Introduction
Cervical cancer is one of the most common
gynecological malignant tumors, and mainly occurs in developing
countries, including China (1,2). It has
been well-established that human papillomavirus (HPV) infection is
significantly associated with the development of cervical cancer
(3). Furthermore, certain oncogenes
and tumor suppressors have also been identified to have key roles
in cervical cancer (4–6). Elucidation of the molecular mechanisms
underlying cervical cancer growth and metastasis is beneficial for
the development of novel therapeutic strategies for this
disease.
MicroRNAs (miRs), a class of small non-coding RNAs,
can directly bind to the 3′ untranslated region (UTR) of their
target mRNAs, and cause RNA degradation or translation inhibition
(7,8). Through mediating the expression of
their target genes, miRs are involved in various cellular
biological processes, including cell proliferation,
differentiation, migration and invasion, as well as tumorigenesis
(9–11). A large number of miRs have been
demonstrated to have promoting or suppressive roles in various
human cancer types, including cervical cancer, and certain miRs are
strongly associated with HPV (12,13). For
instance, HPV16 E7 increases the expression of miR-27b, which
further promotes the proliferation and invasion of cervical
carcinoma cells via directly suppressing the expression of
peroxisome proliferator-activated receptor γ (14).
miR-130a has recently been demonstrated to be
involved in various common human cancer types, including
hepatocellular carcinoma, ovarian cancer, glioblastoma, prostate
carcinoma, leukemia and cervical cancer (15). Feng et al (16) reported that miR-130a was regulated by
nuclear factor (NF)-κB and promoted cervical cancer cell growth by
inhibiting the expression of phosphatase and tensin homolog (PTEN).
However, the exact role of miR-130a in cervical cancer metastasis,
as well as its regulation and the underlying mechanisms, have
remained to be determined.
Tissue inhibitor of metalloproteinases 2 (TIMP2) is
a member of the TIMP gene family, which are natural inhibitors of
the matrix metalloproteinases (MMPs), a group of peptidases
involved in the degradation of the extracellular matrix and thus
cancer metastasis (17). TIMP2 was
reported to be associated with cervical cancer invasion (18). However, the regulatory roles of TIMP2
in cervical cancer have remained to be fully elucidated.
The present study mainly aimed to explore the
regulatory roles of miR-130a in cervical cancer metastasis and the
underlying mechanisms. Furthermore, the possible link between HPV
E6, miR-130a and TIMP2 in cervical cancer cells was assessed.
Materials and methods
Tissue collection
This study was approved by the Ethics Committee of
the First Affiliated Hospital of Xinxiang Medical University
(Weihui, China). Cervical cancer tissues and matched adjacent
normal tissues were collected from 56 cervical cancer patients at
the First Affiliated Hospital of Xinxiang Medical University
(Weihui, China) between September 2014 and May 2016. These cervical
cancer patients were aged between 43 and 67 years (mean age, 55.7
years). Written informed consent was obtained from all patients.
None of these patients received any radiation therapy or
chemotherapy prior to surgery. After resection the tissues were
immediately snap-frozen in liquid nitrogen and stored in liquid
nitrogen until use.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and cell lines
using TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). A High-Capacity cDNA Reverse Transcription kit (cat. no.
4368813; Thermo Fisher Scientific, Inc.) was used to convert 1 µg
RNA into complementary (c)DNA according to the manufacturer's
protocol. For detection of miR-130a expression, the MiRNA qPCR
Detection kit (cat. no. AMPR-0200; GeneCopoeia, Inc., Rockville,
MD, USA) was used for amplification of cDNA on an ABI 7500
fluorescent qPCR machine (Thermo Fisher Scientific, Inc.) according
to the manufacturer's protocol. U6 was used as the internal
reference. The primers for miR-130a (cat. no. HmiRQP0156) and U6
(cat. no. HmiRQP9001) were purchased from Fulengen (Guangzhou,
China). For detecting the mRNA expression, SYBR Green qPCR Master
mix (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used
according to the manufacturer's protocol. GAPDH was utilized as the
internal reference. The primer sequences were as follows: HPV18 E6,
forward 5-AGGCGATTAAGTTGGGTA-3 and reverse 5-CGGTAGGCGTGTACGGTG-3;
TIMP2, forward 5-AAGCGGTCAGTGAGAAGGAAG-3 and reverse
5-GGGGCCGTGTAGATAAACTCTAT-3; GAPDH, forward
5-GGAGCGAGATCCCTCCAAAAT-3 and reverse 5-GGCTGTTGTCATACTTCTCATGG-3.
The thermocycling conditions were as follows: Initial denaturation
at 95°C for 3 min and 35 cycles of denaturation at 95°C for 15 sec
and annealing/elongation at 60°C for 30 sec. A melting curve
analysis was performed to detect products. The relative expression
was analyzed using the 2−ΔΔCq method
(19).
Cell culture
The SiHa (HPV16+), Caski (HPV16+), HeLa (HPV18+) and
C33A (HPV-) human cervical cancer cell lines were purchased from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
C33A cells were cultured in RPMI1640 medium (Thermo Fisher
Scientific, Inc.) with 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.), and CaSki, SiHa and HeLa cells were cultured in
Dulbecco's modified Eagle's Medium (DMEM; Thermo Fisher Scientific,
Inc.) with 10% FBS at 37°C in a humidified atmosphere containing 5%
CO2.
Cell transfection
HeLa cells were transfected with negative control
inhibitor (anti-NC; cat. no. CmiR-AN0001-SN; Fulengen), miR-130a
inhibitor (anti-miR-130a; cat. no. HmiR-AN0156-SN-10; Fulengen),
scrambled miRNA mimics (miR-NC; cat. no. CmiR0001-MR04; Fulengen),
miR-130a mimics (cat. no. HmiR0170-MR04; Fulengen), NC small
interfering (si)RNA (cat. no. sc-37007; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), HPV18 E6 siRNA (cat. no. 3262; Dharmacon;
Thermo Fisher Scientific, Inc.), or co-transfected with miR-130a
inhibitor and TIMP2 siRNA (cat. no. sc-29506; Santa Cruz
Biotechnology, Inc.), or co-transfected with miR-130a inhibitor and
NC siRNA using Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instruction.
Western blot analysis
Cells were lysed in cold (4°C)
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Inc.) for 30 min. The protein concentration was examined using a
Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.).
Subsequently, 50 µg protein was separated by 10% SDS-PAGE and then
transferred to a polyvinylidene fluoride membrane (Thermo Fisher
Scientific, Inc.). The membrane was blocked in 5% non-fat dried
milk in (PBS) at room temperature for 4 h. Subsequently, the
membrane was incubated with mouse anti-HPV18 E6 antibody (1:500
dilution; cat. no. ab20192; Abcam, Cambridge, MA, USA), rabbit
anti-human TIMP2 antibody (1:500 dilution; cat. no. ab180630;
Abcam), or rabbit anti-human GAPDH antibody (1:500 dilution; cat.
no. ab9485; Abcam) for at room temperature 3 h, and then incubated
with goat anti-mouse secondary antibody (1:5,000 dilution; cat. no.
ab97035; Abcam) or goat anti-rabbit secondary antibody (1:5,000
dilution; cat. no. ab7090; Abcam) at room temperature for 1 h.
