Introduction
Coronary heart disease is a generic term for
coronary arteries atherosclerosis lesions caused by a large number
of factors, such as vascular cavity stenosis, occlusion, myocardial
ischemia, hypoxia, necrosis, inflammation and apoptosis of
myocardial cells (1,2). It has been demonstrated that reduced
blood pressure and cholesterol are important factors in reducing
deaths from coronary heart disease (3). In addition, a large number of proteins
are associated with the protective effects for host against
myocardial ischemia and reperfusion injury through modulating
myocardial apoptosis and inflammation (4,5).
Furthermore, inflammation and apoptosis contribute to the
initiation and development of coronary heart disease, which have
been considered as the prognostic indicators for patients with
coronary heart disease following drug treatment in clinical
settings (6,7). Therefore, a number of potential
strategies to relieve or mitigate the apoptosis of myocardial cells
have been proposed to protect the heart against coronary heart
disease, such as ameliorating the apoptosis of cardiac cells
mediated by endothelial stem cell (8), or activating the signaling pathway to
promote the expression of anti-apoptosis proteins and decrease
pro-apoptosis proteins to protect the heart (9).
Coronary heart disease is the leading cause of
mortality worldwide and is closely associated with metabolism
disorders of endogenous substances (10). A growing number of studies have
reported that apoptosis of myocardial cells has a crucial role in
the progression of cardiovascular diseases (11–13).
Apoptosis of leukocytes has been considered as a marker of
neutrophil-endotheliocyte interaction in coronary heart disease
(14). Expression of apoptosis
factors has become an important reference of surgical results
following coronary stent implantation in coronary heart disease
patients determined by characteristics of neutrophil cluster of
differentiation (CD)11b, platelet CD62P, endothelin, monocyte
CD11b, and neutrophil CD178 in patients with coronary heart disease
prior to and following primary coronary stenting (15).
Inflammation as the cause of coronary heart disease
has been reviewed previously (16).
Expression levels of inflammation cytokines including interleukin
(IL)-6, tumor necrosis factor (TNF)-α and IL-1 in patients with
coronary heart disease may also be associated with the progression
pathogenesis (17). These reports
indicate that inhibition of inflammation contributes to the
treatment of coronary heart disease. In the present study, the
inhibitory effects of decoy receptor-3 (DCR-3) on inflammation were
investigated.
It has been demonstrated that DCR-3 can act as a
pleiotropic immunomodulator for enhancing angiogenesis, which may
be associated with the progression of coronary heart disease
(18). DCR-3 is well known as
soluble receptor TR6, which belongs to the TNFR superfamily and is
constituted by a 300-aa polypeptide (19,20).
DCR-3 has been identified as the most common biomarker of tumor
deterioration in cancer patients by a previous meta-analysis
(21). However, no previous report
has focused on the clinical significance of DCR-3 expression levels
in coronary and peripheral blood of coronary heart disease.
In the present study, the DCR-3 concentration levels
were investigated in patients with coronary heart disease. The
efficacy and molecular mechanism of DCR-3 were evaluated in mice
with coronary heart disease. Inflammation and apoptosis of
myocardial cells were investigated in mice with coronary heart
disease following treatment with DCR-3. The phosphoinositide
3-kinase (PI3K)/protein kinase B (AKT) signaling pathway was also
analyzed in DCR-3-treated myocardial cells.
Materials and methods
Ethics statement
A total of 242 patients (age, 27–56 years;
female:male, 1:1.1) with coronary heart disease and 103 healthy
volunteers age, (28–63 years; female:male, 1:1.1) were recruited
from Department of Cardiology, Zhujiang Hospital, Southern Medical
University (Guangzhou, China) between January and May 2016 for
analyzing serum levels of DCR-3. The patients were classified into
four groups. Health group contained participants without angina.
Primary group participants who only suffered angina during intense,
rapid or prolonged physical activity or exercise. Moderate group
were slightly limited in daily activities, as angina occurred
following brisk walking, going upstairs, walking in cold air or
after mood swings. Severe group were under great limitation and
suffered angina after even mild exercise. The present study was
approved by the Ethics Committee of Southern Medical University
(Guangzhou, China). All patients provided written informed consent
prior to experiments. Primary, moderate and severe coronary heart
disease was diagnosed using 2014 ACC/AHA Recommendations for the
management of NSTE ACS (22).
Cell culture
Cardiac fibroblasts were harvested from C57BL/6J
mice as described previously (23).
A total of 50 female C57BL/6J (age, 8 weeks; weight, 28–32 g) mice
were purchased from OrientBio, Inc. (Seongnam, Korea). All mice
were given free access to food and water, and housed at 23°C with
50% humidity and a 12-h artificial light/dark cycle. Briefly, mice
were sacrificed under 20 mg/kg subcutaneous meperidine anesthesia
(24). Harvested hearts were washed
with PBS and heart tissues were digested with digestion buffer
prepared with 0.05% (w/v) collagenase type II (Worthington
Biochemical Corporation, Lakewood, NJ, USA) and 0.06% (w/v)
pancreatin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in PBS
for 10 min at 37°C. The supernatant was collected and filtrated
with a 70-µm filter and collected cells were centrifuged at 100 × g
for 4 min at 25°C and resuspended in culture medium: RPMI-1640
medium (Sigma-Aldrich; Merck KGaA) with penicillin-streptomycin
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C for 12
h. Following washing out digestion buffer with RPMI-1640 medium
twice, cells were seeded on cell culture dishes and incubated for 1
h at 37°C. Following incubation, unattached cells were removed and
attached cells were cultured with fresh culture medium supplemented
with 10% fetal bovine serum (Sigma-Aldrich; Merck KGaA). Cells were
cultured in a 5% CO2 incubator with a humidified
atmosphere at 37°C.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cardiac fibroblasts
using TRIzol reagent (Life Technologies; Thermo Fisher Scientific,
Inc.). Quality of extracted mRNA was identified by Spectroscopy
Elemental Isotope Analysis using NanoDrop Lite (Thermo Fisher
Scientific, Inc.). According to the manufacturer's descriptions,
total RNA (1 µg) was reverse-transcribed to cDNA using the
Transcriptor First Strand cDNA Synthesis kit (Invitrogen; Thermo
Fisher Scientific, Inc.). The qPCR reaction was performed with a
SYBR-Green detection system (Bio SYBR Green Master Mix; Takara
Biotechnology Co., Ltd., Dalian, China). The following primers were
synthesized by Invitrogen; Thermo Fisher Scientific, Inc.: DCR-3,
forward 5′-CTCTTCCTCCCATGACAC-3′ and reverse
5′-CTGGAAAGCCACAAAGTC-3′; and β-actin, forward
5′-CGTGAAAAGATGACCCAGATCA-3′ and reverse
5′-CAGCCTGGATGGCTACGTACA-3′. PCR cycling conditions were performed
at 95°C for 30 sec and 45 cycles of 95°C for 5 sec, 56.5°C for 10
sec and 72°C for 10 sec. Relative mRNA expression changes were
calculated by the 2−ΔΔCq method (25). The results are expressed as a
fold-change compared with the β-actin control.
