Introduction
Renal cell carcinoma (RCC) is the most common type
of primary kidney malignancy and accounts for an estimated 90–95%
of kidney cancer cases (1). At
present, RCC ranks as the 7 and 9th most common cancer type among
men and women, respectively (2). In
~40% of cases, metastasis to the ipsilateral renal vein or inferior
vena cava has typically occurred at the time of RCC diagnosis
(3). Kidney renal clear cell
carcinoma (KIRC) is the most common type of RCC, accounting for
~75% of RCC cases (4). During the
past few decades, a number of treatments have been developed for
KIRC. However, due to tumor metastasis and recurrence, which are
associated with poor prognosis, the therapeutic efficacy if limited
(1,5). Therefore, it is necessary to explore
novel therapeutic options based on the molecular mechanisms of KIRC
and to identify biomarkers that may facilitate the early diagnosis
of KIRC.
In recent years, microRNAs (miRNAs/miRs) have been
studied as important regulators of gene expression in a variety of
cancer types, with different expression patterns observed at
different stages and in different tumor types (6–8). The
miR-183/182/96 cluster is a critical gene located on the short arm
of chromosome 7 (7q32.2), which generates a single polycistronic
transcript that yields three mature miRNAs: miR-183, miRNA-96 and
miR-182 (9). The potential
mechanistic roles of the miR-183/182/96 cluster have been
investigated in numerous studies. For instance, Wnt/β-catenin was
reported to activate miR-183/182/96 expression and promotes cell
invasion in hepatocellular carcinoma (10). The miR-183/96/182 cluster regulates
oxidative apoptosis and sensitizes cells to chemotherapy in gliomas
(11). miR-183 inhibits cell growth
in human non-small cell lung cancer by downregulating metastasis
associated 1 (12). miR-96 regulates
cell proliferation, invasion and migration of pancreatic cancer
(13). Furthermore, miR-182 promotes
proliferation and metastasis by targeting forkhead box (FOX)F2 in
triple-negative breast cancer (14).
The expression of the miR-183/182/96 cluster is upregulated in most
cancer types (15), including
bladder cancer (16), colorectal
cancer (17) and hepatocellular
carcinoma (18). Li et al
(19) reported that miR-96, miR-182
and miR-183 were all upregulated in intestinal-type gastric
cancers. However, Kong et al (20) indicated that miR-182 was
significantly downregulated in human gastric adenocarcinoma tissue
samples. The role of the miR-183/182/96 cluster as a biomarker has
also been investigated in gastric cancer (19,20),
breast cancer (21) and colon cancer
(22); however, no similar studies
have been reported for KIRC.
In recent years, great research efforts have been
made to discover novel miRNAs, identify miRNA targets and decipher
miRNA functions (23). The
application of computational methods may shed light on biological
roles of miRNAs, as bioinformatics may reveal statistically
significant trends based on a hypothesis that can be established
and may then be experimentally confirmed. For instance, identifying
miRNAs associated with tumor development has been achieved via the
use of a comprehensive analysis of miRNAs datasets (17). Furthermore, miRNA-mRNA interaction in
tumors has been identified by integrated transcriptome analyses
(24). In addition, the association
of miRNA expression with a gene or pathway has been explored
through comprehensive bioinformatics calculations (25,26).
The present study was designed to investigate the
differential expression patterns of miR-183/182/96 between KIRC and
normal kidney tissues. Reverse transcription- guantitative
polymerase chain reaction (RT-qPCR) was used to confirm the
reliability of microarray expression data. Furthermore, the
association between miR-183/182/96 expression and
clinicopathological characteristics of patients was analyzed, and
the pathways and functions of the target genes of miR-183/182/96
were further explored, which may provide novel insight into the
potential mechanistic roles of miR-183/182/96 in KIRC.
Materials and methods
Data acquisition and processing
The raw miRNA expression profile and clinical
information were downloaded from the Broad Institute The Cancer
Genome Atlas (TCGA) Genome Data Analysis Center in 2016 (http://gdac.broadinstitute.org/runs/analyses–latest/reports).
A total of 322 samples, comprising 251 KIRC tissues and 71 normal
tissues, were included in the present study. The data of the miRNAs
expression profiles were processed using R software (version 3.5.2;
http://www.r-project.org). The differential
expression analysis of miRNAs between KIRC and normal tissues was
performed with the R limma Bioconductor package (27). miRNAs with an absolute fold-change
(|FC|) of ≥2 and P<0.05 were considered to be significantly
differentially expressed.
Cell culture and RT-qPCR
validation
The human renal tubular epithelial cell line (HKC-5;
BNCC100598) and KIRC cell lines (LoMet-ccRCC and 786-O) were
obtained from the BeNa Culture Collection (Beijing, China), and
were cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% sfetal
bovine serum (ScienCell Research Laboratories, Inc., San Diego, CA,
USA) and 1% antibiotics (streptomycin and penicillin; Hyclone; GE
Healthcare, Little Chalfont, UK) in a humidified atmosphere
containing 5% CO2 at 37°C. Total cellular RNA was isolated using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and
the concentration and quality of total RNA were detected using a
microplate reader. Subsequently, first-strand complementary DNA was
synthesized using TaqMan™ MicroRNA Reverse Tanscription kit (Thermo
Fisher Scientific, Inc.). RT-qPCR was performed with the iCycler
Real Time System (Bio-Rad Laboratories, Hercules, CA, USA) using
mirVana™ qRT-PCR miRNA Detection (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
primers for the miRNAs of interest are listed in Table I. Relative quantification of miRNAs
was performed using the 2−ΔΔCq method using
U6 as the internal control (28).
 | Table I.Sequences of primers used in the
polymerase chain reaction assays for the indicated nucleotides. |
Table I.