According to the manufacturer's instructions, the immune complex on
the polyvinylidene fluoride membrane was detected using an Enhanced
Chemiluminescence Western Blotting Kit (Thermo Fisher Scientific,
Inc.). The protein expression was determined using Image-Pro Plus
software 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Wound healing assay
HeLa cells were cultured to full confluence in
6-well plates. Mitomycin C (10 µg/ml; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was used to treat cells at 37°C for 2 h to
inhibit cell proliferation. Cells were washed with DMEM for 3
times. A wound was created by scraping the cell monolayer with a
200-µl pipette tip. After washing with DMEM twice, the cells were
incubated in DMEM supplemented with 1% FBS for 24 h. Subsequently,
the wound was observed under a microscope (Olympus, Tokyo,
Japan).
Transwell assay
Matrigel® pre-coated Transwell chambers
(BD Biosciences, Franklin Lakes, NJ, USA) were used to study cell
invasion. HeLa cells (105 cells) in serum-free DMEM were
seeded in the upper chambers, and DMEM with 10% FBS was added to
the lower chamber. After incubation at 37°C for 24 h, the Transwell
chambers were rinsed with 1% PBS. Cells on the upper surface were
removed with a cotton-tipped swab, and the chamber was then stained
with 0.1% crystal violet (Thermo Fisher Scientific, Inc.) at room
temperature for 10 min. The invaded cells were counted under an
inverted microscope (Olympus).
Bioinformatics analysis
TargetScan software 7.1 (http://www.targetscan.org) was used to predict the
potential target genes of miR-130a.
Luciferase reporter gene assay
The wild-type (WT) sequence of the 3UTR of TIMP2
containing miR-130a binding sites and the mutant-type (MT) sequence
of the 3UTR of TIMP2 lacking these miR-130a binding sites were
amplified by PCR and individually subcloned into the psiCHECK-2
vector (Promega Corp., Madison, WI, USA). Lipofectamine®
2000 was used to co-transfect HeLa cells with WT or MT TIMP2 3UTR
luciferase reporter gene plasmid, and miR-NC or miR-130a mimics,
respectively. In the control group, HeLa cells were transfected
with WT (or MT) plasmids, without miR mimic (or miR-NC). After
transfection for 48 h, the luciferase activity was determined using
the Dual-Luciferase Reporter Assay System (Promega Corp.). The
firefly luciferase activities were normalized to Renilla luciferase
activity.
Statistical analysis
Values are expressed as the mean ± standard
deviation. SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used to
perform statistical analysis. Differences were analyzed using
Student's t-test or one-way analysis of variance followed by a
post-hoc Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-130a is upregulated in cervical
cancer and induced by HPV18 E6
n the present study, the expression levels of
miR-130a were first examined in cervical cancer tissues. The
results indicated that miR-130a was significantly upregulated in
cervical cancer tissues compared with that in adjacent non-tumorous
tissues (Fig. 1A). The cervical
cancer patients included in the present study were then subdivided
into a high miR-130a expression group and a low miR-130a expression
group by using the mean value of miR-130a expression as the cutoff
value. Further investigation revealed that high expression of
miR-130a was significantly associated with lymph node metastasis
and advanced clinical stage (Table
I). These results suggest that the increased expression of
miR-130a may contribute to the malignant progression of cervical
cancer.
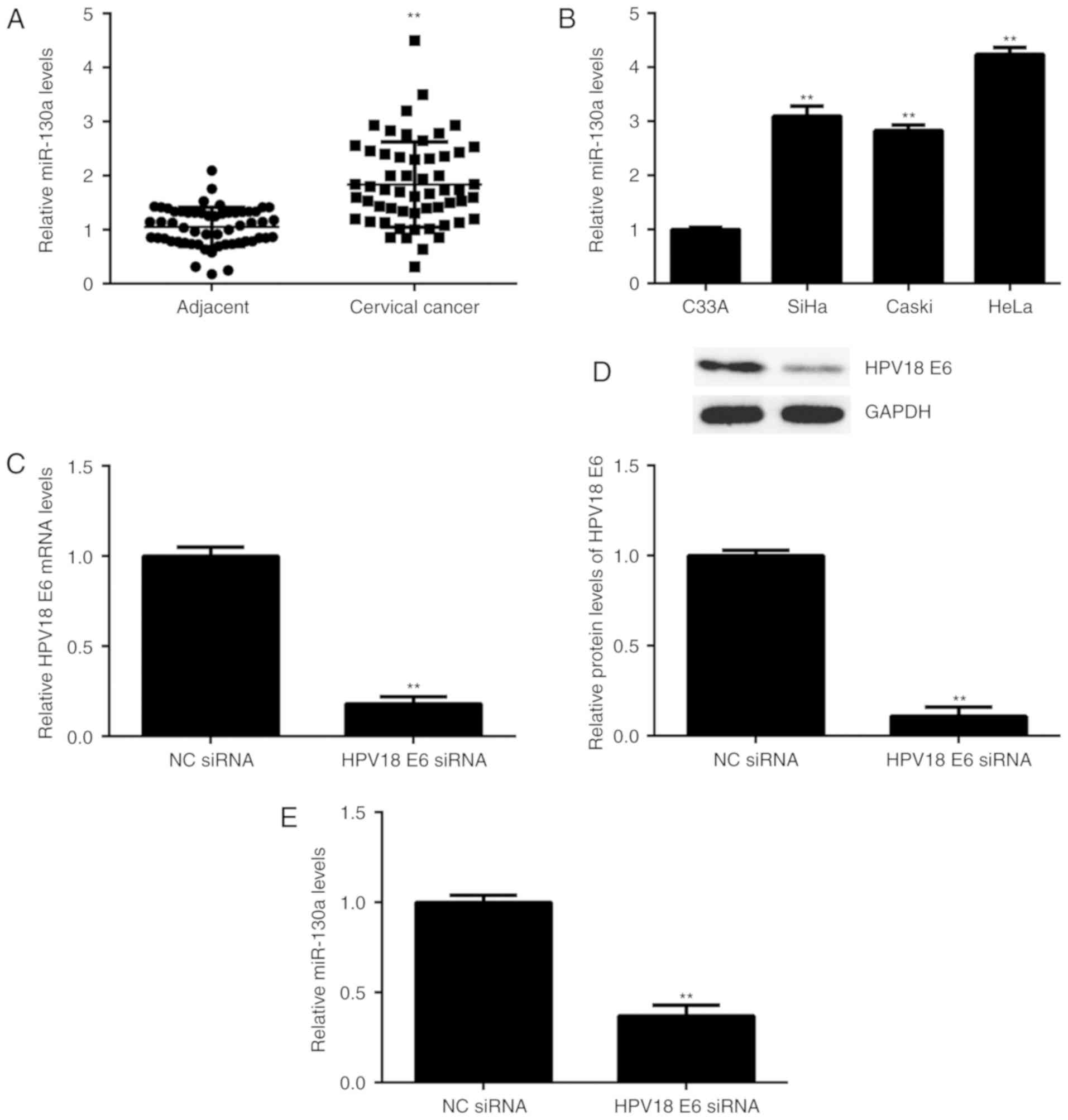 | Figure 1.(A) RT-qPCR was performed to examine
the miR-130a expression in cervical cancer tissues compared with
that in adjacent non-tumorous tissues. **P<0.01 vs. Adjacent.
