Introduction
Obstructive sleep apnoea (OSA) is a very common
sleep and breathing disorder that is characterized by the
repetitive collapse of the upper airway during sleep (1); in addition, OSA has high morbidity in
paediatric and adult populations. In OSA, upper airway obstruction
is usually associated with recurrent hypoxemia interspersed with
periods of reoxygenation and is typically terminated by brief
arousals, resulting in intermittent hypoxia (IH) and sleep
fragmentation (2). The oscillatory
nature of IH, especially chronic IH, may mimic the paradigm of
ischaemia-reperfusion in that tissues and cells are exposed to
periods of low and high O2, which may lead to oxidative
stress and induces lipid peroxidation, apoptosis, stress-responsive
proteins, cell death and inflammation (3–5). Studies
have confirmed that IH via different signalling pathways induces
cell apoptosis (6,7). Mitochondrial separation protein
inhibitor (Mdivi-1) is a selective dynamin-related protein 1
(Drp-1) inhibitor that has been reported to be a potential
therapeutic target for brain, cardiovascular, liver and tumour
angiogenesis (8,9). How Mdivi-1 inhibits lung apoptosis
during IH remains unclear. The aim of the present study was to
investigate the protective effect and possible mechanism of Mdivi-1
in lung cell apoptosis during IH.
Materials and methods
Animals
A total of 32 specific pathogen free 6–8-week-old
male Sprague Dawley (SD) rats (body weight, 180–220 g) were
purchased from the Animal Care and Use Committee of the Army
Medical University (Chongqing, China). The experiments were
conducted in accordance with the Guide for the Care and Use of
Laboratory Animals (10). All
experimental procedures were approved by the Ethics Committee of
Guizhou Provincial People's Hospital (Guiyang, China). All efforts
were made to minimize animal suffering and to reduce the number of
animals used.
In vivo experimental design
All animals were maintained in a controlled
environment (22–25°C, 40–60% relative humidity, with a 12-h
light-dark cycle with the lights on at 08:00 am) with food and
water available. Animals were allowed to acclimatize for 1 week to
the experimental chamber. The rats were allocated randomly to four
groups: Control group (n=8), IH group (n=8), IH+25 mg/kg Mdivi-1
group (n=8) and IH+50 mg/kg Mdivi-1 group (n=8). The rats in the IH
group, IH+25 mg/kg Mdivi-1 group and IH+50 mg/kg Mdivi-1 group were
exposed to IH conditions (21% O2, 5% CO2 and
balanced N2) for 90 sec and hypoxia (8% O2,
5% CO2 and balanced N2 for 30 sec) from 08:00
am to 04:00 pm daily for 4 consecutive weeks (11–13). The
chambers had a rear N2 inlet and a front air extractor,
which enabled recovery to normoxia. A computerized system
controlled the valve inlets and the alternating cycles of the
extractors. The CO2 in the chamber was maintained at a
low level by continuous air extraction (14). Rats in the control group were
maintained under normoxic conditions during the experiment. The
treatment groups were injected intraperitoneally with Mdivi-1
(MedChemExpress, Monmouth Junction, NJ, USA) prior to oxygen
treatment every day (15). A general
clinical pain score for scoring cyanosis condition for decisions
about endpoint was use as presented in Table I (16).
 | Table I.Assessing pain and distress in
animal. |
Table I.
Assessing pain and distress in
animal.
| 1) No indication of
pain and distress | Normal; well
groomed; alert; active; good condition; asleep or calm; normal
appetite; BCS=3, 4 or 5 |
| 2) Mild or
anticipated pain and distress | Not well groomed;
awkward gait; slightly hunched; looks at wound or pulls away when
area touched; mildly agitated; BCS=2 |
| 3) Moderate pain
and distress | Rough hair coat;
dirty incision; squinted eyes; moves slowly; walks hunched and/or
slowly; depressed or moderately agitated; slight dehydration;
pruritic; restless; uncomfortable; not eating or drinking;
BCS=2. |
| 4) Severe pain and
distress | Very rough hair
coat; eyes sunken (severe dehydration); slow to move or
non-responsive when coaxed; hunched; large abdominal mass; dyspnea;
self mutilating; violent reaction to stimuli or when approached;
BCS=1 |
Sample collection and tissue
preparation
Trained personnel euthanized the experimental
animals via CO2 inhalation following IH exposure. Rats
were left in the chamber until clinical death could be ensured. The
flow rate of CO2 displaced 20% of the volume chamber per
minute. If no breath was seen for 60 sec the rodent was removed
from the chamber. To ensure death, the rat's chest was felt to
determine that there is no longer a heartbeat. Death was verified
following euthanasia and prior to disposal. The lungs were
dissected out and the right lower lobes were fixed in 4%
paraformaldehyde at room temperature for 48 h. The remaining lung
tissues were frozen in liquid nitrogen and then stored at −80°C
until further analysis.
Histological examination
The right lower lobes were fixed in 4%
paraformaldehyde at room temperature for 24 h, embedded in paraffin
blocks and slices into 6-µm-thick sections. The sections were then
stained with haematoxylin for 5 min and eosin for 90 sec at room
temperature, and observed by light microscopy. Damage to the lung
tissue was scored by the pathologist on a scale of 1 (no injury) to
4 (worst), as described previously (17).
In vitro experimental design
WTRL1 cells (a rat pulmonary alveolar epithelial
cell line; http://www.cellresource.cn/fdetail.aspx?id=1349) were
purchased from the National Infrastructure of Cell Line Resource,
(Beijing, China). The cells were grown in RPMI-1640 medium
(HyClone, Logan, UT, USA) with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), penicillin (100 units/ml) and
streptomycin (100 µg/ml) and were cultured at 37°C in 5%
CO2 in a humidified incubator. WTRL1 cells in the
logarithmic growth phase were divided randomly into five groups
(3×106 cells/well) in 6-well plates: Control, IH, IH+10
µM Mdivi-1, IH+50 µM Mdivi-1 and IH+100 µM Mdivi-1. The control
group was exposed to air, while the IH group, IH+10 µM Mdivi-1
group, IH+50 µM Mdivi-1 group and IH+100 µM Mdivi-1 group were
placed in a low oxygen culture box containing 5% O2 for
1 h. The box was then reoxygenated with 21% O2 for 30
min and this process was repeated for 12 h. The treatment groups
were incubated with Mdivi-1 for 30 min prior to IH culture.
