Introduction
Diabetes and obesity are reaching pandemic scale
worldwide (1). The targeted and
effective methods for the pharmacological treatment of obesity are
sparse, but there have been several new therapies introduced into
clinical practice to treat type 2 diabetes mellitus [e.g.
glucagon-like peptide 1 (GLP-1) agonists, dipeptidyl peptidase-4
(DPP-4) inhibitors or sodium/glucose cotransporter 2 (SGLT-2)
inhibitors] which may ease weight reduction (SGLT-2 inhibitors,
GLP-1 agonists). Weight loss is crucial to diabetic subjects
(2) and it is rather easy to explain
the mechanism during the therapy with drugs that induce glycosuria
(leading to a glucose loss of up to 300 kcal per day with urine).
However, it is not that clear for the other group of novel
antidiabetic drugs.
GLP-1 is a hormone that is produced in the
gastrointestinal (GI) tract and is liberated after the ingestion of
meals, leading to a myriad of effects (i.e. the incretin effect).
It lowers GI motility, increases insulin secretion, reduces
glucagon level and reduces food intake (3). As a result, a tendency to reduce the
body weight of subjects with type 2 diabetes during the treatment
with GLP-1 agonists was noted (4).
This observation led to exploratory studies, which showed the
weight-reducing potency of those drugs in non-diabetic subjects
(5). Subsequently, liraglutide (a
GLP-1 agonist) has been approved for the treatment of obesity in
subjects without diabetes (6).
Mechanisms behind the weight loss seen during the
therapy with GLP-1 agonists are multifactorial and not well
understood (7). Nevertheless, it
seems that the impact on the central nervous system and eating
habits are crucial. There are several sites in the central nervous
system (e.g. hypothalamus) that are linked with eating habits
(8). Neurons that form those centers
have limited access to GLP-1 agonists due to the existence of the
blood brain barrier (BBB). The BBB is formed from several different
cell types, including astrocytes. Astrocytes not only protect
neurons, but also actively participate in the transduction of
signals from the bloodstream to neurons (9,10), which
suggest that they have paracrine modulating capabilities in
neuronal signaling. A significant positive correlation between
astrogliosis, represented by an increased expression of
glial-fibrillary acidic protein (GFAP), and obesity in animal
studies have been noted (11). The
mediators of astrocyte signaling may include inflammatory pathways
(IL-1β and NFκB) and oxidative stress (reactive oxygen species-ROS;
the expression of proteins associated with oxidative stress-p22
NADPH oxidase; and anti-oxidative enzymes-catalase, superoxide
dismutase and glutathione peroxidase) (12). It is thought that inflammatory
cytokines and excessive oxidative stress may have anorexigenic
properties (13,14). Inflammation and oxidative stress are
exaggerated by variations in glucose concentrations, especially
during prolonged hyperglycemia in diabetes (15). Recent studies have shown that
astrocytes are actively involved in the inflammatory and oxidative
processes associated with the pathogenesis of several diseases
(e.g. major depressive disorder, epilepsy) (16). In addition, a growing body of
evidence suggests their key participation in the pathogenesis of
obesity and diabetes (17,18). Brain magnetic resonance imaging
studies in human subjects showed a significant correlation between
glial activation and obesity (19).
Finally, astrocytes are now considered to be contributors in the
CNS signaling system (20).
Several studies have explored the impact of GLP-1
agonists on astrocytes, but they have been performed on animal
behavioral models (21,22) or animal-derived astrocytes in an
in vitro setting (23). We
have noted that there are few data on the impact of GLP-1 agonists
on human non-malignant astrocytes.
Therefore, we conceived a study to assess the
short-term impact of exenatide (a GLP-1 agonist) on IL-1β, NFκB,
GFAP and redox status in normal human astrocytes (NHA) cultured
in vitro. Due to the clinical use of GLP-1 agonists in the
treatment of type 2 diabetes and obesity without coexisting
diabetes we decided to perform a set of experiments in various
glycemic conditions ranging from hypoglycemia (40 mg/dl), via
normoglycemia (100 mg/dl) to hyperglycemia (400 mg/dl). In order to
explore whether the changes were dependent on PKA activation, which
is one of the major downstream pathways responsible for the
cellular effects (e.g. inflammatory and oxidative) of GLP-1
agonists (24,25), we performed experiments using a
selective PKA inhibitor [PKI (14–22)].
Materials and methods
Cell culture
Normal human astrocytes (NHA) cell line was
purchased from Lonza, cat. no. CC-2565 (Cell lab, Warsaw, Poland).
Cells were thawed accordingly to the supplier's recommendations and
cultivated in the astrocyte growth medium (AGM), which consisted of
astrocyte basal medium (cat. no. CC-3187) supplemented with AGM
SingleQuots (cat. no. CC-4123), both obtained from Lonza. All
experiments were done within 7th cell passages. The day before each
experiment, culture medium was replaced with glucose-containing
medium at predefined concentrations (40, 100 and 400 mg/dl). On the
day of the experiment, exenatide, which belongs to GLP-1 agonists
(Exendin-4, cat. no. E7144, Sigma-Aldrich; Merck KGaA, Poznań,
Poland), was reconstituted in water accordingly to the
manufacturer's recommendations. Afterwards it was diluted in the
culture medium to achieve 1 mM solution of exenatide. In selected
culture dishes, 30 min prior to the addition of exenatide, PKI
(14–22) (a pharmacological inhibitor of protein
kinase A) was added to the medium at the final concentration of 10
µM. NHA were incubated at 37°C in the atmosphere containing 95% of
air and 5% of CO2 in a CO2 incubator
(Hera-Cell, Thermo Fischer Scientific, Inc., Grand Island, NY,
USA). Those culture conditions are referred further as ‘standard
culture conditions’. Experiments were performed for 24 h.
Afterwards samples were collected and stored as described below.
HeLa cell line was a gift from the Department of Biotechnology and
Genetic Engineering, Medical University of Silesia, Sosnowiec,
Poland. HeLa cells were cultivated in the Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% of bovine fetal serum
and 50 µg/ml of gentamycin (cat. no. P04-01550, Pan-Biotech, GmbH,
Immuniq, Żory, Poland). HeLa cells were used as a reference cell
line that shows low expression of GLP-1 receptors (26).
Resazurin assay
In order to estimate the viability of cultured cells
in the experimental setting the resazurin assay was used. The
choice of the assay was based on its reliability, simplicity and
sensitivity in the assessment of cell viability (27). Briefly, one day before the
experiment, 5×104 NHA were seeded on 24-well culture
plate (SPL Life Sciences Co., Ltd., Immuniq) in 0,5 ml of AGM
growth medium. On the next day, 50 µl of resazurin dye solution was
added into each well (cat. no. TOX-8, Sigma-Aldrich; Merck KGaA).
