Introduction
Postoperative cognitive dysfunction (POCD) is a
common postsurgical complication of the central nervous system.
POCD occurs at an early stage of postoperative care and increases
the rates of perioperative mortality (1). The pathogenesis and prevention of POCD
have been extensively studied. The mechanism underlying the
behavioral alterations following surgery in animal models may be
associated with the dysfunction of neurons and synapses (2–6). It has
been hypothesized that the production and aggregation of amyloid β
(Aβ), and the abnormal hyperphosphorylation and aggregation of tau
protein may be involved in the pathogenesis of POCD (7). Aβ may alter the mitochondrial
morphology and affect the steady state of calcium in the neurons
(8). The hyperphosphorylation and
aggregation of tau protein serves an important role in
neurodegeneration (9).
The neuroprotective effect of α-lipoic acid (ALA)
has been previously studied (10)
and it was demonstrated that ALA promotes the secretion of leptin
from adipocytes (11). A number of
studies reported that leptin induces a protective effect on the
cognitive function (12–15). Leptin regulates glucose and fat
metabolism, and the leptin receptor activates brain-derived
neurotrophic factor to regulate the synaptic plasticity and promote
neural differentiation (12,13). Furthermore, leptin reduces the
hyperphosphorylation of tau protein (14–16).
There are no effective treatment methods for
patients with POCD and the underlying mechanism of this conditions
remains to be elucidated. To the best of the authors' knowledge,
the current study is the first to investigate the effect of ALA on
POCD and the underlying mechanism of action. The present study
aimed to investigate the effect of ALA on POCD induced by
hepatectomy in wild type (WT) and leptin receptor-deficient (db/db)
mice. Protein expression levels of Cdk5, phosphorylated tau (p-tau)
and Aβ were determined by western blotting, and the ultrastructure
of hippocampal neurons and synapses was analyzed by transmission
electron microscopy.
Materials and methods
Experimental animals
A total of 60 WT C57BL/6 mice and 60 db/db C57BL/6
mice (all male; weight, 20–25 g; age, 14 weeks) were obtained from
Beijing Vital River Laboratory Animal Technology Co., Ltd.
(Beijing, China). Mice were housed with a 12 h light/dark cycle at
a temperature of 24±1°C with access to food and water ad
libitum. ALA (60 mg/kg; Wyeth Pharmaceutical Co., Ltd., Suzhou,
China) or 1% dimethyl sulfoxide in corn oil (vehicle) was
administrated orally daily. At the end of the experiment, the
animals were euthanized by intraperitoneal administration of a
lethal dose of sodium pentobarbital (150 mg/kg). The present study
was approved by the Institution Animal Care and Use Committee of
Nanjing First Hospital (Nanjing, China).
Animal grouping
C57BL/6 WT and db/db mice were divided randomly into
three groups each, including the control, surgery and ALA + surgery
groups (20 mice/group). Control group mice received anesthesia
only, the surgery group received 70% hepatectomy surgery, and the
ALA + surgery group was intragastrically administrated 100 mg/kg
ALA once daily for 12 weeks (17),
and subsequently received 70% hepatectomy surgery. The control and
surgery groups received the same volume of vehicle intragastrically
(WT, 20 µl/mice; db/db, 30 µl/mice).
Hepatectomy surgery
Mice were anesthetized by an intraperitoneal
injection of 50 mg/kg sodium pentobarbital (1% in saline). Mice
were subsequently placed on a warming blanket and rectal
temperatures were monitored. A roll of gauze was placed under the
right scapula to provide adequate exposure of the liver. The
abdomen of the mice was shaved, sterilized and draped. A 1–1.5-cm
midline incision was made from the xiphoid inferiorly with micro
dissecting scissors. The upper abdomen was opened and the three
anterior lobes of the liver were isolated (~68% of the total liver
weight), including the right upper lobe, left upper lobe and left
lower lobe. Three knots were tied with moistened silk suture at the
base of the lobes near the inferior vena cava. The tied lobes were
subsequently cut immediately distal to the suture. The abdomen was
irrigated with 2 ml of sterile saline (37°C) to ensure the removal
of blood and to decrease contamination. Gentle pressure was applied
on the abdomen with sterile gauze to remove residual irrigation.
