Introduction
Radix Sophorae tonkinensis, the dried roots
and rhizomes of S. tonkinensis Gapnep., is a well-known
traditional Chinese medicine (TCM) herb and is primarily
distributed in the southwest provinces of China (1). It has been widely used in clinics to
treat sore throats, viral hepatitis and jaundice (1,2). Radix
S. tonkinensis was first described in Kaibao Materia Medica,
the earlier Pharmacopoeia of China in Northern Song (968–976 AD)
(2). In addition to studies
investigating the molecular mechanisms of its pharmacological
activities, much attention has recently been focused on the side
effects of radix S. tonkinensis, including neurological
toxicity and liver toxicity. Liver toxicities following
intoxication with radix S. tonkinensis are less well known
compared with its neurological complications (3). However, it has been reported in
clinical settings that liver function is abnormal, as evidenced by
an increase in the levels of serum alanine aminotransferase (ALT),
aspartate aminotransferase (AST) and total bilirubin (TBil), after
treatment with this herb (4). A
recent study by our group revealed that single and repeated oral
administration with extracts of radix S. tonkinensis may
induce hepatotoxicity and even mortality in mice in a
dose-dependent manner (5).
The known major chemical components of radix S.
tonkinensis include quinolizidine alkaloids, flavonoids and
triterpenoids (6). However, it
remains poorly understood which component(s) of this herb is
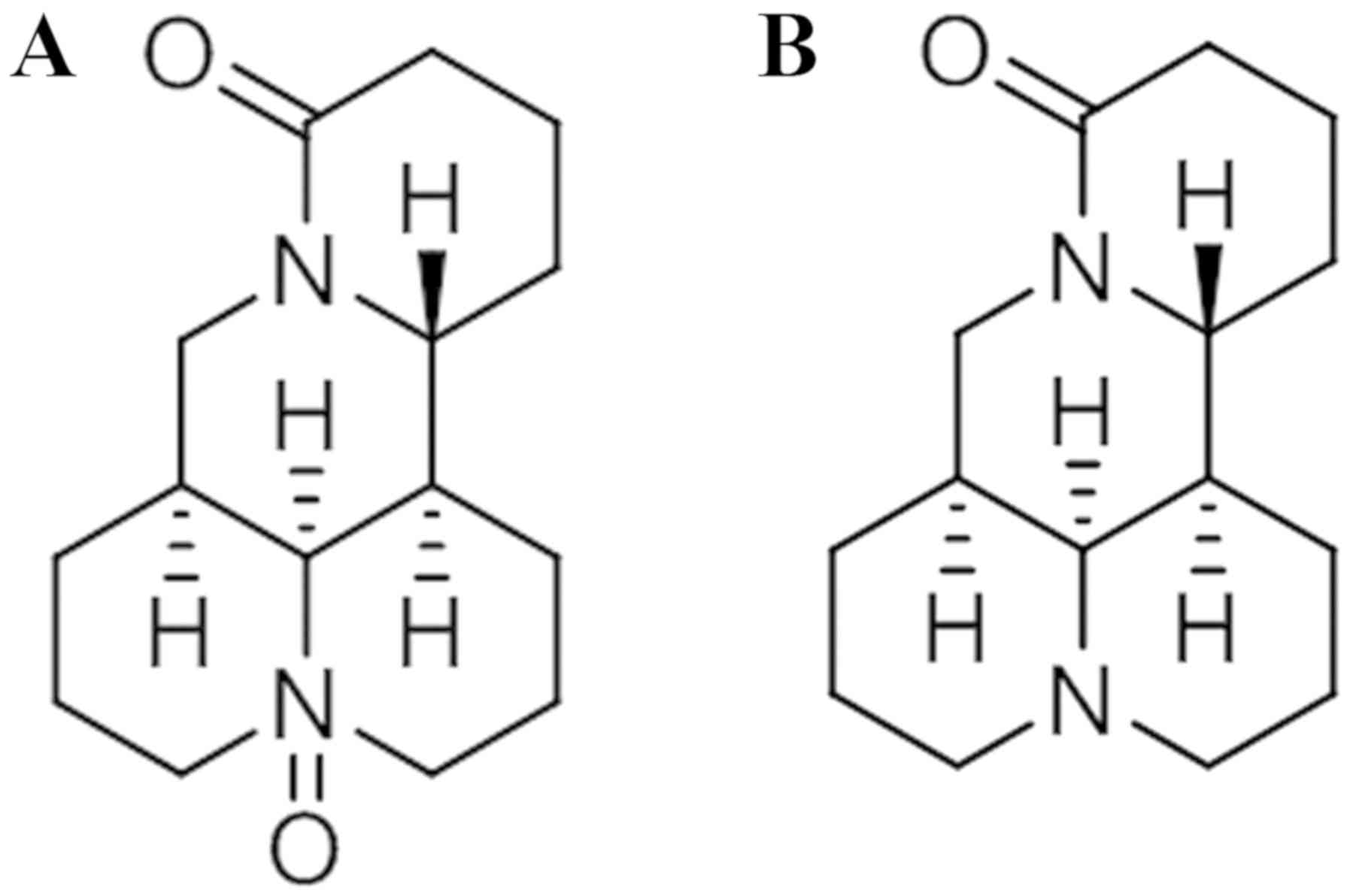
responsible for its hepatotoxic effects. Oxymatrine (OMT; Fig. 1A) and matrine (MT; Fig. 1B) are the two major quinolizidine
alkaloids found in the herb, and have long been regarded as the
main active components contributing to the pharmacological
properties of radix S. tonkinensis (7,8). The
hepatotoxicity of OMT and MT has been gradually documented in the
literature. It was reported that treatment with large doses of OMT
in mice for 7 days caused abnormal liver function (9). Furthermore, OMT worsened liver damage
in patients with hepatitis B (10).
Our group has demonstrated that single oral administration with
large doses of OMT or MT induced hepatotoxicity and even mortality
in mice (11). Taken together, these
results indicate that OMT and MT may be responsible for the
hepatotoxicity of radix S. tonkinensis. In addition to the
alkaloids, it has been reported that the non-alkaloid components of
radix S. tonkinensis, including flavonoids and
triterpenoids, induce marked cytotoxicity in zebrafish and HepG2
cells, with lethal concentration 50 and half maximal inhibitory
concentration (IC50) values of 0.43 and 0.98 mg/ml,
respectively (12). In that study,
the non-alkaloid part of the herb was identified to be the
hepatotoxic constituent, while the alkaloid part did not exhibit
hepatotoxicity (12). To date, no
adverse effects or toxicity caused by the flavonoids or
triterpenoids alone in radix S. tonkinensis have been
reported. Therefore, it is important to verify which components of
this herb may be responsible for its hepatotoxicity.
To the best of our knowledge, previous studies have
examined the effects of administration of a large dose of OMT, MT
or the non-alkaloid components to investigate the cause of radix
S. tonkinensis-induced hepatotoxicity however, no studies
have reported the toxic effects of the components at the equivalent
amounts contained in radix S. tonkinensis that can induce
hepatotoxicity. Since MT and OMT are regarded as the ‘marker
compounds’ of this herb, and the main clinical applications of MT
and OMT are treatment of patients with cancer, viral hepatitis,
cardiac diseases and skin diseases (7,8), the
current study investigated the hepatotoxicity of MT and OMT.