Sequences of primers used in the
polymerase chain reaction assays for the indicated nucleotides.
|
| Primer (5′-3′) |
|---|
|
|
|
|---|
| Name | Forward | Reverse |
|---|
| miR-183 |
GCCGAGUAUGGCACUGGUAGAAUUCACU |
CTCAACTGGTGTCGTGGA |
| miR-182 |
GCCGAGUUUGGCACUAGCACAUUUUUGCU |
CTCAACTGGTGTCGTGGA |
| miR-96 |
GCCGAGUUUGGCAAUGGUAGAACUCACACU |
CTCAACTGGTGTCGTGGA |
| U6 |
CTCGCTTCGGCAGCACATA |
CGAATTTGCGTGTCATCCT |
Clinical significance analysis
The miRNA expression data were normalized by log2
transformation. The clinical features of the 235 KIRC patients,
including age at diagnosis, sex, tumor laterality, histological
grade, pathological stage and tumor-nodes-metastasis stage were
evaluated to analyze the association between these features and the
expression of miR-183, miR-96 and miR-182. Furthermore, the
predictive and prognostic value of miR-183, miR-96 and miR-182 was
evaluated using receiver operating characteristic curve (ROC) and
Kaplan-Meier (KM) analysis, respectively.
Target gene prediction and functional
analysis
The target genes of miR-183/182/96 were predicted
using the miRDB (http://www.mirdb.org/miRDB/) and TargetScan
(http://www.targetscan.org/) online
analysis tools. In order to enhance the reliability of the
bioinformatics analysis, the overlapping target genes were
identified using a Venn diagram. To then explore the biological
mechanisms associated with the target genes, the Database for
Annotation, Visualization and Integrated Discovery (DAVID) online
analysis tool (https://david.ncifcrf.gov/) was used, and Gene
Ontology (GO) and Panther pathway enrichment analysis were
performed. P<0.05 and ≥3 genes enriched in the pathway were set
as the cut-off criteria.
Statistical analysis and
visualization
All data were expressed as the mean ± standard
deviation and were processed with GraphPad Prism 6.0 (GraphPad
Software Inc., La Jolla, CA, USA). The heatmap was generated using
R software with the ggplot2 package (29). The bar plots, as well as the ROC and
KM curves were prepared with GraphPad Prism 6.0. The volcano plot,
Venn diagram and GO enrichment plots were prepared using the
ImageGP online tools (http://www.ehbio.com/ImageGP). Comparisons between two
groups were made using the Student's t-test. The association
between clinicopathological features and miRNA expression was
performed using an independent-samples t-test. The Cox proportional
hazard regression model was used for uni- and multivariate
analysis. The KM method was used to estimate survival, and the
log-rank test was used to assess differences between the survival
curves. P<0.05 was considered to indicate statistical
significance.
Results
Differentially expressed miRNAs
In total, 322 samples were evaluated in this study,
including 251 KIRC tissues and 71 normal tissues. Based on the
cut-off criteria (|FC| ≥2 and P<0.05), 127 miRNAs were
identified that were differentially expressed between KIRC tissues
and normal tissues, including 47 upregulated and 80 downregulated
miRNAs. Hierarchical clustering revealed that mRNA expression
patterns between KIRC tissues and matched normal tissues were
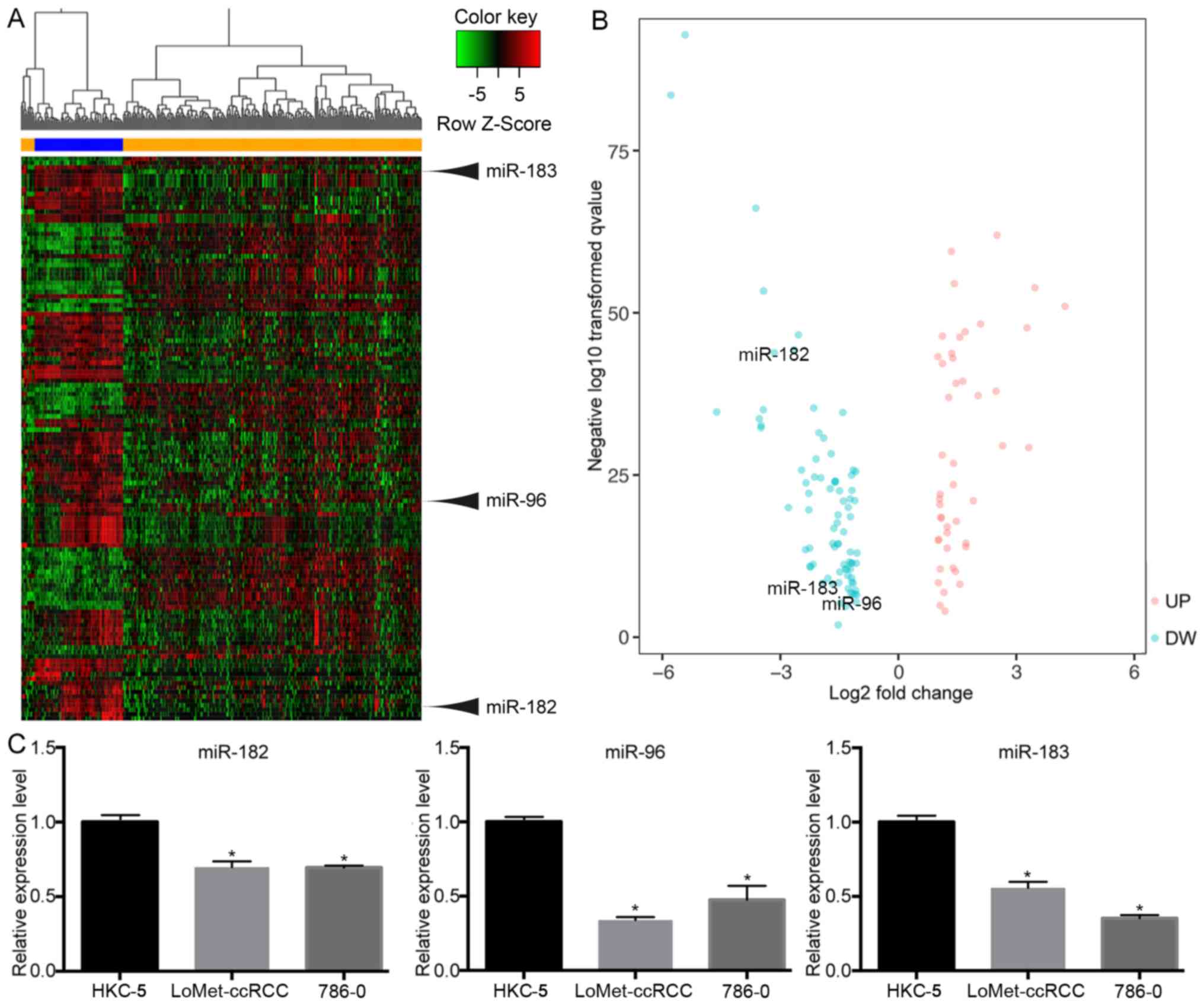
distinguishable (Fig. 1A). In order
to visualize and assess the variation in mRNA expression, the data
were presented in a volcano plot (Fig.