(B) RT-qPCR was performed to examine the miR-130a expression in
several cervical cancer cell lines, including SiHa (HPV16+), Caski
(HPV16+), HeLa (HPV18+) and C33A (HPV-). **P<0.01 vs. C33A. (C
and D) HeLa cells were transfected with HPV18 E6 siRNA or NC siRNA.
After transfection, (C) RT-qPCR and (D) western blot analysis were
performed to examine the mRNA and protein expression of HPV18 E6,
respectively, and (E) qPCR was performed to examine the expression
of miR-130a. **P<0.01 vs. NC siRNA. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; HPV, human
papillomavirus; siRNA, small interfering RNA; miR, microRNA; NC,
negative control. |
 | Table I.Association between miR-130a
expression and clinicopathological characteristics of patients with
cervical cancer. |
Table I.
Association between miR-130a
expression and clinicopathological characteristics of patients with
cervical cancer.
|
|
| miR-130a
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | Total (n=56) | Low (n=30) | High (n=26) | P-value |
|---|
| Age (years) |
|
|
| 0.786 |
|
<55 | 22 | 11 | 11 |
|
| ≥55 | 34 | 19 | 15 |
|
| Tumor size (cm) |
|
|
| 0.399 |
| ≤4
cm | 38 | 22 | 16 |
|
| >4
cm | 18 | 8 | 10 |
|
| Differentiation |
|
|
| 0.191 |
|
Well/moderate | 44 | 26 | 18 |
|
|
Poor | 12 | 4 | 8 |
|
| Clinical stage |
|
|
| 0.025 |
|
I/II | 37 | 24 | 13 |
|
|
III/IV | 19 | 6 | 13 |
|
| Lymph node
metastasis |
|
|
| 0.009 |
| No | 40 | 26 | 14 |
|
|
Yes | 16 | 4 | 12 |
|
| Distant
metastasis |
|
|
| 0.086 |
| No | 50 | 29 | 21 |
|
|
Yes | 6 | 1 | 5 |
|
The expression of miR-130a was then examined in 4
common cervical cancer cell lines, namely SiHa (HPV16+), Caski
(HPV16+), HeLa (<?__anchored_object__
“ro_u237cins3387”?><?__anchored_object__
“ro_u237cins3388”?>HPV18+) and C33<?__anchored_object__
“ro_u237cins3397”?><?__anchored_object__
“ro_u237cins3398”?>A (HPV-). The re<?__anchored_object__
“ro_u237cins33a8”?><?__anchored_object__
“ro_u237cins33a9”?>sults indicated th<?__anchored_object__
“ro_u237cins33bb”?><?__anchored_object__
“ro_u237cins33bc”?>at the expression of miR-130a was
significantly higher in HPV+ cervical cancer cell lines when
compared with that in HPV-C33A cells (Fig. 1B). To further examine the association
between HPV and miR-130a, HeLa cells were transfected with HPV18 E6
siRNA to knockdown its expre<?__anchored_object__
“ro_u237cins34ce”?><?__anchored_object__
“ro_u237cins34cf”?>ssion. After transfection, the mRNA and
protein levels of HPV18 E6 were significantly decreased compared
with those in the NC siRNA group (Fig.
1C and D). Of note, the miR-130a levels were also reduced after
inhibition of HPV18 E6 (Fig. 1E).
These results suggest that in HeLa cells, the expression of
miR-130a is mediated by HPV18 E6.
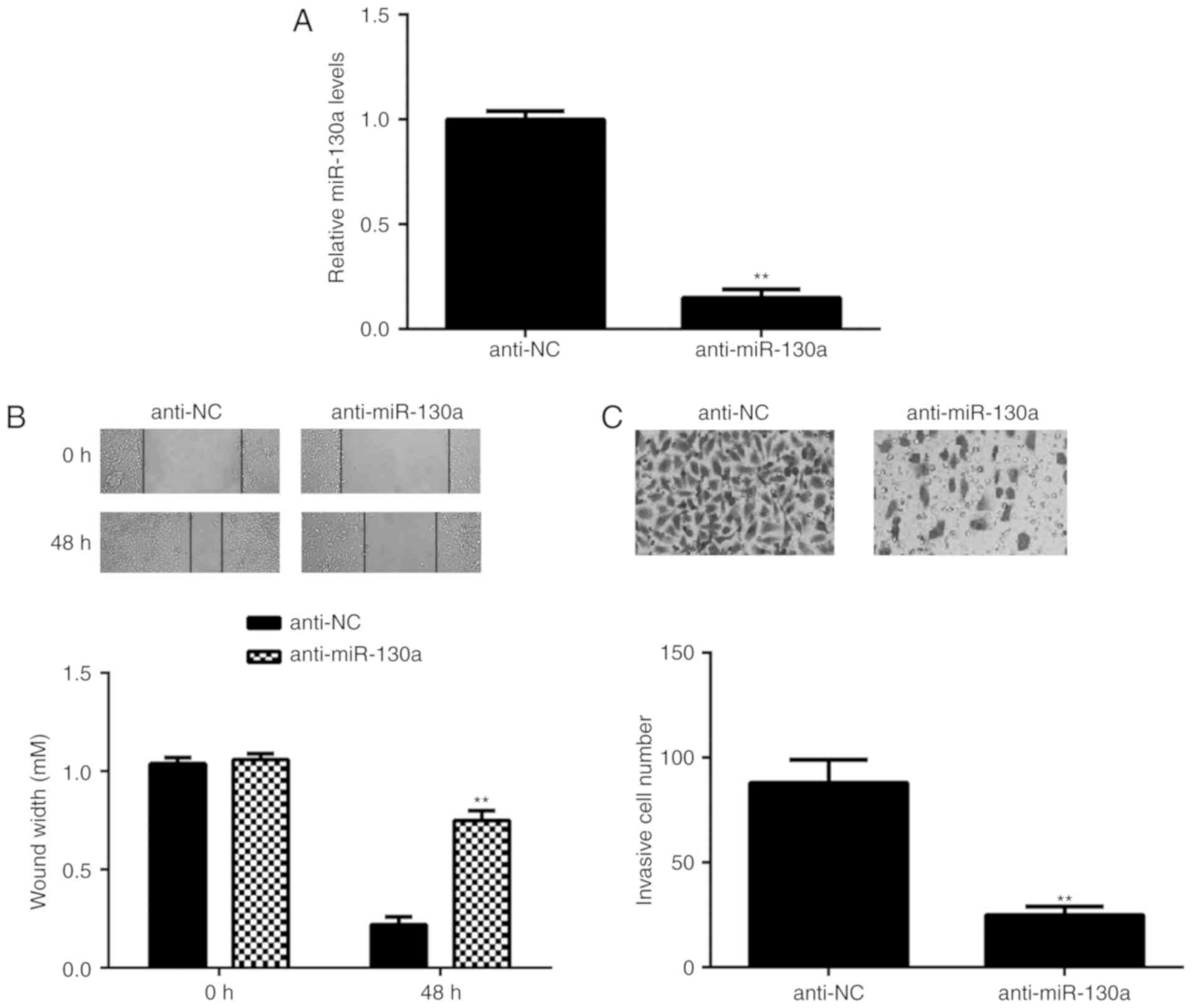
Knockdown of miR-130a inhibits
HeLa-cell migration and invasion
As miR-130a was highly expressed in HeLa cells, this
cell line was transfected with miR-130a inhibitor to reduce its
expression. After transfection, the expression of miR-130a was
significantly downregulated in the anti-miR-130a group compared
with tha<?__anchored_object__
“ro_u237cins375c”?><?__anchored_object__
“ro_u237cins375d”?>t in the anti-NC group (Fig. 2A). The role of miR-130a in HeLa-cell
migration and invasion was then studied. A wound healing assay
indicated that knockdown of miR-130a caused a significant decrease
in HeLa-cell migration (Fig. 2B).