Metabolism is an important pharmacokinetic process
that influences the biological activity and toxicity of drugs in
vivo (13). Certain components
of a drug can be converted to toxic metabolites, while other
components are transformed to non-toxic metabolites (14). It has been reported that when taken
orally, the majority of OMT is transformed into the more absorbable
metabolite MT by intestinal bacteria in the gastrointestinal tract
(15). The metabolite MT may have
pharmacological and toxicological implications (16,17).
However, there is little information on the role of the active
metabolite MT in the toxicity of OMT after oral administration.
Therefore, it is necessary to compare the hepatic toxicity of OMT
with its active metabolite MT.
The primary aims of this study were to investigate:
i) Whether or not MT and OMT are responsible for the hepatotoxicity
of radix S. tonkinensis; and ii) whether the hepatotoxicity
induced by OMT in mice is primarily caused by OMT itself or by the
subsequent transformation to its active metabolite MT.
Materials and methods
Materials
OMT (cat. no. 150629) and MT (cat. no. 160930) were
purchased from Shanghai Winherb Medical Technology Co., Ltd.
(Shanghai, China) with a purity of ≥99%. Stock solutions of OMT (10
mg/ml), MT (10 mg/ml) and OMT + MT (10 + 10 mg/ml) were prepared
with distilled water and stored at −20°C for the subchronic
toxicity study. For cell cytotoxicity studies, OMT, MT and OMT + MT
were separately dissolved with phosphate-buffered saline (pH 7.2),
and then filtered (0.22 µm) to obtain stock solutions (≤400 mM).
Immediately before use, the stock solution was diluted in cell
culture medium (DMEM/F-12 supplemented with heat-inactivated 10%
fetal bovine serum; each, Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) to the expected concentrations. Commercially
biochemical identification kits for determining ALT [Reagent (R)-I:
cat. no. F661, R-II: cat. no. H657], AST (R-I: cat. no. I663, R-II:
cat. no. G656), TBil (R-I: cat. no. DR066, R-II: cat. no. DR067)
and lactic dehydrogenase (LDH; R-I: cat. no. K553, R-II: cat. no.
K554) were provided by Shino-Test Corporation (Tokyo, Japan).
Isoflurane (cat. no. 130302) was obtained from Hebei Yipin
Pharmaceutical Co., Ltd. (Shijiazhuang, China). Dulbecco's modified
Eagle's medium/nutrient mixture F-12 (DMEM/F-12; cat. no.
11330-032), penicillin-streptomycin (cat. no. 15140-122) and fetal
bovine serum (FBS; cat. no. 10099-141) were purchased from Gibco
(Thermo Fisher Scientific, Inc.). Insulin, transferrin and sodium
selenite (ITS) liquid media supplement (cat. no. I3146) and
dexamethasone (cat. no. D4902) were from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). The Cell Counting Kit-8 (CCK-8; cat. no.
C0037) assay was purchased from Beyotime Institute of Biotechnology
(Shanghai, China).
Animals
A total of 44 male specific-pathogen-free C57BL/6
mice (certificate no. 2008001653092; age, 35 days; weight, 18–20
g), were obtained from Shanghai SIPPR-BK Laboratory Animal Co.,
Ltd. (Shanghai, China). Mice were housed in cages (5 or 6
animals/cage) with ad libitum access to standard diet and
drinking water under controlled conditions (temperature, 23±1°C;
relative humidity, 53–65%; 12-h light-dark cycle) for at least 2
days prior to the experiments at the Center for Laboratory Animals
of the Center for Drug Safety Evaluation and Research, Shanghai
University of Traditional Chinese Medicine (Shanghai, China). The
experimental procedures were approved by the Animal Ethics
Committee of Shanghai University of Traditional Chinese Medicine
(certificate no. SZY201504021) and performed in accordance with the
Guidelines for the Care and Use of Laboratory Animals (National
Institutes of Health, Bethesda, MD, USA) (18). The experiments were also conducted
according to the standards of The Guidelines of Test Technology for
Long-Term Toxicity of Chemical Drugs (19).
Subchronic toxicity study
A total of 44 male C57BL/6 mice used in this study
were randomly divided into four experimental groups, with 11
mice/group. In a previous study by our group, moderate
centrilobular hypertrophy was observed in the mice treated with 2.5
g/kg radix S. tonkinensis extracts, in which the calculated
contents of OMT and MT were 40.5 and 69.1 mg/kg, respectively
(5). Therefore, these were used as
the working dosages in the current study. The stock solution was
diluted immediately prior to the experiment in distilled water to
the expected concentrations: OMT (2.025 mg/ml), MT (3.455 mg/ml)
and OMT + MT (2.025+3.455 mg/ml). Each group was scheduled for
90-day oral gavage (20 ml/kg body weight). Finally, groups were
orally treated with OMT (40.5 mg/kg), MT (69.1 mg/kg) and OMT + MT
(40.5+69.1 mg/kg) for 90 days, while the control group was treated
with distilled water. During the treatment period, mice were
weighed each week. At the end of the administration period, animals
were fasted for 4 h, with water ad libitum. They were
subsequently anesthetized with isoflurane, and blood samples were
collected from the abdominal vein of all mice for analysis of serum
biochemistry. After blood collection, the animals were sacrificed
and their livers were collected for histopathological
examination.
Analysis of serum biochemistry
The serum was obtained from whole blood centrifuged
at 2,010 × g for 15 min at room temperature. For the
assessment of liver function, ALT, AST and TBil levels were
determined using a Hitachi 7080 automatic biochemistry analyzer
(Hitachi, Ltd., Tokyo, Japan) following use of the aforementioned
kits.
Histopathological examination
Livers were fixed in 10% neutral buffered formalin
for 3 days at room temperature, embedded in paraffin, cut into
4-µm-thick sections, deparaffinized in xylene at room temperature
and serially rehydrated in decreasing concentrations of ethanol at
room temperature. Sections were stained with hematoxylin (10 min)
and eosin (3 min) at room temperature and examined under a light
microscope to evaluate tissue structure.
Cell culture
The mouse hepatocyte cell line AML12 was generously
provided by Dr Qin Feng from Shuguang Hospital, Shanghai University
of Traditional Chinese Medicine. AML12 liver cells were cultured in
DMEM/F12 medium supplemented with 10% FBS, 100 IU/ml of penicillin,
100 µg/ml of streptomycin, ITS liquid media supplement and 40 ng/ml
dexamethasone. All cells were incubated at 37°C under a humidified
atmosphere of 5% CO2.
Cell viability and cytotoxicity
The inhibitory effects of OMT, MT and OMT + MT on
the growth of AML12 cells were tested using the CCK-8 assay, may be
more suitable compared with MTT for analyzing cell proliferation,
as it can be reduced to soluble formazan by dehydrogenase in
mitochondria and induces little toxicity to cells (20). Briefly, cells were seeded at a
density of 5×103 cells/well in a 96-well flat-bottomed
plate and incubated for 24 h at 37°C with 5% CO2. Cells
were then incubated with culture medium containing various
concentrations of OMT (10, 20, 30 and 40 mM), MT (10, 14, 18, 22,
26 and 30 mM) or OMT + MT (10, 14, 18, 22, 26 and 30 mM each) for
further 24 h. After 10 µl CCK-8 dye was added to each well, cells
were incubated at 37°C for 2 h and the absorbance was finally
determined at 450 nm using a microplate reader (Synergy2; BioTek
Instruments, Inc., Winooski, VT, USA). Results were expressed as a
percentage calculated from the ratio of the absorbance of treated
cells to untreated cells. The concentration that caused a 50% loss
of cell growth, IC50, was used as an indication of OMT,
MT and OMT + MT growth inhibition potency.