Flow cytometry
WTRL1 cells were grown logarithmically and placed on
60-mm Petri dishes in a low oxygen culture box containing 5%
O2 for 1 h. The box was reoxygenated with 21%
O2 for 30 min and this process was repeated for 6, 12,
24 and 48 h. Next, the cells were resuspended in Annexin V-PI from
a dual-staining assay kit (Nanjing KeyGen Biotech. Co. Ltd.,
Nanjing, China) for 10 min at room temperature. The fluorescence
signals from Annexin V and PI were measured by flow cytometry with
a FACSCalibur™ flow cytometer (BD Bioscience), and the
data were analysed using Cell Quest Pro software (version 5.0; BD
Bioscience). The flow cytometry results were measured in 1 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from lung tissues or cells
with TRIzol (Thermo Fisher Scientific, Inc.). The D260/D280 ratio
was measured and samples with a ratio between 1.8 and 2.0 were used
for reverse transcription with a High Capacity cDNA Archive kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The reverse
transcription protocol were as follows: 25°C for 10 min, 37°C for
120 min and 85°C for 5 min. qPCR was applied to analyze the cDNAs
by using the 2X Maxima SYBR-Green/ROX qPCR Master Mix kit (Thermo
Fisher Scientific, Inc.) following the manufacturer's protocol. The
amplification conditions were as follows: 95°C for 10 min for
denaturation, followed by 40 cycles of 95°C for 15 sec, 60°C for 30
sec and 72°C for 60 sec. qPCR for Bcl-2, caspase-3, caspase-9,
Cyt-C and Drp-1 was performed using TaqMan gene expression assays
and the 2−ΔΔCq method with GAPDH as the endogenous
control (18). The primers were
synthesized by Sheng Gong (Shanghai) Co., Ltd. (Shanghai, China)
and the sequences are presented in Table II.
 | Table II.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table II.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Primer name | Primer sequence
(5′-3′) |
|---|
| GAPDH | F:
AGCCACATCGCTCAGACAC |
|
| R:
GCCCAATACGACCAATCC |
| B-cell lymphoma
2 | F:
TCCTTCCAGCCTGAGAGCAACC |
|
| R:
TCACGACGGTAGCGACGAGAG |
| Caspase-3 | F:
GTAGCAGTGTGCCGCTGTGTC |
|
| R:
ACTCTTGTCCATCGCCTCTCCTC |
| Caspase-9 | F:
ATGGAGGAGGCTGACCGGCAACTCCTG |
|
| R:
TCATGAAGTTTTAAAGAACAGCTTCTTC |
| Cytochrome C | F:
AGGAGGCAAGCATAAGAC |
|
| R:
ATTAGGTCTGCCCTTTCT |
| Dynamin-related
protein 1 | F:
TTCGCCGCCTGGAGGACC |
|
| R:
CACTTCGTTGCCACAATGCTGAA |
Western blot analysis
Total protein cell lysates were isolated from rat
lungs or from cells as described previously (19). Protein quantification was assessed
using a bicinchoninic acid protein quantification assay and
absorbance read at 562 nm was used to determine sample volumes for
further analysis. A total of 50 µg total protein from each tissue
homogenate was separated by 30% SDS-PAGE and then transferred to
polyvinylidene difluoride membranes, which were blocked with 5%
skim milk at room temperature for 1 h. Membranes were washed twice
with TBST and incubated with primary antibodies at 4°C overnight
against Bcl-2 polyclonal antibody (cat. no. YT0470), caspase-3
polyclonal antibody (cat. no. YT0656), caspase-9 polyclonal
antibody (cat. no. YT0662), Drp1 Polyclonal antibody (cat. no.
YT1414; all 1:1,000; ImmunoWay Biotechnology Company, Plano, TX,
USA), Cyt-C antibody (cat. no. 10993-1-AP; 1:1,000; ProteinTech,
Group, Inc., Chicago, IL, USA), mouse monoclonal β-actin antibody
(1:4,000; cat. no. YM3138; ImmunoWay Biotechnology Company).
Membranes were washed twice and incubated with horseradish
peroxidase-conjugated goat anti-mouse IgG (1:6,000; cat. no.
PMK-014-091; Bioprimacy, Wuhan, China) and goat anti-rabbit IgG
(1:8,000; cat. no. PMK-014-090; Bioprimacy, Wuhan, China) for 2 h
at room temperature. The blots were visualized with eECL western
blot kit (Beyotime Institute of Biotechnology, Jiangsu, China). The
intensities of the bands were measured using ImageLab 5.1 Software
(Bio-Rad Laboratories, Inc.). Rat β-actin was used as the
loading control in western blotting.
Statistical analysis
All experiments were repeated three times. All data
are presented as mean ± standard deviation. Comparisons of two or
three experimental conditions were evaluated using an unpaired
Student's t-test or one-way analysis of variance and the Bonferroni
t-test followed by the Bonferroni post-test using GraphPad Prism
5.0 software (GraphPad Software Corp, San Diego, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Lung histopathology
Histopathological examinations were performed for
the lungs of rats subjected to normoxia, IH, IH+25 mg/kg Mdivi-1
and IH+50 mg/kg Mdivi-1. IH caused severe interstitial pneumonia
and a large number of inflammatory cells, while following Mdivi-1
intervention, interstitial pneumonia and inflammatory cells were
reduced. The effect in the IH+50 mg/kg Mdivi-1 was more obvious
compared with in the IH+25 mg/kg Mdivi-1 group (Fig. 1). Consistent with these
histopathological observations, the lung injury score in the IH
group was significantly increased compared with the control group
(P<0.005) and the score in the IH+50 mg/kg Mdivi-1 group was
significantly decreased compared with in the IH group
(P<0.005).
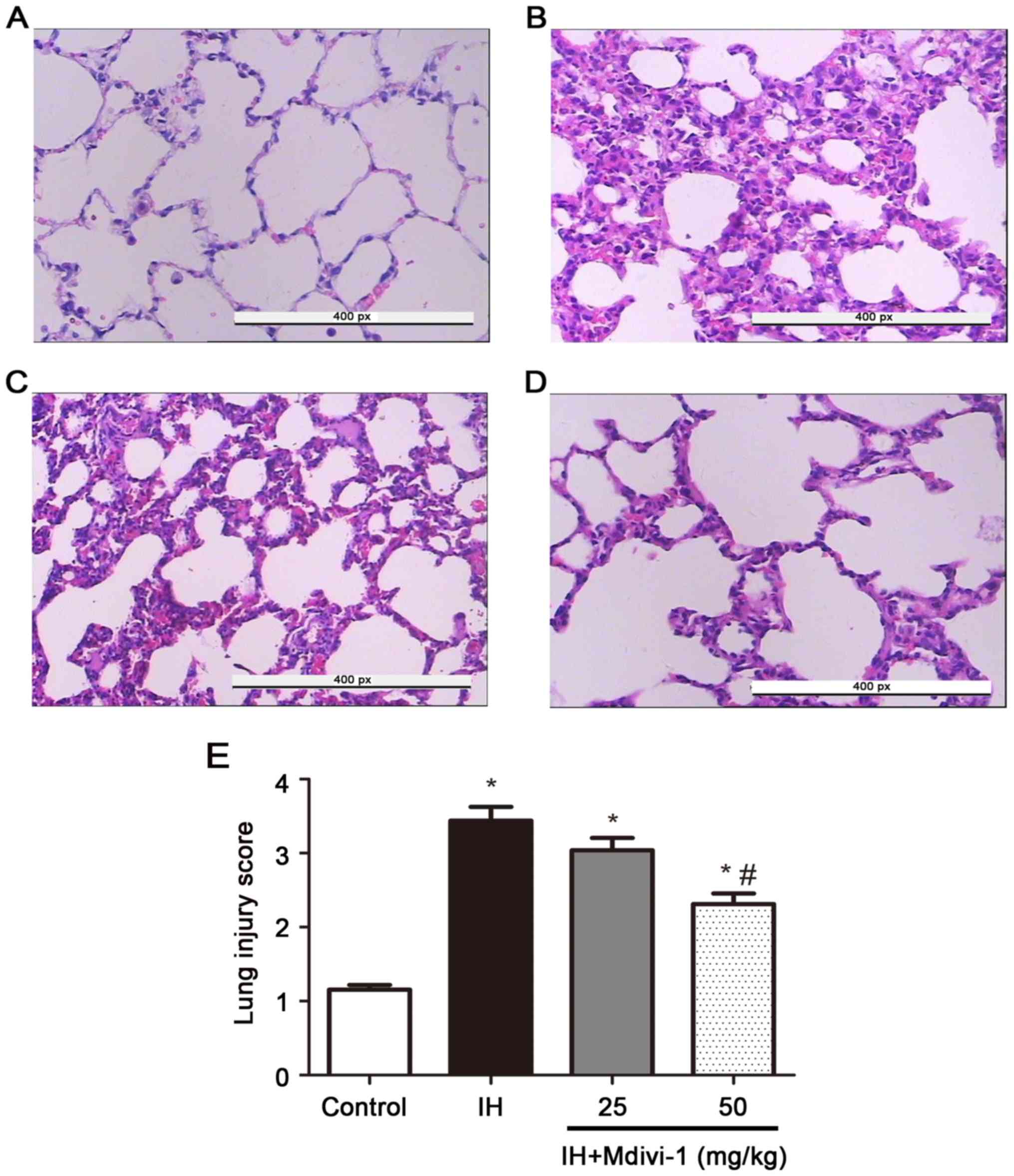 | Figure 1.Detection of variations in the lung
media of rats in each group using haematoxylin and eosin staining
(magnification, 400; scale bar, 50 µm). (A) The control group, n=8.