Similarly, the Transwell assay demonstrated that inhibition of
miR-130a significantly repressed HeLa-cell invasion (Fig. 2C). These results suggest that
miR-130a has a promoting role in cervical cancer metastasis.
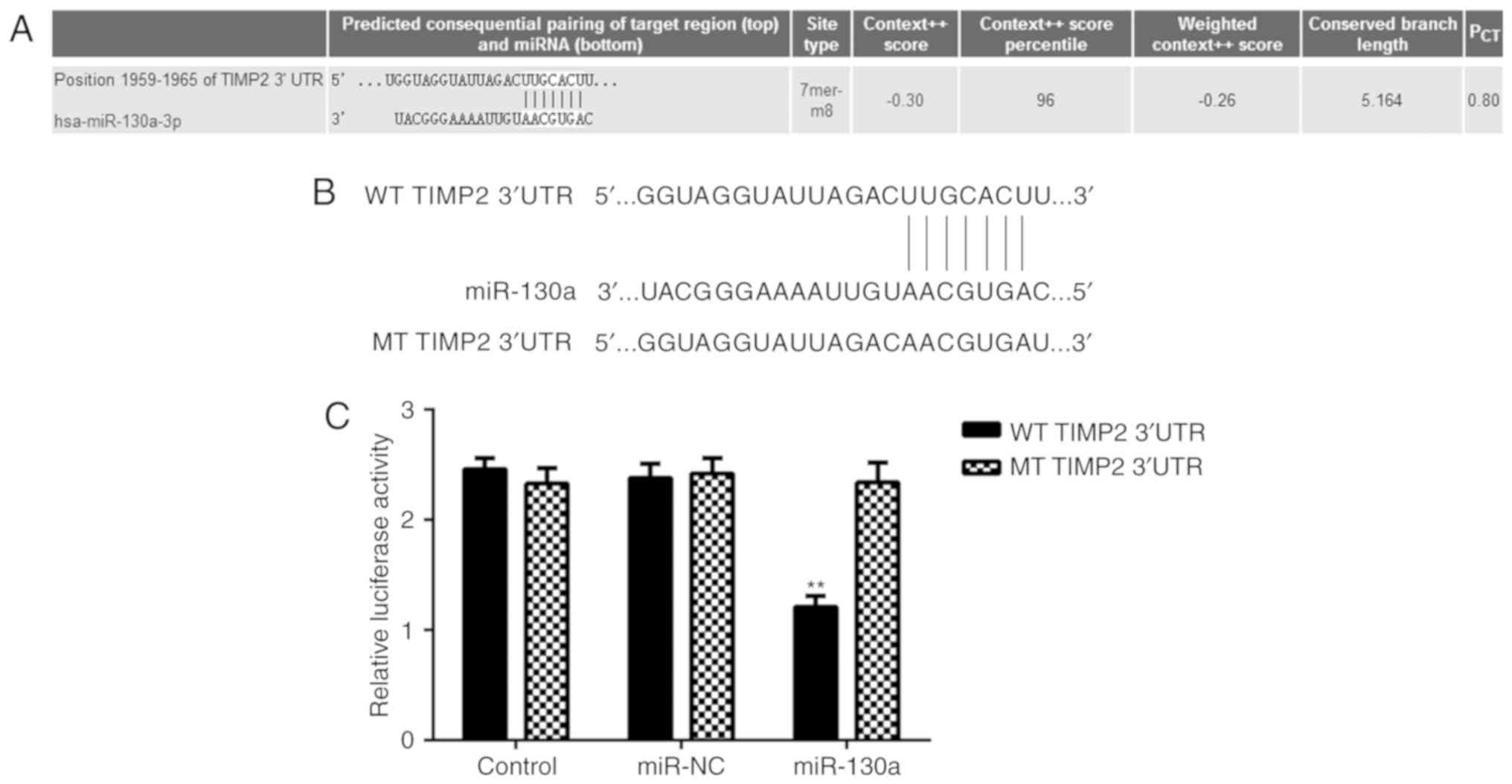
TIMP2 is a direct target gene of
miR-130a
Potential target genes of miR-130a in HeLa cells
were then investigated. By using a Targetscan bioinformatics
<?__anchored_object__
“ro_u237cins39b4”?><?__anchored_object__
“ro_u237cins39b5”?>prediction, TIMP2 was identified as a
putative target gene of miR-130a (Fig.
3A). To verify this prediction, luciferase reporter gene
plasmids driven by a WT or MT sequence of the TIMP2 3UTR were
constructed (Fig. 3B), which were
employed in a luciferase reporter gene assay. As presented in
Fig. 3C, the luciferase activity in
HeLa cells transfected with WT TIMP2 3UTR luciferase reporter
pla<?__anchored_object__
“ro_u237cins3b40”?><?__anchored_object__
“ro_u237cins3b41”?>smid was significantly reduced in the
presence of miR-130a, while it was not reduced in the group
transfected with the MT TIMP2 3UTR luciferase reporter plasmid and
miR-130a (Fig. 3C). These results
indicate that TIMP2 is a direct target gene of miR-130a in HeLa
cells.