ALT, AST and LDH leakage
The levels of extracellular ALT, AST and LDH in the
culture medium were measured using a Hitachi 7080 automatic
biochemistry analyzer. Briefly, AML12 liver cells (5×103
cells/well) in a 96-well flat-bottomed plate were incubated for 24
h, then the cells were incubated with culture medium containing
various concentrations of OMT (18 mM), MT (6, 12 and 18 mM) or OMT
+ MT (4, 10 and 16 mM each) for 3, 6, 12 and 24 h. Control cells
received only solvent (cell culture medium) instead of OMT, MT or
OMT + MT. Following 3, 6, 12 and 24 h of treatment, cell culture
media from each of the 96 wells were collected and centrifuged at
1,000 × g for 10 min at 4°C. The supernatant was collected
for further tests (ALT, AST and LDH determination as
aforementioned) by a Hitachi 7080 automatic biochemistry analyzer
(Hitachi, Ltd., Tokyo, Japan).
Cell morphology observation
AML12 liver cells were seeded into 6-well plate
(5×104 cells/well) and cultured at 37°C for 24 h. The
culture medium was then removed and subsequently replaced with
fresh culture medium containing MT (18 mM), OMT (18 mM) or MT + MT
(16 mM) and incubated for a further 6 h, while cells cultured in
fresh culture medium only served as a control. At 6 h time points,
cells were observed and photographed immediately with an Olympus
X51 inverted microscope (Olympus Corporation, Tokyo, Japan).
Statistical analysis
SPSS (version 24; IBM Corp., Armonk, NY, USA) and
SigmaPlot (version 11.0; Systat Software Inc., Chicago, IL, USA)
software were used for statistical analysis. Data are presented as
the mean ± standard error. When equal variance was assumed, the
data were analyzed using one-way analysis of variance followed by
least significant difference (LST) post-hoc test for multiple
comparisons. P<0.05 was considered as statistically significant.
When equal variance was not assumed, the data were compared using
the non-parametric test. To avoid false positives caused by
multiple comparisons, a Bonferroni's correction was performed to
adjust the test level (0.05/n). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of OMT, MT and OMT + MT on
histopathology, biochemistry and body weight in mice
Toxicity induced by 90-day oral administration of
OMT and/or MT in mice was studied by evaluating body weight,
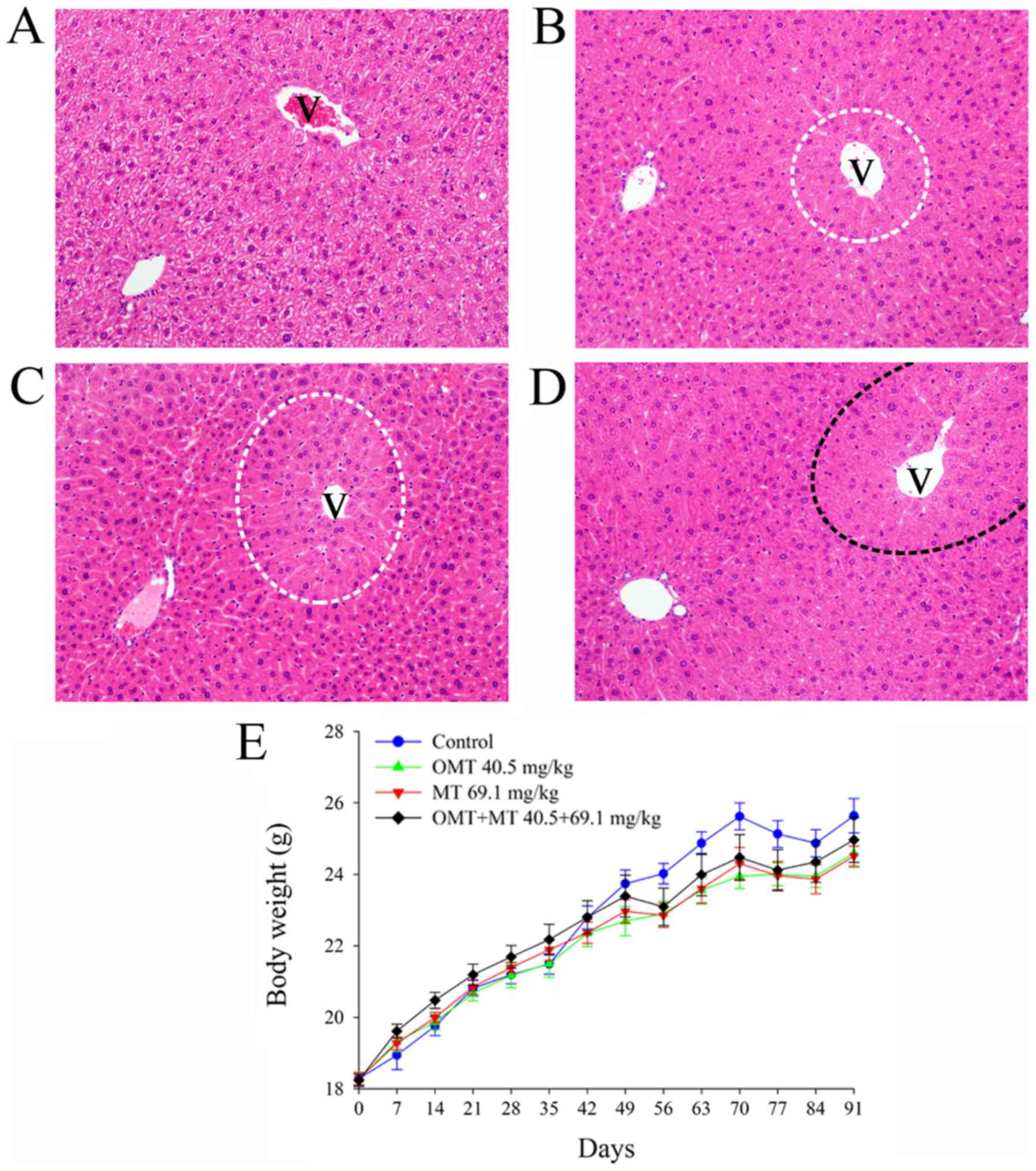
histopathology and biochemistry (Fig.
2). No signs of toxicity (including hypoactivity and
alterations in locomotor activity) or cases of mortality were
observed during the administration of OMT, MT and OMT + MT at doses
of 40.5, 69.1 and 40.5+69.1 mg/kg, respectively. Since body weight
changes and growth rate in prolonged toxicity studies are important
indicators of adverse effects of drugs and chemicals on laboratory
animals (21), body weight was
monitored in the mice used in the current study. The body weight of
the control group was slightly lower prior to day 35 and then
exceeded the treated groups following day 49, but the differences
were not statistically significant (P>0.05; Fig. 2E), which suggested that the 90-day
oral administration of OMT, MT and OMT + MT had no obvious effect
on the normal growth of mice. Serum biochemistry serves as an
important indicator of physiological abnormalities in liver
intoxication (22). The values of
biochemical indicators following 90 days of exposure to OMT, MT and
OMT + MT are presented in Table I.