Small interfering RNA (siRNA)
transfection
siRNA to target PI3K (Si-PI3K) and scrambled siRNA
(Si-vector) were designed and synthesized by Shanghai GenePharma
Co., Ltd. (Shanghai, China). The sequences were as follows:
Si-PI3K, forward 5′-CCAACAACAGCAUGAACAAdTdT-3′ and reverse
5′-UUGUUCAUGCUGUUGUUGGdTdT-3′; and Si-vector, forward
5′-AUGAACGUGAAUUGCUCAAdTdT-3′ and reverse
5′-UUGAGCAAUUCACGUUCAUdTdT-3′. Cardiac fibroblasts isolated from
experimental mice were seeded in 24-well plates and were cultured
to 80% confluence. Then 1.25 µl 25 µM Si-PI3K or Si-vector were
transfected to cells using Lipofectamine™ RNAi MAX (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. Si-PI3K and Si-vector were from Shanghai GenePharma
Co., Ltd. (Shanghai, China). The interval between transfection and
subsequent experiments was 24 h.
Syntax score
Syntax score of patients with coronary heart disease
were calculated according to a previous study (26). The DCR-3 expression and syntax score
were analyzed in patients with coronary heart disease with healthy
volunteers as controls.
ELISA
In the protein detection assay, serum levels of
DCR-3 were measured using an ELISA kit (DY142; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The operating steps were
conducted according to the manufacturer's instructions. The final
results were recorded at 450 nm on an ELISA plate reader.
Western blotting
Myocardial cells harvested from mice were lysed in
radioimmunoprecipitation assay buffer containing a phosphatase
inhibitor and the protease inhibitor cocktail (Pierce; Thermo
Fisher Scientific, Inc.). Protein concentrations were determined
via bicinchoninic protein assay (Pierce; Thermo Fisher Scientific,
Inc.). Equal amounts of proteins (40 µg/lane) were loaded and
separated by 12% SDS-PAGE. The proteins were transferred to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were then blocked for 1 h at 37°C with 10%
bovine serum albumin (cat. no. 10735108001; Roche Applied Science,
Penzburg, Germany). The following rabbit anti-mouse antibodies were
used to incubate protein for 12 h at 4°C: DCR-3 (1:1,000, cat. no.
ab8405), cardiac troponin (cTn)T (1:1,000; cat. no. ab45932), cTn1
(1:1,000; cat. no. ab210798), IL-6 (1:1,000; cat. no. ab83053),
C-reactive protein 1 (CRP-1) (1:1,000; cat. no. ab211631), albumin
(1:1,000; cat. no. ab207327), intercellular adhesion molecule
(ICAM)-1 (1:1,000; cat. no. ab119817), vascular cell adhesion
molecule (VCAM)-1 (1:1,000; cat. no. ab134047), PI3K (1:1,000; cat.
no. ab40776), AKT (1:1,000; cat. no. ab8805), phosphorylated (p)AKT
(1:1,000; cat. no. ab81283) and β-actin (1:2,000; cat. no. ab8226;
all Abcam, Cambridge, UK). Horseradish peroxidase (HRP)-conjugated
goat anti-rabbit antibody (1:5,000; cat. no. HAF019; Bio-Rad
Laboratories, Inc.) was used as a secondary antibody for 2 h at
37°C and protein was detected using a western blotting Luminol
reagent (cat. no. 12015218001; Sigma-Aldrich; Merck KGaA) for
enhanced chemiluminescence. Lanes were observed using light
microscopy (magnification, ×4). The density of the bands was
analyzed using Quantity One software, version 4.62 (Bio-Rad
Laboratories, Inc.).
Immunofluorescence
Myocardial cells were fixed with formaldehyde
solution (10%) for 2 h at 37°C and processed according to standard
procedures. The sections are blocked with 5% bovine serum albumin
(Sigma-Aldrich; Merck KGaA) at 37°C for 15 min. Cells were
incubated with primary antibody against DCR-3 (1:1,000, cat. no.
ab8405) for 12 h at 4°C. Cells were then incubated with
HRP-conjugated anti-rabbit antibodies (1:200; cat. no. 71623,
Bio-Rad Laboratories, Inc.) for 2 h at 37°C. Cells were washed and
mounted with VectaShield mounting media with DAPI (Vector
Laboratories, Inc., Burlingame, CA, USA) and kept in the dark at
4°C prior to microscopic analysis. All images (magnification, ×10)
were captured with a confocal microscope (Fluoview1000; Olympus
Corporation, Tokyo, Japan).
Animal experiments
A total of 50 female C57BL/6J (age, 8 weeks; weight,
28–32 g) mice were purchased from OrientBio, Inc. (Seongnam,
Korea). All mice were given free access to food and water, and
housed at 23°C with 50% humidity with a 12-h artificial light/dark
cycle. A total of 45 mice were fed with a high-fat diet to
establish coronary heart disease model according to a previous
study (27); 5 healthy mice and 5
model mice were used to demonstrated the changes in DCR-3 level due
to coronary heart disease. The remaining 40 model mice were divided
into two groups (n=20/group) and received an intravenous injection
of DCR-3 (10 mg/kg; Sigma-Aldrich; Merck KGaA) or PBS (Control).
The treatments were repeated once daily for 10 days. Following 3
weeks, animals were sacrificed by cervical dislocation under
anesthesia with 3% isoflurane (Sigma-Aldrich; Merck KGaA). The
present study has been approved by the Committee for Experimental
Animal Studies of Southern Medical University.
Histological assay
Cardiac slices isolated from mice with cardiac
fibrosis were prepared and fixed in 4% paraformaldehyde for 30 min
at 37°C. Cardiac slices were performed using an
avidin-biotin-peroxidase technique. Paraffin-embedded tissue
sections were prepared, heated at 65°C to melt the paraffin and
dewaxed to water with xylene, ethyl alcohol (100, 95, 85 and 75%)
and water successively. Epitope retrieval was performed for further
analysis. The paraffin sections (4 µm) were incubated with hydrogen
peroxide (3%) for 10 min, and were blocked with 5% bovine serum
albumin (Sigma-Aldrich; Merck KGaA) for 10 min at 37°C. Finally,
the sections were incubated with rabbit anti-mouse anti-DCR-3 (cat.
no. ab8405; Abcam) diluted with PBS (1:1,000) or anti-major
histocompatibility complex І antibodies (cat. no. ab185706; Abcam)
diluted with PBS (1:1,000), for 12 h at 4°C following blocking with
5% bovine serum albumin (Sigma-Aldrich; Merck KGaA). Following
rinsing, sections were incubated in the presence of a biotinylated
horse anti-rabbit antibody (cat. no. a0545; 1:500; Chemicon; Merck
KGaA) for 2 h at 37°C. Sections were washed and observed
(magnification, ×10) using fluorescent video microscopy (BZ-9000;
Keyence Corporation, Osaka, Japan).
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay
The TUNEL assay was used for the analysis of
apoptosis of myocardial tissue in experimental mice at 37°C
following simvastatin treatment (10 mg/kg/day; Sigma-Aldrich; Merck
KGaA) or an equivalent dose of PBS. Procedures were performed as
previously described (27). Briefly,
4-µm tissue sections were fixed with 4% paraformaldehyde at 37°C
followed by permeabilization with 0.1% Triton X-100 for 1 h at
37°C. Subsequently, tissue sections or myocardial cells
(1×106) were stained with TUNEL reaction mixture
(Sigma-Aldrich; Merck KGaA) at 37°C for 2 h. Tissue sections and
myocardial cells were washed 3 times in TBS-Tween-20. TUNEL assays
were conducted using a TUNEL fluorescence FITC kit (Roche
Diagnostics, Indianapolis, IN, USA) according to the manufacturer's
instructions. Samples were mounted with neutral gum. Images in 6
fields of view (magnification, ×400) were captured using a Zeiss
LSM 510 confocal microscope (Zeiss AG, Oberkochen, Germany) at a
wavelength of 488 nm.