(B) The IH group, n=8. (C) The IH+25 mg/kg Mdivi-1 group, n=8. (D)
IH+50 mg/kg Mdivi-1 group, n=8. (E) Compared with normoxia, IH
caused interstitial alterations in the lung tissues of SD rats,
while Mdivi-1 significantly reduced the pulmonary interstitial
lesions. The effects in the IH+50 mg/kg Mdivi-1 group were more
obvious than those in the IH+25 mg/kg Mdivi-1 group. IH,
intermittent hypoxia; SD, Sprague Dawley; Mdivi, mitochondrial
division inhibitor-1. *P<0.05 vs. group C; #P<0.05
vs. group IH. |
Detection of Bcl-2, caspase-3,
caspase-9, Cyt-C and Drp-1 expression levels via RT-qPCR
After SD rats were exposed to IH for 4 weeks and
after WTRL1 cells were exposed to IH for 12 h in culture, the
expression of apoptosis markers was increased at the gene level in
the rat lung tissue cells and WRTL1 cells. In addition, Mdivi-1
treatment effectively reduced the apoptosis rate of the
hypoxia-exposed cells. After SD rats were exposed to IH for 4
weeks, Bcl-2 mRNA levels were significantly decreased (P<0.05;
Fig. 2A), but caspase-3 and
caspase-9 mRNA levels were significantly increased compared with
the control group rats (P<0.05; Fig.
2B and C). After Mdivi-1 intervention, Bcl-2 mRNA levels were
significantly increased and caspase-3, and Drp-1 mRNA levels were
significantly decreased in the IH+50 mg/kg Mdivi-1 group compared
with the IH group (P<0.05; Fig. 2A, B
and D). However, no significant difference in Cyt C levels was
identified between the groups (Fig.
2E). After WTRL1 cells were exposed to IH for 12 h, Bcl-2 mRNA
levels were significantly decreased, but caspase-3, caspase-9,
Cyt-C and Drp-1 mRNA levels were significantly increased compared
with the control group (P<0.05; Fig.
3A-E). After Mdivi-1 intervention, Bcl-2 mRNA levels were
significantly increased and caspase-3, caspase-9, Cyt-C and Drp-1
mRNA levels were significantly decreased compared with in the IH
group (P<0.05).
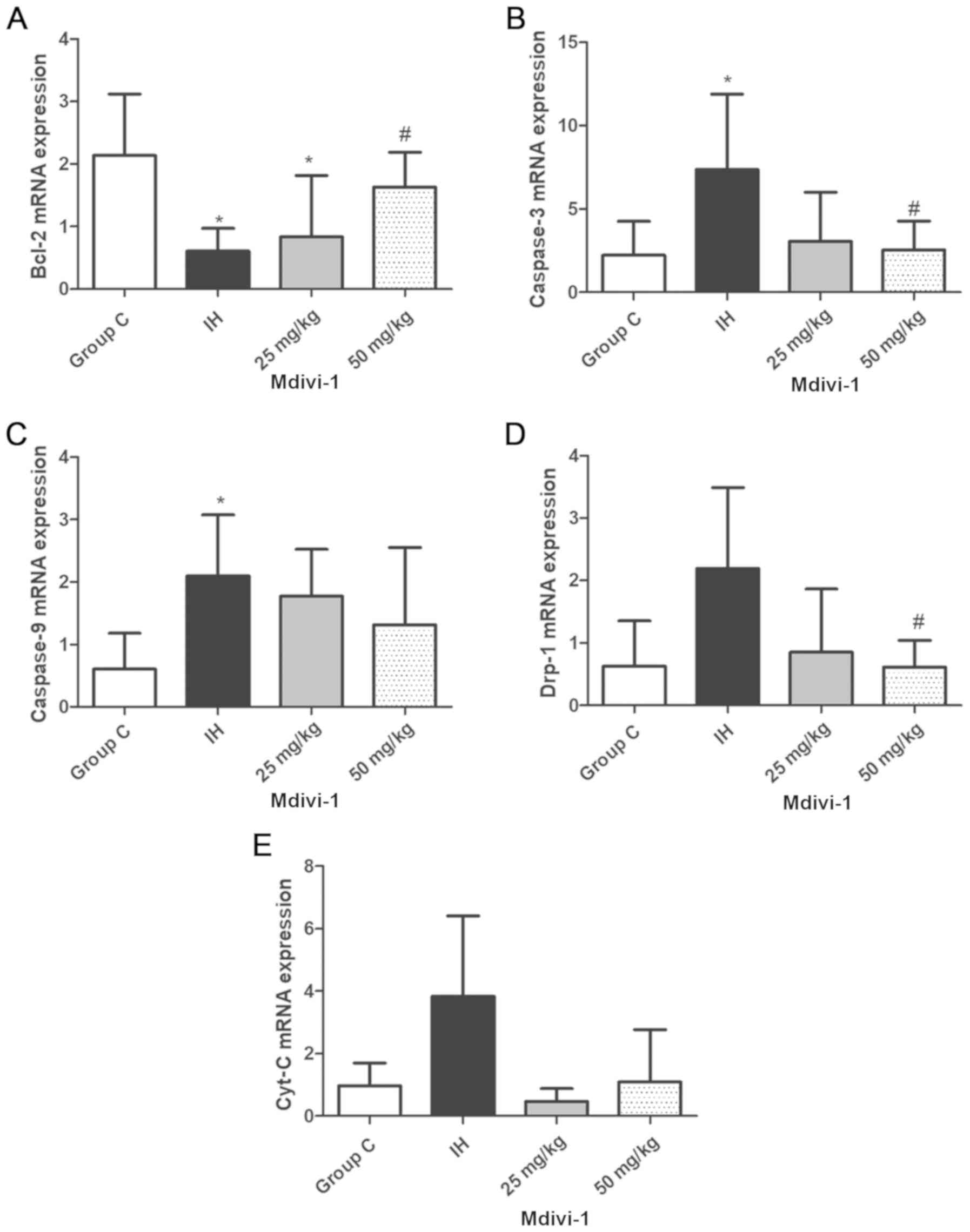 | Figure 2.Detection of Bcl-2, caspase-3,
caspase-9, Drp-1 and Cyt-C expression levels in rats lungs via
reverse transcription-quantitative polymerase chain reaction. (A)
Bcl-2 levels. (B) Caspase-3 levels. (C) Caspase-9 levels. (D) Drp-1
levels. (E) Cyt-C levels. Compared with those of group C, Bcl-2
mRNA levels were decreased, but caspase-3 and caspase-9 mRNA levels
were increased in the IH group, and these differences were
statistically significant *P<0.05 vs. group C. Compared with
those of the IH group, Bcl-2 mRNA levels were increased and