It was observed levels of serum ALT were significantly higher in
OMT-treated mice and levels of AST were significantly higher in
MT-treated mice compared with control mice (P<0.05; Table I). Although animals treated with OMT
showed higher ALT and treated with MT exhibited higher AST,
compared with the control group, these parameters were within
normal range for the species utilized in the current study, which
was also congruent to a previous study (23). Compared with the control, no
significant alterations in TBil levels were observed in OMT, MT or
OMT + MT groups (Table I). At the
end of the sub-chronic toxicity study, there were no histological
changes observed in the control group (Fig. 2A), while mild centrilobular
hypertrophy (areas circled with white dotted lines) in the liver
was observed in the OMT (Fig. 2B)
and MT (Fig. 2C) groups, and
moderate centrilobular hypertrophy (area circled with a black
dotted line) was observed in the OMT + MT group (Fig. 2D). These histopathological results
are consistent with our previous study on radix S.
tonkinensis-induced hepatotoxicity (5).
 | Table I.Comparison of serum biochemistry
after 90 days of treatment with OMT, MT or OMT + MT in mice. |
Table I.
Comparison of serum biochemistry
after 90 days of treatment with OMT, MT or OMT + MT in mice.
| Group | ALT, IU/l | AST, IU/l | TBil, mmol/l |
|---|
| Control (n=10) | 21.50±0.82 | 38.10±1.57 | 1.57±0.09 |
| OMT, 40.5 mg/kg
(n=9) |
25.11±1.23a | 43.33±2.41 | 1.28±0.07 |
| MT, 69.1 mg/kg
(n=11) | 22.09±0.84 |
44.82±2.55a | 1.56±0.22 |
| OMT + MT, 40.5+69.1
mg/kg (n=11) | 22.27±1.20 | 39.82±1.55 | 1.52±0.28 |
Cytotoxic effects of OMT, MT and OMT +
MT on AML12 liver cells
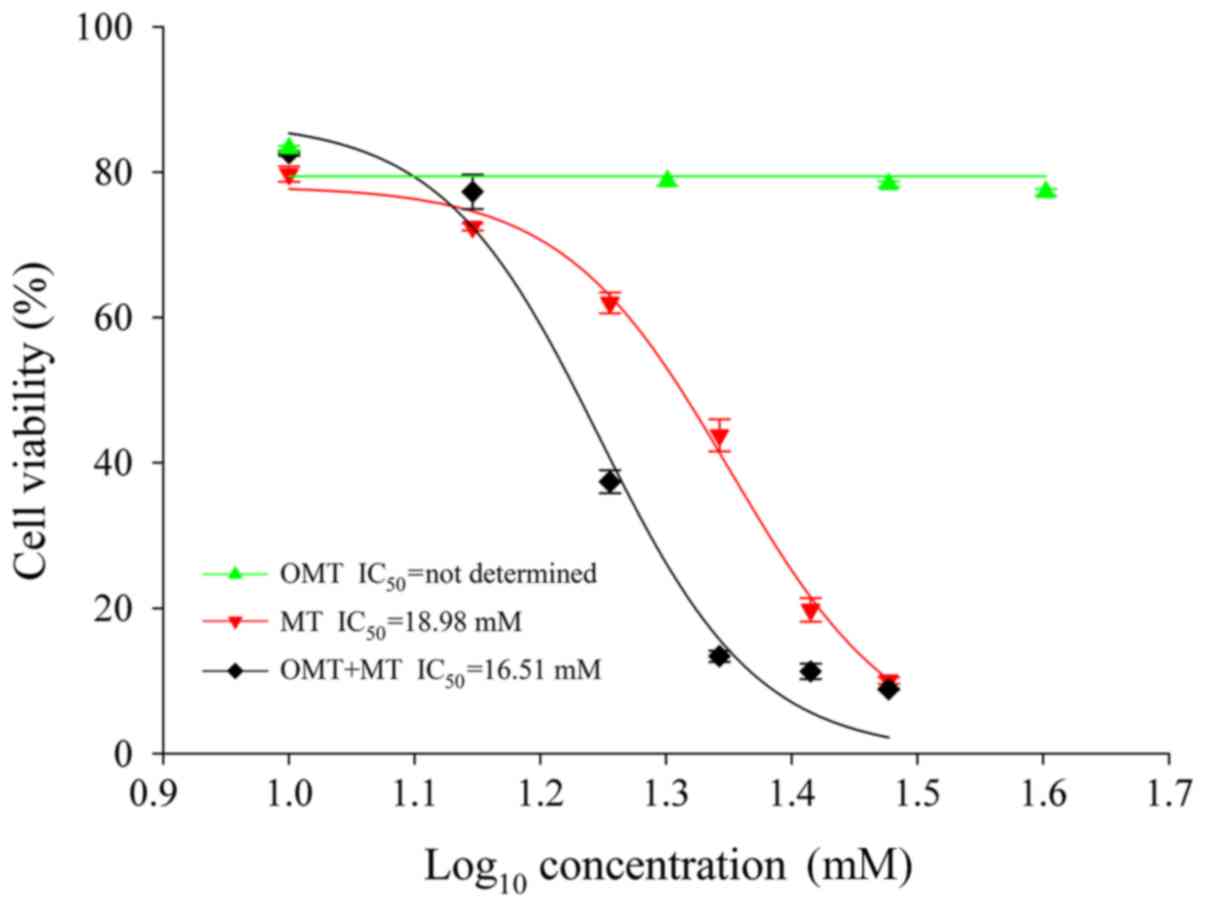
To determine the cytotoxicity of treatment with OMT,
MT and OMT + MT, a CCK-8 assay was used in AML12 cells treated with
different concentrations of OMT (10, 20, 30 and 40 mM), MT (10, 14,
18, 22, 26 and 30 mM) or OMT + MT (10, 14, 18, 22, 26 and 30 mM
each) for 24 h. A modified log10 [dose]-response curve
was applied to fit the data and obtain the IC50 values.
IC50 value is defined as the concentration of a molecule
that inhibits 50% of the measured response (24). The data indicated that cell viability
was not significantly altered by incubation with OMT for 24 h (10,
20, 30 and 40 mM; 83.27±0.35, 78.77±0.65, 78.35±0.39 and
77.24±0.46%, respectively; expressed as the percentage of control
cells; Fig. 3). Treatment with MT
and OMT + MT induced cytotoxicity in a dose-dependent manner. At
the end of the 24-h treatment period, incubation with 10, 14, 18,
22, 26 and 30 mM of MT reduced cell viability (expressed as
percentage of control cells) to 79.73±1.04, 72.45±0.49, 62.00±1.42,
43.78±2.21, 19.77±1.61 and 10.02±0.44%, respectively (Fig. 3). Under the same concentration
conditions, cell viability of OMT + MT-treated cells decreased to
82.59±0.37, 77.28±2.36, 37.39±1.60, 13.38±0.77, 11.31±1.04 and
8.84±0.09%, respectively (Fig. 3).
The IC50 values of MT and OMT + MT in this assay,
calculated using the SigmaPlot 11.0 software, were 18.98 and 16.51
mM, respectively. However, the IC50 value for OMT could
not be determined with the treatment concentrations used in the
present study. These results suggest that OMT had no marked
cytotoxic effect on AML12 liver cells compared with MT and OMT + MT
groups. The left shift in the IC50 value for treatment
with OMT + MT implied a relatively high toxicity compared with the
MT group, suggesting that OMT with MT may have a synergistic effect
and enhance the hepatotoxicity in AML12 liver cells.