Afterwards cells were incubated for 2 h under standard culture
conditions and shaked gently by using rotation platform set at 100
rpm. The decrease in absorbance at a wavelength of 600 nm was
measured spectrophotometrically using xMark Microplate Absorbance
Spectrophotometer (Bio-Rad Laboratories, Inc., Warsaw, Poland).
Additional measurements were done at a reference wavelength of 690
nm and were subtracted from the previous that were obtained at 600
nm.
ROS assessment
As shown by other researchers, a spectrometry-based
assay for determining intracellular ROS production and its level at
the final phase of experiments was performed using Nitrotetrazolium
Blue chloride (NBT) dye (No. cat. N6876, Sigma-Aldrich; Merck KGaA)
(28). A stock solution (1% NBT) was
added into cell culture to the final concentration of 0,1%. Cells
were then incubated for 24 h under standard conditions in 24-well
plates (5×104). Thereafter, supernatant was completely
removed and 200 µl of PBS solution was added into the cells. A
sonication procedure at 80% power intensity for 10 sec. was done in
order to disrupt the cells and liberate NBT into the solution.
Finally, absorbance at the wavelength of 550 nm was measured using
xMark Microplate Absorbance Spectrophotometer (Bio-Rad
Laboratories, Inc.).
Western blot analysis
In the western blot analysis, human-specific
antibodies were used as follows: For GLP-1 receptor (GLP-1R):
Anti-GLP-1R, (Bioss Antibodies Inc. Woburn, MA, USA); for nuclear
factor κB (NF-κB p65): Anti-NF-κB (Cell Signaling Technology,
Lab-JOT Ltd., Warsaw, Poland); for NADPH oxidase (p22): Anti-CYBA;
for GFAP: GFAP Polyclonal Antibody (Thermo Fischer Scientific,
Inc., Warsaw, Poland); for glutathione peroxidase (GPx): Anti-GPX1;
for catalase (Cat): Anti-Catalase and for superoxide dismutase-1
(SOD1): Anti-SOD1 (all from Sigma-Aldrich; Merck KGaA). For
quantitative analysis, a reference glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) protein, β-actin or lamin B1 were estimated
in every sample using an anti-GAPDH antibody, Actin-beta antibody
(both obtained from Thermo Fischer Scientific, Inc.) or Anti-Lamin
B1 antibody (Abcam, Symbiosis, Rotmanka, Poland).
Cells were cultured on 12-well culture plates
(1×105 cells per well) (SPL Life Sciences Co., Ltd.).
Prior to cell lysis, plates were placed on ice and cells were
washed briefly with 500 µl of ice-cold PBS. Protein extraction was
done using 150 µl of cold RIPA buffer supplemented with 1.5 µl of
Halt Protease Inhibition Cocktail (1:100 v/v) per well (both
chemicals from Thermo Fischer Scientific, Inc.). The amount of
total protein was measured in each sample by bicinchoninic acid
assay (BCA assay) technique and total protein concentration was
calculated according to the standard curve based on bovine serum
albumin (BSA) solutions of known protein concentration (Thermo
Fischer Scientific, Inc.). Proteins from cell lysates were
separated by means of electrophoresis in polyacrylamide gel in the
presence of ColorPlus Prestained Protein Marker (New England
Biolabs, Lab-Jot, Warsaw, Poland). 20 µg of total protein was
loaded into gel slots. After separation, proteins were right away
electroblotted onto PVDF membrane (Merck Millipore, Poznań,
Poland). Membranes were blocked by incubation in 3% bovine serum
albumin (BSA) solution in Tris-buffered saline (1X TBS) for two
hours and then membranes were placed in 3% BSA/1X TTBS (TBS
supplemented with 0,05% of Tween-20) containing one type of
antibody at a final dilution of 1:1,000, except for anti-GAPDH
antibody that was diluted to a greater extent (1:2,000).
Incubations were performed for 1 h at ambient temperature with
continuous rocking. Then, after two washes in TTBS for 10-min each,
an Anti-rabbit IgG (whole molecule)-peroxidase antibody (No. cat.
A0545, Sigma-Aldrich; Merck KGaA) was added (antibody dilution:
1:10,000 in 3% BSA/TTBS). Incubation was performed for one hour
under continuous rocking. Finally, after three washes (2X TTBS for
5 min. each and 1X TBS for 5 min.), a specific chemiluminescent
signal was developed (Pierce ECL Western Blotting Substrate, Thermo
Fischer Scientific, Inc.). After development, membranes were
digitalized using ChemiDoc-It Imaging System (Analytik Jena, Jena,
Germany). Measurements of integrated optical density representing
the amount of the protein of interest in a sample were done using
ImageJ software.
ELISA
Cell supernatants were sampled from cell culture
directly before cell lysis for protein analysis was performed
(1×105 cells per well). After removing from cell culture
plate, supernatants were aliquoted in 500 µl and frozen at −80°C
for ELISA analysis. The concentration of interleukin-1β in NHA cell
supernatant was estimated using Human IL-1β ELISA kit (Diaclone,
cat. no. 850.006, Immuniq)-according to the supplier's instructions
for use. The level of IL-1β showed the level of expression and
secretion of this cytokine to the culture media. Every sample was
analyzed in triplicate. The measurements of absorbance at the
wavelength of 450 nm were done using xMark Microplate Absorbance
Spectrophotometer (Bio-Rad Laboratories, Inc.). A second
measurement at a reference wavelength of 620 nm was done and this
value was subtracted from that of 450 nm.
Statistical analysis
The normality of distribution of data was evaluated
using Shapiro-Wilk's test. Afterwards data were analyzed using
one-way ANOVA test with post-hoc Tukey test and were reported as
mean ± SE. The P level below 0.05 was considered as
statistically significant.