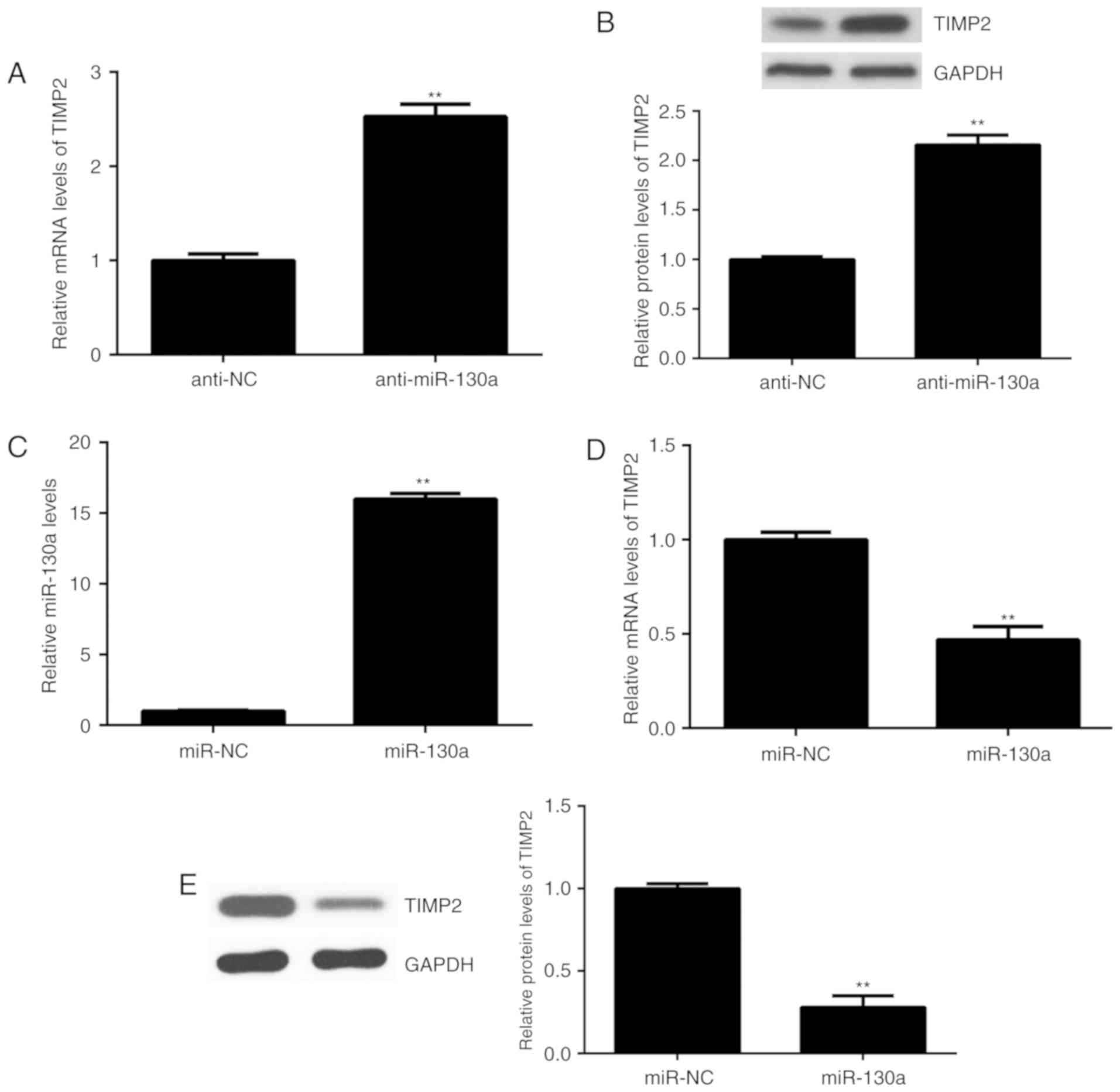
The expression of TIMP2 is regulated
by miR-130a in HeLa cells
Next, the effect of miR-130a on the expression of
TIMP2 in HeLa cells was assessed. The results indicated that the
mRNA and protein levels of TIMP2 were significantly increased after
knockdown of miR-130a in HeLa cells (Fig. 4A and B). To further confirm these
results, HeLa cells were transfected with miR-130a to upregulate
its expression. After transfection, the expression of miR-130a was
significantly higher than that in the miR-NC group (Fig. 4C). Of note, the mRNA and protein
levels of TIMP2 were significantly reduced after overexpression of
miR-130a (Fig. 4D and E). Therefore,
the expression of TIMP2 is negatively regulated by miR-130a in HeLa
cells.
HPV18 E6 regulates TIMP2 expression
through miR-130a
The effect of HPV18 E6 knockdown on the expression
of TIMP2 expression in HeLa cells was then assessed. As presented
in Fig. 5A and B, the mRNA and
protein levels of TIMP2 were significantly increased in the HPV18
E6 siRNA group, when compared with<?__anchored_object__
“ro_u237cins4056”?><?__anchored_object__
“ro_u237cins4057”?> those in the NC siRNA group. Based on these
and t<?__anchored_object__
“ro_u237cins4089”?><?__anchored_object__
“ro_u237cins408a”?>he above results, it was suggested that HPV18
E6 inhibits the expression of TIMP2 via upregulating miR-130a. To
further confirm this speculation, HeLa cells were co-transfected
with HPV18 E6 siRNA and miR-133a mimics.
Afte<?__anchored_object__
“ro_u237cins4168”?><?__anchored_object__
“ro_u237cins4169”?>r transfection, the expression of miR-130a
was significantly increased in the HPV18 E6 siRNA+miR-133a group
compared with that in the HPV18 E6 siRNA+miR-NC group (Fig. 5C). Furthermore, the mRNA and protein
expression of TIMP2 was significantly reduced in the HPV18 E6
siRNA+miR-133a group compared with that in the HPV18 E6
siRNA+miR-NC group (Fig. 5D and E).
Therefore, HPV18 E6 negatively regulates the expression of TIMP2
through mediation of miR-130a in HeLa cells.