Effects of OMT, MT and OMT + MT on
ALT, AST and LDH leakage in the culture medium of AML12 liver
cells
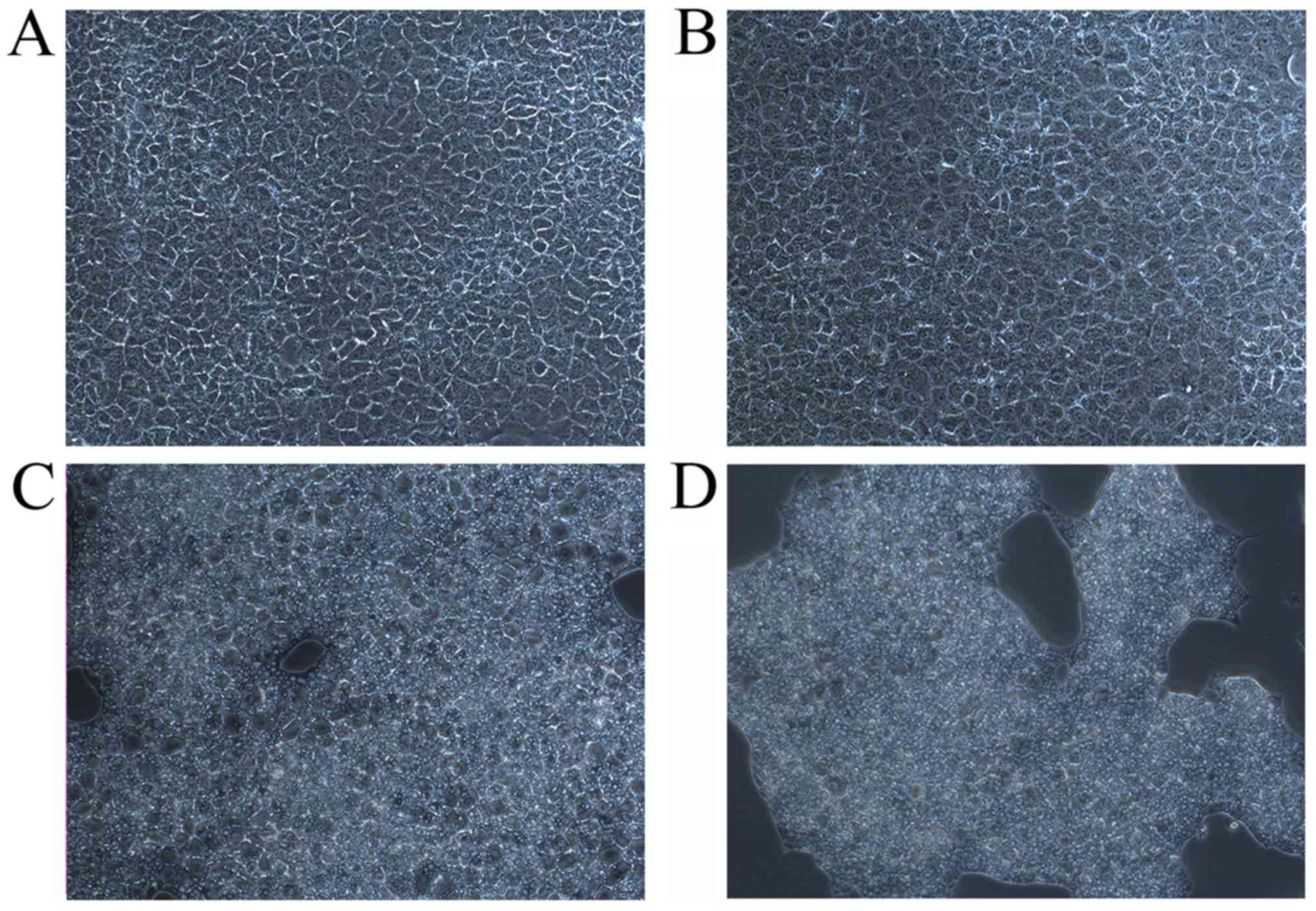
Light microscopy examination revealed different
histological alterations in AML12 cells after 6 h of treatment with
OMT, MT and OMT + MT. Cells exhibited intact morphology and
structure in the control group (Fig.
4A) and following treatment with 18 mM OMT (Fig. 4B). Certain pathological alterations,
including shrinkage, unclear contour and vacuolar degeneration,
were observed in the 18 mM MT group (Fig. 4C). In the group treated with 16 mM
OMT + MT, the cell number was visibly reduced (Fig. 4D). These results suggest that MT
alone or combined with OMT may increase cell injury and cause cell
death.
Hepatotoxicity in vitro can be directly
determined by measuring the levels of the aforementioned enzymes
released into the culture medium (25). Cytotoxic results may provide
important preliminary data for selecting a dosage for enzyme
leakage tests in vitro (26).
As indicated in Table II, treatment
with 12 and 18 mM MT significantly increased ALT, AST and LDH
release (P<0.05 or P<0.01) in a dose-dependent manner at 3,
6, 12 and 24 h, with the exception of ALT release following
treatment with 12 mM MT at 24 h. Furthermore, treatment with 4 mM
OMT + MT was observed to have no effect on ALT, AST and LDH release
(Table III). In the cells treated
with 16 mM OMT + MT, the levels of ALT, AST and LDH significantly
increased (P<0.01) at 3 h, peaked at 6 h, and declined
thereafter (Table III). However,
in the 10 mM OMT + MT group, the levels of ALT, AST and LDH
(P<0.05 or P<0.01) peaked at 3 h, and then decreased
gradually over time (Table III).
By contrast, when the cells were treated with OMT (18 mM) only, no
significant increases in the three enzymes were observed over
exposure periods of 3, 6, 12 or 24 h (P>0.05), with the
exception of the level of AST, which significantly decreased
compared with the control at 24 h (P<0.01; Table IV). The above toxic effects of MT
and OMT + MT may reflect cellular leakage and loss of functional
integrity of liver cell membranes in a dose-dependent manner in
AML12 liver cells subjected to 3, 6, 12 and 24 h of incubation.
Consistent with previous studies, the increased release of these
intracellular enzymes indicate membrane damage and instability,
owing to injury induced by xenobiotics (25,27). The
results of the current study indicate that MT and OMT + MT, but not
OMT, may induce cell membrane damage.
 | Table II.Comparison of enzyme activities after
3, 6, 12 and 24 h treatment with MT in AML12 liver cells. |
Table II.
Comparison of enzyme activities after
3, 6, 12 and 24 h treatment with MT in AML12 liver cells.
| Group | Time, h | ALT, IU/l | AST, IU/l | LDH, IU/l |
|---|
| Control (n=6) | 3 | 0.00±0.00 | 3.33±0.21 | 15.83±0.70 |
|
| 6 | 0.17±0.17 | 3.33±0.21 | 13.83±0.54 |
|
| 12 | 0.17±0.17 | 3.17±0.17 | 13.33±0.42 |
|
| 24 | 0.67±0.21 | 2.83±0.17 | 13.33±0.33 |
| MT, 6 mM (n=4) | 3 | 0.00±0.00 | 2.75±0.63 | 14.50±1.32 |
|
| 6 | 0.00±0.00 | 3.25±0.48 | 15.25±0.85 |
|
| 12 | 0.00±0.00 | 3.00±0.41 | 14.25±0.63 |
|
| 24 | 0.00±0.00 | 2.75±0.25 | 12.25±0.25 |
| MT, 12 mM
(n=4) | 3 |
3.75±1.25b |
12.00±2.68b |
75.75±17.88a |
|
| 6 |
1.00±0.00a |
5.50±0.65a |
37.00±6.26a |
|
| 12 |
2.75±1.49a |
10.25±4.27b |
76.75±36.44a |
|
| 24 | 1.25±0.25 |
5.00±0.41a |
32.75±5.76b |
| MT, 18 mM
(n=4) | 3 |
10.5±1.71b |
25.50±3.01b |
188.00±23.28a |
|
| 6 |
10.00±0.91b |
22.50±1.19b |
147.50±10.97a |
|
| 12 |
14.5±1.49b |
25.75±2.75b |
151.00±32.45a |
|
| 24 |
17.75±2.95b |
26.75±1.55b |
176.50±20.39b |
 | Table III.Comparison of enzyme activities after
3, 6, 12 and 24 h treatment with OMT + MT in AML12 liver cells. |
Table III.