1B). The results indicated that miR-183 (|FC|=2.3, P<0.001),
miR-96 (|FC|=8.9, P<0.001) and miR-182 (|FC|=2.09, P<0.001)
were significantly downregulated in tumor tissues. To confirm these
results, RT-qPCR was used to verify the miRNA expression in a human
renal tubular epithelial cell line and in KIRC cell lines. The
RT-qPCR results were consistent with those obtained from the miRNA
expression profile (Fig. 1C),
demonstrating that the miRNA expression results were relatively
reliable.
Correlation with clinicopathological
features
The association between the expression levels of the
three miRNAs and the individual clinicopathological features of
KIRC patients was evaluated (Table
II). The results indicated that miR-183 was significantly
associated with the histological grade (P<0.001), pathological
stage (P=0.018), T stage (P<0.016) and metastasis (P=0.007). In
addition, miR-96 was significantly associated with the histological
grade (P=0.006), pathological stage (P=0.011), T stage (P=0.023),
and metastasis (P=0.001). Furthermore, miR-182 was significantly
associated with age at diagnosis (P<0.001), sex (P=0.037),
histological grade (P<0.001), pathological stage (P=0.085) and
metastasis (P=0.02), but no significant association was observed
between miR-182 and T stage (P=0.071).
 | Table II.Association of clinicopathological
characteristics with miR-183, miR-96 and miR-182 expression. |
Table II.
Association of clinicopathological
characteristics with miR-183, miR-96 and miR-182 expression.
| Variable | n | miR-183 | P-value | miR-96 | P-value | miR-182 | P-value |
|---|
| Age at diagnosis
(years) |
|
| 0.3490 |
| 0.5307 |
| 0.0002 |
|
<60 | 118 | 9.393±0.1511 |
| 1.239±0.1627 |
| 11.410±0.1456 |
|
|
≥60 | 117 | 9.189±0.1572 |
| 1.098±0.1561 |
| 10.090±0.3041 |
|
| Sex |
|
| 0.0865 |
| 0.0960 |
| 0.0373 |
|
Male | 160 | 9.420±0.1288 |
| 1.297±0.1353 |
| 11.430±0.1265 |
|
|
Female | 75 | 9.019±0.2003 |
| 0.895±0.2007 |
| 10.960±0.1848 |
|
| Tumor
laterality |
|
| 0.8083 |
| 0.7620 |
| 0.8398 |
|
Left | 110 | 9.312±0.1610 |
| 1.196±0.1675 |
| 11.301±0.1563 |