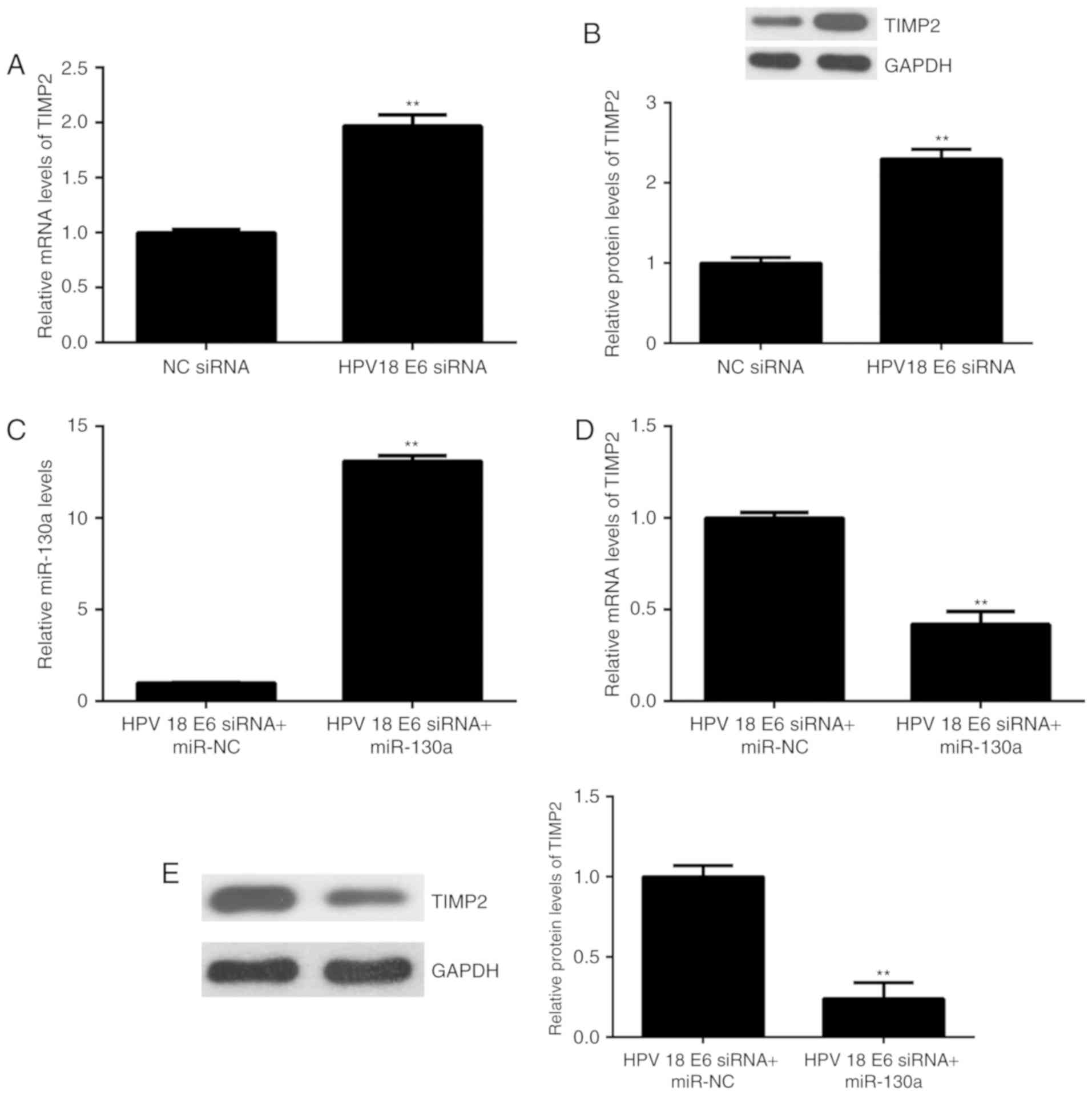 | Figure 5.(A and B) HeLa cells were transfected
with HPV18 E6 siRNA or NC siRNA, and (A) RT-qPCR and (B) western
blot analysis were performed to examine the mRNA and protein
expression of TIMP2, respectively. **P<0.01 vs. NC siRNA. (C-E)
HeLa cells were co-transfected with HPV18 E6 siRNA and miR-133a
mimics, or with HPV18 E6 siRNA and miR-NC. (C) RT-qPCR was
performed to examine the expression of miR-130a. (D) RT-qPCR and
(E) western blot analysis were applied to examine the mRNA and
protein expression of TIMP2, respectively. **P<0.01 vs. HPV18 E6
siRNA+miR-NC. NC, negative control; TIMP, tissue inhibitor of
metalloproteinases; miR, microRNA; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; HPV, human
papillomavirus; siRNA, small interfering RNA. |
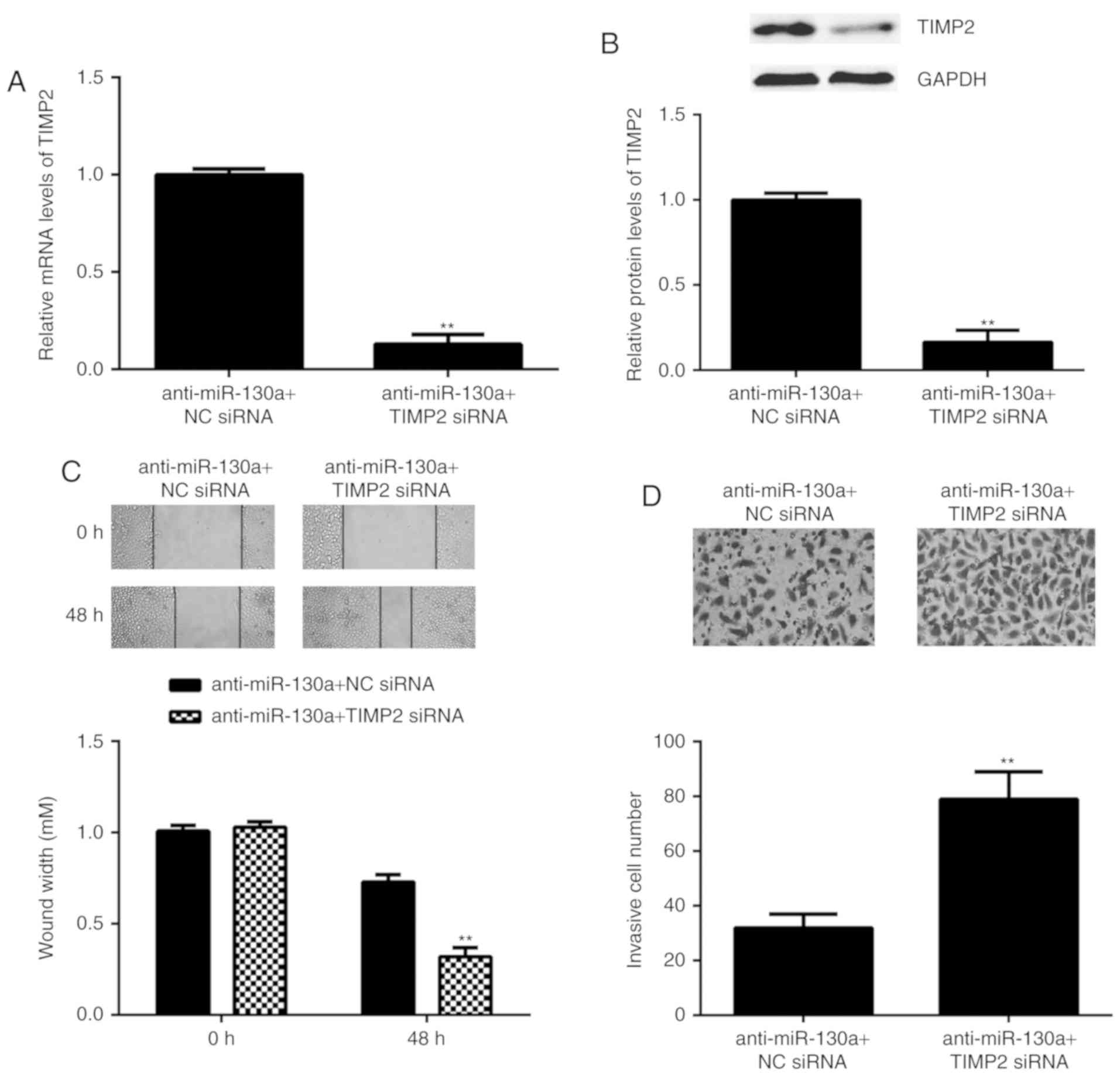
TIMP2 is involved in the
miR-130a-induced migration and invasion of HeLa cells
As TIMP2 is an antagonist of MMP2, it was speculated
that miR-130a may exert its promoting effect on HeLa cell migration
and invasion via inhibition of TIMP2 expression. To verify this
speculation, HeLa cells were co-transfected with miR-130a inhibitor
and TIMP2 siRNA. After transfection, the mRNA and protein levels of
TIMP2 were significantly reduced in the anti-miR-130a+TIMP2 siRNA
group when com<?__anchored_object__
“ro_u237cins4521”?><?__anchored_object__
“ro_u237cins4522”?>pared with those in the anti-miR-130a+NC
siRNA group (Fig. 6A
an<?__anchored_object__
“ro_u237cins4562”?><?__anchored_object__
“ro_u237cins4563”?>d B). Furthermore, the migration and invasion
of HeLa cells was significantly increased in the
anti-miR-130a+TIMP2 siRNA group compared with that in the
anti-miR-130a+NC siRNA group (Fig. 6C
and D). These results demonstrate that knockdown of TIMP2
attenuated, at least partly, the suppressive effects of miR-130a
inhibition on the migration and invasion of HeLa cells, suggesting
that the promoting role of miR-130a in HeLa cell migration and
invasion is mediated through inhibition of the expression of
TIMP2.