caspase-3 and Drp-1 mRNA levels were decreased in the IH+50 mg/kg
Mdivi-1 group, #P<0.05 vs. the IH group. IH,
intermittent hypoxia; Mdivi, mitochondrial division inhibitor-1;
Bcl, B-cell lymphoma; Cyt-c, cytochrome C; group C, control
group. |
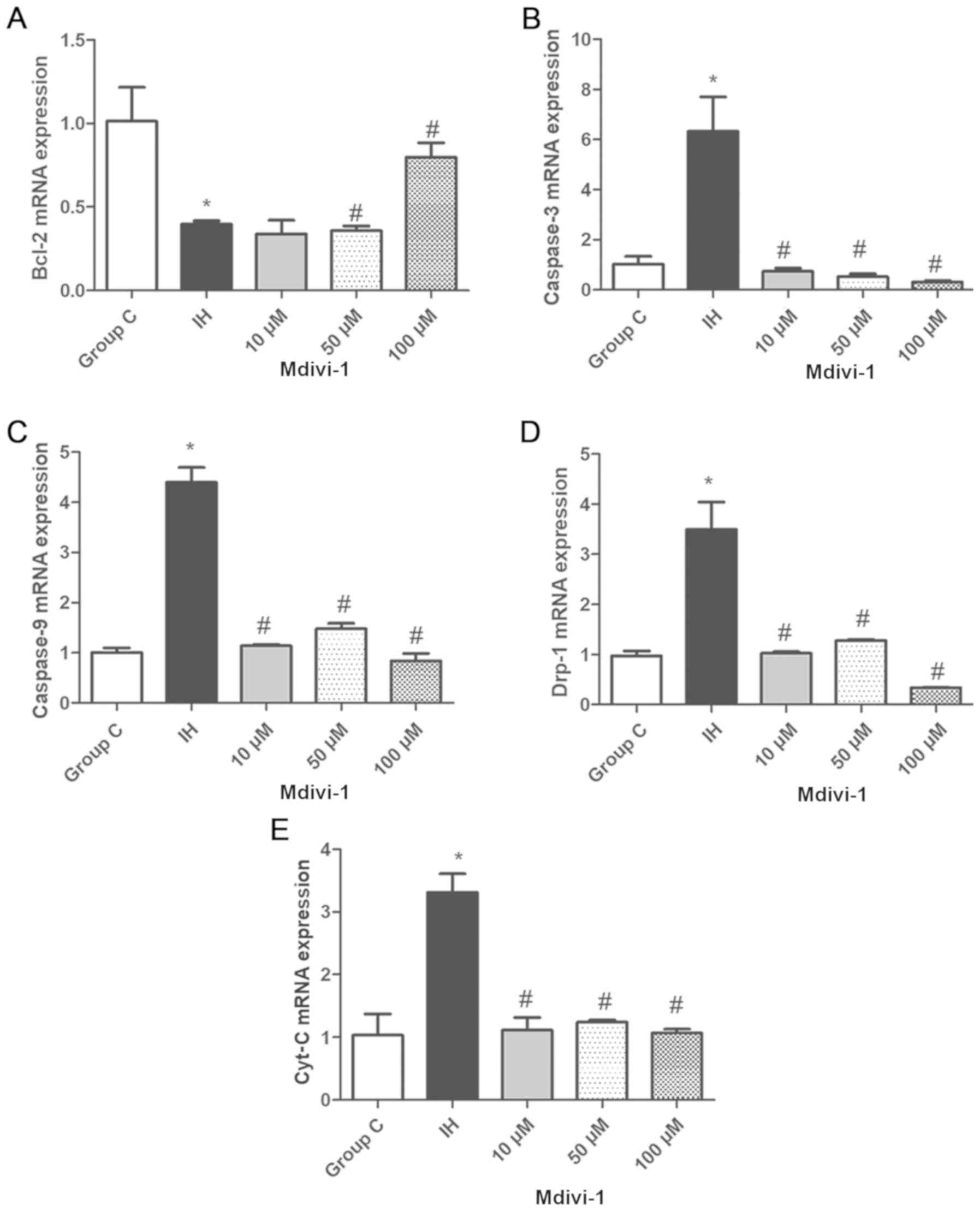 | Figure 3.Detection of Bcl-2, caspase-3,
caspase-9, Drp-1 and Cyt-C expression levels in WTRL1 cells via
reverse transcription-quantitative polymerase chain reaction. (A)
Bcl-2 levels. (B) Caspase-3 levels. (C) Caspase-9 levels. (D) Drp-1
levels. (E) Cyt-C levels. Compared with those in group C, Bcl-2
mRNA levels were lower, but caspase-3 and caspase-9 mRNA levels
were higher in the IH group, and these differences were
statistically significant *P<0.05 vs. group C. Compared with
those in the IH group, Bcl-2 mRNA expression levels were increased
and caspase-3, and Drp-1 mRNAs were decreased After Mdivi-1
intervention, #P<0.05 vs. the IH group. IH,
intermittent hypoxia; Mdivi, mitochondrial division inhibitor-1;
Bcl, B-cell lymphoma; Cyt-c, cytochrome C; group C, control
group. |
Western blotting results for Bcl-2,
caspase-3, caspase-9, Drp-1 and Cyt-C
After SD rats were exposed to IH for 4 weeks and
after WTRL1 cells were exposed to IH for 12 h in culture, the
expression of apoptosis markers was increased at the protein level
in rat lung tissue cells and WRTL1 cells. In addition, Mdivi-1
treatment reduced the apoptosis rate of the hypoxia-exposed cells.
Western blotting results for Bcl-2, caspase-3, caspase-9, Cyt-C and
Drp-1 protein expression in rat lungs are presented in Fig. 4. After SD rats were exposed to IH for
4 weeks, the protein expression of Bcl-2 in the IH group was
significantly decreased, but the protein expression of caspase-3,
caspase-9 and Drp-1 was significantly increased compared with the
control group (P<0.05; Fig.
4A-E). After Mdivi-1 intervention, the protein expression of
Bcl-2 was significantly increased and the protein expression of
caspase-3, caspase-9, and Drp-1 was significantly decreased in the
IH+50 mg/kg Mdivi-1 group compared with the IH group (P<0.05).
After WTRL1 cells were exposed to IH for 12 h, Bcl-2 protein
expression was significantly decreased in the IH group and
caspase-3, caspase-9 and Drp-1 protein expression was significantly
increased (P<0.05; Fig. 5A-E).
After Mdivi-1 intervention, Bcl-2 protein expression was
significantly increased and caspase-9, Cyt-C, and Drp-1 protein
expression was significantly decreased in the IH+100 µM Mdivi-1
group (P<0.05; Fig. 5 A, B and
D-F).