The wound was infiltrated with 0.25% bupivacaine to relieve pain.
The peritoneum and the skin were subsequently closed separately
with silk suture. The mice were placed under warming lights while
waking up from anesthesia, and subsequently housed individually
(18).
Morris water maze (MWM)
Cognitive function was assessed using a 5-day MWM
test as previously described (19).
A round tub was used for the MWM task and filled with water mixed
with nontoxic black paint to submerge a platform 1 cm below the
water surface (water temperature, 19–24°C). Visual distal cues were
located on the walls. During the first four days, mice were given
four trials/day using a random starting location. If mice did not
reach the platform within 60 sec, they were gently guided to the
hidden platform. The latency to reach the platform was recorded
from all sessions, and averaged to calculate the escape latency for
each day. On day 5, the platform was removed for probe testing.
Mice were allowed to swim freely for 60 sec. The number of
crossings over the platform that had served as the target on days
1–4 was recorded. The time spent in the quadrant where the target
platform was located was recorded. Data were collected and analyzed
using motion detection software (DigBehv-MM; Shanghai Jiliang
Software Technology Co., Ltd., Shanghai, China).
Transmission electron microscopy
Mice were intraperitoneally injected with 1%
pentobarbital sodium (35 mg/kg) and then fixed on a foam plate. A
large U-shaped incision was cut into the chest to expose the heart.
The perfused needle was inserted into the apical part with 5 mm
depth and fixed by hemostatic clamp. Heparin saline was infused
until liver became pale, followed with 3% glutaraldehyde perfusion.
The brain was removed, and the hippocampus was isolated and
post-fixed in 2.5% glutaraldehyde solution for 2 h in 4°C, followed
by embedding in the epoxy resin for 12 h at 45°C. The embedded
tissues were cut into 50–70-nm-thick sections and mounted on 150
mesh copper grids. Following staining with uranyl acetate and lead
citrate for 12 h in 4°C, the specimens were observed under a
transmission electron microscope (HITACHI-7650; Hitachi, Ltd.,
Tokyo, Japan).
Western blotting
The hippocampal tissue was homogenized with Tris-HCl
(pH 7.4; 50 mM), 1% Triton X-100, 0.2% sodium deoxycholate, 0.2%SDS
and 1 mM EDTA (Beyotime Institute of Biotechnology, Shanghai,
China), and centrifuged at 8,000 × g for 20 min at 4°C. The protein
concentration from each mouse was tested using the bicinchoninic
acid method. Protein (40 µg/lane) was separated by SDS-PAGE (12%
gel) and transferred to a nitrocellulose membrane
(Hybond® ECL™; GE Healthcare, Chicago, IL, USA).
Membranes were blocked with 5% milk and 0.1% Tween-20 in PBS for 1
h at room temperature. Membranes were then incubated with primary
antibodies against Cdk5 (cat. no. ab21249), tau (cat. no. ab80579),
β-actin (cat. no. ab8227; all 1:1,000), Aβ (cat. no. ab10148;
1:7,000; all Abcam, Cambridge, MA, USA) and p-tau (cat. no.