|
|
Right | 124 | 9.258±0.1490 |
| 1.127±0.1527 |
| 11.260±0.1432 |
|
| Histologic
grade |
|
| 0.0002 |
| 0.0064 |
| 0.0005 |
|
G1+G2 | 100 | 8.841±0.1687 |
| 0.817±0.1755 |
| 10.870±0.1665 |
|
|
G3+G4 | 133 | 9.641±0.1353 |
| 1.435±0.1430 |
| 11.601±0.1287 |
|
| Pathologic
stage |
|
| 0.0184 |
| 0.0114 |
| 0.0850 |
|
I+II | 144 | 9.092±0.1414 |
| 0.938±0.1475 |
| 11.141±0.1376 |
|
|
III+IV | 90 | 9.621±0.1677 |
| 1.524±0.1691 |
| 11.520±0.1617 |
|
| Tumor stage |
|
| 0.0157 |
| 0.0234 |
| 0.0712 |
|
T1+T2 | 152 | 9.098±0.1358 |
| 0.981±0.1418 |
| 11.140±0.1321 |
|
|
T3+T4 | 83 | 9.647±0.1771 |
| 1.514±0.1801 |
| 11.540±0.1708 |
|
| Lymph node
status |
|
| 0.7177 |
| 0.9162 |
| 0.8927 |
| N0 | 93 | 9.437±0.1819 |
| 1.316±0.1693 |
| 11.440±0.1716 |
|
| N1 | 8 | 9.667±0.4228 |
| 1.253±0.5192 |
| 11.520±0.4263 |
|
| Metastasis
status |
|
| 0.0074 |
| 0.0011 |
| 0.0202 |
| M0 | 163 | 9.244±0.1286 |
| 1.019±0.1376 |
| 11.270±0.1235 |
|
| M1 | 40 | 10.010±0.2237 |
| 1.992±0.1952 |
| 11.901±0.2088 |
|
Predictive and prognostic
evaluation
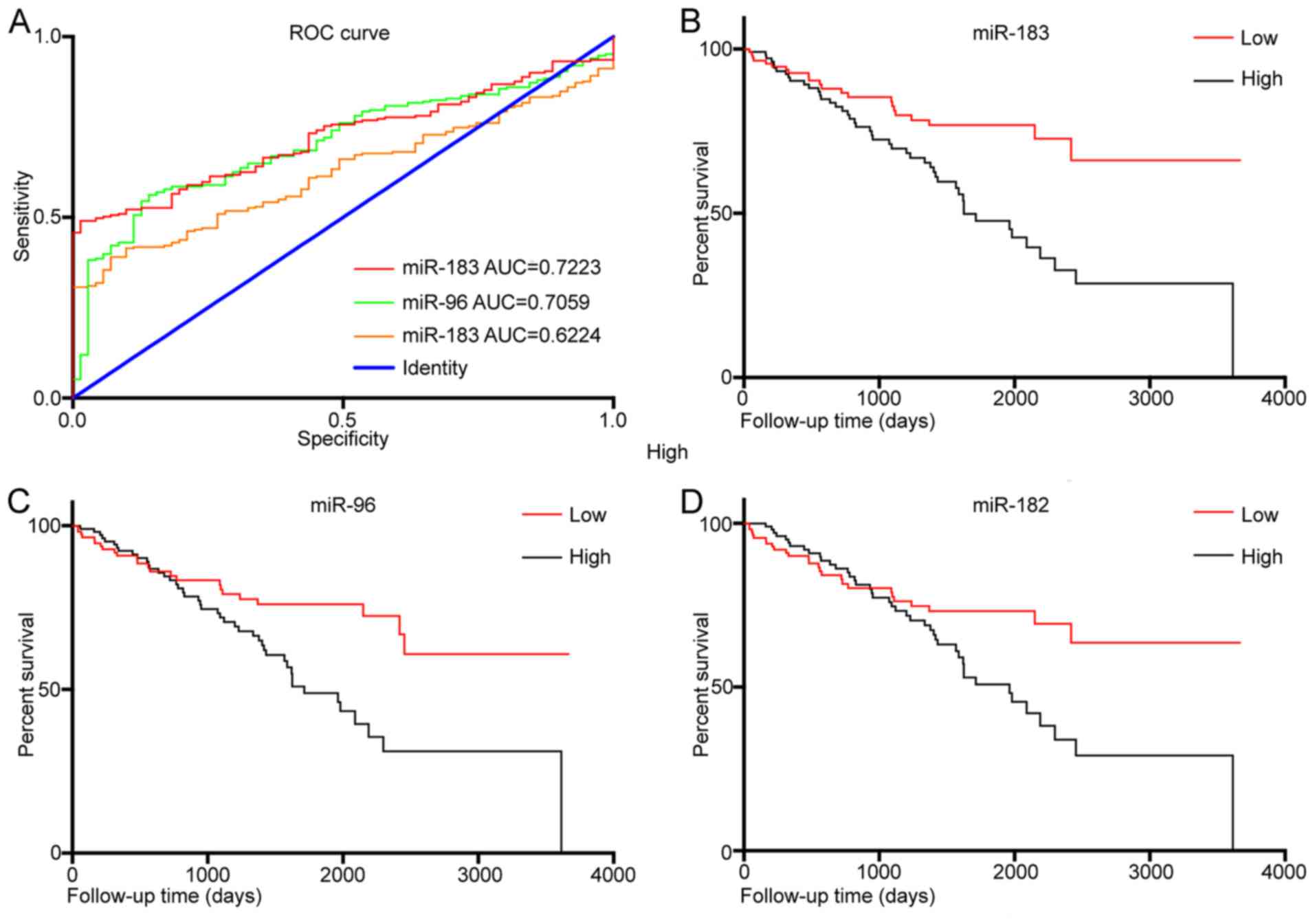
In order to explore the diagnostic value of miR-183,
miR-96 and miR-182 expression levels in KIRC tissues, a ROC
analysis was performed (Fig. 2A).
The results revealed that the area under curve (AUC) for miR-183
was 0.722 [95% confidence interval (CI): 0.668–0.777, P<0.001]
and that for and miR-96 was 0.706 (95% CI, 0.647–0.765,
P<0.001). However, the AUC for miR-182 was 0.622 (95% CI:
0.562–0.683, P=0.002). In addition, KM curves were drawn to
evaluate the association between the expression of the three miRNAs
and the overall survival of KIRC patients. The results indicated
that a higher expression of miR-183 (P<0.001; Fig. 2B), miR-96 (P=0.004; Fig. 2C) and miR-182 (P=0.023; Fig. 2B) was significantly associated with
worse overall survival. Uni- and multivariate Cox regression
analysis identified that miR-183 may be an independent prognostic
factor in KIRC (P=0.001; Tables
III and IV).
 | Table III.Univariate analysis of factors
associated with overall survival. |
Table III.