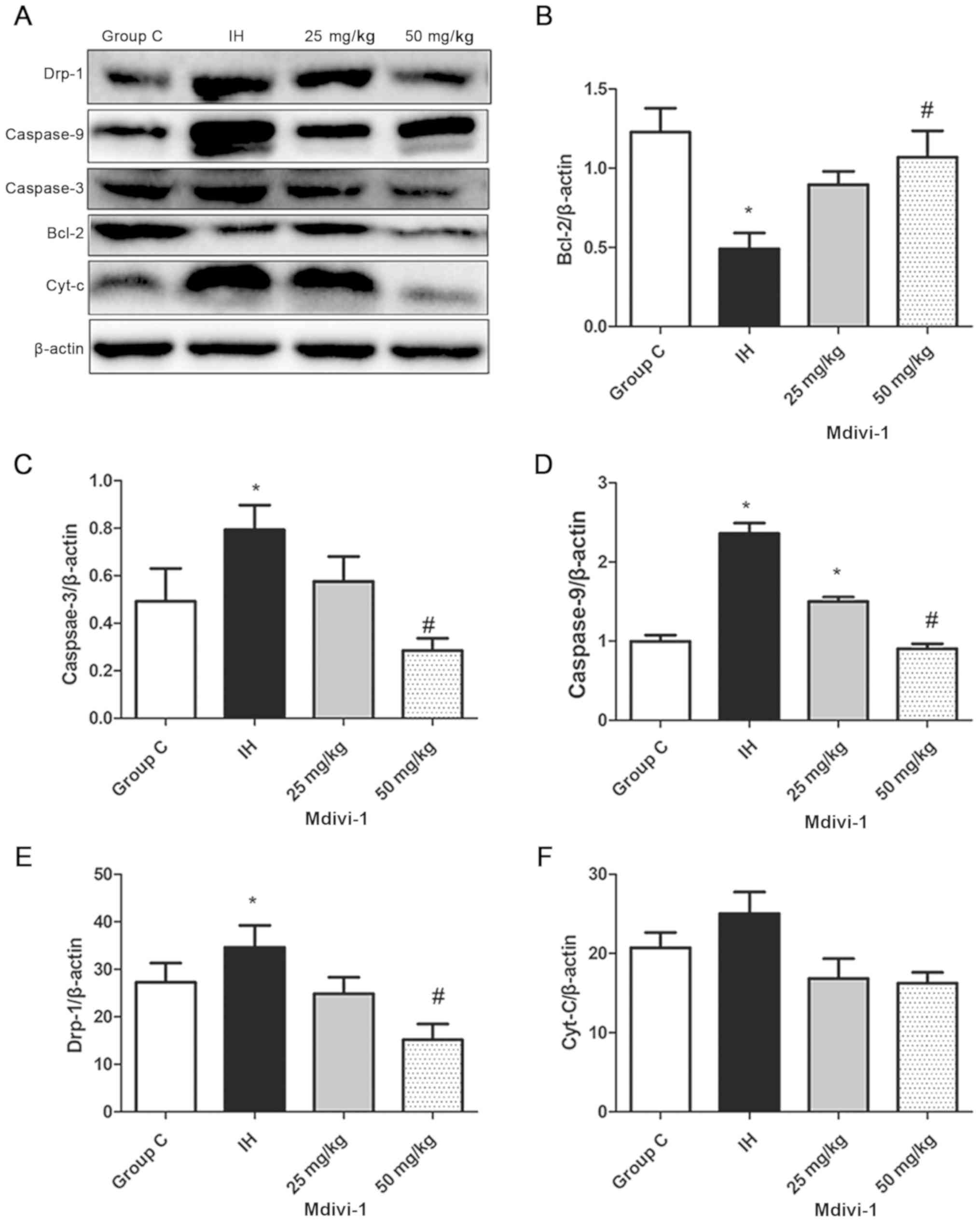 | Figure 4.Detection of Bcl-2, caspase-3,
caspase-9, Cyt-C and Drp-1 protein expression levels in rat lungs
via western blotting assays. (A) (Lane 1) Group C, n-8. (Lane 2) IH
group, n=8. (Lane 3) IH+25 mg/kg Mdivi-1 group, n=8. (Lane 4) IH+50
mg/kg Mdivi-1 group, n=8. Compared with that in the group C, the
protein expression of (B) Bcl-2 in the IH group was decreased and
that of (C) caspase-3, (D) caspase-9 and (E) Drp-1 was increased in
the IH group. (F) The changes in protein expression of Cyt C were
not significant. *P<0.05 vs. group C. Compared with the IH
group, the protein expression of Bcl-2 was increased and caspase-3,
caspase-9, and Drp-1 was decreased in the IH+50 mg/kg Mdivi-1
group, #P<0.05 vs. IH. IH, intermittent hypoxia;
Mdivi, mitochondrial division inhibitor-1; Bcl, B-cell lymphoma;
Cyt-c, cytochrome C; group C, control group. |
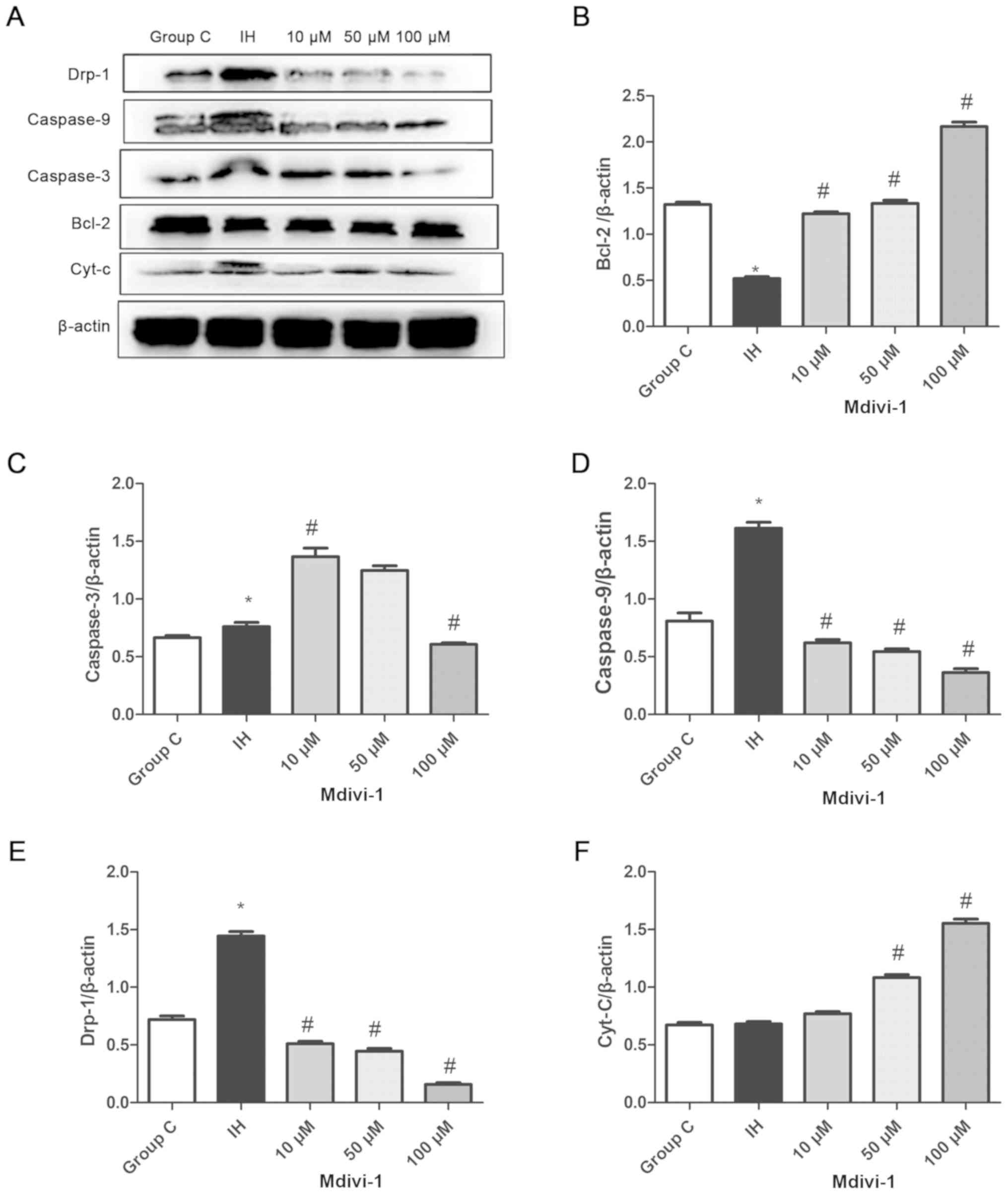 | Figure 5.Detection of Bcl-2, caspase-3,
caspase-9, Cyt-C and Drp-1 expression levels in WTRL1 cells via