44-750G; 1:1,000; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
overnight at 4°C. After washing with tris buffered saline with
Tween-20 (TBST) three times, membranes were incubated with
anti-rabbit secondary antibodies (cat. no. ab6721; 1:2,000; Abcam)
for 2 h at room temperature. Between steps, blots were washed with
TBST. Immunodetection was performed using a LumiGLO
chemiluminescence kit (Amersham; GE Healthcare). Bands were
analyzed by Quantity One software (version 4.6.2; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation. Data were analyzed using SPSS software (version 17.0;
SPSS, Inc., Chicago, IL, USA) or GraphPad Prism (version 5.0;
GraphPad Software, Inc., La Jolla, CA, USA). Two-way analysis of
variance (ANOVA) was used to analyze the spatial memory data
obtained from the MWM test. The levels of protein and gene
expression were analyzed using one-way ANOVA. ANOVA analyses were
followed by the Bonferroni post-hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
ALA rescues the impaired cognitive
function in WT but not db/db mice
To study cognitive function, MWM was used to examine
spatial learning and memory in both WT and db/db mice with or
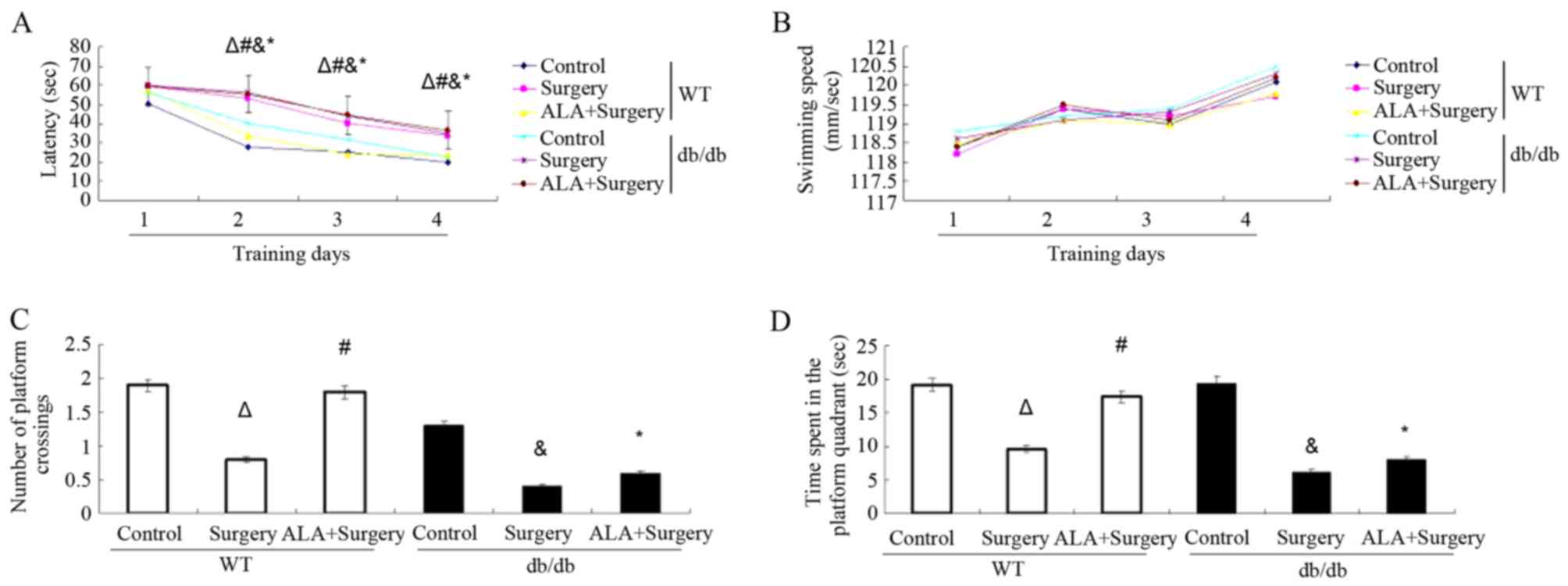
without treatment with ALA. Among the WT mice, the surgery group
(day 2, 53.0±5.3; day 3, 40.1±6.7; day 4, 33.8±4.1) exhibited
significantly longer latency to find the platform compared with the
control group (day 2, 27.7±3.7; day 3, 25.0±5.9; day 4, 19.9±3.9;
all P<0.01). Among the db/db mice, the surgery group (day 2,
56.3±2.1; day 3, 43.8±3.0; day 4, 35.0±1.5) exhibited significantly
longer latency to find the platform compared with the control group
(day 2, 40.3±6.9; day 3, 31.8±6.3; day 4, 22.3±3.9; P<0.01). The
db/db ALA + surgery group (day 2, 55.4±2.2; day 3, 44.5±4.8; day 4:
36.7±4.3) did not exhibit altered latency compared with the surgery
only group (P>0.05; Fig. 1A).