Univariate analysis of factors
associated with overall survival.
| Variable | Hazard ratio (95%
CI) | P-value |
|---|
| Age at diagnosis
(≥60 vs. <60 years) | 0.762
(0.568–1.214) | 0.113 |
| Sex (male vs.
female | 2.235
(0.714–7.233) | 0.124 |
| Tumor laterality
(left vs. right) | 1.436
(0.346–5.941) | 0.617 |
| miR-183 expression
(high vs. low) | 2.433
(1.105–5.833) | 0.043 |
| miR-96 expression
(high vs. low) | 1.375
(1.915–3.122) | 0.048 |
| miR-182 expression
(high vs. low) | 1.892
(1.332–3.437) | 0.039 |
| Histological grade
(G3/G4 vs. G1/G2) | 2.564
(1.142–5.760) | 0.023 |
| Pathological stage
(III/IV vs. I/II) | 1.840
(1.056–3.301) | 0.034 |
| Metastasis status
(M1 vs. M0) | 1.245
(0.860–1.903) | 0.392 |
| Tumor stage (T3/T4
vs. T1/T2) | 1.609
(1.583–1.859) | 0.022 |
| Lymph node status
(N1 vs. N0) | 1.188
(1.292–2.563) | 0.021 |
 | Table IV.Multivariate analysis of factors
associated with overall survival. |
Table IV.
Multivariate analysis of factors
associated with overall survival.
| Variable | Hazard ratio (95%
CI) | P-value |
|---|
| miR-183 expression
(high vs. low) | 5.126
(2.385–11.021) | <0.001 |
| miR-96 expression
(high vs. low) | 2.078
(0.464–3.138) | 0.061 |
| miR-182 expression
(high vs. low) | 1.312
(0.508–3.132) | 0.070 |
| Histological grade
(G3/G4 vs. G1/G2) | 2.556
(1.139–5.736) | 0.023 |
| Pathological stage
(III/IV vs. I/II) | 1.160
(0.282–4.769) | 0.837 |
| Metastasis status
(M1 vs. M0) | 1.819
(0.961–3.274) | 0.068 |
| Tumor stage (T3/T4
vs. T1/T2) | 1.713
(1.067–3.033) | 0.028 |
| Lymph node status
(N1 vs. N0) | 2.230
(1.774–3.118) | 0.045 |
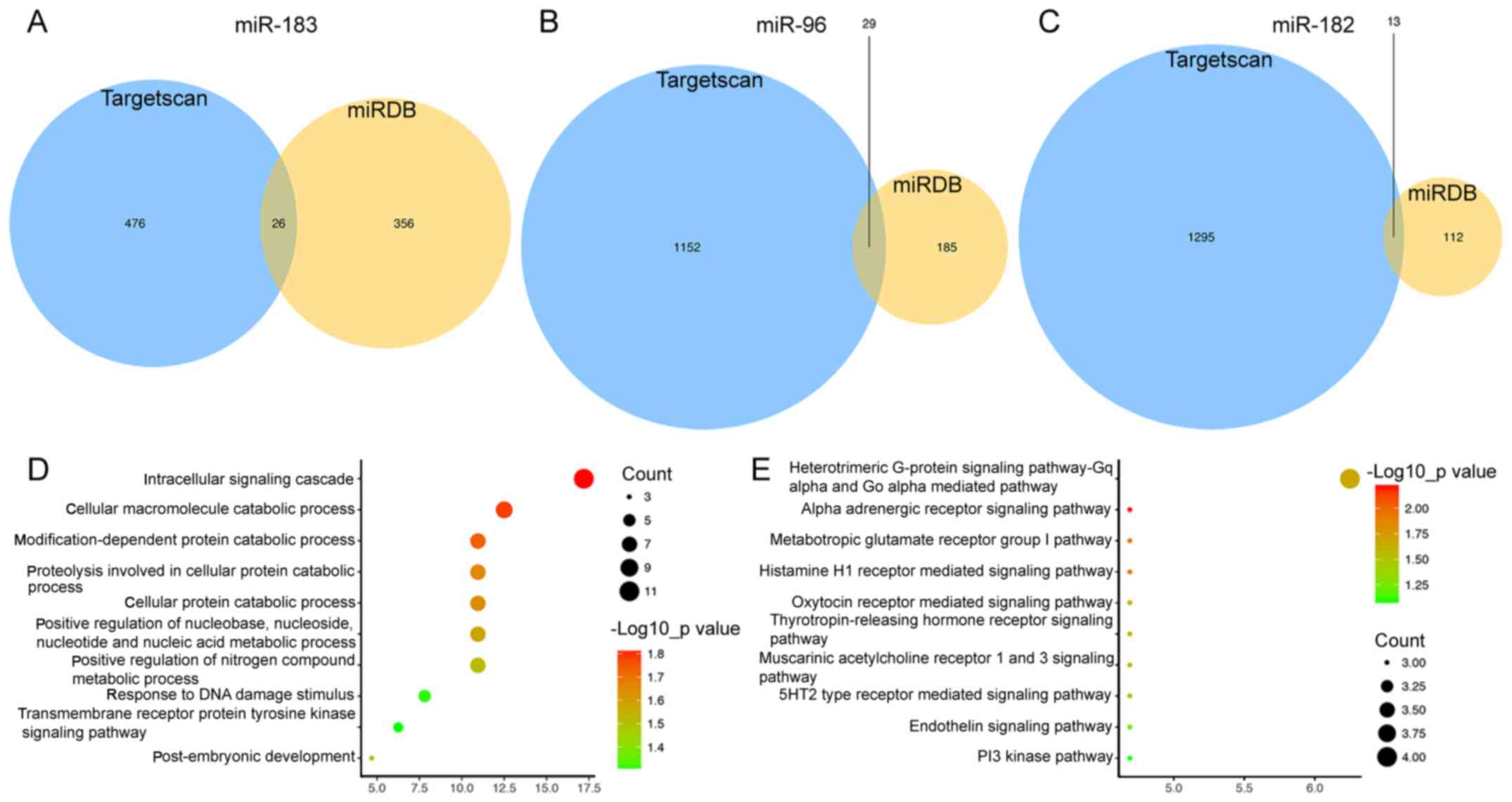
Target gene prediction and functional
analysis
The target genes of the three miRNAs were predicted
using the miRDB and TargetScan online analysis tools. In total, 26
genes targeted by miR-183 (Fig. 3A),
29 targeted by miR-96 (Fig. 3B) and
13 targeted by miR-182 were identified by miRDB and TargetScan
combined (Fig. 3C). Subsequently, a
functional enrichment analysis was performed to elucidate the
biological functions of the consensus target genes. The genes were
mainly enriched in the GO terms in the category biological process
(BP) of the intracellular signaling cascade (P=0.015), cellular
macromolecule catabolic process (P=0.016) and response to DNA
damage stimulus (P=0.040; Fig. 3D).