Results
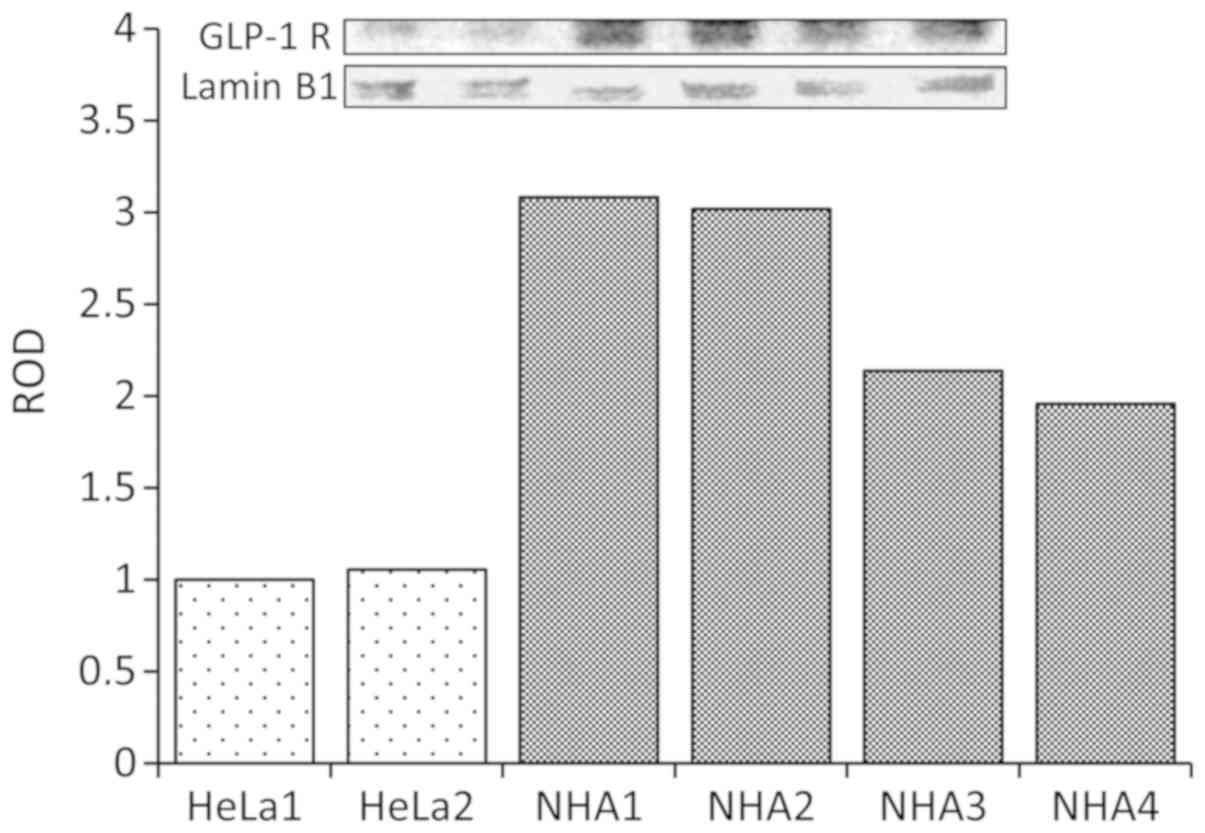
The expression of GLP-1R
The first objective of the study was to examine the
presence of potential targets of the therapy by confirming the
expression of GLP-1 receptors in NHA. The experiments showed that
these cells expressed substantial amount of GLP-1 receptors
(Fig. 1).
The viability of NHA in culture
conditions
In the next step of the study, the viability of
cells was assessed in all selected glycemic conditions and in the
absence or presence of exenatide in culture media. We estimated
that the viability of NHA in all culture conditions ranged between
98.76 and 108.7%. No statistically significant differences between
treatment groups were observed. Therefore, data were not presented
in the figure.
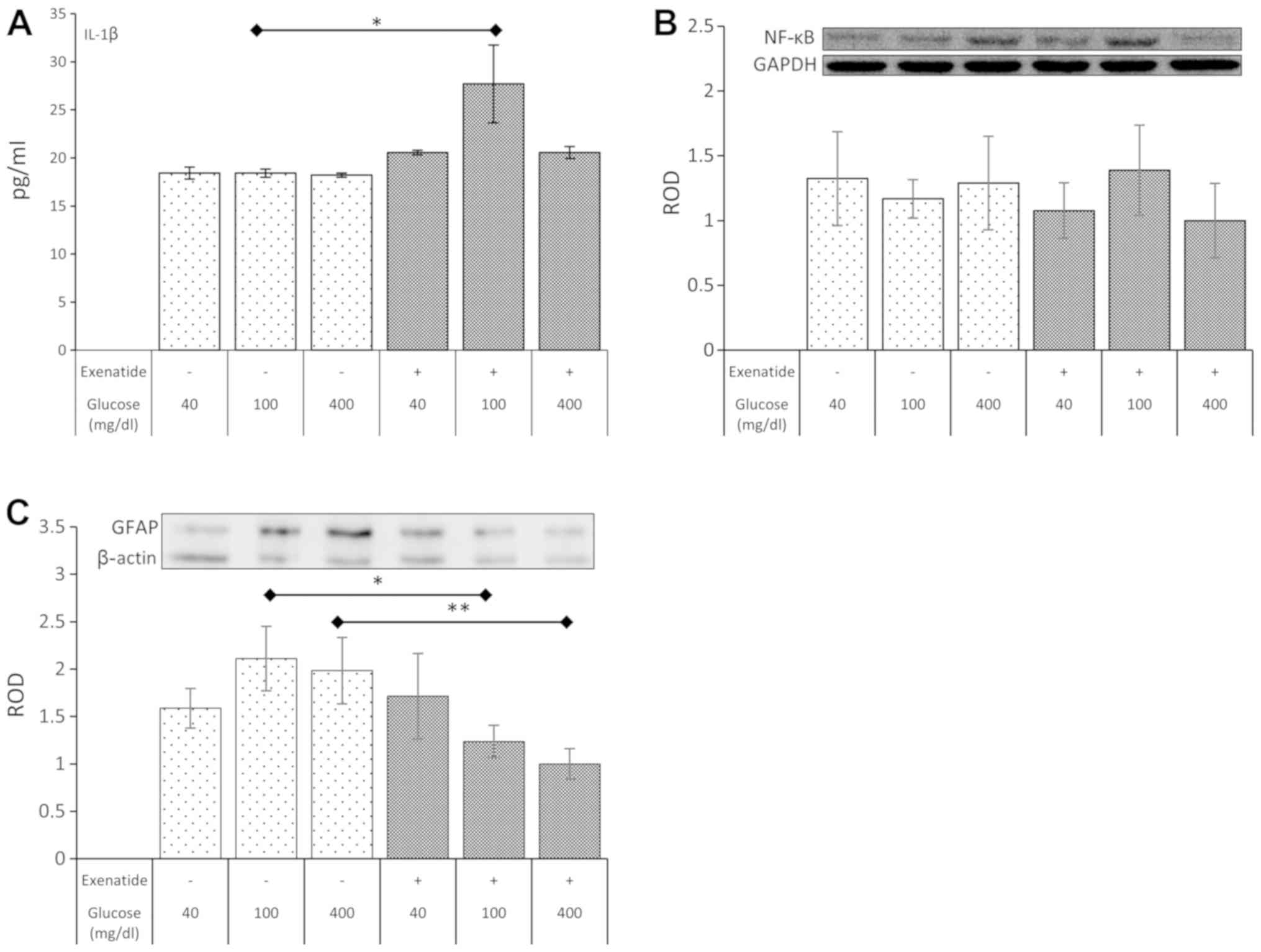
The impact of various glycemic
conditions and exenatide on the level of interleukin 1β (IL-1β) in
the culture medium
In the next step of the experiment, the impact of
selected glycemic conditions and exenatide on a marker of
inflammation (IL-1β) was estimated. The IL-1β level was not altered
in any of the selected glycemic conditions without exenatide.