However, the db/db ALA + surgery group exhibited significantly
longer latency compared with the WT ALA + surgery group (P<0.01;
Fig. 1A). These results suggest that
hepatectomy surgery prolonged the escape latency during the MWM
test in both WT and db/db mice. Treatment with ALA alleviated the
effect induced by surgery in WT mice but not in db/db mice. The
swimming speed was recorded during the leaning sessions in the MWM
test. The results revealed that there were no differences in the
swimming speed among the control, surgery and ALA + surgery groups
in WT and db/db mice (Fig. 1B).
On probe testing day, WT mice in the surgery group
crossed the target quadrant fewer times (0.8±0.5 vs. 1.9±0.8;
P<0.01) and spent less time in the platform quadrant (9.6±7.7
vs. 19.2±6.3; P<0.01) compared with the control group. Treatment
with ALA increased the time spent in the platform quadrant
(17.4±8.0 vs. 9.6±7.7; P<0.05) and crossing number (1.8±0.6 vs.
0.8±0.5; P<0.05) in the target quadrant compared with the
surgery group. Among the db/db mice, the surgery group crossed the
target quadrant fewer times (0.4±0.3 vs. 1.3±0.9; P<0.01) and
spent less time in the platform quadrant (6.2±3.0 vs. 19.5±6.9;
P<0.01) compared with the control group. However, the crossing
number (0.6±0.4 vs. 0.4±0.3; P>0.05) and time spent in the
platform quadrant (8.0±5.4 vs. 6.2±3.0; P>0.05) remained
unaltered following administration of ALA compared with the surgery
group among the db/db mice (Fig. 1C and
D). The ALA + surgery group db/db mice spent less time in the
platform quadrant (8.0±3.1 vs. 17.4±8.0; P<0.01) and crossed the
platform fewer times (0.6±0.3 vs. 1.8±0.6; P<0.05) compared to
ALA + surgery group WT mice (P<0.01; Fig. 1C and D).
Hepatectomy increases Cdk5, p-tau and
Aβ protein levels in both WT and db/db mice, and this effect is
reversed following treatment with ALA among WT mice but not db/db
mice
Western blotting was used to assess the expression
levels of cognitive function-associated proteins Cdk5 and Aβ and