In addition, the genes were significantly enriched Panther pathways
including α-adrenergic receptor signaling pathway (P=0.006),
metabotropic glutamate receptor group I pathway (P=0.012),
histamine H1 receptor-mediated signaling pathway (P=0.012) and
thyrotropin-releasing hormone receptor signaling pathway (P=0.025;
Fig. 3E).
Discussion
In recent decades, the development and
implementation of targeted therapies has greatly improved the
prognosis of patients with KIRC (30,31).
However, KIRC is a multifaceted and therapeutically challenging
disease, and is prone to developing resistance against therapeutics
(32). The prognosis and therapeutic
outcomes for KIRC patients may be significantly improved if
reliable predictive biomarkers were available at the time of
initial diagnosis. Therefore, it is necessary to expand the current
understanding of the molecular mechanisms underlying KIRC
progression and identify novel biomarkers. In the present study, a
total of 127 differentially expressed miRNAs were identified in
KIRC vs. normal renal tissues. Of note, the miR-183/182/96 cluster
has not been previously reported in KIRC, and the present study
indicated that this cluster was downregulated in KIRC. Hence, the
association of the three miRNAs miR-183, miR-96 and miR-182 with
clinicopathological parameters and survival was analyzed, and they
were identified to be predictive and prognostic biomarkers for KIRC
patients. Furthermore, the target genes of the three miRNAs were
predicted, and the GO terms in the category BP and pathways
enriched by the target genes were determined.
In the last decade, a vast amount of evidence has
been provided to demonstrate that miRNAs are regulators of gene
expression and complex pathways in KIRC (33,34).
Furthermore, previous studies have demonstrated that numerous
miRNAs are critical for the initiation, progression and metastasis,
as well as prognostic indicators and therapeutic targets in KIRC,
including miRNA-34a, miRNA-143 and miRNA-21 (35,36).
However, most of the previous studies had a small sample size and
assessed a relatively limited number of miRNAs. In the present
study, the microarray data of 322 samples, including 251 KIRC
tissues and 71 normal renal tissues were subjected to a
bioinformatics analysis to identify that miR-183, miR-96 and
miR-182 among the deregulated miRNAs in KIRC; furthermore, they
were determined to be associated with the clinicopathological
characteristics and the prognosis of KIRC patients. The present
results indicated that the overexpression of miR-183, miR-96 and
miR-182 was associated with poor prognosis, and miR-183 was
revealed to be an independent prognostic factor for KIRC patients.
Furthermore, miR-183, miR-96 and miR-182 were significantly
associated with histological grade and pathological stage,
indicating they are involved in the progression of KIRC. Studies
exploring the roles and mechanisms of miR-183, miR-96, and miR-182
in KIRC are rare. Ge et al (37) reported that miR-183 and miR-182 were
differentially expressed between BRCA1-associated protein-1 mutant
and wild-type KIRC tumors. Qiu et al (38) reported that miR-183 has an oncogenic
role by increasing cell proliferation, migration and invasion via
targeting protein phosphatase 2A. Xu et al (39) reported that miR-182 contributes to
RCC proliferation via activating the AKT/FOXO3a signaling pathway.
Furthermore, dicer was indicated to suppress tumor growth and
angiogenesis by inhibiting the expression of hypoxia-inducible
factor-2α, a direct target of miR-182, in KIRC patients (40). In addition, Yu et al (41) suggested that miR-96 modulates ezrin
expression and promotes RCC invasion.
The results of the present study indicated that the
expression of miR-183, miR-96, and miR-182 in KIRC tissue was lower
compared with that in normal tissues. The results of the RT-qPCR
validation in cell lines were consistent with those obtained from
the miRNA expression profile. However, previous studies on the
miR-183/182/96 cluster have indicated that it is highly expressed
in breast, colon and liver cancer (10,22,42).
This indicates that the expression of miR-183, miR-96 and miR-182
is dependent on the tissue/cancer type. Typically, an oncogene or
oncogenic factor exhibits higher expression in cancer than in
normal tissues and is correlated with worse overall survival of
cancer patients. However, the present results indicated that
compared to normal patients, low expression of miR-183/182/96 in
KIRC patients indicated a better overall survival rate. In other
words, the present results suggested that the expression of
miR-183/182/96 has a promoting effect on tumor development in KIRC
patients. Similar studies have also reported that miR-18a is highly
expressed in colorectal cancer, but it restrains cell proliferation
by inhibiting cell division cycle 42 and the phosphoinositide-3
kinase signaling pathway (43). This
may indicate that the low expression of miR-183/182/96 in KIRC is
associated with other factors that have a role in its development,
including the tumor microenvironment and metabolism. Future studies
by our group will focus on this point and investigate the causes,
implications and effects of low expression of miR-183, miR-96 and
miR-182 in KIRC.