However, exenatide led to a rise (51%; P=0.022) in the
concentration of IL-1β in normoglycemic cultures (Fig. 2A). The impact of the GLP-1 agonist in
hypo- and hyperglycemia was statistically insignificant.
The impact of various glycemic
conditions and exenatide on the expression of nuclear factor kappa
B (NFκB)
Despite the impact of exenatide on IL-1β levels the
expression of NFκB remained unaffected in all experimental
conditions (Fig. 2B).
The impact of various glycemic
conditions and exenatide on the expression of GFAP
The GFAP expression was not affected by different
glycemic culture conditions. However, a significant reduction in
the expression of GFAP was noted in cells exposed to exenatide
under normoglycemic (42%; P=0.014) and hyperglycemic (56%; P=0.005)
conditions. Exenatide failed to impact the expression of GFAP in
hypoglycemic conditions (Fig.
2C).
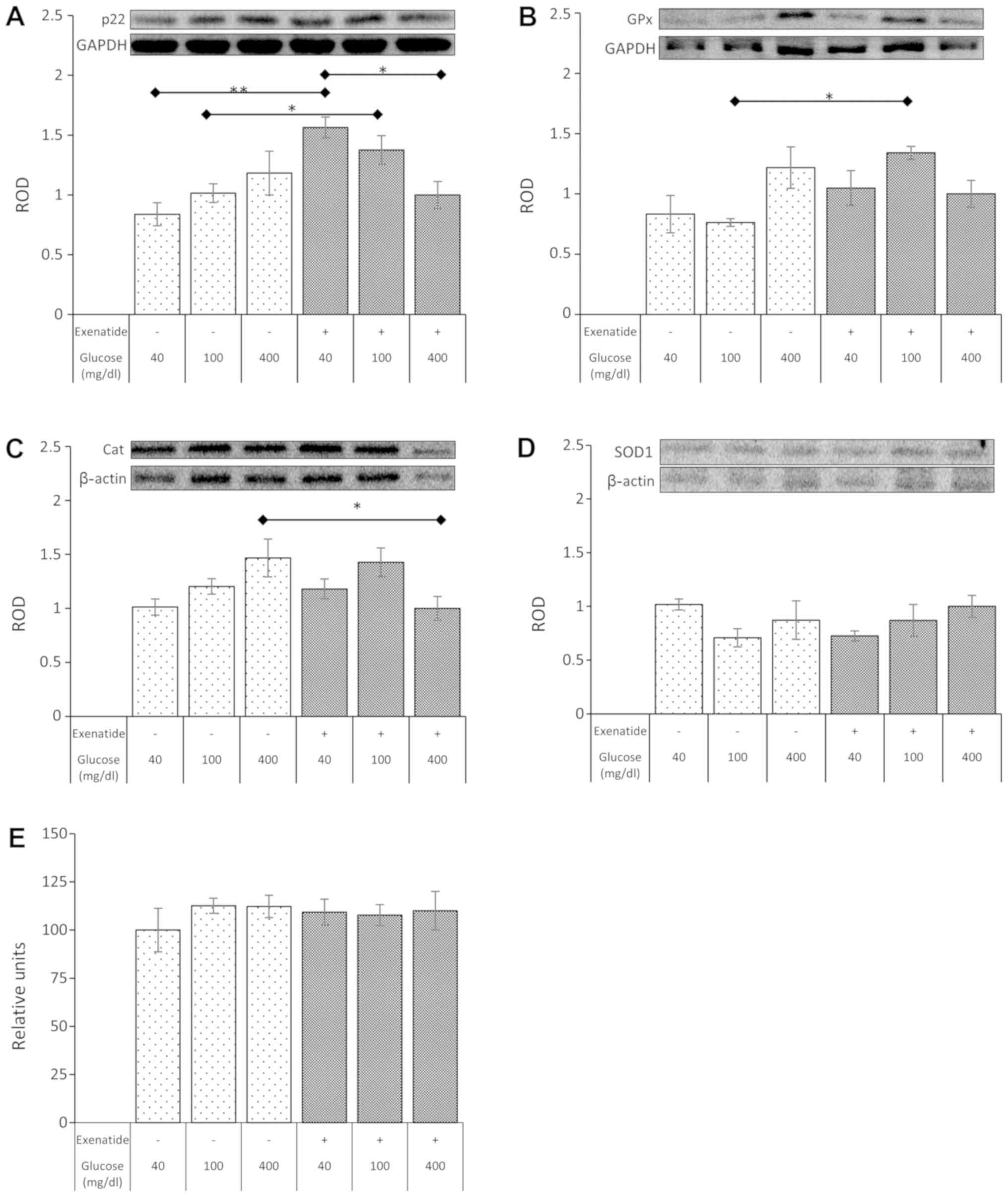
The impact of various glycemic
conditions and exenatide on the expression of NADPH oxidase
(p22)
In the next step of our study, a set of experiments
estimating the oxidative stress was performed. The first results
(without a GLP-1 agonist) showed a positive trend toward higher
expression of p22 that was concurrent with the increasing
concentration of glucose in the culture medium, but it was not
statistically significant. On the other hand, exenatide showed a
strong influence on the p22 expression. GLP-1 agonist nearly
doubled the expression of p22 in hypoglycemic conditions (94%
increase in p22 expression; P=0.001), while the impact on p22 in
normoglycemia resulted in an increase, reaching 47% (P=0.021). No
impact of exenatide was noted in hyperglycemic culture conditions
(Fig. 3A). Of note, the expression
of p22 in astrocytes in the presence of exenatide decreased with
increasing concentrations of glucose. The magnitude of the
reduction between hyperglycemia and hypoglycemia reached 36%
(P=0.011).
The impact of various glycemic
conditions and exenatide on the expression of glutathione
peroxidase (GPx)
Several other experiments focused on the expression
of proteins connected with the protection against oxidative stress.
Glutathione peroxidase expression was not affected by changes in
the glucose concentration without exenatide. However the GLP-1
agonist was able to significantly elevate the level of GPx (76%
increase; P=0.015) in NHA cultured in normoglycemic conditions.
However, the GPx expression after exposure to exenatide in
hyperglycemia and hypoglycemia remained unaffected (Fig. 3B).
The impact of various glycemic
conditions and exenatide on the expression of catalase (Cat)
Regardless of the concentration of glucose in
culture media, the expression of catalase, the second most
important antioxidative enzyme, did not change. However, exenatide
led to a surprising decrease (42%; P=0.022) in catalase expression
in NHA cultured under hyperglycemic conditions (Fig. 3C).
