study was approved by The Animal Care and Use
Review Committee of Wujiang Hospital Affiliated to Nantong
University (Suzhou, China).
Patient consent for publication
No patients participated in this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu Y: New insights into
epithelial-mesenchymal transition in kidney fibrosis. J Am Soc
Nephrol. 21:212–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zeisberg M and Neilson EG: Mechanisms of
tubulointerstitial fibrosis. J Am Soc Nephrol. 21:1819–1834. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bohle A, Christ H, Grund KE and Mackensen
S: The role of the interstitium of the renal cortex in renal
disease. Contrib Nephrol. 16:109–114. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johnson A and DiPietro LA: Apoptosis and
angiogenesis: An evolving mechanism for fibrosis. FASEB J.
27:3893–3901. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Docherty NG, O'Sullivan OE, Healy DA,
Fitzpatrick JM and Watson RW: Evidence that inhibition of tubular
cell apoptosis protects against renal damage and development of
fibrosis following ureteric obstruction. Am J Physiol Renal
Physiol. 290:F4–F13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Y: Hepatocyte growth factor in kidney
fibrosis: Therapeutic potential and mechanisms of action. Am J
Physiol Renal Physiol. 287:F7–F16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang G, Oldroyd SD, Huang LH, Yang B, Li
Y, Ye R and El Nahas AM: Role of apoptosis and Bcl-2/Bax in the
development of tubulointerstitial fibrosis during experimental
obstructive nephropathy. Exp Nephrol. 9:71–80. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu CF, Liu H, Fang Y, Jiang SH, Zhu JM
and Ding XQ: Rapamycin reduces renal hypoxia, interstitial
inflammation and fibrosis in a rat model of unilateral ureteral
obstruction. Clin Invest Med. 37:E1422014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miyajima A, Chen J, Lawrence C, Ledbetter
S, Soslow RA, Stern J, Jha S, Pigato J, Lemer ML, Poppas DP, et al:
Antibody to transforming growth factor-beta ameliorates tubular
apoptosis in unilateral ureteral obstruction. Kidney Int.
58:2301–2313. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao X, Mae H, Ayabe N, Takai T, Oshima K,
Hattori M, Ueki T, Fujimoto J and Tanizawa T: Hepatocyte growth
factor gene therapy retards the progression of chronic obstructive
nephropathy. Kidney Int. 62:1238–1248. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morrissey J, Hruska K, Guo G, Wang S, Chen
Q and Klahr S: Bone morphogenetic protein-7 improves renal fibrosis
and accelerates the return of renal function. J Am Soc Nephrol. 13
Suppl 1:S14–S21. 2002.PubMed/NCBI
|
|
12
|
Fu JH, Yang S, Nan CJ, Zhou CC, Lu DQ, Li
S and Mu HQ: MiR-182 affects renal cancer cell proliferation,
apoptosis, and invasion by regulating PI3K/AKT/mTOR signaling
pathway. Eur Rev Med Pharmacol Sci. 22:351–357. 2018.PubMed/NCBI
|
|
13
|
Harvey SJ, Jarad G, Cunningham J, Goldberg
S, Schermer B, Harfe BD, McManus MT, Benzing T and Miner JH:
Podocyte-specific deletion of dicer alters cytoskeletal dynamics
and causes glomerular disease. J Am Soc Nephrol. 19:2150–2158.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ho JJ and Marsden PA: Dicer cuts the
kidney. J Am Soc Nephrol. 19:2043–2046. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y and Lee CG: MicroRNA and cancer -
focus on apoptosis. J Cell Mol Med. 13:12–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hermeking H: The miR-34 family in cancer
and apoptosis. Cell Death Differ. 17:193–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qi R, An H, Yu Y, Zhang M, Liu S, Xu H,
Guo Z, Cheng T and Cao X: Notch1 signaling inhibits growth of human
hepatocellular carcinoma through induction of cell cycle arrest and
apoptosis. Cancer Res. 63:8323–8329. 2003.PubMed/NCBI
|
|
18
|
Bommer GT, Gerin I, Feng Y, Kaczorowski
AJ, Kuick R, Love RE, Zhai Y, Giordano TJ, Qin ZS, Moore BB, et al:
p53-mediated activation of miRNA34 candidate tumor-suppressor
genes. Curr Biol. 17:1298–1307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ratajczak J, Wysoczynski M, Hayek F,
Janowska-Wieczorek A and Ratajczak MZ: Membrane-derived
microvesicles: Important and underappreciated mediators of
cell-to-cell communication. Leukemia. 20:1487–1495. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Graves LE, Ariztia EV, Navari JR, Matzel
HJ, Stack MS and Fishman DA: Proinvasive properties of ovarian
cancer ascites-derived membrane vesicles. Cancer Res. 64:7045–7049.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Skog J, Würdinger T, van Rijn S, Meijer
DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky
AM and Breakefield XO: Glioblastoma microvesicles transport RNA and
proteins that promote tumour growth and provide diagnostic
biomarkers. Nat Cell Biol. 10:1470–1476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Taylor DD and Gercel-Taylor C: MicroRNA
signatures of tumor-derived exosomes as diagnostic biomarkers of
ovarian cancer. Gynecol Oncol. 110:13–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bryant RJ, Pawlowski T, Catto JW, Marsden
G, Vessella RL, Rhees B, Kuslich C, Visakorpi T and Hamdy FC:
Changes in circulating microRNA levels associated with prostate
cancer. Br J Cancer. 106:768–774. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cole KA, Attiyeh EF, Mosse YP, Laquaglia
MJ, Diskin SJ, Brodeur GM and Maris JM: A functional screen
identifies miR-34a as a candidate neuroblastoma tumor suppressor
gene. Mol Cancer Res. 6:735–742. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen CC and Lau LF: Deadly liaisons: Fatal
attraction between CCN matricellular proteins and the tumor
necrosis factor family of cytokines. J Cell Commun Signal. 4:63–69.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Borges FT, Melo SA, Özdemir BC, Kato N,
Revuelta I, Miller CA, Gattone VH II, LeBleu VS and Kalluri R:
TGF-β1-containing exosomes from injured epithelial cells activate
fibroblasts to initiate tissue regenerative responses and fibrosis.
J Am Soc Nephrol. 24:385–392. 2013. View Article : Google Scholar : PubMed/NCBI
|