Abnormal metabolic pathways and BPs have crucial
roles in the pathogenesis and progression of KIRC. Since the
kidneys secrete a variety of hormones participating in multiple
metabolic processes, the target genes of miR-183, miR-96 and
miR-182 were predicted and the metabolic that may be regulated by
them were explored. It was revealed that several key metabolic
pathways were regulated by the three miRNAs, including the
α-adrenergic receptor signaling pathway, metabotropic glutamate
receptor group I pathway, histamine H1 receptor-mediated signaling
pathway and thyrotropin-releasing hormone receptor signaling
pathway. To further explore the molecular functions, GO annotations
were also analyzed. The genes were mainly enriched in BP terms
including the intracellular signaling cascade, cellular
macromolecule catabolic process and response to DNA damage
stimulus. If these predictions are confirmed by further molecular
studies, those results may provide novel targets for therapeutic
interventions for KIRC.
In conclusion, despite advances in KIRC research,
this disease remains a challenge. In the present study, a
bioinformatics analysis identified miR-183, miR-96 and miR-182 as
potential carcinogenic factors and prognostic predictors in KIRC.
Further studies should be performed to validate the present
results, and to explore the roles of miR-183, miR-96 and miR-182,
as well as the underlying mechanisms, in KIRC progression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the Broad Institute The Cancer Genome
Atlas Genome Data Analysis Center in 2016 (firebrowse.org/).
Authors' contributions
NW conceived and designed the study. JY also
participated in the design of the study and performed
bioinformatics analysis. RD, FL, LZ, YL and JW provided their
advice during the process of the research. JY and RD performed the
data analysis and wrote the manuscript. LZ and YL performed the
cell line validation assay. NW, FL and JW reviewed and edited the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kuthi L, Jenei A, Hajdu A, Németh I, Varga
Z, Bajory Z, Pajor L and Iványi B: Prognostic factors for renal
cell carcinoma subtypes diagnosed according to the 2016 WHO renal
tumor classification: A study involving 928 patients. Pathol Oncol
Res. 23:689–698. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rini BI, Campbell SC and Escudier B: Renal
cell carcinoma. Lancet. 373:1119–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oto A, Herts BR, Remer EM and Novick AC:
Inferior vena cava tumor thrombus in renal cell carcinoma: Staging
by MR imaging and impact on surgical treatment. AJR Am J
Roentgenol. 171:1619–1624. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grignon DJ and Che M: Clear cell renal
cell carcinoma. Clin Lab Med. 25:305–316. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McGuire A, Brown JA and Kerin MJ:
Metastatic breast cancer: The potential of miRNA for diagnosis and
treatment monitoring. Cancer Metastasis Rev. 34:145–155. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Romero-Cordoba SL, Salido-Guadarrama I,
Rodriguez-Dorantes M and Hidalgo-Miranda A: miRNA biogenesis:
Biological impact in the development of cancer. Cancer Biol Ther.
15:1444–1455. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rocci A, Hofmeister CC and Pichiorri F:
The potential of miRNAs as biomarkers for multiple myeloma. Expert
Rev Mol Diagn. 14:947–959. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leung AK and Sharp PA: microRNAs: A
safeguard against turmoil? Cell. 130:581–585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leung WK, He M, Chan AW, Law PT and Wong
N: Wnt/β-catenin activates MiR-183/96/182 expression in
hepatocellular carcinoma that promotes cell invasion. Cancer Lett.
362:97–105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang H, Bian Y, Tu C, Wang Z, Yu Z, Liu Q,
Xu G, Wu M and Li G: The miR-183/96/182 cluster regulates oxidative
apoptosis and sensitizes cells to chemotherapy in gliomas. Curr
Cancer Drug Targets. 13:221–231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang CL, Zheng XL, Ye K, Ge H, Sun YN, Lu
YF and Fan QX: MicroRNA-183 acts as a tumor suppressor in human
non-small cell lung cancer by down-regulating MTA1. Cell Physiol
Biochem. 46:93–106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou Y, Chen Y, Ding W, Hua Z, Wang L, Zhu
Y, Qian H and Dai T: LncRNA UCA1 impacts cell proliferation,
invasion, and migration of pancreatic cancer through regulating
miR-96/FOXO3. IUBMB Life. 70:276–290. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang X, Ma G, Liu J and Zhang Y:
MicroRNA-182 promotes proliferation and metastasis by targeting
FOXF2 in triple-negative breast cancer. Oncol Lett. 14:4805–4811.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang QH, Sun HM, Zheng RZ, Li YC, Zhang
Q, Cheng P, Tang ZH and Huang F: Meta-analysis of microRNA-183
family expression in human cancer studies comparing cancer tissues
with noncancerous tissues. Gene. 527:26–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Han Y, Zhang H, Nie L, Jiang Z, Fa
P, Gui Y and Cai Z: Synthetic miRNA-mowers targeting miR-183-96-182
cluster or miR-210 inhibit growth and migration and induce
apoptosis in bladder cancer cells. PLoS One. 7:e522802012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Falzone L, Scola L, Zanghì A, Biondi A, Di
Cataldo A, Libra M and Candido S: Integrated analysis of colorectal
cancer microRNA datasets: Identification of microRNAs associated
with tumor development. Aging (Albany NY). 10:1000–1014. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Anwar SL, Krech T, Hasemeier B, Schipper
E, Schweitzer N, Vogel A, Kreipe H, Buurman R, Skawran B and
Lehmann U: hsa-mir-183 is frequently methylated and related to poor
survival in human hepatocellular carcinoma. World J Gastroenterol.