The impact of various glycemic
conditions and exenatide on the expression of superoxide dismutase
1 (SOD1)
Experiments on the expression of SOD1 did not show
any statistically significant impact of various glycemic
conditions, regardless of the presence or absence of exenatide
(Fig. 3D).
The impact of various glycemic
conditions and exenatide on the level of reactive oxygen species
(ROS)
Noteworthy, despite changes in the expression of p22
and antioxidative enzymes, experiments did not show any significant
influence of culture conditions on the amount of ROS generated by
NHA (Fig. 3E).
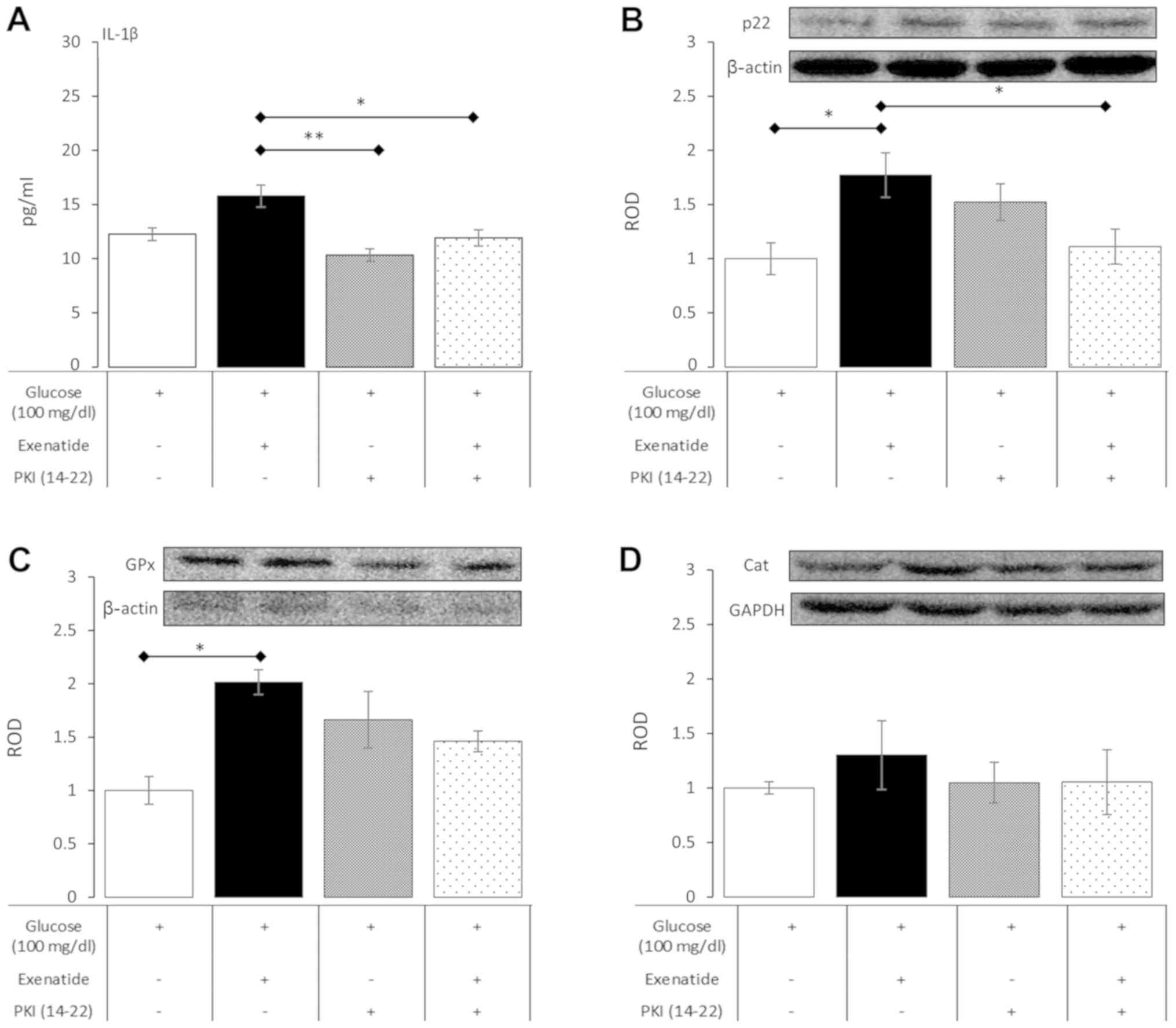
Experiments with protein kinase A
inhibition
The activation of protein kinase A is the main
mechanism of intracellular downstream signaling from GLP-1
receptors. Therefore, in order to explore whether alterations in
concentration of IL-1β and the expression of p22, catalase and
glutathione peroxidase were the result of GLP-1 activation, a set
of experiments with PKI (14–22) (a
pharmacological inhibitor of protein kinase A) was performed under
normoglycemic conditions. PKI (14–22), as
a sole addition to the culture medium, did not affect the measured
variables compared to control cultures, which were supplemented
only with glucose at a final concentration of 100 mg/dl. However, a
significant dampening of the influence of exenatide was noted when
PKI (14–22) was added to culture media (Fig. 4). In detail, PKI (14–22)
significantly reduced IL-1β concentration by 24% (P=0.047) in
exenatide-treated NHA, resulting in levels comparable to those seen
in control cultures. PKI (14–22) was
also able to inhibit p22 expression by 37% (P=0.048) in cells
treated with PKI (14–22) plus exenatide compared to cells
treated with exenatide alone. The impact of PKI (14–22) on
the effects of exenatide on GPx and catalase showed only a trend
between PKA activation and their expression, but this did not reach
statistical significance. These experiments showed that the impact
of exenatide on NHA relies to some extent on the activation of
protein kinase A, which is a major signaling transduction pathway
from GLP-1 receptors.
Discussion
Our study was focused on the assessment of
inflammatory status and redox potential of NHA subjected to hypo-,
normo- and hyperglycemia in the absence or presence of exenatide (a
GLP-1 agonist). According to our results, short-term exposure only
to varying glycemic conditions was not connected with changes in
the studied parameters of the NHA. Noteworthy, we noted increased
inflammatory activity (expressed by an elevated level of IL-1β in
culture media) in cells subjected to the treatment with exenatide
in normoglycemic conditions, which was accompanied by the reduced
expression of GFAP in normoglycemic and hyperglycemic conditions.
We also observed that exenatide affected redox status in NHA. There
was an increase in the expression of p22 in NHA cells treated with
exenatide in hypoglycemic and normoglycemic conditions. These
changes were accompanied by the increased expression of the
antioxidative enzyme GPx under normoglycemic conditions and a
decrease in Cat expression in cells subjected to both hyperglycemia
and exenatide. Finally, we confirmed that the observed effects were
partially reversible by a pharmacological inhibitor of PKA (PKI
14–22) in normoglycemic cultures.