the phosphorylation levels of tau. Among the WT mice, surgery
significantly increased the protein expression of Cdk5 and Aβ and
the p/total (t) tau ratio in the hippocampus compared with the
control group (P<0.01). Administration pf ALA following surgery
significantly reduced the protein expression of Cdk5 and Aβ, and
the p/t tau ratio in the hippocampus compared with the surgery
group (P<0.05; Fig. 2).
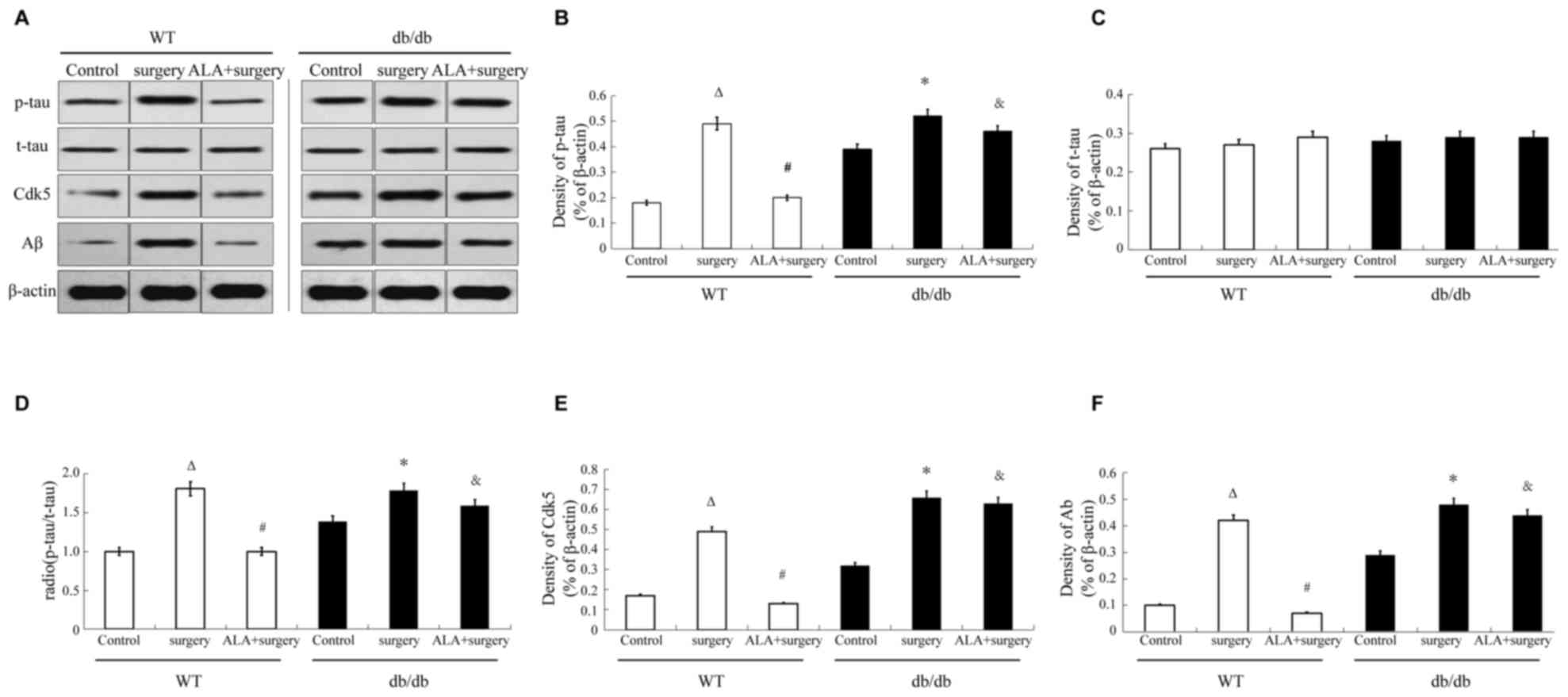 | Figure 2.Effects of ALA on surgery-induced
alterations in Cdk5 and Aβ protein expression levels and p/t tau
ratio in the hippocampus. (A) Representative images of western
blotting for the analysis of p-tau, t-tau, Cdk5, Aβ and β-actin
protein expression. (B) Quantitative analysis of p-tau protein
expression relative to β-actin. (C) Quantitative analysis of t-tau
protein expression relative to β-actin. (D) Quantitative analysis
of p-tau to t-tau protein expression ratio. (E) Quantitative
analysis of Cdk5 protein expression relative to β-actin. (F)
Quantitative analysis of Aβ protein expression relative to β-actin.
Data are presented as the mean ± standard deviation (n=5/group).
∆P<0.01 compared with the WT control group;
#P<0.05 compared with the surgery group; *P<0.01
compared with the db/db control group; &P<0.01
compared with the WT ALA + surgery group. ALA, α-lipoic acid; WT,
wild type; db/db, leptin receptor-deficient mice; p,
phosphorylated; t, total; Cdk5, cyclin-dependent kinase 5; Aβ,
amyloid β. |
Among the db/db mice, surgery significantly
increased the protein expression of Cdk5 and Aβ and the p/total (t)
tau ratio in the hippocampus compared with the control group
(P<0.01). However, treatment with ALA did not alter the protein
expression of Cdk5 and Aβ or the p/t tau ratio compared with the
surgery group. The db/db ALA + surgery group mice exhibited
significantly increased expression of Cdk5 and Aβ, and p/t tau
ratio in the hippocampus compared with the WT ALA + surgery group
in mice (P<0.01; Fig. 2).