23:1568–1575. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li X, Luo F, Li Q, Xu M, Feng D, Zhang G
and Wu W: Identification of new aberrantly expressed miRNAs in
intestinal-type gastric cancer and its clinical significance. Oncol
Rep. 26:1431–1439. 2011.PubMed/NCBI
|
|
20
|
Kong WQ, Bai R, Liu T, Cai CL, Liu M, Li X
and Tang H: MicroRNA-182 targets cAMP-responsive element-binding
protein 1 and suppresses cell growth in human gastric
adenocarcinoma. FEBS J. 279:1252–1260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song C, Zhang L, Wang J, Huang Z, Li X, Wu
M, Li S, Tang H and Xie X: High expression of microRNA-183/182/96
cluster as a prognostic biomarker for breast cancer. Sci Rep.
6:245022016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Q, Ren W, Huang B, Yi L and Zhu H:
MicroRNA-183/182/96 cooperatively regulates the proliferation of
colon cancer cells. Mol Med Rep. 12:668–674. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tutar Y: miRNA and cancer; computational
and experimental approaches. Curr Pharm Biotechnol. 15:4292014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang J and Zeng Y: Identification of
miRNA-mRNA crosstalk in pancreatic cancer by integrating
transcriptome analysis. Eur Rev Med Pharmacol Sci. 19:825–834.
2015.PubMed/NCBI
|
|
25
|
Falzone L, Candido S, Salemi R, Basile MS,
Scalisi A, McCubrey JA, Torino F, Signorelli SS, Montella M and
Libra M: Computational identification of microRNAs associated to
both epithelial to mesenchymal transition and NGAL/MMP-9 pathways
in bladder cancer. Oncotarget. 7:72758–72766. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hafsi S, Candido S, Maestro R, Falzone L,
Soua Z, Bonavida B, Spandidos DA and Libra M: Correlation between
the overexpression of Yin Yang 1 and the expression levels of
miRNAs in Burkitt's lymphoma: A computational study. Oncol Lett.
11:1021–1025. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ito K and Murphy D: Application of ggplot2
to pharmacometric graphics. CPT Pharmacometrics Syst Pharmacol.
2:e792013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu SS, Quinn DI and Dorff TB: Clinical use
of cabozantinib in the treatment of advanced kidney cancer:
Efficacy, safety, and patient selection. Onco Targets Ther.
9:5825–5837. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Coppin C, Kollmannsberger C, Le L,
Porzsolt F and Wilt TJ: Targeted therapy for advanced renal cell
cancer (RCC): A Cochrane systematic review of published randomised
trials. BJU Int. 108:1556–1563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
van der Mijn JC, Mier JW, Broxterman HJ
and Verheul HM: Predictive biomarkers in renal cell cancer:
Insights in drug resistance mechanisms. Drug Resist Updat.
17:77–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rydzanicz M, Wrzesiński T, Bluyssen HA and
Wesoły J: Genomics and epigenomics of clear cell renal cell
carcinoma: Recent developments and potential applications. Cancer
Lett. 341:111–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xing T and He H: Epigenomics of clear cell
renal cell carcinoma: Mechanisms and potential use in molecular
pathology. Chin J Cancer Res. 28:80–91. 2016.PubMed/NCBI
|
|
35
|
He YH, Chen C and Shi Z: The biological
roles and clinical implications of microRNAs in clear cell renal
cell carcinoma. J Cell Physiol. 233:4458–4465. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ran L, Liang J, Deng X and Wu J: miRNAs in
prediction of prognosis in clear cell renal cell carcinoma. Biomed
Res Int. 2017:48329312017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ge YZ, Xu LW, Zhou CC, Lu TZ, Yao WT, Wu
R, Zhao YC, Xu X, Hu ZK, Wang M, et al: A BAP1 mutation-specific
MicroRNA signature predicts clinical outcomes in clear cell renal
cell carcinoma patients with wild-type BAP1. J Cancer. 8:2643–2652.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qiu M, Liu L, Chen L, Tan G, Liang Z, Wang
K, Liu J and Chen H: microRNA-183 plays as oncogenes by increasing
cell proliferation, migration and invasion via targeting protein
phosphatase 2A in renal cancer cells. Biochem Biophys Res Commun.
452:163–169. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu X, Wu J, Li S, Hu Z, Xu X, Zhu Y, Liang
Z, Wang X, Lin Y, Mao Y, et al: Downregulation of microRNA-182-5p
contributes to renal cell carcinoma proliferation via activating
the AKT/FOXO3a signaling pathway. Mol Cancer. 13:1092014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fan Y, Li H, Ma X, Gao Y, Bao X, Du Q, Ma
M, Liu K, Yao Y, Huang Q, et al: Dicer suppresses the malignant
phenotype in VHL-deficient clear cell renal cell carcinoma by
inhibiting HIF-2α. Oncotarget. 7:18280–18294. 2104.
|
|
41
|
Yu N, Fu S, Liu Y, Xu Z, Liu Y, Hao J,
Wang B and Zhang A: miR-96 suppresses renal cell carcinoma invasion
via downregulation of Ezrin expression. J Exp Clin Cancer Res.
34:1072015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li P, Sheng C, Huang L, Zhang H, Huang L,
Cheng Z and Zhu Q: MiR-183/−96/−182 cluster is up-regulated in most
breast cancers and increases cell proliferation and migration.
Breast Cancer Res. 16:4732014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Humphreys KJ, McKinnon RA and Michael MZ:
miR-18a inhibits CDC42 and plays a tumour suppressor role in
colorectal cancer cells. PLoS One. 9:e1122882014. View Article : Google Scholar : PubMed/NCBI
|