western blotting assays. (A) (Lane 1) Group C, n-8. (Lane 2) IH
group, n=8. (Lane 3) IH+10 µM Mdivi-1 group, n=8. (Lane 4) IH+50 µM
Mdivi-1 group, n=8. (Lane 5) IH+100 µM Mdivi-1 group, n=8. Compared
with the group C, (B) Bcl-2 protein expression in the IH group was
decreased, but (C) caspase-3, (D) caspase-9 and (E) Drp-1 protein
expression was increased, *P<0.05 vs. group C. Compared with the
IH group, Bcl-2 protein expression was increased and caspase-9
Drp-1 and (F) Cyt-C protein expression was decreased in the IH+100
µM Mdivi-1 group, #P<0.05 vs. IH. Mdivi,
mitochondrial division inhibitor-1; Drp-1, dynamin-related protein
1; IH, intermittent hypoxia; Bcl, B-cell lymphoma; group C, Control
group. |
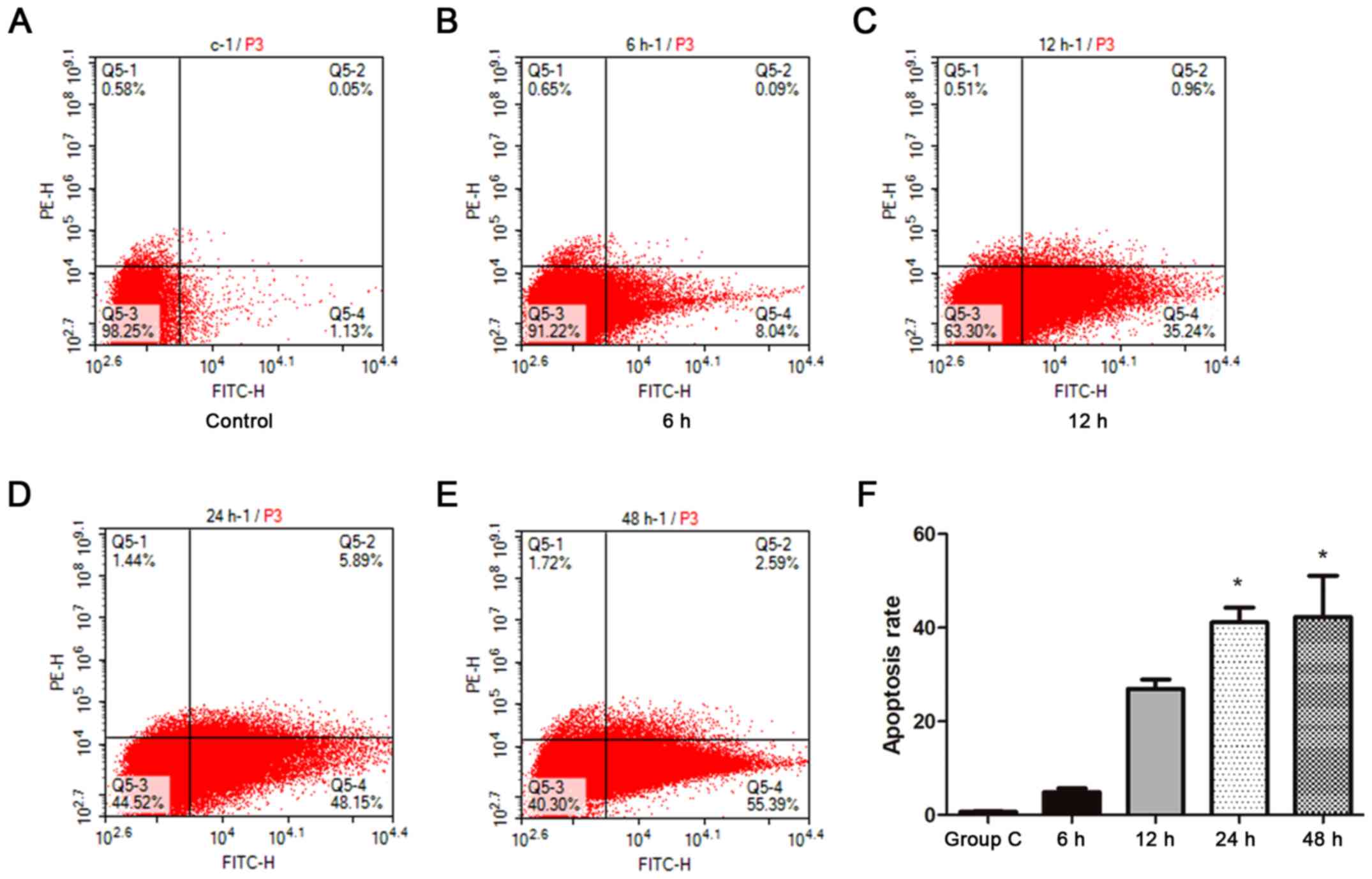
Detection of apoptosis levels
The apoptosis rate of WTRL1 cells was positively
correlated with the time of exposure to IH. After WTRL1 cell
exposure to IH for 6, 12, 24 and 48 h, the rate of apoptosis in
WTRL1 cells increased gradually with the IH exposure time, and the
apoptosis rate was the highest at 48 h (P<0.05; Fig. 6).