Comparison of enzyme activities after
3, 6, 12 and 24 h treatment with OMT + MT in AML12 liver cells.
| Group | Time, h | ALT, IU/l | AST, IU/l | LDH, IU/l |
|---|
| Control (n=6) | 3 | 0.00±0.00 | 3.33±0.21 | 15.83±0.70 |
|
| 6 | 0.17±0.17 | 3.33±0.21 | 13.83±0.54 |
|
| 12 | 0.17±0.17 | 3.17±0.17 | 13.33±0.42 |
|
| 24 | 0.67±0.21 | 2.83±0.17 | 13.33±0.33 |
| OMT + MT, 4 mM
(n=4) | 3 | 0.00±0.00 | 3.00±0.00 | 15.50±0.65 |
|
| 6 | 0.00±0.00 | 3.25±0.25 | 14.75±1.11 |
|
| 12 | 0.00±0.00 | 3.25±0.25 | 15.25±0.95 |
|
| 24 | 0.00±0.00 | 3.00±0.00 | 13.00±0.41 |
| OMT + MT, 10 mM
(n=4) | 3 |
4.75±1.55b |
13.75±3.71b |
98.75±26.73a |
|
| 6 |
2.00±0.41b |
6.75±0.48b |
40.25±2.25b |
|
| 12 |
1.25±0.25a |
6.25±0.48b |
38.00±2.48a |
|
| 24 | 0.25±0.25 | 3.25±0.25 |
17.00±0.71a |
| OMT + MT, 16 mM
(n=4) | 3 |
14.00±0.91b |
33.50±1.19b |
271.50±4.66b |
|
| 6 |
17.50±1.50b |
43.00±3.76b |
269.25±9.93b |
|
| 12 |
10.50±2.50b |
27.50±4.66b |
168.00±33.34b |
|
| 24 |
10.00±2.65b |
20.75±6.20b |
140.00±44.56b |
 | Table IV.Comparison of enzyme activities after
3, 6, 12 and 24 h treatment with OMT in AML12 liver cells. |
Table IV.
Comparison of enzyme activities after
3, 6, 12 and 24 h treatment with OMT in AML12 liver cells.
| Group | Time, h | ALT, IU/l | AST, IU/l | LDH, IU/l |
|---|
| Control (n=4) | 3 | 2.50±0.50 | 6.00±0.41 | 32.25±1.44 |
|
| 6 | 2.25±0.25 | 6.00±0.41 | 43.50±4.33 |
|
| 12 | 2.50±0.29 | 7.25±0.25 | 61.50±15.20 |
|
| 24 | 1.25±0.25 | 8.00±0.00 | 24.25±0.25 |
| OMT, 18 mM
(n=4) | 3 | 2.50±0.29 | 6.00±0.41 | 36.00±3.39 |
|
| 6 | 2.50±0.29 | 6.75±0.48 | 59.50±5.36 |
|
| 12 | 2.75±0.25 | 7.50±0.50 | 68.75±5.62 |
|
| 24 | 1.00±0.00 |
5.50±0.29a | 21.25±1.11 |
Discussion
Several notable observations were made in the
current study: i) Treatment with OMT, MT and OMT + MT in the
equivalent amounts contained in radix S. tonkinensis induced
hepatotoxicity; ii) the toxicity of OMT was weaker compared with MT
in vitro; however, OMT-induced hepatotoxicity was consistent
with that following treatment with MT in mice; and iii) OMT and MT
in combination may serve a synergistic role in hepatotoxicity.
These results support the hypothesis that MT and OMT are the toxic
constituents responsible for the hepatotoxicity of radix S.
tonkinensis, and the active metabolite MT transformed from OMT
may be responsible for the toxic effect of OMT.
The increased release of liver enzymes, including
ALT, AST and LDH, as well as TBiL, is recognized as an initial
indicator of physiological dysfunction and liver toxicity (22,28).
However, hepatic histopathological evaluation is currently the
standard method for determining the degree of liver injury during
exposure to xenobiotics (29).
Levels of serum biochemical parameters may be used to predict the
level of histological damage in the liver (30,31).
However, the alterations in the levels of these enzymes and TBil in
the mice were not dose-dependent and were within the normal range
for the species utilized in the present study (23). Therefore, pathological changes in the
liver were also evaluated to verify this result. Centrilobular
hypertrophy, characterized by the enlargement of both the cytoplasm
and nucleus in the affected hepatocytes (32), was consistent with the
histopathological results reported previously in radix S.
tonkinensis-induced hepatotoxicity (5). This supported the hypothesis that OMT
and MT are responsible for the hepatotoxicity of radix S.
tonkinensis. It has also been reported previously that
hepatocellular hypertrophy, in the absence of other histological
findings, is not always associated with changes in serum ALT
activity or other measured serum hepatic enzymes (33).
Centrilobular hepatocellular hypertrophy, the most
common histological change associated with enzyme induction in
animals, may be considered as an adaptive effect (33). However, this adaptive response may be
overcome following exposure to intense or prolonged stimuli,
leading to hepatocellular degeneration, necrosis or proliferation
(34). The extent and degree of
hepatotoxicity may depend on the duration of exposure (35). Indeed, the duration of exposure in
the present study may have affected the results. Treatment with MT
and OMT over 90 days may induce more severe liver injury compared
with that of centrilobular hepatocellular hypertrophy presented in
MT, OMT or MT + OMT treated groups in the current study. OMT and MT
were identified in radix S. tonkinensis, Sophorae
flavescentis and the above ground portion of Sophora
alopecuroides, and these herbal remedies are also commonly
prescribed for a number of illnesses (36,37).
Therefore, it is recommended that increased attention is paid to
the hepatotoxicity of herbs containing MT and OMT when applying
them for an extended period of time, as it is generally believed
that herbal products are harmless and can be taken for a long
period of time (38,39).
Having identified that the hepatotoxic effect of
radix S. tonkinensis is primarily caused by OMT or its
metabolite MT, the effects on cell viability and the release of
three enzymes in vitro were subsequently evaluated. The
AML12 cell line was originally established from normal hepatocytes
obtained from a CD1 male mouse strain, and the cells exhibit
typical hepatocyte features, including peroxisomes and bile
canalicular-like structures (40,41). It
was observed that MT and OMT + MT induced cell membrane damage and
instability in vitro, while OMT had marked cytotoxic effect.
It has been previously reported that following administration of
OMT oral solution, the concentration of MT was significantly
greater compared with OMT, indicating that only a small proportion
of the oral solution is absorbed by the gastrointestinal tract,
while most of the OMT is absorbed after it arrives in the
intestines and is rapidly transformed into the metabolite MT
(16,42). By contrast, it was reported that only
a small amount of OMT is transformed into metabolite MT following
intravenous administration of OMT, as indicated by the area under
the plasma concentration-time curve (17). These results indicate that the active
metabolite MT, originating from OMT, may be the actual toxic
substance in radix S. tonkinensis.
The combined toxicity of OMT and MT was also of
interest in the current study. Previous evidence indicated that
combined treatment with OMT and MT could increase the mortality
rate in mice during a median lethal dose test, suggesting that
combined treatment with OMT and MT exhibited a synergistic effect
on toxicity (43). Therefore, it may
be hypothesized that OMT combined with MT is more toxic for liver
compared with OMT or MT alone.