Discussion
The molecular mechanisms underlying miR-130a
expression in cervical cancer as well as the regulatory roles of
miR-130a in cervical cancer metastasis have remained largely
elusive. In the present study, it was observed that miR-130a was
significantly upregulated in cervical cancer tissues compared with
that in adjacent non-tumor tissues. High expression of miR-130a was
significantly associated with lymph node metastasis and advanced
clinical stage in cervical cancer patients. Furthermore, the
expression of miR-130a was also higher in HPV+ cervical cancer cell
lines compared with that in HPV-cervical cancer cells. Knockdown of
HPV18 E6 significantly inhibited the expression of miR-130a in HeLa
cervical cancer cells. In addition, knockdown of miR-130a reduced
the migration and invasion of HeLa cells. TIMP2, an antagonist of
MMP2, was identified as a novel and direct target gene of miR-130a.
The expression of TIMP2 was negatively regulated by miR-130a, and
HPV18 E6 inhibited the expression of TIMP2 in HeLa cells at least
partly by upregulating miR-130a. Furthermore, knockdown of TIMP2
rescued the suppressive effects of miR-130a inhibition on the
migration and invasion of HeLa cells.
HPV infection has been demonstrated to modulate the
expression of certain miRs in cervical cancer (20,21). For
instance, HPV E5, E6 and E7 directly or indirectly lead to the
dysregulation of multiple miRs, including miR-34a, miR-218, miR-29a
and miR-146a, which contributes to the initiation and progression
of cervical cancer (22). Kong et
al (20) reported that HPV E7
promotes the expression of miR-21 in HeLa cervical cancer cells,
which further potentiated HeLa cell proliferation and invasion. In
turn, certain miRs also regulate the expression of HPV genes
(22,23). For instance, Zhang et al
(23) reported that miR-129-5p
reduces the expression of HPV18 E6 and E7 by targeting signaling
protein 1 in cervical cancer cells. In the present study, it was
indicated that the expression of miR-130a was significantly
increased in cervical cancer tissues compared with that in adjacent
normal tissues, and in HPV+ cervical cancer cell lines compared
with that in HPV-cervical cancer cells. In addition, the present
study was the first, to the best of our knowledge, to demonstrate
that the expression of miR-130a was induced by HPV18 E6 in HeLa
cells. This interaction should be assessed further in future
studies. In addition, high expression of miR-130a was revealed to
be significantly associated with lymph node metastasis and advanced
clinical stage in cervical cancer patients. Consistent with the
clinical data, knockdown of miR-130a significantly decreased the
migration and invasion of HeLa cells, which further suggests that
miR-130a may contribute to cervical cancer metastasis.
miRs function through mediating the expression of
their target genes (7). Zhang et
al (24) reported that low
concentrations of tumor necrosis factor (TNF)-α stimulated NF-κB
activity and then induced miR-130a expression, while miR-130a
directly targets TNF-α, and thus forms a negative feedback
regulation of NF-κB/miR-130a/TNF-α/NF-κB in cervical cancer. Feng
et al (16) demonstrated that
miR-130a promoted cervical cancer cell growth by targeting PTEN.
However, other target genes of miR-130a in cervical cancer still
remain to be elucidated. In the present study, TIMP2 was identified
as a novel target gene of miR-130a in HeLa cells. MMPs have a key
role in cancer metastasis (25). As
an endogenous inhibitor of MMPs, TIMP2 has been reported to be
frequently deregulated in certain human cancer types, including
cervical cancer (17,26,27).
MMP2 and TIMP2 expression was significantly associated with an
advanced stage and poor survival of cervical cancer patients
(26). The serum levels of TIMP2
were also reported to be associated with the outcome of cervical
cancer patients undergoing radiochemotherapy (28). In addition, the present study
indicated that the expression of TIMP2 was regulated by HPV18 E6.
Similarly, Cardeal et al (29) also reported that HPV 16 oncoproteins
induced an imbalance between MMPs and TIMP2 in primary
keratinocytes, which was suggested to be a possible implication in
cervical carcinogenesis.
Further investigation in the present study indicated
that knockdown of TIMP2 partly attenuated the suppressive effects
of miR-130a inhibition on the migration and invasion of HeLa cells,
suggesting that miR-130a promotes HeLa cell migration and invasion
at least in part through inhibiting the expression of TIMP2. In
addition to miR-130a, miR-20a was also reported to promote cervical
cancer proliferation and metastasis in vitro and in
vivo by targeting TIMP2 (30).
In summary, the results of the present study suggest
that HPV18 E6 promotes the expression of miR-130a, which in turn
directly inhibits the expression of TIMP2 and promotes cervical
cancer cell invasion. Therefore, HPV/miR-130a/TIMP2 signaling may
regarded as a potential target for the prevention of cervical
cancer metastasis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SY processed tissue samples and wrote the
manuscript; QZ and YW performed the experiments; SL performed
statistical analysis; and RH designed the present study and revised
the manuscript. The final version of the manuscript was read and
approved by all authors, and each author believes that the
manuscript represents their honest work.
Ethical approval and consent to
participate
This study was approved by the Ethics Committee of
the First Affiliated Hospital of Xinxiang Medical University
(Weihui, China). All patients provided written informed
consent.