Statistical analysis
Results are expressed as the mean + standard
deviation. All data were analyzed using SPSS software (version
19.0; IBM Corp., Armonk, NY, USA). Comparisons between two groups
and among multiple groups were conducted by Student's t-test and
one-way analysis of variance followed by Tukey's post hoc test,
respectively. P<0.05 was considered to indicate a statistically
significant difference.
Results
DCR-3 expression levels in myocardial
cells of patients with coronary heart disease
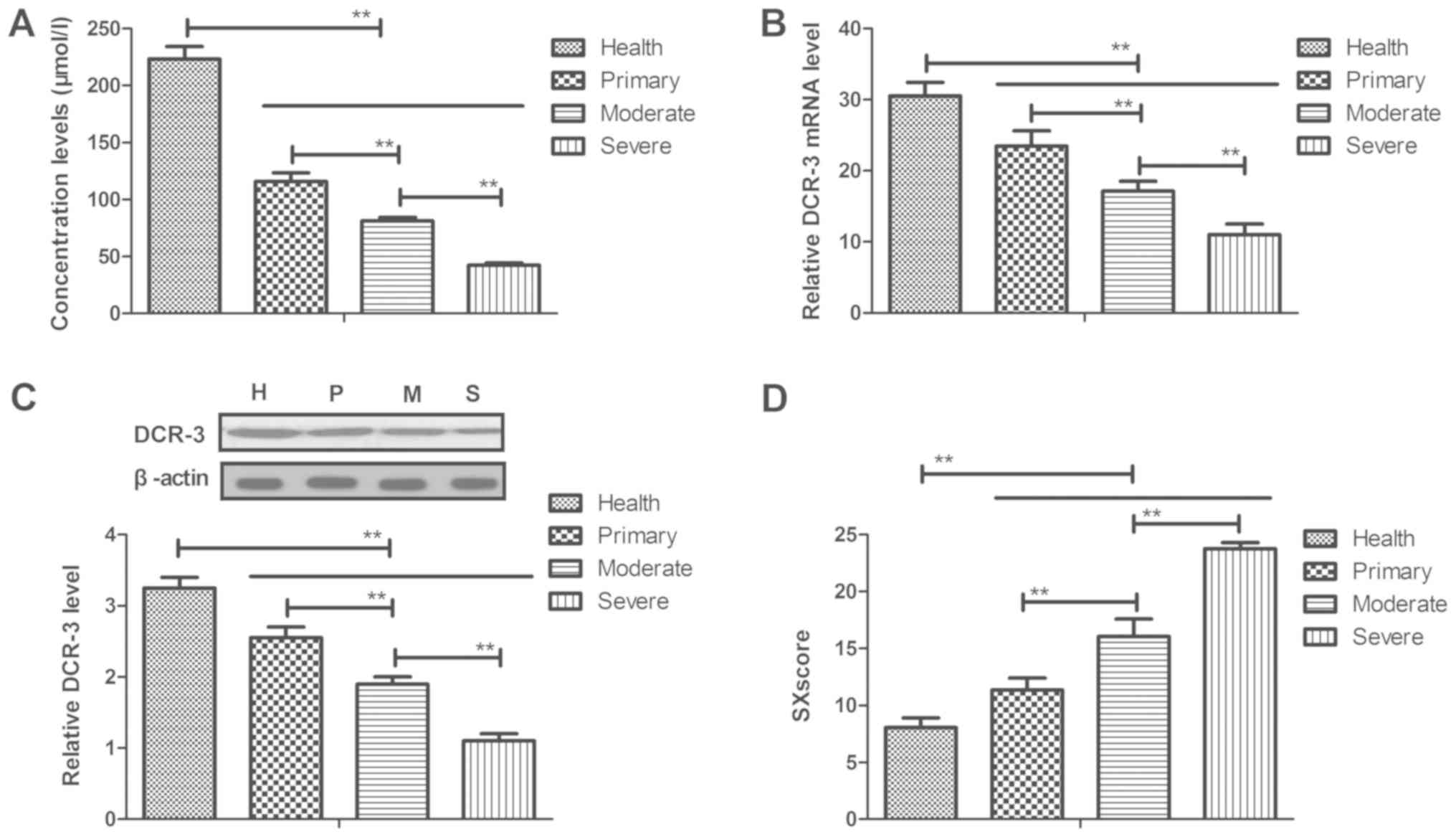
In the present study, the plasma concentration
levels of DCR-3 in patients with coronary heart disease were
analyzed. As presented in Fig. 1A,
it was observed that plasma concentration levels of DCR-3 were
downregulated in peripheral blood with the severity of the disease
increasing, as determined by ELISA. In addition, it was also
demonstrated that mRNA and protein expression in myocardial cells
were decreased with increasing disease severity, as determined by
RT-qPCR and western blotting, respectively (Fig. 1B and C). Furthermore, circulating
DCR-3 levels were associated with the Syntax score and severity of
coronary heart disease, which was demonstrated by the increase in
SXscore along with more serious disease condition (Fig. 1D). Taken together, these results
suggest DCR-3 expression levels can predict the events and severity
of coronary heart disease.