Hepatectomy impairs cellular structure
of the hippocampus of WT and db/db mice, and this effect is
reversed following treatment with ALA among WT mice but not db/db
mice
The ultrastructure of the CA3 hippocampal region was
analyzed using transmission electron microscopy (Fig. 3). The images revealed that among the
WT mice, surgery (Fig. 3B) impaired
the ultrastructure of hippocampal neurons, which exhibited
pyknosis, shrinkage of nuclear membrane, broadened perinuclear
space and reduced synaptic density. Administration of ALA after
surgery (Fig. 3C) improved the
neuronal structure in the hippocampus, which appeared similar to
the control group (Fig. 3A),
exhibiting a smooth nuclear membrane and normal synaptic density
and structure.
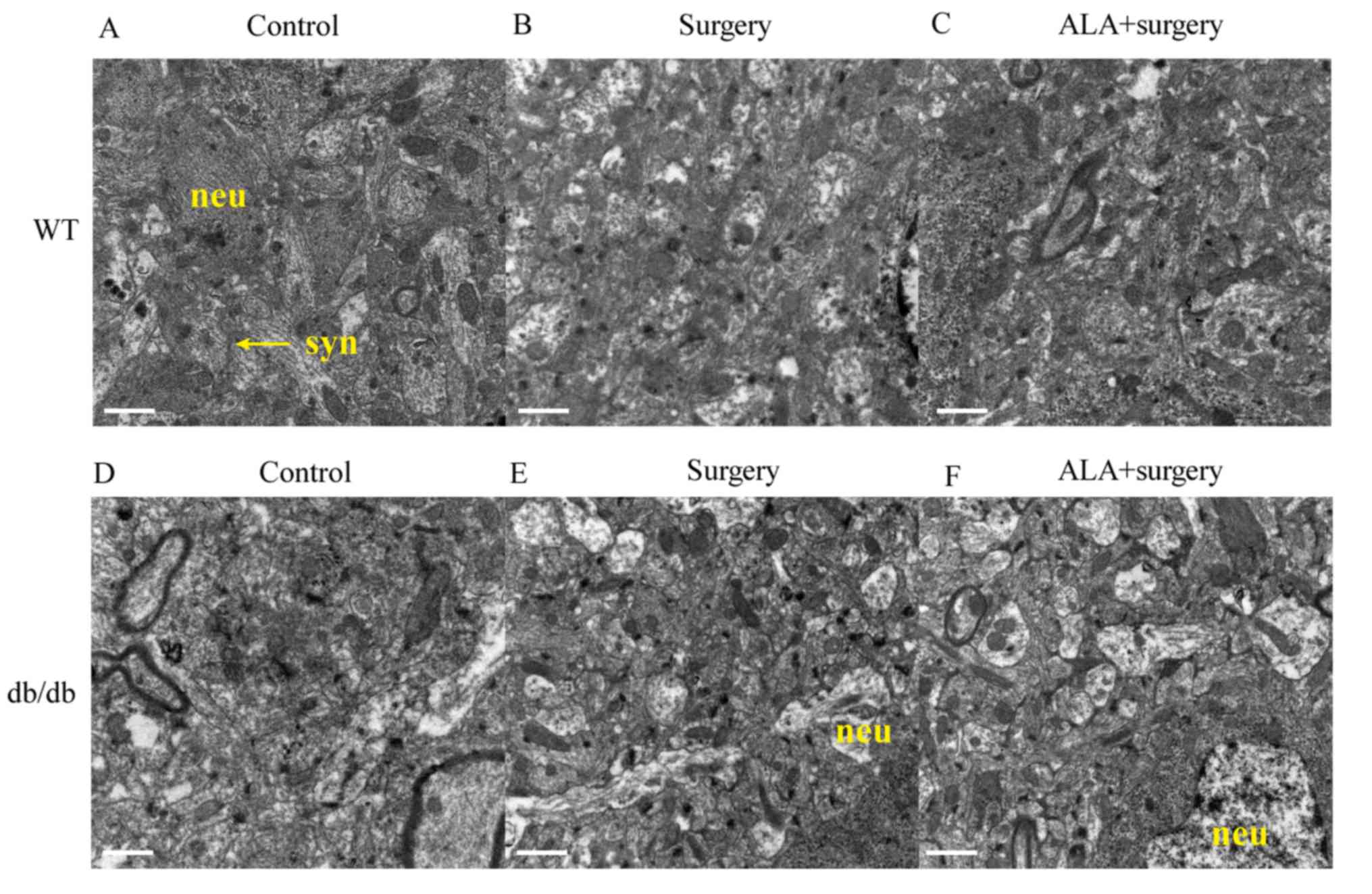 | Figure 3.Ultrastructure of neurons and synapses
in the hippocampus. The ultrastructure images from electron
microscopy presenting the results for (A) WT control group, (B) WT
surgery group, (C) WT ALA + surgery group, (D) db/db control group,
(E) db/db surgery group, (F) db/db ALA+surgery group. Scale bar=1
µm. Neu, neuron; syn, synapse; ALA, α-lipoic acid; WT, wild type;
db/db, leptin receptor-deficient mice. |
Among the db/db mice, surgery (Fig. 3E) impaired the ultrastructure of
hippocampal neurons and synapses compared with the control group.
