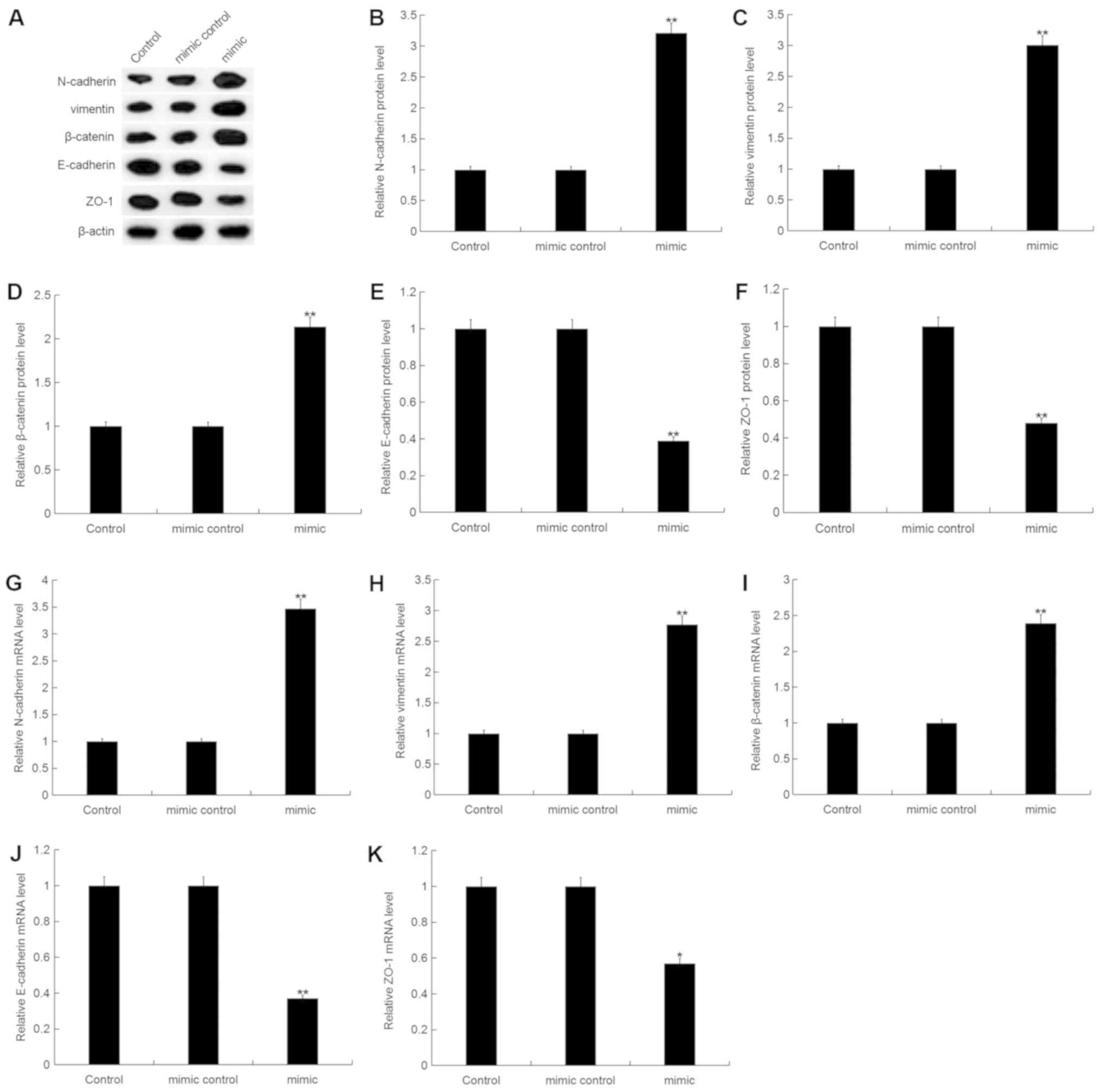
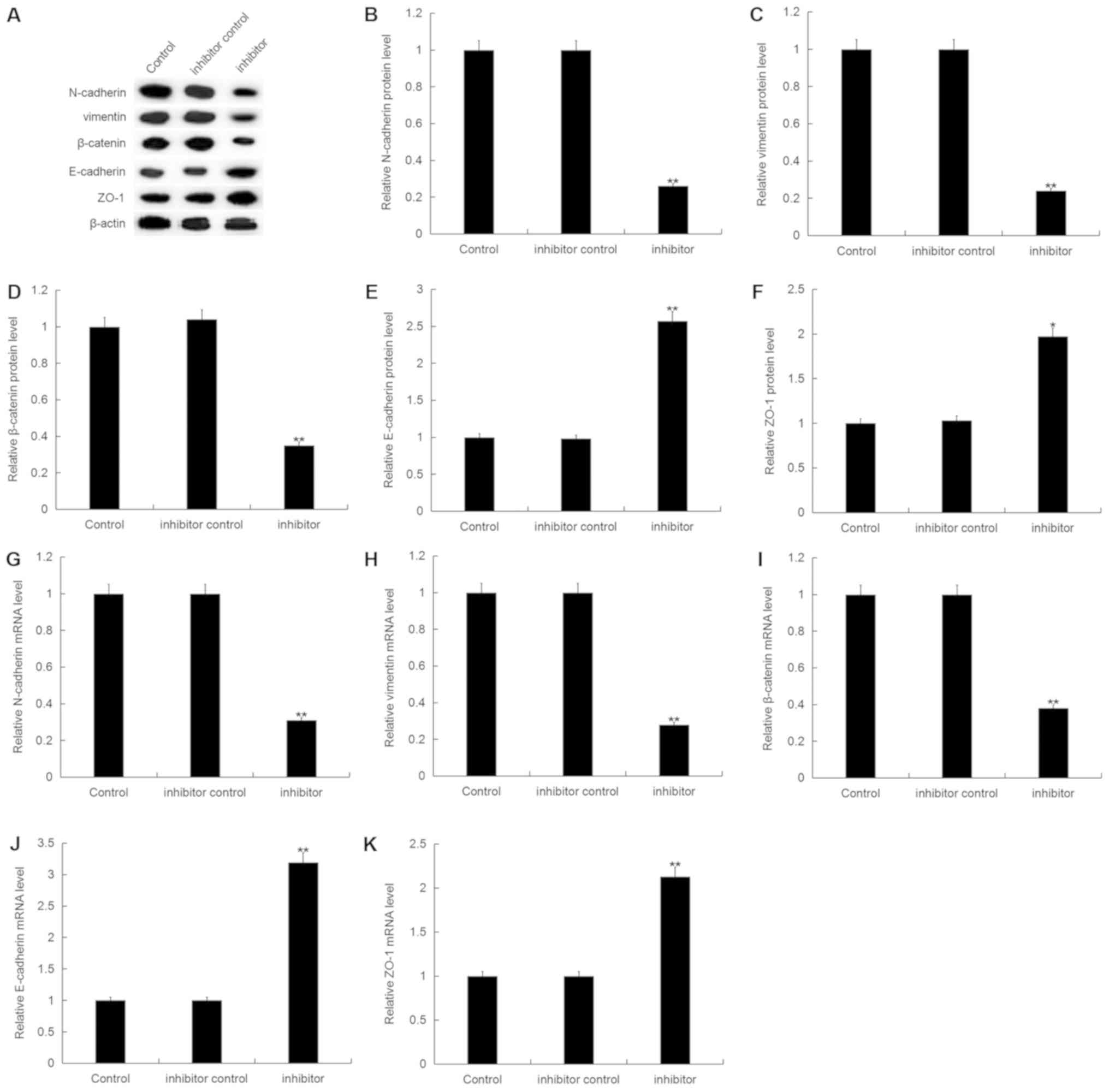
|
1
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Althuis MD, Dozier JM, Anderson WF, Devesa
SS and Brinton LA: Global trends in breast cancer incidence and
mortality 1973–1997. Int J Epidemiol. 34:405–412. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martin AM, Cagney DN, Catalano PJ, Warren
LE, Bellon JR, Punglia RS, Claus EB, Lee EQ, Wen PY, Haas-Kogan DA,
et al: Brain metastases in newly diagnosed breast cancer: A
population-based study. JAMA Oncol. 3:1069–1077. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de la Mare JA, Contu L, Hunter MC, Moyo B,
Sterrenberg JN, Dhanani KC, Mutsvunguma LZ and Edkins AL: Breast
cancer: Current developments in molecular approaches to diagnosis
and treatment. Recent Pat Anticancer Drug Discov. 9:153–175. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Liu F, Lin B, Luo H, Liu M, Wu J, Li
C, Li R, Zhang X, Zhou K and Ren D: miR-150 inhibits proliferation
and tumorigenicity via retarding G1/S phase transition in
nasopharyngeal carcinoma. Int J Oncol. 10:Mar;2017.(Epub ahead of
print).
|
|
6
|
Khew-Goodall Y and Goodall GJ:
Myc-modulated miR-9 makes more metastases. Nat Cell Biol.