Discussion
OSA is characterized by intermittent airway
obstruction and systemic hypoxia during sleep. OSA affects 5–25% of
the general population. However, previous studies have illustrated
a rising prevalence of moderate-to-severe sleep disordered
breathing in men (ranging from 10–49%, depending on age and cohort)
and women (ranging from 10–23%) in western countries (20,21). OSA
frequently goes unnoticed by people who are at highest risk for
developing the disease (22–25). OSA is recognized as a low-grade
systemic inflammatory disease; it induces inflammatory responses
and can be an indication of metabolic deregulation, in addition to
posing a great risk for apoptotic cell death (26). Apoptosis can be triggered by two
distinct signalling pathways, namely, the intrinsic and extrinsic
pathways (27,28). The intrinsic apoptotic pathway is
elicited by a wide range of intracellular stress conditions,
including cytokine deprivation, DNA damage, oxidative stress,
cytosolic Ca2+ overload and endoplasmic reticulum
stress. In the extrinsic pathway, apoptosis is triggered by death
receptors, including FAS-associated death domain protein, therefore
activating caspases-8 and 10 (the initiator caspases), which in
turn activate executioner caspases-3, 6 and 7. In the intrinsic
pathway, the mitochondrial permeability transition pore serves a
pivotal role in regulating the release of pro-apoptotic proteins,
including Cyt-C. The Cyt-C released from mitochondria initiates
apoptosome assembly, activating factor 1 and caspase-9, an
initiator caspase that cleaves and activates caspases-3 and 7
(29).
IH causes mitochondrial and endoplasmic reticulum
system damage and produces inflammatory factors that cause lung
injury. Sustained and IH-induced cell apoptosis mediates the
mitochondrial apoptosis signalling pathway (30). A number of hypoxia and inflammatory
factors activate caspases via the mitochondrial apoptosis
signalling pathway. Mitochondrial morphology is now recognized as
an important determinant of the energetic state of mitochondria.
IH/hypercapnia leads to oxidative stress due to the mitochondrial
response. Mitochondria constantly undergo fusion and fission, which
are essential for organelle fidelity. Lung cell apoptosis occurs
through a highly regulated proteolytic process that eliminates
unwanted, damaged, or altered cells. However, abnormal
mitochondrial fission is involved in regulating apoptosis (31).
Mdivi-1 has emerged as a critical regulator of
cellular function and differentiation under hypoxic conditions.
However, the role of mitochondrial dynamics in hypoxia-induced
angiogenesis remains to be elucidated. The aim of the present study
was to investigate the protective effect and possible mechanism of
Mdivi-1 in lung cell apoptosis induced by IH.
Several studies have revealed that Drp-1
participates in mitochondrial fission and the subsequent apoptosis
induced by various stimuli (32,33).
Drp-1 assembles from the cytosol onto mitochondria at focal sites
of mitochondrial fission; emerging evidence indicates that it is
concomitantly involved in apoptosis with mitochondrial outer
membrane permeabilization and that Drp-1 inhibition prevents
partially intrinsic apoptosis (34,35). The
activity of the prototypical Bcl-2 protein is usually considered
anti-apoptotic (36). The Bcl-2
family is classified into three subgroups: Pro-apoptotic proteins
(Bax, BAD, Bak and Bok); anti-apoptotic proteins (Bcl-2, Bcl-xL and
Bcl-w) and BH3-only members [Bad, Bid, Bim, Noxa and PUMA (a
p53-upregulated modulator of apoptosis)]. The caspase family of
cysteine-aspartic proteases serves an important role in the
initiation and completion of cell apoptosis and executes cellular
apoptosis. For instance, caspase-3 is the primary activator of
apoptotic DNA fragmentation. Certain reports have demonstrated that
the pro-apoptotic signalling of Cyt-C triggers the caspase cascade
amplification reaction, which leads to the cleavage of a set of
proteins (37,38).
Mitochondria are autonomous and morphologically
dynamic organelles that structurally reflect a precise balance of
ongoing fission and fusion within a cell. In the two models, in
vitro and in vivo, it was observed that Mdivi-1
decreases mitochondrial fission and inhibits cell apoptosis in the
lungs. These results agree with evidence from previous studies
(39,40). In this study, it was demonstrated
that inhibiting mitochondrial fission by Mdivi-1 prevents lung
apoptosis during IH and it was demonstrated that IH induced
mitochondrial fission and apoptosis via a mitochondrial mechanism
involving the transcriptional activation of caspase-3, caspase-9
and Drp-1. The results of the present study provide a
well-characterized mechanism explaining how IH stimulates
mitochondrial fission and consequent apoptosis, which may offer a
novel therapeutic strategy for treating OSA via the mitochondrial
apoptosis pathway.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Youth Fund
Project of Guizhou Provincial People's Hospital (GZSYQN [2016]
grant no. 16); the Technology Project of Guizhou Province (contract
[2016] No. 2907); the Major Disease Prevention and Control Research
of Guizhou Province (contract [2015] grant no. 78); the Science and
Technology Project of a tobacco corporation (Guizhou Province
division) of China (contract [201608]).
Availability of data and materials
The datasets used and/or analysed in the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
DZ and XY contributed to the conception and design
of the study. CY and ZW collected the data and revised the
manuscript for important intellectual content. DZ and XZ analysed
and interpreted the data and drafted the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving
animals were in accordance with the ethical standards of the
institution or practice at which the studies were conducted. The
study was approved by the Ethics Committee of Guizhou Provincial
People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Young T, Evans L, Finn L and Palta M:
Estimation of the clinically diagnosed proportion of sleep apnea
syndrome in middle-aged men and women. Sleep. 20:705–706. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Punjabi NM: The epidemiology of adult
obstructive sleep apnea. Proc Am Thorac Soc. 5:136–143. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakao S, Taraseviciene-Stewart L, Lee JD,
Wood K, Cool CD and Voelkel NF: Initial apoptosis is followed by
increased proliferation of apoptosis-resistant endothelial cells.
FASEB J. 19:1178–1180. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haslip M, Dostanic I, Huang Y, Zhang Y,
Russell KS, Jurczak MJ, Mannam P, Giordano F, Erzurum SC and Lee
PJ: Endothelial Ucp2 regulates mitophagy and pulmonary hypertension
during intermittent-hypoxia. Arterioscler Thromb Vasc Biol.
35:1166–1178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou S, Yin X, Zheng Y, Miao X, Feng W,
Cai J and Cai L: Metallothionein prevents intermittent
hypoxia-induced cardiac endoplasmic reticulum stress and cell death
likely via activation of Akt signaling pathway in mice. Toxicol
Lett. 227:113–123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu KX, Chen GP, Lin PL, Huang JC, Lin X,
Qi JC and Lin QC: Detection and analysis of apoptosis- and
autophagy-related miRNAs of mouse vascular endothelial cells in
chronic intermittent hypoxia model. Life Sci. 193:194–199. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ren J, Liu W, Deng Y, Li GC, Pan YY, Xie
S, Jin M and Liu HG: Losartan attenuates aortic endothelial
apoptosis induced by chronic intermittent hypoxia partly via the
phospholipase C pathway. Sleep Breath. 21:679–689. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang J, Hansen K, Edwards R, Van Houten B
and Qian W: Mitochondrial division inhibitor 1 (mdivi-1) enhances
death receptor-mediated apoptosis in human ovarian cancer cells.
Biochem Biophys Res Commun. 456:7–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim DY, Jung SY, Kim YJ, Kang S, Park JH,
Ji ST, Jang WB, Lamichane S, Lamichane BD, Chae YC, et al:
Hypoxia-dependent mitochondrial fission regulates endothelial
progenitor cell migration, invasion, and tube formation. Korean J
Physiol Pharmacol. 22:203–213. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gonder JC and Laber K: A renewed look at
laboratory rodent housing and management. ILAR J. 48:29–36. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shortt CM, Fredsted A, Chow HB, Williams
R, Skelly JR, Edge D, Bradford A and O'Halloran KD: Reactive oxygen
species mediated diaphragm fatigue in a rat model of chronic
intermittent hypoxia. Experiment physiology. 99:688–700. 2014.