Patient consent for publication
All written informed consents were obtained.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
King T: HPV, cervical cancer, and you. J
Midwifery Womens Health. 53:263–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang JJ, Wang DD, Du CX and Wang Y: Long
noncoding RNA ANRIL promotes cervical cancer development by acting
as a sponge of miR-186. Oncol Res. May 22–2017.(Epub ahead of
print).
|
|
5
|
Yang M, Zhai X, Ge T, Yang C and Lou G:
MiR-181a-5p promotes proliferation and invasion, and inhibits
apoptosis of cervical cancer cells via regulating inositol
polyphosphate-5-phosphatase A (INPP5A). Oncol Res. 26:703–712.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang C, Zhou B, Liu M, Liu Y and Gao R:
miR-126-5p restoration promotes cell apoptosis in cervical cancer
by targeting Bcl2l2. Oncol Res. 25:463–470. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Human MicroRNA targets. PLoS Biol.
2:e3632004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu K, He Y, Xia C, Yan J, Hou J, Kong D,
Yang Y and Zheng G: MicroRNA-15a inhibits proliferation and induces
apoptosis in CNE1 nasopharyngeal carcinoma cells. Oncol Res.
24:145–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou Y, Yang C, Wang K, Liu X and Liu Q:
MicroRNA-33b inhibits the proliferation and migration of
osteosarcoma cells via targeting hypoxia-inducible factor-1α. Oncol
Res. 25:397–405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang S, Hui Y, Li X and Jia Q: WITHDRAWN:
Silencing of lncRNA-CCDC26 restrains the growth and migration of
glioma cells in vitro and in vivo via targeting miR-203. Oncol Res.
Jun 9–2017.(Epub ahead of print).
|
|
12
|
Luo C and Qiu J: MiR-181a inhibits
cervical cancer development via downregulating GRP78. Oncol Res.
25:1341–1348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao YL, Zhao ZS, Zhang MY, Han LJ, Dong YJ
and Xu B: Long noncoding RNA PVT1 facilitates cervical cancer
progression via negative regulating of miR-424. Oncol Res.
25:1391–1398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang S, Liu F, Mao X, Huang J, Yang J,
Yin X, Wu L, Zheng L and Wang Q: Elevation of miR-27b by HPV16 E7
inhibits PPARγ expression and promotes proliferation and invasion
in cervical carcinoma cells. Int J Oncol. 47:1759–1766. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang HD, Jiang LH, Sun DW, Li J and Ji
ZL: The role of miR-130a in cancer. Breast Cancer. 24:521–527.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng Y, Zhou S, Li G, Hu C, Zou W, Zhang H
and Sun L: Nuclear factor-κB-dependent microRNA-130a upregulation
promotes cervical cancer cell growth by targeting phosphatase and
tensin homolog. Arch Biochem Biophys. 598:57–65. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yi X, Guo J, Sun S, Yang P, Wang J, Li Y,
Xie L, Cai J and Wang Z: EZH2-mediated epigenetic silencing of
TIMP2 promotes ovarian cancer migration and invasion. Sci Rep.
7:35682017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang Y, Li J, Xiang L, Han D, Shen X and
Wu X: 17β-Oestradiol activates proteolysis and increases invasion
through phosphatidylinositol 3-kinase pathway in human cervical
cancer cells. Eur J Obstet Gynecol Reprod Biol. 165:307–312. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kong Q, Wang W and Li P: Regulator role of
HPV E7 protein on miR-21 expression in cervical carcinoma cells and
its functional implication. Int J Clin Exp Pathol. 8:15808–15813.
2015.PubMed/NCBI
|
|
21
|
Shi M, Du L, Liu D, Qian L, Hu M, Yu M,
Yang Z, Zhao M, Chen C, Guo L, et al: Glucocorticoid regulation of
a novel HPV-E6-p53-miR-145 pathway modulates invasion and therapy
resistance of cervical cancer cells. J Pathol. 228:148–157. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu YK, Cheng N, Hu Y and Cen YZ: The role
of microRNAs in the pathogenesis of cervical cancer and its
relationship to HPV. Sheng Li Ke Xue Jin Zhan. 43:251–256. 2012.(In
Chinese). PubMed/NCBI
|
|
23
|
Zhang J, Li S, Yan Q, Chen X, Yang Y, Liu
X and Wan X: Interferon-β induced microRNA-129-5p down-regulates
HPV-18 E6 and E7 viral gene expression by targeting SP1 in cervical
cancer cells. PLoS One. 8:e813662013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang J, Wu H, Li P, Zhao Y, Liu M and
Tang H: NF-κB-modulated miR-130a targets TNF-α in cervical cancer
cells. J Transl Med. 12:1552014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miao Y, Lu M, Yan Q, Li S and Feng Y:
Inhibition of proliferation, migration, and invasion by knockdown
of pyruvate kinase-M2 (PKM2) in ovarian cancer SKOV3 and OVCAR3
cells. Oncol Res. 24:463–475. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Braicu EI, Fotopoulou C, Chekerov R,
Richter R, Blohmer J, Kümmel S, Stamatian F, Yalcinkaya I, Mentze
M, Lichtenegger W and Sehouli J: Role of serum concentration of
VEGFR1 and TIMP2 on clinical outcome in primary cervical cancer:
Results of a companion protocol of the randomized, NOGGO-AGO phase
III adjuvant trial of simultaneous cisplatin-based
radiochemotherapy vs.carboplatin and paclitaxel containing
sequential radiotherapy. Cytokine. 61:755–758. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pietruszewska W, Bojanowska-Poźniak K and
Kobos J: Matrix metalloproteinases MMP1, MMP2, MMP9 and their
tissue inhibitors TIMP1, TIMP2, TIMP3 in head and neck cancer: An
immunohistochemical study. Otolaryngol Pol. 70:32–43. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Braicu EI, Gasimli K, Richter R, Nassir M,
Kümmel S, Blohmer JU, Yalcinkaya I, Chekerov R, Ignat I, Ionescu A,
et al: Role of serum VEGFA, TIMP2, MMP2 and MMP9 in monitoring
response to adjuvant radiochemotherapy in patients with primary
cervical cancer-results of a companion protocol of the randomized
NOGGO-AGO phase III clinical trial. Anticancer Res. 34:385–391.
2014.PubMed/NCBI
|
|
29
|
Cardeal LB, Boccardo E, Termini L,
Rabachini T, Andreoli MA, di Loreto C, Longatto Filho A, Villa LL
and Maria-Engler SS: HPV16 oncoproteins induce MMPs/RECK-TIMP-2
imbalance in primary keratinocytes: Possible implications in
cervical carcinogenesis. PLoS One. 7:e335852012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao S, Yao D, Chen J, Ding N and Ren F:
MiR-20a promotes cervical cancer proliferation and metastasis in
vitro and in vivo. PLoS One. 10:e01209052015. View Article : Google Scholar : PubMed/NCBI
|