The current study contributed to the better
understanding the hepatotoxicity of radix S. tonkinensis and
may have implications for its application in clinical settings.
However, the present results are limited to the administration of
MT and OMT for evaluating hepatotoxicity of radix S.
tonkinensis. The effects of the non-alkaloid part of this herb
on the liver require further investigation. It will also be
important to evaluate long-term effects (>3 months) of
administration of MT and OMT on histological changes in the liver.
Following prolonged exposure, the liver damage may be more severe
compared with that observed in the current study. In addition, a
number of studies reported that the increased centrilobular
hepatocellular hypertrophy is likely due to the observed induction
of hepatic cytochrome P450 (CYP450) enzymes, notably cytochrome
P450 2B (CYP2B) (44–46). CYP450 participates in liver injury on
various levels, including hepatocellular apoptosis, necrosis or
aberrant proliferation (47,48). Therefore, an evaluation of the change
in CYP450 and CYP2B may be valuable to comprehensively understand
the pathogenesis of exposure to MT and OMT in our future study.
To the best of our knowledge, the current study
demonstrated for the first time that administration of OMT and MT
alone or simultaneously can induce hepatotoxicity at the dose
equivalent to that contained in radix S. tonkinensis at a
hepatotoxic dose, and MT and OMT are likely the hepatotoxic
components of this herb. The results revealed that OMT induced
hepatotoxicity in vivo, and this toxic effect may be
primarily exerted by active metabolite MT. In addition, OMT in
combination with MT was more toxic compared with MT or OMT alone.
Since OMT may be transformed into the active metabolite MT by
intestinal bacteria, the hepatotoxicity of OMT should be closely
monitored when OMT is administered orally instead of intravenously.
The current results improve the understanding of hepatotoxicity
induced by radix S. tonkinensis, and provide insight into
the actual hepatotoxic components among its main active
ingredients.
Acknowledgements
Not applicable.
Funding
The present study was supported by funds from
Shanghai University of Traditional Chinese Medicine (grant no.
2014YSN24), National Natural Science Foundation of China (grant
nos. 81574078 and 81072646) and National Key Research and
Development Program of China (grant no. 2017YFC1702000).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
XT designed the experiments and wrote the
manuscript. ZZ performed hepatic histopathological evaluation and
wrote the histopathological result section of the manuscript. YG,
JL, WS, RJ and TO performed the experiments and analyzed the data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of Shanghai University of Treditional Chinese Medicine
(Shanghai China; certificate no. SZY201504021), and performed in
accordance with the Guidelines for the Care and Use of Laboratory
Animals (National Institutes of Health, Bethesda, MD, USA).
Patient consent for publication
Not applicable.
Competing interests
Toko Ohira is currently an employee of Shanghai
Innostar Biotech Co., Ltd. (Shanghai, China), but had no competing
interests. The remaining authors declare that they have no
competing interests.
References
|
1
|
The State pharmacopoeia commission of P.R.
China. Pharmacopoeia of the People's Republic of China. Chin Med
Sci Press. (Beijing). pp272015.(In Chinese).
|
|
2
|
Tian XS: Research progress on toxicity of
alkaloids in sophorae tonkinensis radix et rhizoma. Zhongguo Shi
Yan Fang Ji Xue Za Zhie. 22:230–234. 2016.(In Chinese).
|
|
3
|
Wang XP and Yang RM: Movement disorders
possibly induced by traditional Chinese herbs. Eur Neurol.
50:153–159. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li X, Luan Y, Li X and Sun R: Study on
anti-inflammatory efficacy accompanied by side effects of different
components of Sophorae Tonkinensis Radix et Rhizoma. Zhongguo Zhong
Yao Za Zhi. 37:2232–2237. 2012.(In Chinese). PubMed/NCBI
|
|
5
|
Wang L, Lu J, Sun W, Gu Y, Zhang C, Jin R,
Li L, Zhang Z and Tian X: Hepatotoxicity induced by radix Sophorae
tonkinensis in mice and increased serum cholinesterase as a
potential supplemental biomarker for liver injury. Exp Toxicol
Pathol. 69:193–202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JW, Lee JH, Lee C, Jin Q, Lee D, Kim
Y, Hong JT, Lee MK and Hwang BY: Inhibitory constituents of Sophora
tonkinensis on nitric oxide production in RAW 264.7 macrophages.
Bioorg Med Chem Lett. 25:960–962. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu ML, Xiang XH and Xia SH: Potential
signaling pathways involved in the clinical application of
oxymatrine. Phytother Res. 30:1104–1112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang J and Xu H: Matrine: Bioactivities
and structural modifications. Curr Top Med Chem. 16:3365–3378.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu H, Zhang L, Gu LL, Hou BY and Du GH:
Oxymatrine induces liver injury through JNK signalling pathway
mediated by TNF-α in vivo. Basic Clin Pharmacol Toxicol.
119:405–411. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao ZW, Zhang RQ and Liao XH: Two cases of
aggravating liver damage caused by oxymatrine injection in the
patients with chronic hepatitis B. Yao Wu Bu Liang Fan Ying Za Zhi.
2002:120–121. 2002.(In Chinese).
|
|
11
|
Guo QP and Jin RM: Comparison of liver
toxicity of matrine and oxymatrine in mice. Zhongguo Yao Li Yu Du
Li Xue Za Zhi. 30:736–740. 2016.(In Chinese).
|
|
12
|
Chen Y, Zhang Q, Han SX, Han FM, Chen LM,
Tong Y and You Y: Study on toxicity of different extractions of
Sophorae tonkinensis. Zhongguo Yao Wu Jing Jie. 14:582–586.
2017.(In Chinese).
|
|
13
|
Miners JO, Knights KM, Houston JB and
Mackenzie PI: In vitro-in vivo correlation for drugs and other
compounds eliminated by glucuronidation in humans: Pitfalls and
promises. Biochem Pharmacol. 71:1531–1539. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen P, Zhang X, Huang T, Yu Q and Cheng
N: Metabolism of the hepatotoxic compound Sophoraflavanone G in rat
liver microsomes. J Food Sci. 79:T1462–T1468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie MZ, Zhou WZ and Zhang YD: The
metabolic fate of oxymatrine. Zhongguo Yao Li Xue Bao. 16:481–487.
1981.(In Chinese).
|
|
16
|
Wang ML, Zhou QL and Wang BX: Studies on
metabolism of oxymatrine by human intestinal bacteria. Zhongguo
Zhong Yao Za Zhi. 26:272–274. 2001.(In Chinese). PubMed/NCBI
|
|
17
|
Wu XL, Hang TJ, Shen JP and Zhang YD:
Determination and pharmacokinetic study of oxymatrine and its
metabolite matrine in human plasma by liquid chromatography tandem
mass spectrometry. J Pharm Biomed Anal. 41:918–924. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
National Research Council (US) committee
for the update of the guide for the care use of laboratory animals.
Guide for the care and use of laboratory animals. (8th). National
Academies Press (US). (Washington, DC). 2011.
|
|
19
|
Anon: The guidelines of test technology
for long-term toxicity of chemical drugs. http://www.sfda.gov.cn/directory/web/WS01/images/u6Rp9Kpzu+zpMbatr7Q1MrU0em8vMr11ri1vNSt1PIucGRm.pdf(In
Chinese).
|
|
20
|
Hou G, Xue L, Lu Z, Fan T, Tian F and Xue
Y: An activated mTOR/p70S6K signaling pathway in esophageal
squamous cell carcinoma cell lines and inhibition of the pathway by
rapamycin and siRNA against mTOR. Cancer Lett. 253:236–248. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Amenya HZ, Gathumbi PK, Mbaria JM, Thaiyah
AG and Thoithi GN: Sub-acute toxicity of the chloroformic extract
of Rapanea melanophloeos (L.) Mez in rats. J Ethnopharmacol.