View Article : Google Scholar
|
|
12
|
Briancon-Marjollet A, Monneret D, Henri M,
Hazane-Puch F, Pepin JL, Faure P and Godin-Ribuot D: Endothelin
regulates intermittent hypoxia-induced lipolytic remodelling of
adipose tissue and phosphorylation of hormone-sensitive lipase. J
Physiol. 594:1727–1740. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Poorhassan M, Navae F, Mahakizadeh S,
Bazrafkan M, Nikmehr B, Abolhassani F, Ijaz S, Yamini N, Dashti N,
Mehrannia K, et al: Flaxseed can reduce hypoxia-induced damages in
rat testes. Int J Fertil Steril. 12:235–241. 2018.PubMed/NCBI
|
|
14
|
Krause BJ, Casanello P, Dias AC, Arias P,
Velarde V, Arenas GA, Preite MD and Iturriaga R: Chronic
intermittent hypoxia-Induced vascular dysfunction in rats is
reverted by N-Acetylcysteine supplementation and arginase
inhibition. Front Physiol. 9:9012018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park SW, Kim KY, Lindsey JD, Dai Y, Heo H,
Nguyen DH, Ellisman MH, Weinreb RN and Ju WK: A selective inhibitor
of drp1, mdivi-1, increases retinal ganglion cell survival in acute
ischemic mouse retina. Invest Ophthalmol Vis Sci. 52:2837–2843.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Burkholder T, Foltz C, Karlsson E, Linton
CG and Smith JM: Health evaluation of experimental laboratory mice.
Curr Protoc Mouse Biol. 2:145–165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kostopanagiotou G, Avgerinos E,
Costopanagiotou C, Arkadopoulos N, Andreadou I, Diamantopoulou K,
Lekka M, Smyrniotis V and Nakos G: Acute lung injury in a rat model
of intestinal ischemia-reperfusion: The potential time depended
role of phospholipases A(2). J Surg Res. 147:108–116. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Method. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Nicole NL, Satriotomo I, Harrigan DJ and
Mitchell GS: Acute intermittent hypoxia induced phrenic long-term
facilitation despite increased SOD1 expression in a rat model of
ALS. Exp Neurol. 273:138–150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peppard PE, Young T, Barnet JH, Palta M,
Hagen EW and Hla KM: Increased prevalence of sleep-disordered
breathing in adults. Am J Epidemiol. 177:1006–10014. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heinzer R, Vat S, Marques-Vidal P,
Marti-Soler H, Andries D, Tobback N, Mooser V, Preisig M, Malhotra
A, Waeber G, et al: Prevalence of sleep-disordered breathing in the
general population: The HypnoLaus study. Lancet Respir Med.
3:310–318. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Drager LF, Togeiro SM, Polotsky VY and
Lorenzi-Filho G: Obstructive sleep apnea: A cardiometabolic risk in
obesity and the metabolic syndrome. J Am Coll Cardiol. 62:569–576.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Drager LF, Polotsky VY and Lorenzi-Filho
G: Obstructive sleep apnea: An emerging risk factor for
atherosclerosis. Chest J. 140:534–542. 2011. View Article : Google Scholar
|
|
24
|
Sahlin C, Sandberg O, Gustafson Y, Bucht
G, Carlberg B, Stenlund H and Franklin KA: Obstructive sleep apnea
is a risk factor for death in patients with stroke: A 10-year
follow-up. Arch Intern Med. 168:297–301. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie C, Zhu R, Tian Y and Wang K:
Association of obstructive sleep apnoea with the risk of vascular
outcomes and all-cause mortality: A meta-analysis. BMJ Open.
7:e0139832017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zychowski KE, Sanchez B, Pedrosa RP,
Lorenzi-Filho G, Drager LF, Polotsky VY and Campen MJ: Serum from
obstructive sleep apnea patients induces inflammatory responses in
coronary artery endothelial cells. Atherosclerosis. 254:59–66.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Galluzzi L, Vitale I, Abrams JM, Alnemri
ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry
WS, Fulda S, et al: Molecular definitions of cell death
subroutines: Recommendations of the nomenclature committee on cell
death 2012. Cell Death Differ. 19:107–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mariño G, Niso-Santano M, Baehrecke EH and
Kroemer G: Self-consumption: The interplay of autophagy and
apoptosis. Nat Rev Mol Cell Biol. 15:81–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Douglas RM, Ryu J, Kanaan A, Del Carmen
Rivero M, Dugan LL, Haddad GG and Ali SS: Neuronal death during
combined intermittent hypoxia/hypercapnia is due to mitochondrial
dysfunction. Am J Physiol Cell Physiol. 298:C1594–C1602. 2012.
View Article : Google Scholar
|
|
31
|
Li J, Donath S, Li Y, Qin D, Prabhakar BS
and Li P: miR-30 regulates mitochondrial fission through targeting
p53 and the dynamin-related protein-1 pathway. PLoS Genet.
6:e10007952010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen Y, Lin JR and Gao PJ: Mitochondrial
division inhibitor Mdivi-1 ameliorates angiotensin II-induced
endothelial dysfunction. Sheng Li Xue Bao. 68:669–676.
2016.PubMed/NCBI
|
|
33
|
Oettinghaus B, D'Alonzo D, Barbieri E,
Restelli LM, Savoia C, Licci M, Tolnay M, Frank S and Scorrano L:
DRP1-dependent apoptotic mitochondrial fission occurs independently
of BAX, BAK and APAF1 to amplify cell death by BID and oxidative
stress. Biochim Biophys Acta 1857. 1267–1276. 2016.
|
|
34
|
Tanaka A and Youle RJ: A chemical
inhibitor of DRP1 uncouples mitochondrial fission and apoptosis.
Mol Cell. 29:409–410. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Frank S, Gaume B, Bergmann-Leitner ES,
Leitner WW, Robert EG, Catez F, Smith CL and Youle RJ: The role of
dynamin-related protein 1, a mediator of mitochondrial fission, in
apoptosis. Dev Cell. 1:515–525. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Di Pietro R and Zauli G: Emerging
non-apoptotic functions of tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL)/Apo2L. J Cell Physiol.
201:331–340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sikora E, Bielak-Zmijewska A, Magalska A,
Piwocka K, Mosieniak G, Kalinowska M, Widlak P, Cymerman IA and
Bujnicki JM: Curcumin induces caspase-3-dependent apoptotic pathway
but inhibits DNA fragmentation factor 40/caspase-activated DNase
endonuclease in human Jurkat cells. Mol Cancer Ther. 5:927–934.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Han XJ, Yang ZJ, Jiang LP, Wei YF, Liao
MF, Qian Y, Li Y, Huang X, Wang JB, Xin HB and Wan YY:
Mitochondrial dynamics regulates hypoxia-induced migration and
antineoplastic activity of cisplatin in breast cancer cells. Int J
Oncol. 46:691–700. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sanderson TH, Raghunayakula S and Kumar R:
Neuronal hypoxia disrupts mitochondrial fusion. Neuroscience.
301:71–78. 2015. View Article : Google Scholar : PubMed/NCBI
|