The CNS inflammatory signaling has recently been
connected with the pathogenesis of obesity (29). For several years, it has been known
that astrocytes actively participate in the process of signal
modulation and transduction in the CNS. They gather inputs from the
bloodstream, transport particles through the BBB and convey their
signaling by the secretion of cytokines and changing local redox
status (30). According to our
results, varying the glucose concentration in the culture media did
not change the level of IL-1β secreted and did not affect NFκB
expression. However, we noted a remarkable increase in IL-1β level
in normoglycemic cultures treated with exenatide We believe that
the increased level of IL-1β might be a way to convey signals in
the CNS by astrocytes. The increased expression of IL-1β was
accompanied by a reduced glial activation in normoglycemic and
hyperglycemic condition, which was shown by a reduction in the GFAP
expression. Of note, we did not see a significant change in the
expression of NFκB under similar conditions. This observation seems
to be in contrast to previously published experiments with GLP-1
agonists in human macrophages. Human macrophages exposed to
exenatide expressed less IL-1β (31). However, the NFκB binding activity
remained unaffected in exenatide-treated macrophages (32). GLP-1 agonist reduced IL-1β expression
and inflammatory cytokine levels even more extensively in
macrophages that were concurrently subjected to lipopolysaccharide
(LPS). Similar results were observed in LPS-treated astrocytes,
showing a significant reduction in IL-1β levels in cells treated
with LPS (33). Moreover, in mice
receiving intrahippocampal LPS injection, a significant reduction
in NFκB and IL-1β was reported in the presence of exenatide
(21). However, others published
data showing that the GLP-1 agonist without strong proinflammatory
stimuli actually increased the expression of proinflammatory
cytokines (34). Therefore, it seems
that the discrepancy may be a result of a different cellular
response in severe inflammation (e.g. infection) and in
physiological conditions (which should be reflected in culture
conditions that is devoid of strong inflammatory stimuli). In our
study, which was conceived without strong proinflammatory stimulus,
we showed that exenatide increased the expression of IL-1β, which,
in paracrine way, may be responsible for altered feeding behaviors.
Previously, it was shown that IL-1β had anorexigenic properties and
could affect body weight at the hypothalamic level (35). Of note, we noted that exenatide had
the ability to elevate the level of IL-1β under normoglycemic
culture conditions, which is concordant with the extended
therapeutic indications of GLP-1 agonists to treat obesity even
without coexisting diabetes. Remarkably, in contrast to the
findings in animal-derived astrocytes, we did not observe a
concurrent rise in NFκB expression with IL-1β (36). However, there are several differences
between the experimental settings. Astrocytes in animal studies
were purified cells derived from mice brain samples. The culture
consisted of >95% astrocytes, but the remaining population might
contain highly reactive microglial cells that significantly altered
IL-1 expression due to their great ability to amplify IL-1β
expression on external stimuli (37). Furthermore, in the study by Wang
et al (36), it was
established that NFκB activation and subsequent IL-1β expression
was dependent on the coincident aggravated oxidative stress. In our
experiments on NHA, we did not observe any significant changes in
the ROS level, which may have prevented the activation of NFκB. The
possible explanation for an increase in IL-1β by exenatide is the
impact of the GLP-1 agonist on mitogen-associated protein kinases
(MAPKs) (38). MAPKs are downstream
kinases that are activated by PKA; in our experiments, we showed
that PKA inhibitor was able to reduce the IL-1β levels in the
culture media to levels comparable to those of control control.
Finally, it seems that not only interspecies differences may lead
to unexpected results. It was shown that the inflammatory
responsiveness of cells derived from patients with diabetes and
healthy volunteers differed significantly (39). IL-1β was very low in peripheral blood
mononuclear cells (PBMCs) that were derived from healthy population
and their response to GLP-1 receptor stimulation was negligible;
however, in cells from patients with diabetes (hyperglycemic), the
basal IL-1β level was high and strongly reduced by GLP-1 agonists.
This may be a peculiar type of a legacy effect that results in
varied responses to stimuli in different background conditions. The
issue of different response of NHA to exenatide in our experiments
is still not completely clear, but the strength of our experimental
setting and the use of commercially available human non-malignant
cell line should reduce potential confounders. We speculate that
the direct impact of exenatide on NHA IL-1β signaling may be at
least partly responsible for the mechanisms behind weight reduction
during GLP-1-based therapies, even without coexisting diabetes.
Furthermore we showed reduced astrogliosis, which seems to be
connected with beneficial effects in the treatment of diet-induced
obesity in animal models (40).
Diabetes and obesity are connected with increased
systemic and local oxidative stress (15). More importantly, endoplasmic
reticulum (ER) stress in astrocytes has been connected to several
diseases, including obesity (41).
At the cellular level, ER stress led to the increased generation of
ROS in mitochondria. Astrocytes may play a dual role in the
pathogenesis of obesity and diabetes. These cells may protect
neurons from hostile environment (42) or may become a source and transducer
of detrimental stimuli (43). Our
results showed that the short-term exposure of astrocytes to
varying glucose concentrations in culture media was not sufficient
to change the ROS level. On the other hand, exenatide in
hypoglycemic and normoglycemic conditions increased the expression
of p22, which is a major source of ROS. This change in the
expression of p22 might lead to a local increase in ROS generation.
However, it seems that exenatide action on astrocytes is
multifactorial, while it simultaneously affected the expression of
anti-oxidative enzymes (GPx in normoglycemic cultures and catalase
in hyperglycemic conditions). As a result of the above-mentioned
changes in protein expression, there have been no changes in the
level of ROS. These results might suggest that exenatide, in
normoglycemic conditions, affected the redox status by altering the
anti-oxidative capacity, expressed by the elevated GPx level. This
resulted in an unchanged level of ROS, despite a significant
increase in the expression of major ROS donor. ROS are considered
sensitizers of behavior, which may lead to over-nutrition (44). Recent studies show that exenatide may
have neuroprotective properties in an in vivo model of
neurodegenerative diabetic rats by a reduction of oxidative stress
(45). Excessive oxidative stress
was also abolished by exenatide in the ischemic model of cardiac
muscle injury (46). Noteworthy, it
was also noted that excessive oxidative stress that occurred in rat
model of cerebral stroke led to a significant reduction in the
level of GLP-1 receptors (47). This
loss might be at least partly compensated by the exogenously
administered exenatide. Those results show a significant impact of
exenatide on markers of oxidative stress in animal or in
vitro models of various acute injuries. On the other hand, we
performed a study that was deprived of strong inflammatory (e.g.
LPS, TNF alpha) or oxidative stressors (e.g.