154:593–599. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akanda MR, Kim IS, Ahn D, Tae HJ, Tian W,
Nam HH, Choo BK and Park BY: In vivo and in vitro hepatoprotective
effects of Geranium koreanum methanolic extract via downregulation
of MAPK/Caspase-3 pathway. Evid Based Complement Alternat Med.
2017:81376272017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Serfilippi LM, Pallman DR and Russell B:
Serum clinical chemistry and hematology reference values in outbred
stocks of albino mice from three commonly used vendors and two
inbred strains of albino mice. Contemp Top Lab Anim Sci. 42:46–52.
2003.PubMed/NCBI
|
|
24
|
Tse DY, Chung I and Wu SM: Pharmacological
inhibitions of glutamate transporters EAAT1 and EAAT2 compromise
glutamate transport in photoreceptor to ON-bipolar cell synapses.
Vision Res. 103:49–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi C, Chen X, Liu Z, Meng R, Zhao X, Liu
Z and Guo N: Oleuropein protects L-02 cells against
H2O2-induced oxidative stress by increasing
SOD1, GPx1 and CAT expression. Biomed Pharmacother. 85:740–748.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sofi MS, Sateesh MK and Bashir M:
Screening of the Ethnobotanicals against MDA-MB-231 and MCF-7
breast cancer cell lines. Int J of Phytopharm. 4:140–147. 2014.
|
|
27
|
Mani S, Mondal D, Sarma K and Singh K:
Experimentally induced liver cirrhosis with ascites by carbon
tetrachloride and phenobarbital sodium in wistar rats. Adv Anim Vet
Sci. 2:159–163. 2014. View Article : Google Scholar
|
|
28
|
Gowda S, Desai PB, Hull VV, Math AA,
Vernekar SN and Kulkarni SS: A review on laboratory liver function
tests. Pan Afr Med J. 3:172009.PubMed/NCBI
|
|
29
|
Acton QA: Drugs-advances in research and
application: 2012 edition. Scholarly Editions; Atlanta, GA: pp.
6452012
|
|
30
|
Kleiner DE, Chalasani NP, Lee WM, Fontana
RJ, Bonkovsky HL, Watkins PB, Hayashi PH, Davern TJ, Navarro V,
Reddy R, et al: Hepatic histological findings in suspected
drug-induced liver injury: Systematic evaluation and clinical
associations. Hepatology. 59:661–670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kirisci O, Paksoy T, Caliskan A, Analan A,
Ozkaya E, Kirmaci B, Tumer S, Citil R, Cikim G, Agirbas S, et al:
The relationship between serum DNA levels and serological markers,
Alt and Ast with liver histology in chronic hepatitis B patients.
Acta Medica Mediterr. 32:1805–1811. 2016.
|
|
32
|
Senoh H, Katagiri T, Arito H, Nishizawa T,
Nagano K, Yamamoto S and Matsushima T: Toxicity due to 2- and 13-wk
inhalation exposures of rats and mice to N, N-dimethylformamide. J
Occup Health. 45:365–375. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ennulat D, Walker D, Clemo F, Magid-Slav
M, Ledieu D, Graham M, Botts S and Boone L: Effects of hepatic
drug-metabolizing enzyme induction on clinical pathology parameters
in animals and man. Toxicol Pathol. 38:810–828. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Williams GM and Iatropoulos MJ: Alteration
of liver cell function and proliferation: Differentiation between
adaptation and toxicity. Toxicol Pathol. 30:41–53. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cataudella E, Malaguarnera G, Gagliano C,
Condorelli G, Antic T, Rampello L, Erdogan O, Rampello L and
Malaguarnera M: Pesticides exposure and the management of acute
hepatic injury. Acta Medica Mediterr. 28:245–252. 2012.
|
|
36
|
He X, Fang J, Huang L, Wang J and Huang X:
Sophora flavescens Ait: Traditional usage, phytochemistry and
pharmacology of an important traditional Chinese medicine. J
Ethnopharmacol. 172:10–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang J, Zhang L, Zhu G and Li L:
Separation and enrichment of major quinolizidine type alkaloids
from Sophora alopecuroides using macroporous resins. J Chromatogr B
945–946. 17–22. 2014. View Article : Google Scholar
|
|
38
|
Firenzuoli F and Gori L: Herbal medicine
today: Clinical and research issues. Evid Based Complement Alternat
Med. 4 (Suppl 1):S37–S40. 2007. View Article : Google Scholar
|
|
39
|
Welz AN, Emberger-Klein A and Menrad K:
Why people use herbal medicine: Insights from a focus-group study
in Germany. Bmc Complem Altern Med. 18:922018. View Article : Google Scholar
|
|
40
|
Wu JC, Merlino G and Fausto N:
Establishment and characterization of differentiated,
nontransformed hepatocyte cell lines derived from mice transgenic
for transforming growth factor alpha. Proc Natl Acad Sci USA.
91:674–678. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hassoun E and Mettling C: Dichloroacetate
and trichloroacetate toxicity in AML12 cells: Role of oxidative
stress. J Biochem Mol Toxicol. 29:508–512. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fan R, Liu R, Ma R, Bi K and Li Q:
Determination of oxymatrine and its active metabolite matrine in
human plasma after administration of oxymatrine oral solution by
high-performance liquid chromatography coupled with mass
spectrometry. Fitoterapia. 89:271–277. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Song B, Han CX and Zhang HL: Toxicity of
three Sophora flavescens Ait alkaloids to mice. Xi Bei Zhi Wu Xue
Bao. 29:818–823. 2009.(In Chinese).
|
|
44
|
Sakamoto Y, Yoshida M, Tamura K, Takahashi
M, Kodama Y and Inoue K: Dose-dependent difference of nuclear
receptors involved in murine liver hypertrophy by piperonyl
butoxide. J Toxicol Sci. 40:787–796. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Deguchi Y, Yamada T, Hirose Y, Nagahori H,
Kushida M, Sumida K, Sukata T, Tomigahara Y, Nishioka K, Uwagawa S,
et al: Mode of action analysis for the synthetic pyrethroid
metofluthrin-induced rat liver tumors: Evidence for hepatic CYP2B
induction and hepatocyte proliferation. Toxicol Sci. 108:69–80.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
van der Ven LT, van de Kuil T, Verhoef A,
Leonards PE, Slob W, Cantón RF, Germer S, Hamers T, Visser TJ,
Litens S, et al: A 28-day oral dose toxicity study enhanced to
detect endocrine effects of a purified technical pentabromodiphenyl
ether (pentaBDE) mixture in Wistar rats. Toxicology. 245:109–122.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nebert DW and Dalton TP: The role of
cytochrome P450 enzymes in endogenous signalling pathways and
environmental carcinogenesis. Nat Rev Cancer. 6:947–960. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang X, Zong C, Zhang L, Garner E, Sugie
S, Huang C, Wu W, Chang J, Sakurai T, Kato M, et al: Exposure of
mice to 1,2-dichloropropane induces CYP450-dependent proliferation
and apoptosis of cholangiocytes. Toxicol Sci. 162:559–569. 2018.
View Article : Google Scholar : PubMed/NCBI
|