DCR-3 has benefits for the treatment
of mice with coronary heart disease by improvement of coronary
lesions
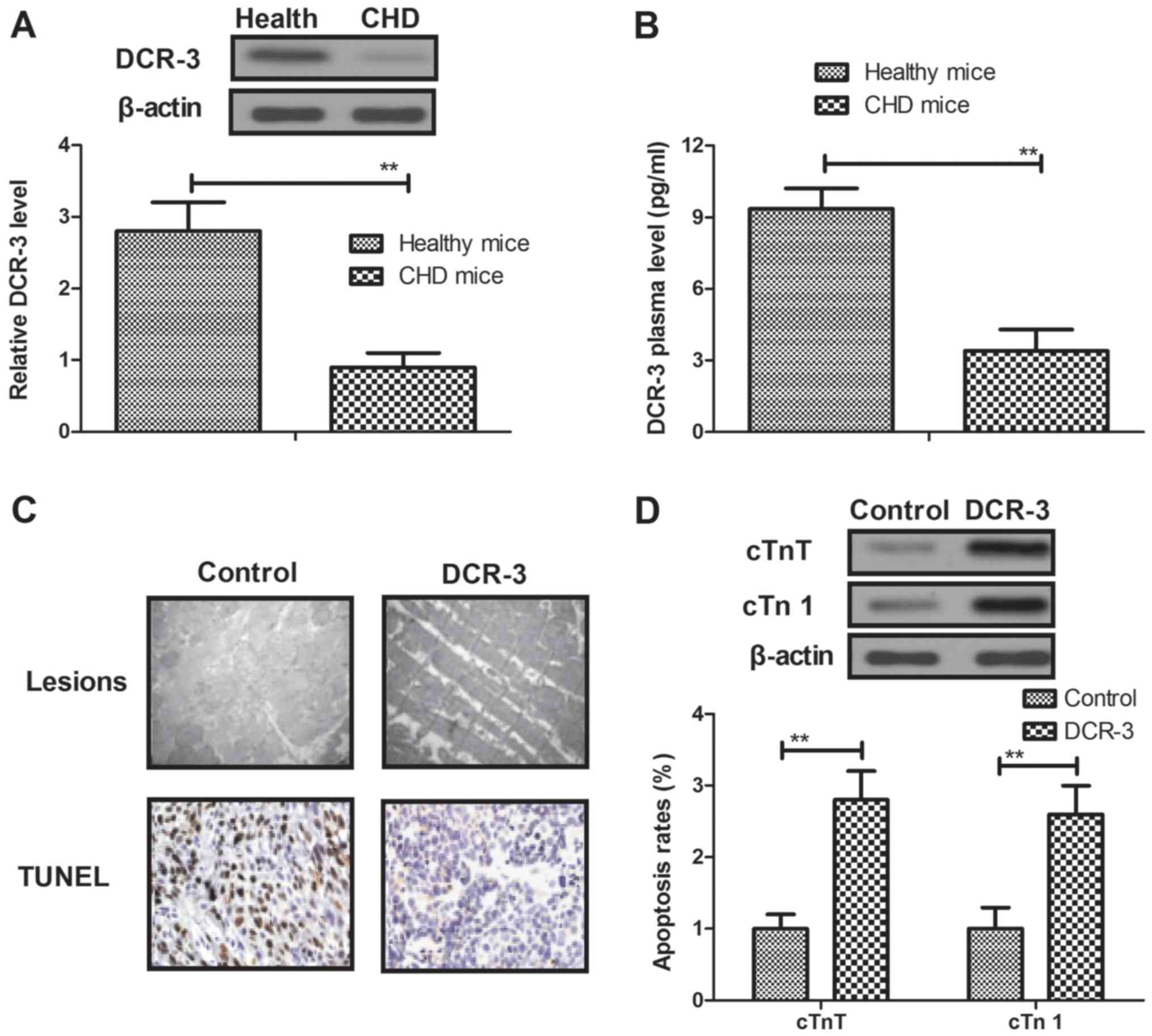
To investigate the efficacy of DCR-3 for coronary
heart disease, the therapeutic effects of DCR-3 were analyzed in
mice with coronary heart disease. By analyzing the DCR-3 expression
in myocardial cells, it was observed that DCR-3 expression in
myocardial cells was analyzed. It was observed that DCR-3
expression levels were downregulated in myocardial cells isolated
from mice with coronary heart disease (Fig. 2A). It was also observed that plasma
concentration levels were downregulated in the coronary heart
disease mice (Fig. 2B). In addition,
DCR-3 treatment affected the lesions and inhibited the apoptosis of
arterial vascular smooth muscle, as determined by histological
analysis (Fig. 2C). Furthermore,
results indicated that myocardial expression levels of
injury-associated proteins cTnT and cTn І were upregulated in
myocardium after treatment with DCR-3 (Fig. 2D). Taken together, these results
suggest that DCR-3 is beneficial for the remission of coronary
heart disease induced by TGF-β1 through improvement of coronary
lesions.
DCR-3 inhibits expression levels of
inflammatory factors in myocardial cells
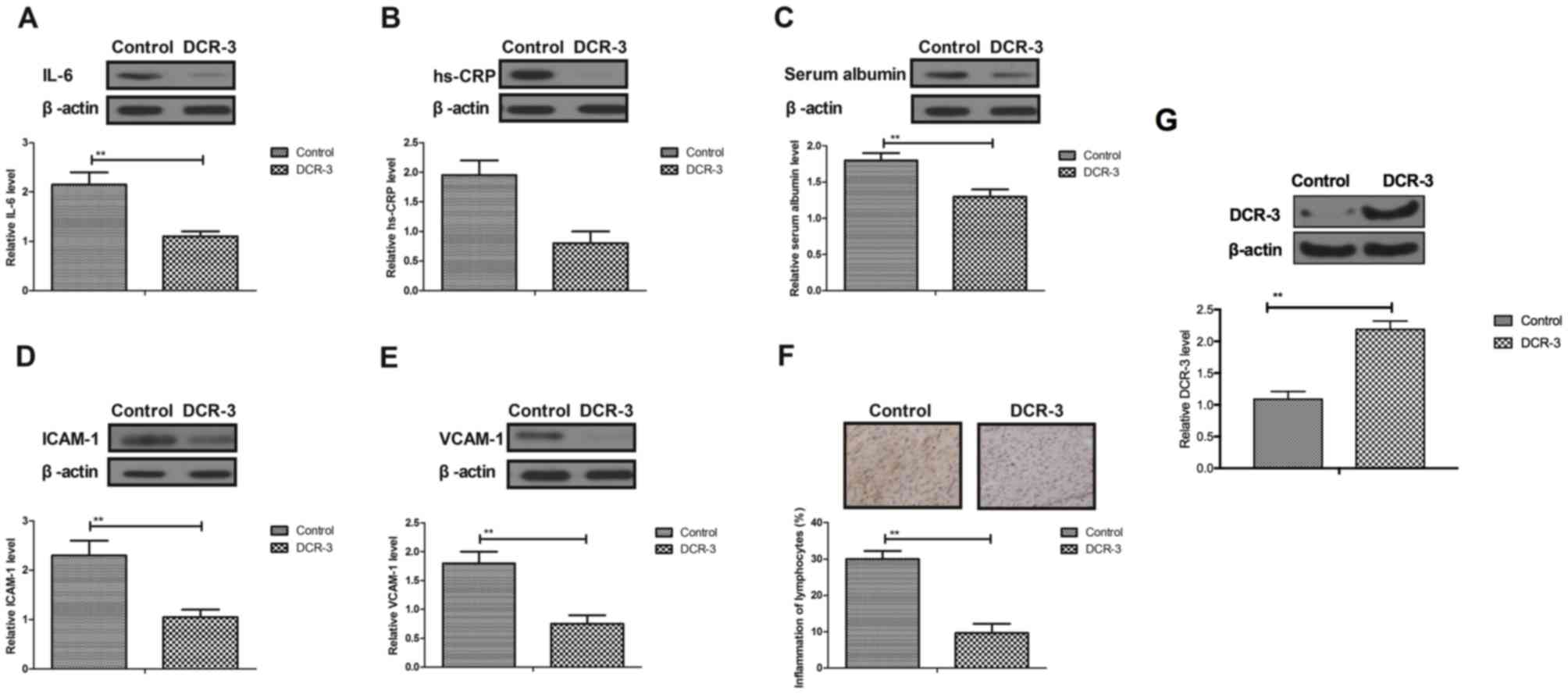
A previous study indicated that inflammatory
responses are associated with the progression of coronary heart
disease (28). Therefore, the
inflammatory factor expression was analyzed in myocardial cells and
tissues. As presented in Fig. 3A-E,
following treatment with DCR-3, IL-6, CRP-1, serum albumin, ICAM-1
and VCAM-1 were all decreased in myocardial cells in mice with
coronary heart disease, which indicated that DCR-3 suppressed the
expression levels of these inflammatory factors. Furthermore, DCR-3
could significantly ameliorate the myocardial inflammation of
lymphocytes as well (Fig. 3F). In
addition, the increased expression of DCR-3 was confirmed by
western blotting in myocardial cells treated with DCR-3 (Fig. 3G). Collectively, these results
suggest that DCR-3 can inhibit expression levels of inflammatory
factors in myocardial cells in experimental mice with coronary
heart disease.