12:209–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen K and Rajewsky N: The evolution of
gene regulation by transcription factors and microRNAs. Nat Rev
Genet. 8:93–103. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ren D, Wang M, Guo W, Huang S, Wang Z,
Zhao X, Du H, Song L and Peng X: Double-negative feedback loop
between ZEB2 and miR-145 regulates epithelial-mesenchymal
transition and stem cell properties in prostate cancer cells. Cell
Tissue Res. 358:763–778. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baranwal S and Alahari SK: miRNA control
of tumor cell invasion and metastasis. Int J Cancer. 126:1283–1290.
2010.PubMed/NCBI
|
|
10
|
Ren D, Wang M, Guo W, Zhao X, Tu X, Huang
S, Zou X and Peng X: Wild-type p53 suppresses the
epithelial-mesenchymal transition and stemness in PC-3 prostate
cancer cells by modulating miR-145. Int J Oncol. 42:1473–1481.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in Cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Liu J, Zang D, Wu S, Liu A, Zhu
J, Wu G, Li J and Jiang L: Upregulation of miR-572
transcriptionally suppresses SOCS1 and p21 and contributes to human
ovarian cancer progression. Oncotarget. 6:15180–15193.
2015.PubMed/NCBI
|
|
13
|
Wang M, Ren D, Guo W, Wang Z, Huang S, Du
H, Song L and Peng X: Loss of miR-100 enhances migration, invasion,
epithelial-mesenchymal transition and stemness properties in
prostate cancer cells through targeting Argonaute 2. Int J Oncol.
45:362–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang S, Guo W, Tang Y, Ren D, Zou X and
Peng X: miR-143 and miR-145 inhibit stem cell characteristics of
PC-3 prostate cancer cells. Oncol Rep. 28:1831–1837. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang D, Qiu C, Zhang H, Wang J, Cui Q and
Yin Y: Human microRNA oncogenes and tumor suppressors show
significantly different biological patterns: From functions to
targets. PLoS One. 5(pii): e130672010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng CJ, Bahal R, Babar IA, Pincus Z,
Barrera F, Liu C, Svoronos A, Braddock DT, Glazer PM, Engelman DM,
et al: MicroRNA silencing for cancer therapy targeted to the tumour
microenvironment. Nature. 518:107–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Calin GA, Ferracin M, Cimmino A, Di Leva
G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, et
al: A MicroRNA signature associated with prognosis and progression
in chronic lymphocytic leukemia. N Engl J Med. 353:1793–1801. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Ye Y, Tu H, Hildebrandt MA, Zhao
L, Heymach JV, Roth JA and Wu X: MicroRNA-related genetic variants
in iron regulatory genes, dietary iron intake, microRNAs and lung
cancer risk. Ann Oncol. 28:1124–1129. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Di Stefano P, Leal MP, Tornillo G, Bisaro
B, Repetto D, Pincini A, Santopietro E, Sharma N, Turco E, Cabodi S
and Defilippi P: The adaptor proteins p140CAP and p130CAS as
molecular hubs in cell migration and invasion of cancer cells. Am J
Cancer Res. 1:663–673. 2011.PubMed/NCBI
|
|
22
|
Damiano L, Di Stefano P, Camacho Leal MP,
Barba M, Mainiero F, Cabodi S, Tordella L, Sapino A, Castellano I,
Canel M, et al: p140Cap dual regulation of E-cadherin/EGFR
cross-talk and Ras signalling in tumour cell scatter and
proliferation. Oncogene. 29:3677–3690. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Di Stefano P, Damiano L, Cabodi S, Aramu
S, Tordella L, Praduroux A, Piva R, Cavallo F, Forni G, Silengo L,
et al: p140Cap protein suppresses tumour cell properties,
regulating Csk and Src kinase activity. EMBO J. 26:2843–2855. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun W, Wang X, Li J, You C, Lu P, Feng H,
Kong Y, Zhang H, Liu Y, Jiao R, et al: MicroRNA-181a promotes
angiogenesis in colorectal cancer by targeting SRCIN1 to promote
the SRC/VEGF signaling pathway. Cell Death Dis. 9:4382018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen R, Liao JY, Huang J, Chen WL, Ma XJ
and Luo XD: Downregulation of SRC kinase signaling inhibitor 1
(SRCIN1) expression by MicroRNA-32 promotes proliferation and
epithelial-mesenchymal transition in human liver cancer cells.