H2O2). This was intentional, as such
experimental conditions may more accurately reflect naturally
occurring changes in the microenvironment. Our previous experiments
on human macrophages also failed to show a spectacular impact of
exenatide on ROS levels, while a parallel minute increase in p22
expression was noted (48). Only
after strong pro-inflammatory stimulation was a clear protective
effect of GLP-1 agonism against oxidative stress noted. In the
current experiment, we did not wish to expose astrocytes to
supraphysiological stimuli and also chose the NHA cell line instead
of animal or human neoplastic lines, which may be less or more
responsive than other cell lines. However, we consider that these
experimental conditions should more closely reflect naturally
occurring processes. While the lack of impact on the global ROS
level in our experimental setting may be surprising, it should be
kept in mind that local changes in redox status resulting from the
altered expression of NADPH oxidase and antioxidative enzymes may
also take part in cell signaling.
In summary, we have found that astrocytes were
susceptible to exenatide stimulation and that the net effect seemed
to rely on background glucose concentration. Exenatide
significantly increased the secretion of IL-1β in normoglycemic
conditions and reduced GFAP expression in normo- and hyperglycemic
cultures. The expression of p22 NADPH oxidase was also elevated in
normoglycemic and hypoglycemic conditions. Those alterations were
accompanied by changes in the expression of antioxidative enzymes
(GPx and Cat) under normoglycemic and hyperglycemic conditions. The
current findings showed some novel aspects of paracrine signaling
in normal human astrocytes exposed to exenatide in selected
glycemic condition. While it is tempting to attribute them as being
responsible for the impact on eating habits and reduced food
intake, further studies in different experimental conditions (e.g.
co-culture, in vivo settings) are warranted to explore such
an assumption.
The limitation of our study must also be taken into
account. The in vitro setting definitely does not reflect
the complexity of cellular interplay in the CNS and entire
organism. The relatively short-term culture in contrast to the
long-lasting burden of hyper- or hypoglycemia may have a slightly
different impact on astrocytes. Therefore further testing in a more
complex setting, as above-mentioned co-cultures, are vital to
attempt the transition of cellular into clinical research. However,
we believe that the use of human astrocytes and the
non-inflammatory culture conditions contribute substantially to the
currently available data.
Acknowledgements
Authors express their gratitude to Mrs. Jaroslawa
Sprada (Department of Pharmacology, School of Medicine in Katowice,
Medical University of Silesia, Katowice, Poland) for her expert
technical support.
Funding
The study was supported by research grant from
Medical University of Silesia (grant no. KNW-1-095/N/8/0).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ŁB conceived the study, performed data analysis, and
wrote the manuscript. GM conducted the in vitro cultures and
laboratory assays and reviewed the manuscript. ES conducted the
in vitro cultures and laboratory assays and reviewed
manuscript. AB analyzed data and reviewed manuscript. BO overviewed
the experiments and was responsible for the interpretation of data,
and critically revising the manuscript for important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Le Magueresse-Battistoni B, Labaronne E,
Vidal H and Naville D: Endocrine disrupting chemicals in mixture
and obesity, diabetes and related metabolic disorders. World J Biol
Chem. 8:108–119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaul S: Mitigating cardiovascular risk in
type 2 diabetes with antidiabetes drugs: A review of principal
cardiovascular outcome results of EMPA-REG OUTCOME, LEADER, and
SUSTAIN-6 trials. Diabetes Care. 40:821–831. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Andersen A, Lund A, Knop FK and Vilsbøll
T: Glucagon-like peptide 1 in health and disease. Nat Rev
Endocrinol. 14:390–403. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Santilli F, Simeone PG, Guagnano MT, Leo
M, Maccarone MT, Di Castelnuovo A, Sborgia C, Bonadonna RC,
Angelucci E, Federico V, et al: Effects of liraglutide on weight
loss, fat distribution, and β-cell function in obese subjects with
prediabetes or early type 2 diabetes. Diabetes Care. 40:1556–1564.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Astrup A, Carraro R, Finer N, Harper A,
Kunesova M, Lean ME, Niskanen L, Rasmussen MF, Rissanen A, Rössner
S, et al: Safety, tolerability and sustained weight loss over 2
years with the once-daily human GLP-1 analog, liraglutide. Int J
Obes (Lond). 36:843–854. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nuffer WA and Trujillo JM: Liraglutide: A
new option for the treatment of obesity. Pharmacotherapy.
35:926–934. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martin-Jiménez CA, Gaitán-Vaca DM,
Echeverria V, González J and Barreto GE: Relationship between
obesity, Alzheimer's disease, and Parkinson's disease: An
astrocentric view. Mol Neurobiol. 54:7096–7115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grill HJ and Hayes MR: The nucleus tractus
solitarius: A portal for visceral afferent signal processing,
energy status assessment and integration of their combined effects
on food intake. Int J Obes (Lond). 33 (Suppl 1):S11–S15. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Croft W, Dobson KL and Bellamy TC:
Plasticity of neuron-glial transmission: Equipping glia for
long-term integration of network activity. Neural Plast.
2015:7657922015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Z, Nelson AR, Betsholtz C and
Zlokovic BV: Establishment and dysfunction of the blood-brain
barrier. Cell. 163:1064–1078. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buckman LB, Thompson MM, Moreno HN and
Ellacott KL: Regional astrogliosis in the mouse hypothalamus in
response to obesity. J Comp Neurol. 521:1322–1333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moretti R, Pansiot J, Bettati D,
Strazielle N, Ghersi-Egea JF, Damante G, Fleiss B, Titomanlio L and
Gressens P: Blood-brain barrier dysfunction in disorders of the
developing brain. Front Neurosci. 9:402015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Git KC and Adan RH: Leptin resistance
in diet-induced obesity: The role of hypothalamic inflammation.
Obes Rev. 16:207–224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
García MC, Wernstedt I, Berndtsson A, Enge
M, Bell M, Hultgren O, Horn M, Ahrén B, Enerback S, Ohlsson C, et
al: Mature-onset obesity in interleukin-1 receptor I knockout mice.