DCR-3 improves inflammatory and
apoptosis in myocardial cells through the PI3K/AKT signaling
pathway
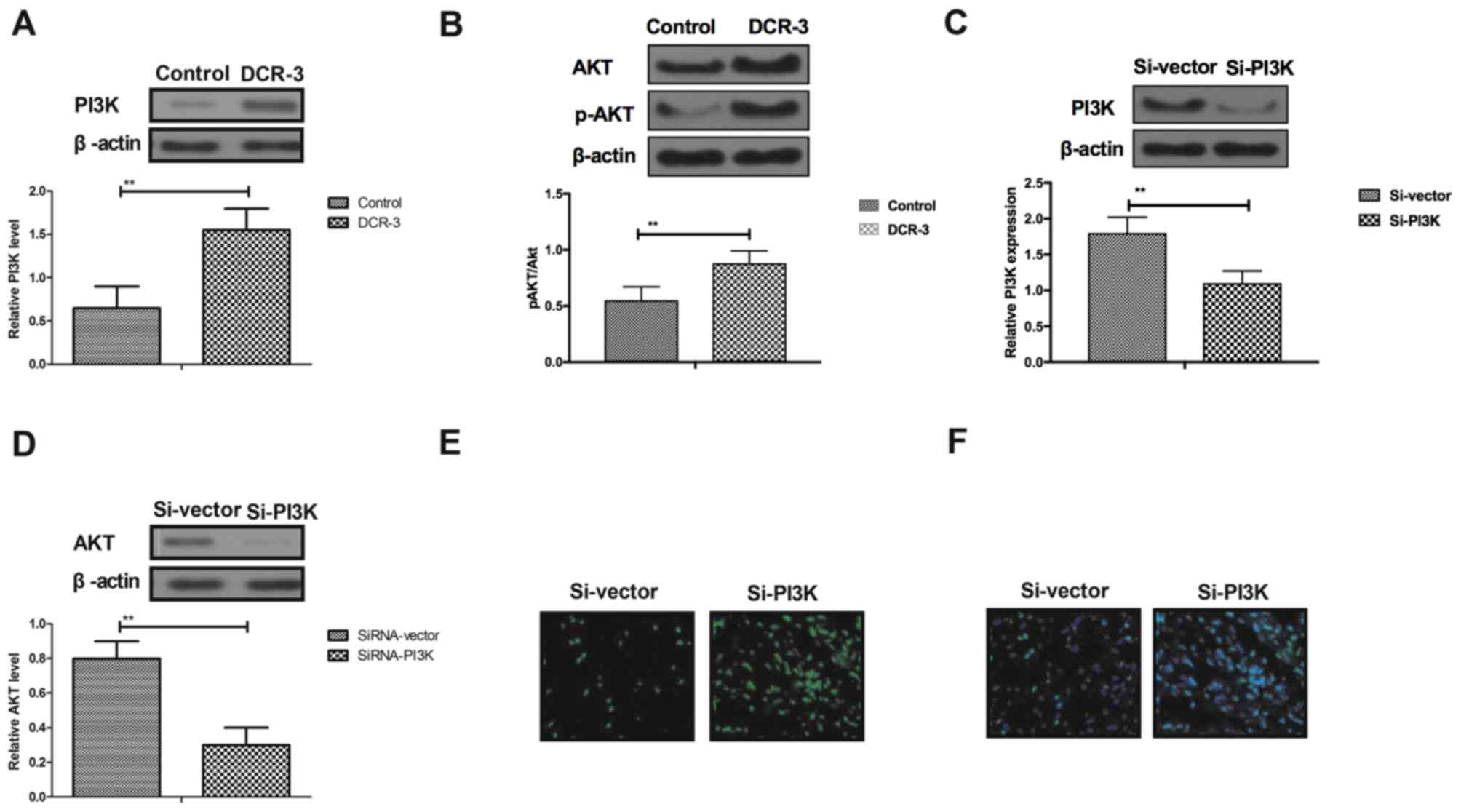
It has been demonstrated previously that
pretreatment of inflammatory factors by activating the PI3K/AKT
signaling pathway contributes to recovery of coronary heart
disease-induced myocardial ischemia and injury (29). In the present study, the PI3K/AKT
signaling pathway in myocardial cells was analyzed in experimental
mice with coronary heart disease treated by DCR-3. As presented in
Fig. 4A and B, DCR-3 in myocardial
cells upregulated the expression levels of PI3K and p-AKT/AKT,
compared with the control group. The results in Fig. 4C demonstrated that endogenous
inhibition of PI3K expression by Si-PI3K was successfully achieved
in myocardial cells. In vitro assays demonstrated that
endogenous inhibition of PI3K expression by Si-PI3K suppressed AKT
expression in myocardial cells (Fig.
4D). Furthermore, representative histological images revealed
that Si-PI3K also markedly inhibited improvement of DCR-3-mediated
apoptosis in myocardial cells, as determined by TUNEL and
immunofluorescence (Fig. 4E and F).
These findings suggest that PI3K/AKT signaling pathway involves the
anti-inflammatory and anti-apoptosis potential of DCR-3 in
myocardial cells in the progression of coronary heart disease
induced by TGF-β1.
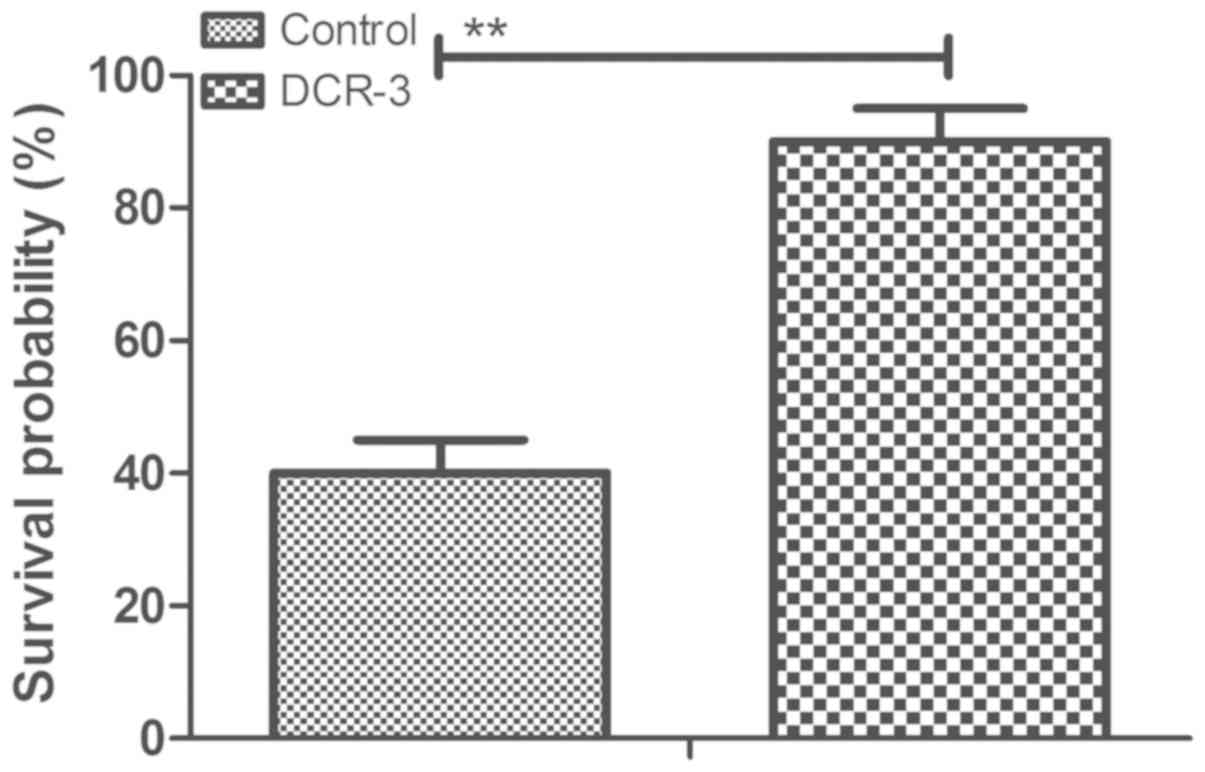
DCR-3 prolongs survival of mice with
coronary heart disease
The efficacy for the survival of mice with coronary
heart disease following treatment with DCR-3 was then determined.