However, treatment with ALA + surgery (Fig. 3F) did not repair the neuronal
structure in the hippocampus, which was different compared with the
normal structure of the control group (Fig. 3D).
Discussion
A previous study reported that ALA can increase the
level of leptin in serum through the regulation of Cdk5. Cdk5
serves an important role in neuron differentiation and synaptic
plasticity by phosphorylating cytoskeleton proteins and signaling
pathway molecules (16). Cdk5 is a
serine/threonine protein kinase that forms complexes with p35 or
p39 and is essential for neural development and function (20). Downregulation of CDK5 has been
demonstrated to mitigate hippocampal degeneration and cognitive
dysfunction (16).
The effects of ALA on the cognitive function
following hepatectomy and the possible underlying mechanism were
studied using db/db and WT mice. The results revelated that surgery
impaired postoperative cognitive function, increased the
hippocampal expression of Cdk5 and Aβ proteins and the
phosphorylation of tau, and damaged the structure of hippocampal
neurons and synapses in both WT and db/db mice. ALA rescued the
cognitive function of WT mice after surgery, as revealed by the
results of the MWM learning and test.
In addition, analysis of protein expression in the
hippocampus revealed that treatment with ALA decreased the elevated
protein expression levels of Cdk5 and Aβ and the p/t tau ratio in
WT mice subjected to surgery. However, this effect was not observed
among the db/db mice. The ultrastructure of neurons and synapses in
the hippocampus was observed in mice following surgery and
treatment with ALA. Hepatectomy markedly damaged the structure of
neurons and synapses in both WT and db/db mice. However, treatment
with ALA repaired the structure of neurons and synapses in WT mice,
but not the db/db mice. These results suggested that the
improvement in cognitive function following administration of ALA
may be associated with reduced protein expression levels of Cdk5
and Aβ, and the phosphorylation level of tau. Furthermore,
treatment with ALA enabled maintenance of the normal structure of
hippocampal neurons and synapses.
Treatment with ALA did not improve the cognitive
function of db/db mice following hepatectomy, which may suggest
that the leptin signaling pathway may be a potential target of ALA.
ALA may regulate the expression of leptin or regulate the biding to
leptin receptor to alter the expression levels of Cdk5 and Aβ, and
the phosphorylation level of tau to maintain the normal structure
of hippocampal neurons and synapses. Future studies should
investigate the effect of ALA on the leptin signaling pathway and
cognitive function in WT mice with hepatectomy.
Acknowledgements
Not applicable.
Funding
The current study was supported by grants from the
National Natural Science Foundation (grant no. 81873954) and the
Medicine Development Project of Nanjing Scientific Committee (grant
no. YKK15089).
Availability of data and materials
All data generated or analyzed during this study are
available from the corresponding author on reasonable request.
Authors' contributions
YZ, HGB conceived the study design. YZ and YLL
performed the experiments. YNS, JWZ and YNQ participated in the
data analysis and interpretation.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Krenk L and Rasmussen LS: Postoperative
delirium and postoperative cognitive dysfunction in the
elderly-what are the differences? Minerva Anestesiol. 77:742–749.