Oncol Res. 26:573–579. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cao M, Hou D, Liang H, Gong F, Wang Y, Yan
X, Jiang X, Wang C, Zhang J, Zen K, et al: miR-150 promotes the
proliferation and migration of lung cancer cells by targeting SRC
kinase signalling inhibitor 1. Eur J Cancer. 50:1013–1024. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen T, You Y, Jiang H and Wang ZZ:
Epithelial-mesenchymal transition (EMT): A biological process in
the development, stem cell differentiation, and tumorigenesis. J
Cell Physiol. 232:3261–3272. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shenoy AK, Jin Y, Luo H, Tang M, Pampo C,
Shao R, Siemann DW, Wu L, Heldermon CD, Law BK, et al:
Epithelial-to-mesenchymal transition confers pericyte properties on
cancer cells. J Clin Invest. 126:4174–4186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
DeMichele A, Yee D and Esserman L:
Mechanisms of resistance to neoadjuvant chemotherapy in breast
cancer. N Engl J Med. 377:2287–2289. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Watanabe A, Tagawa H, Yamashita J, Teshima
K, Nara M, Iwamoto K, Kume M, Kameoka Y, Takahashi N, Nakagawa T,
et al: The role of microRNA-150 as a tumor suppressor in malignant
lymphoma. Leukemia. 25:1324–1334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun W, Zhang Z, Wang J, Shang R, Zhou L,
Wang X, Duan J, Ruan B, Gao Y, Dai B, et al: microRNA-150
suppresses cell proliferation and metastasis in hepatocellular
carcinoma by inhibiting the GAB1-ERK axis. Oncotarget.
7:11595–11608. 2016.PubMed/NCBI
|
|
35
|
Ito M, Teshima K, Ikeda S, Kitadate A,
Watanabe A, Nara M, Yamashita J, Ohshima K, Sawada K and Tagawa H:
MicroRNA-150 inhibits tumor invasion and metastasis by targeting
the chemokine receptor CCR6, in advanced cutaneous T-cell lymphoma.
Blood. 123:1499–1511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma Y, Zhang P, Wang F, Zhang H, Yang J,
Peng J, Liu W and Qin H: miR-150 as a potential biomarker
associated with prognosis and therapeutic outcome in colorectal
cancer. Gut. 61:1447–1453. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Srivastava SK, Bhardwaj A, Singh S, Arora
S, Wang B, Grizzle WE and Singh AP: MicroRNA-150 directly targets
MUC4 and suppresses growth and malignant behavior of pancreatic
cancer cells. Carcinogenesis. 32:1832–1839. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wuerkenbieke D, Wang J, Li Y and Ma C:
miRNA-150 downregulation promotes pertuzumab resistance in ovarian
cancer cells via AKT activation. Arch Gynecol Obstet.
292:1109–1116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stamatopoulos B, Van Damme M, Crompot E,
Dessars B, Housni HE, Mineur P, Meuleman N, Bron D and Lagneaux L:
Opposite prognostic significance of cellular and serum circulating
MicroRNA-150 in patients with chronic lymphocytic leukemia. Mol
Med. 21:123–133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yin QW, Sun XF, Yang GT, Li XB, Wu MS and
Zhao J: Increased expression of microRNA-150 is associated with
poor prognosis in non-small cell lung cancer. Int J Clin Exp
Pathol. 8:842–846. 2015.PubMed/NCBI
|
|
41
|
Wu Q, Jin H, Yang Z, Luo G, Lu Y, Li K,
Ren G, Su T, Pan Y, Feng B, et al: MiR-150 promotes gastric cancer
proliferation by negatively regulating the pro-apoptotic gene EGR2.
Biochem Biophys Res Commun. 392:340–345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Summy JM and Gallick GE: Src family
kinases in tumor progression and metastasis. Cancer Metastasis Rev.
22:337–358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang F, Luo LJ, Zhang L, Wang DD, Yang SJ,
Ding L, Li J, Chen D, Ma R, Wu JZ and Tang JH: MiR-346 promotes the
biological function of breast cancer cells by targeting SRCIN1 and
reduces chemosensitivity to docetaxel. Gene. 600:21–28. 2017.
View Article : Google Scholar : PubMed/NCBI
|