As presented in Fig. 5, DCR-3
significantly prolonged the survival of mice with coronary heart
disease induced by TGF-β1 in a 90-day observation (n=20 in each
group). Survival analysis suggests that DCR-3 may be an efficient
agent for the treatment of coronary heart disease.
Discussion
DCR-3 acts as a soluble receptor of Fas ligand
(FasL), TNF superfamily member 14 (LIGHT) and TNF superfamily
member 15 that is reported highly expressed in cancer cells, but is
expressed less in myocardial cells (30). A previous study has indicated that
expression of DCR-3 is notable in chronic liver disease (31). However, the significance of
expression of DCR-3 in myocardial cells in progression of coronary
heart disease has not been reported in a previous study (32). The present study investigated the
molecular mechanism of DCR-3 in myocardial cells in the progression
of coronary heart disease. Although previous reports have reported
that plasma DCR-3 levels may indicate the severity of coronary
heart disease (33), the potential
mechanism mediated by DCR-3 has not been reported in myocardial
cells. The present results demonstrated that DCR-3 is downregulated
in patients with coronary heart disease and increasing DCR-3
expression can prevent inflammation and apoptosis in myocardial
cells induced by coronary heart disease. DCR-3 also mediate
anti-inflammatory and anti-apoptosis potential through the PI3K/AKT
signaling pathway in myocardial cells in the progression of
coronary heart disease induced by TGF-β1. These findings may
elucidate the function of DCR-3 in processes of apoptosis and
inflammation for patients with coronary heart disease.
Inflammation is one of the most common symptoms of
patients with acute coronary heart disease and the marked
inflammation during acute coronary syndrome contributes to later
depression in a subset of patients (34). Inflammatory response may be used as a
prognostic indicator for borderline lesion coronary heart disease
patients, who were treated with naoxintong (35). In addition, El-Mesallamy et al
(36) have indicated that
inflammation is one of factors in the imitation of coronary heart
disease. Myocardial tissue has a higher level of inflammatory
factors and lymphocytes. However, a stable DCR-3 analogue can
reduce FasL-induced murine pulmonary inflammation (37). At the same time, DCR-3 may improve
experimental autoimmune encephalomyelitis by directly counteracting
local inflammation, suggesting that DCR-3 is the potential agent
for treating human multiple sclerosis (38). All of these reports suggest that
DCR-3 is associated with inflammatory responses in patients with
cardiovascular disease. Additionally, although previous data
demonstrated that slightly elevated cTnS levels may not be a
sensitive prognostic marker for the Chinese population (39), a number of studies have indicated
that serum cTnT levels are also regarded as an indicator of
myocardial injury in ischemic and hemorrhagic stroke patients
(40–42). The present results demonstrated that
DCR-3 increased serum levels of cTnT and cTn1 in mice with coronary
heart disease and that DCR-3 can inhibit inflammation responses and
expression levels of inflammatory responses in myocardial cells in
mice with coronary heart disease.
Apoptosis of myocardial cells is another crucial
factor in initiating coronary heart disease (7). Considerable evidence has indicated that
apoptosis has an important role in hepatocyte death in chronic
liver disease (43). Research also
demonstrated that apoptosis of leukocytes acts as a disease marker
of neutrophil-endotheliocyte interaction in patients with coronary
heart disease (14). Liu et
al (15) have previously
investigated the expression levels of inflammatory and apoptosis
factors in coronary stent implantation in coronary heart disease
patients, and demonstrated that downregulation of inflammatory and
apoptosis factors is beneficial to the recovery of coronary heart
disease. DCR-3, as a pleiotropic immunomodulator for enhancing
angiogenesis, has been observed to downregulate cell apoptosis by
binding with its receptor of FasL and LIGHT, and subsequently
improved survival of many types of cell, such as human IPF
fibroblasts (44) and mouse
lymphocytes (45). Furthermore,
DCR-3 also suppressed FasL-induced apoptosis via extracellular
signal-regulated kinase 1/2 activation in various cancer cells
(46–48). Furthermore, a number of studies have
reported DCR-3 function in lymphocytes and dendritic cells
(45,49). The present study indicated that DCR-3
could inhibit apoptosis of myocardial cells in mice with coronary
heart disease, which contributed to protect myocardial cells
against injury in the process of coronary heart disease. Notably,
injury-associated protein levels of cTnT and cTn І were markedly
upregulated in myocardium by the treatment of DCR-3. These results
suggest that inhibition of apoptosis is the essential function of
DCR-3 for the treatment of coronary heart disease. However, the
present study did not use a healthy or sham control to identify the
role of DCR-3 for the treatment of coronary heart disease, which is
a limitation of this research.
The PI3K/AKT signaling pathway is involved in the
progression of myocardial infarction in the process of coronary
heart disease (50). Hu et al
(51) presented the protective
effect of proanthocyanidins on anoxia-reoxygenation injury of
myocardial cells and results have indicated that the
PI3K/AKT/glycogen synthase kinase-3β pathway mediates mitochondrial
ATP-sensitive potassium channel to regulate oxidative stress. It
has also been indicated that the PI3K/AKT pathway-regulated
recruiting T cells could attenuate myocardial ischemia-reperfusion
injury in a mice model (52). In the
present study, by investigating the association between DCR-3 and
the PI3K/AKT signal pathway in myocardial cells in mice with
coronary heart disease, it was observed that DCR-3 mediated the
improvement of myocardial ischemia via the PI3K/AKT signaling
pathway. Notably, it also demonstrated that endogenous inhibition
of PI3K expression by Si-PI3K abolished DCR-3-mediated
anti-inflammation and anti-apoptosis in myocardial cells in mice
with coronary heart disease.
In conclusion, the present study investigated the
efficacy of DCR-3 in the progression of coronary heart disease and
the molecular mechanism of DCR-3-mediated anti-inflammation and
anti-apoptosis in myocardial cells in mice with coronary heart
disease. Results have demonstrated that patients and mice with
coronary heart disease have lower DCR-3 expression levels in
peripheral blood. DCR-3 can inhibit inflammatory factor expression
and apoptosis of myocardial cells and that the PI3K/AKT signaling
pathway is associated with anti-inflammation and anti-apoptosis in
the process of coronary heart disease. These results confirm that
DCR-3 treatment may be beneficial for the recovery of coronary
heart disease by anti-inflammation and anti-apoptosis in myocardial
cells through the PI3K/AKT signaling pathway, suggesting that DCR-3
may be able to predict the extent and severity of cardiovascular
diseases.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from
Fujian Provincial Health and Family Planning Young and Middle-aged
Key Members Talent Training Project (grant no. 2017-ZQN-9), Fujian
Provincial Natural Science Funding Project (grant no. 2018J01244),
The Key Clinical Specialty Discipline Construction Program of
Fujian (Program of Vasculocardiology) and Fujian Provincial Youth
Scientific Research Project of Health Department (grant no.