2011.PubMed/NCBI
|
|
2
|
Hovens IB, Schoemaker RG, van der Zee EA,
Absalom AR, Heineman E and van Leeuwen BL: Postoperative cognitive
dysfunction: Involvement of neuroinflammation and neuronal
functioning. Brain Behav Immun. 38:202–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mézière A, Paillaud E and Plaud B:
Anesthesia in the elderly. Presse Med. 42:197–201. 2013.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miyoshi E, Wietzikoski EC, Bortolanza M,
Boschen SL, Canteras NS, Izquierdo I and Da Cunha C: Both the
dorsal hippocampus and the dorsolateral striatum are needed for rat
navigation in the Morris water maze. Behav Brain Res. 226:171–178.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sawamura S: Postoperative care of the
elderly. Nihon Rinsho. 71:1060–1064. 2013.(In Japanese). PubMed/NCBI
|
|
6
|
Wuri G, Wang DX, Zhou Y and Zhu SN:
Effects of surgical stress on long-term memory function in mice of
different ages. Acta Anaesthesiol Scand. 55:474–485. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie Z and Tanzi RE: Alzheimer's disease
and post-operative cognitive dysfunction. Exp Gerontol. 41:346–359.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hovens IB, Schoemaker RG, van der Zee EA,
Heineman E, Nyakas C and van Leeuwen BL: Surgery-induced behavioral
changes in aged rats. Exp Gerontol. 48:1204–1211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gustafson DR: Adiposity and cognitive
decline: Underlying mechanisms. J Alzheimers Dis. 30 Suppl
2:S97–S112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deng C, Sun Z, Tong G, Yi W, Ma L, Zhao B,
Cheng L, Zhang J, Cao F and Yi D: α-Lipoic acid reduces infarct
size and preserves cardiac function in rat myocardial
ischemia/reperfusion injury through activation of PI3K/Akt/Nrf2
pathway. PLoS One. 8:e583712013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kandeil MA, Amin KA, Hassanin KA, Ali KM
and Mohammed ET: Role of lipoic acid on insulin resistance and
leptin inexperimentally diabetic rats. J Diabetes Complications.
25:31–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta A: Leptin as a neuroprotective agent
in glaucoma. Med Hypotheses. 81:797–802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Folch J, Pedrós I, Patraca I, Sureda F,
Junyent F, Beas-Zarate C, Verdaguer E, Pallàs M, Auladell C and
Camins A: Neuroprotective and anti-ageing role of leptin. J Mol
Endocrinol. 49:R149–R156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Irving AJ and Harvey J: Leptin regulation
of hippocampal synaptic function in health and disease. Philos
Trans R Soc Lond B Biol Sci. 369:201301552014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sánchez JC, Ospina JP and González MI:
Association between leptin and delirium in elderly inpatients.
Neuropsychiatr Dis Treat. 9:659–666. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shukla V, Skuntz S and Pant HC:
Deregulated Cdk5 activity is involved in inducing Alzheimer's
disease. Arch Med Res. 43:655–662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen WL, Kang CH, Wang SG and Lee HM:
α-Lipoic acid regulates lipid metabolism through induction of
sirtuin 1 (SIRT1) and activation of AMP-activated protein kinase.
Diabetologia. 55:1824–1835. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Higgins GM and Anderson RM: Experimental
pathology of the liver. I. Restoration of the liver of the white
rat following partial surgical removal. Arch Pathol. 12:186–202.
1931.
|
|
19
|
Ku S, Yan F, Wang Y, Sun Y, Yang N and Ye
L: The blood-brain barrier penetration and distribution of
PEGylated fluorescein-doped magnetic silica nanoparticles in rat
brain. Biochem Biophys Res Commun. 394:871–876. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wilkaniec A, Gąssowska-Dobrowolska M,
Strawski M, Adamczyk A and Czapski GA: Inhibition of
cyclin-dependent kinase 5 affects early neuroinflammatory
signalling in murine model of amyloid beta toxicity. J
Neuroinflammation. 15:12018. View Article : Google Scholar : PubMed/NCBI
|