2012-2-10).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XJC, RHW and WC designed the experiments. XJC and
RHW performed the experiments and analysis. RHW and LL helped
analyze the data. RHW, WC and ZLL wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee and the Committee for Experimental Animal Studies of
Southern Medical University (Guangzhou, China). All patients
provided written informed consent.
Patient consent for publication
All patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tully PJ and Baumeister H: Collaborative
care for comorbid depression and coronary heart disease: A
systematic review and meta-analysis of randomised controlled
trials. BMJ Open. 5:e0091282015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heikkilä K, Koskinen OA, Agarwal A,
Tikkinen KA, Mäki M and Kaukinen K: Associations of coeliac disease
with coronary heart disease and cerebrovascular disease: A
systematic review and meta-analysis. Nutr Metab Cardiovasc Dis.
25:816–831. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mayor S: Reduced blood pressure and
cholesterol are main factors in fall in deaths from coronary heart
disease. BMJ. 350:h4152015. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Niermann C, Gorressen S, Klier M, Gowert
NS, Billuart P, Kelm M, Merx MW and Elvers M: Oligophrenin1
protects mice against myocardial ischemia and reperfusion injury by
modulating inflammation and myocardial apoptosis. Cell Signal.
28:967–978. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo CX, Jiang X, Zeng XJ, Wang HX, Li HH,
Du FH and Chen BX: Soluble receptor for advanced glycation
end-products protects against ischemia/reperfusion-induced
myocardial apoptosis via regulating the ubiquitin proteasome
system. Free Radic Biol Med. 94:17–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
O'Neal WT, Soliman EZ, Howard G, Howard
VJ, Safford MM, Cushman M and Zakai NA: Inflammation and hemostasis
in atrial fibrillation and coronary heart disease: The REasons for
geographic and racial differences in stroke study. Atherosclerosis.
243:192–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Geng YJ: Molecular mechanisms for
cardiovascular stem cell apoptosis and growth in the hearts with
atherosclerotic coronary disease and ischemic heart failure. Ann N
Y Acad Sci. 1010:687–697. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fang Y, Chen S, Liu Z, Ai W, He X, Wang L,
Xie P, Jiang B and Fang H: Endothelial stem cells attenuate cardiac
apoptosis via downregulating cardiac microRNA-146a in a rat model
of coronary heart disease. Exp Ther Med. 16:4246–4252.
2018.PubMed/NCBI
|
|
9
|
Xu L, Jiang X, Wei F and Zhu H: Leonurine
protects cardiac function following acute myocardial infarction
through anti-apoptosis by the PI3K/AKT/GSK3β signaling pathway. Mol
Med Rep. 18:1582–1590. 2018.PubMed/NCBI
|
|
10
|
Wang Z, Zhang J, Ren T and Dong Z:
Targeted metabolomic profiling of cardioprotective effect of Ginkgo
biloba L.extract on myocardial ischemia in rats. Phytomedicine.
international journal of phytotherapy and phytopharmacology.
23:621–631. 2016. View Article : Google Scholar
|
|
11
|
Wang L, Niu X, Hu J, Xing H, Sun M, Wang
J, Jian Q and Yang H: After myocardial ischemia-reperfusion,
mir-29a, and let7 could affect apoptosis through regulating IGF-1.
BioMed Research International. 2015:2454122015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wakiyama H, Cowan DB, Toyoda Y, Federman
M, Levitsky S and McCully JD: Selective opening of mitochondrial
ATP-sensitive potassium channels during surgically induced
myocardial ischemia decreases necrosis and apoptosis. Eur J
Cardiothorac Surg. 21:424–433. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Elsasser A, Suzuki K, Lorenz-Meyer S, Bode
C and Schaper J: The role of apoptosis in myocardial ischemia: A
critical appraisal. Basic Res Cardiol. 96:219–226. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salmina AB, Shul'man VA, Nikulina SY,
Trufanova LV, Fursov AA, But'yanov PA, Kuskaev AP, Bol'shakova EV
and Kotlovskii MY: Apoptosis of leukocytes as a marker of
neutrophil-endotheliocyte interaction in coronary heart disease.
Bull Exp Biol Med. 144:39–41. 2007.(In English, Russian).
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu LL, Lin LR, Lu CX, Fu JG, Chao PL, Jin
HW, Zhang ZY and Yang TC: Expression of inflammatory and apoptosis
factors following coronary stent implantation in coronary heart
disease patients. Int Immunopharmacol. 11:1850–1854. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Azambuja MI: Inflammation as the cause of
coronary heart disease. Lancet Infect Dis. 10:142–143. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu T, Sun QF, Yang PS, Feng W and Liu XL:
Levels of inflammation cytokines in patients with coronary heart
disease and periodontal disease. Zhonghua Kou Qiang Yi Xue Za Zhi.
45:265–268. 2010.(In Chinese). PubMed/NCBI
|
|
18
|
Yan Y, Song D, Liu L, Meng X, Qi C and
Wang J: The relationship of plasma decoy receptor 3 and coronary
collateral circulation in patients with coronary artery disease.
Life Sci. 189:84–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marriott HM, Daigneault M, Thompson AA,
Walmsley SR, Gill SK, Witcher DR, Wroblewski VJ, Hellewell PG,
Whyte MK and Dockrell DH: A decoy receptor 3 analogue reduces
localised defects in phagocyte function in pneumococcal pneumonia.
Thorax. 67:985–992. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu SF, Liu TM, Lin YC, Sytwu HK, Juan HF,
Chen ST, Shen KL, his SC and Hsieh SL: Immunomodulatory effect of
decoy receptor 3 on the differentiation and function of bone
marrow-derived dendritic cells in nonobese diabetic mice: From
regulatory mechanism to clinical implication. J Leukoc Biol.
75:293–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang M, Lin X, He R, Lin X, Liang L, Tang
R, Xiong D, Wei K, Dang Y, Feng Z and Chen G: Decoy receptor 3
(DcR3) as a biomarker of tumor deterioration in female reproductive
cancers: A meta-analysis. Med Sci Monit. 22:1850–1857. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dietz R and Rauch B: German Society of
Cardiology-Heart Circulation Research; German Society for
Prevention Rehabilitation of Cardiac Diseases; German Society for
Thoracic CardiovascularSurgery: Guidelines for diagnosis and
treatment of chronic coronary heart disease. Issued by the
executive committee of the German Society of Cardiology - Heart
Circulation Research in cooperation with the German Society for
Prevention and Rehabilitation of Cardiac Diseases and the German
Society for Thoracic and Cardiovascular Surgery. Z Kardiol.
92:501–521. 2003.(In German). PubMed/NCBI
|
|
23
|
Golden HB, Gollapudi D, Gerilechaogetu F,
Li J, Cristales RJ, Peng X and Dostal DE: Isolation of cardiac
myocytes and fibroblasts from neonatal rat pups. Methods Mol Biol.
843:205–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee I, Chalon J, Ramanathan S, Gross S and
Turndorf H: Analgesic properties of meperidine, amitriptyline and
phenelzine in mice. Can Anaesth Soc J. 30:501–505. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Börekçi A, Gür M, Şeker T, Baykan AO,
Özaltun B, Karakoyun S, Karakurt A, Türkoğlu C, Makça I and Çaylı
M: Coronary collateral circulation in patients with chronic
coronary total occlusion; its relationship with cardiac risk
markers and SYNTAX score. Perfusion. 30:457–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu ZW, Liu YF, Wang S and Li B: miRNA-146a
induces vascular smooth muscle cell apoptosis in a rat model of
coronary heart disease via NF-κB pathway. Genet Mol Res.
14:18703–18712. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Werba JP, Veglia F, Amato M, Baldassarre
D, Massironi P, Meroni PL, Riboldi P, Tremoli E and Camera M:
Patients with a history of stable or unstable coronary heart
disease have different acute phase responses to an inflammatory
stimulus. Atherosclerosis. 196:835–840. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rong R and Xijun X: Erythropoietin
pretreatment suppresses inflammation by activating the PI3K/Akt
signaling pathway in myocardial ischemia-reperfusion injury. Exp
Ther Med. 10:413–418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Funke B, Autschbach F, Kim S, Lasitschka
F, Strauch U, Rogler G, Gdynia G, Li L, Gretz N, Macher-Goeppinger
S, et al: Functional characterisation of decoy receptor 3 in
Crohn's disease. Gut. 58:483–491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim S, Kotoula V, Hytiroglou P, Zardavas D
and Zhang L: Significance of increased expression of decoy receptor
3 in chronic liver disease. Dig Liver Dis. 41:591–598. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chang TY, Hsu CY, Huang PH, Chiang CH, Leu
HB, Huang CC, Chen JW and Lin SJ: Usefulness of circulating decoy
receptor 3 in predicting coronary artery disease severity and
future major adverse cardiovascular events in patients with
multivessel coronary artery disease. Am J Cardiol. 116:1028–1033.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim S, McAuliffe WJ, Zaritskaya LS, Moore
PA, Zhang L and Nardelli B: Selective induction of tumor necrosis
receptor factor 6/decoy receptor 3 release by bacterial antigens in
human monocytes and myeloid dendritic cells. Infect Immun.
72:89–93. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Steptoe A, Wikman A, Molloy GJ,
Messerli-Bürgy N and Kaski JC: Inflammation and symptoms of
depression and anxiety in patients with acute coronary heart
disease. Brain Behav Immun. 31:183–188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li SR, Wang TH and Zhang BJ: Effects of
naoxintong capsule on the inflammation and prognosis in borderline
lesion coronary heart disease patients. Zhongguo Zhong Xi Yi Jie He
Za Zhi. 32:607–611. 2012.(In Chinese). PubMed/NCBI
|
|
36
|
El-Mesallamy HO, Hamdy NM, Salman TM and
Ibrahim SM: Adiponectin and sE-selectin concentrations in relation
to inflammation in obese type 2 diabetic patients with coronary
heart disease. Angiology. 63:96–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wortinger MA, Foley JW, Larocque P,
Witcher DR, Lahn M, Jakubowski JA, Glasebrook A and Song HY: Fas
ligand-induced murine pulmonary inflammation is reduced by a stable
decoy receptor 3 analogue. Immunology. 110:225–233. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen SJ, Wang YL, Kao JH, Wu SF, Lo WT, Wu
CC, Tao PL, Wang CC, Chang DM and Sytwu HK: Decoy receptor 3
ameliorates experimental autoimmune encephalomyelitis by directly
counteracting local inflammation and downregulating Th17 cells. Mol
Immunol. 47:567–574. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang M, He H, Wang ZM, Xu Z, Zhou N, Tao
Z, Chen B, Li C, Zhu T, Yang D, et al: Diagnostic and prognostic
value of minor elevated cardiac troponin levels for percutaneous
coronary intervention-related myocardial injury: A prospective,
single-center and double-blind study. J Biomed Res. 28:98–107.
2014.PubMed/NCBI
|
|
40
|
Apak I, Iltumur K, Tamam Y and Kaya N:
Serum cardiac troponin T levels as an indicator of myocardial
injury in ischemic and hemorrhagic stroke patients. Tohoku J Exp
Med. 205:93–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nesher N, Zisman E, Wolf T, Sharony R,
Bolotin G, David M, Uretzky G and Pizov R: Strict thermoregulation
attenuates myocardial injury during coronary artery bypass graft
surgery as reflected by reduced levels of cardiac-specific troponin
I. Anesth Analg. 96:328–335. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Boriani G, Biffi M, Cervi V, Bronzetti G,
Magagnoli G, Zannoli R and Branzi A: Evaluation of myocardial
injury following repeated internal atrial shocks by monitoring
serum cardiac troponin I levels. Chest. 118:342–347. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu ZW, Liu YF, Wang S and Li B:
Corrigendum miRNA-146a induces vascular smooth muscle cell
apoptosis in a rat model of coronary heart disease via NF-kappaB
pathway - Genet. Mol. Res. 14(4): 18703–18712, Genet Mol Res 15.
2016. View Article : Google Scholar
|
|
44
|
Im J, Kim K, Hergert P and Nho RS:
Idiopathic pulmonary fibrosis fibroblasts become resistant to Fas
ligand-dependent apoptosis via the alteration of decoy receptor 3.
J Pathol. 240:25–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liang D, Hou Y, Lou X and Chen H: Decoy
receptor 3 improves survival in experimental sepsis by suppressing
the inflammatory response and lymphocyte apoptosis. PLoS One.
10:e01316802015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang Y, Li D, Zhao X, Song S, Zhang L,
Zhu D, Wang Z, Chen X and Zhou J: Decoy receptor 3 suppresses
FasL-induced apoptosis via ERK1/2 activation in pancreatic cancer
cells. Biochem Biophys Res Commun. 463:1144–1151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Koksal IT, Sanlioglu AD, Karacay B,
Griffith TS and Sanlioglu S: Tumor necrosis factor-related
apoptosis inducing ligand-R4 decoy receptor expression is
correlated with high Gleason scores, prostate-specific antigen
recurrence, and decreased survival in patients with prostate
carcinoma. Urol Oncol. 26:158–165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou J, Song S, He S, Wang Z, Zhang B, Li
D and Zhu D: Silencing of decoy receptor 3 (DcR3) expression by
siRNA in pancreatic carcinoma cells induces Fas ligand-mediated
apoptosis in vitro and in vivo. Int J Mol Med. 32:653–660. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
You RI, Chang YC, Chen PM, Wang WS, Hsu
TL, Yang CY, Lee CT and Hsieh SL: Apoptosis of dendritic cells
induced by decoy receptor 3 (DcR3). Blood. 111:1480–1488. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang Z, Li S, Cui M, Gao X, Sun D, Qin X,
Narsinh K, Li C, Jia H, Li C, et al: Rosuvastatin enhances the
therapeutic efficacy of adipose-derived mesenchymal stem cells for
myocardial infarction via PI3K/Akt and MEK/ERK pathways. Basic Res
Cardiol. 108:3332013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hu Y, Li L, Yin W, Shen L, You B and Gao
H: Protective effect of proanthocyanidins on anoxia-reoxygenation
injury of myocardial cells mediated by the PI3K/Akt/GSK-3beta
pathway and mitochondrial ATP-sensitive potassium channel. Mol Med
Rep. 10:2051–2058. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fang J, Hu F, Ke D, Yan Y, Liao Z, Yuan X,
Wu L, Jiang Q and Chen L: N,N-dimethylsphingosine attenuates
myocardial ischemia-reperfusion injury by recruiting regulatory T
cells through PI3K/Akt pathway in mice. Basic Res Cardiol.
111:322016. View Article : Google Scholar : PubMed/NCBI
|