Diabetes. 55:1205–1213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boyer F, Vidot JB, Dubourg AG, Rondeau P,
Essop MF and Bourdon E: Oxidative stress and adipocyte biology:
Focus on the role of AGEs. Oxid Med Cell Longev. 2015:5348732015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dossi E, Vasile F and Rouach N: Human
astrocytes in the diseased brain. Brain Res Bull. 136:139–156.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chowen JA, Argente-Arizón P,
Freire-Regatillo A, Frago LM, Horvath TL and Argente J: The role of
astrocytes in the hypothalamic response and adaptation to metabolic
signals. Prog Neurobiol. 144:68–87. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chowen JA, Argente J and Horvath TL:
Uncovering novel roles of nonneuronal cells in body weight
homeostasis and obesity. Endocrinology. 154:3001–3007. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thaler JP, Yi CX, Schur EA, Guyenet SJ,
Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, et
al: Obesity is associated with hypothalamic injury in rodents and
humans. J Clin Invest. 122:153–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Papouin T, Dunphy J, Tolman M, Foley JC
and Haydon PG: Astrocytic control of synaptic function. Philos
Trans R Soc Lond B Biol Sci. 372(pii): 201601542017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang HJ, Chen YH, Liang KC, Jheng YS,
Jhao JJ, Su MT, Lee-Chen GJ and Hsieh-Li HM: Exendin-4 protected
against cognitive dysfunction in hyperglycemic mice receiving an
intrahippocampal lipopolysaccharide injection. PLoS One.
7:e396562012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reiner DJ, Mietlicki-Baase EG, McGrath LE,
Zimmer DJ, Bence KK, Sousa GL, Konanur VR, Krawczyk J, Burk DH,
Kanoski SE, et al: Astrocytes regulate GLP-1 receptor-mediated
effects on energy balance. J Neurosci. 36:3531–3540. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gullo F, Ceriani M, D'Aloia A, Wanke E,
Constanti A, Costa B and Lecchi M: Plant polyphenols and exendin-4
prevent hyperactivity and TNF-α release in LPS-treated in vitro
neuron/astrocyte/microglial networks. Front Neurosci. 11:5002017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bao Y, Jiang L, Chen H, Zou J, Liu Z and
Shi Y: The neuroprotective effect of liraglutide is mediated by
glucagon-like peptide 1 receptor-mediated activation of
cAMP/PKA/CREB pathway. Cell Physiol Biochem. 36:2366–2378. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee YS and Jun HS: Anti-inflammatory
effects of GLP-1-based therapies beyond glucose control. Mediators
Inflamm. 2016:30946422016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vinet L, Lamprianou S, Babič A, Lange N,
Thorel F, Herrera PL, Montet X and Meda P: Targeting GLP-1
receptors for repeated magnetic resonance imaging differentiates
graded losses of pancreatic beta cells in mice. Diabetologia.
58:304–312. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Czekanska EM: Assessment of cell
proliferation with resazurin-based fluorescent dye. Methods Mol
Biol. 740:27–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nitti M, Furfaro AL, Traverso N, Odetti P,
Storace D, Cottalasso D, Pronzato MA, Marinari UM and Domenicotti
C: PKC delta and NADPH oxidase in AGE-induced neuronal death.
Neurosci Lett. 416:261–265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Douglass JD, Dorfman MD and Thaler JP:
Glia: Silent partners in energy homeostasis and obesity
pathogenesis. Diabetologia. 60:226–236. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Etchegoyen M, Nobile MH, Baez F,
Posesorski B, González J, Lago N, Milei J and Otero-Losada M:
Metabolic syndrome and neuroprotection. Front Neurosci. 12:1962018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bułdak Ł, Machnik G, Bułdak RJ, Łabuzek K,
Bołdys A, Belowski D, Basiak M and Okopień B: Exenatide (a GLP-1
agonist) expresses anti-inflammatory properties in cultured human
monocytes/macrophages in a protein kinase A and B/Akt manner.
Pharmacol Rep. 68:329–337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bułdak Ł, Machnik G, Bułdak RJ, Łabuzek K,
Bołdys A and Okopień B: Exenatide and metformin express their
anti-inflammatory effects on human monocytes/macrophages by the
attenuation of MAPKs and NFκB signaling. Naunyn Schmiedebergs Arch
Pharmacol. 389:1103–1115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iwai T, Ito S, Tanimitsu K, Udagawa S and
Oka J: Glucagon-like peptide-1 inhibits LPS-induced IL-1beta
production in cultured rat astrocytes. Neurosci Res. 55:352–360.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shirazi R, Palsdottir V, Collander J,
Anesten F, Vogel H, Langlet F, Jaschke A, Schürmann A, Prévot V,
Shao R, et al: Glucagon-like peptide 1 receptor induced suppression
of food intake, and body weight is mediated by central IL-1 and
IL-6. Proc Natl Acad Sci USA. 110:16199–16204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schéle E, Benrick A, Grahnemo L, Egecioglu
E, Anesten F, Pálsdóttir V and Jansson JO: Inter-relation between
interleukin (IL)-1, IL-6 and body fat regulating circuits of the
hypothalamic arcuate nucleus. J Neuroendocrinol. 25:580–589. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang J, Li G, Wang Z, Zhang X, Yao L, Wang
F, Liu S, Yin J, Ling EA, Wang L and Hao A: High glucose-induced
expression of inflammatory cytokines and reactive oxygen species in
cultured astrocytes. Neuroscience. 202:58–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Y, Biarnés Costa M and Gerhardinger C:
IL-1β is upregulated in the diabetic retina and retinal vessels:
cell-specific effect of high glucose and IL-1β autostimulation.
PLoS One. 7:e369492012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Varga ZV, Giricz Z, Liaudet L and Haskó G:
Ferdinandy P and Pacher P: Interplay of oxidative,
nitrosative/nitrative stress, inflammation, cell death and
autophagy in diabetic cardiomyopathy. Biochim Biophys Acta.
1852:232–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
He L, Wong CK, Cheung KK, Yau HC, Fu A,
Zhao HL, Leung KM, Kong AP, Wong GW, Chan PK, et al:
Anti-inflammatory effects of exendin-4, a glucagon-like peptide-1
analog, on human peripheral lymphocytes in patients with type 2
diabetes. J Diabetes Investig. 4:382–392. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nerurkar PV, Johns LM, Buesa LM, Kipyakwai
G, Volper E, Sato R, Shah P, Feher D, Williams PG and Nerurkar VR:
Momordica charantia (bitter melon) attenuates high-fat
diet-associated oxidative stress and neuroinflammation. J
Neuroinflammation. 8:642011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Martin-Jiménez CA, García-Vega Á, Cabezas
R, Aliev G, Echeverria V, González J and Barreto GE: Astrocytes and
endoplasmic reticulum stress: A bridge between obesity and
neurodegenerative diseases. Prog Neurobiol. 158:45–68. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tanaka J, Toku K, Zhang B, Ishihara K,
Sakanaka M and Maeda N: Astrocytes prevent neuronal death induced
by reactive oxygen and nitrogen species. Glia. 28:85–96. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Maragakis NJ and Rothstein JD: Mechanisms
of disease: Astrocytes in neurodegenerative disease. Nat Clin Pract
Neurol. 2:679–689. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jang EY, Ryu YH, Lee BH, Chang SC, Yeo MJ,
Kim SH, Folsom RJ, Schilaty ND, Kim KJ, Yang CH, et al: Involvement
of reactive oxygen species in cocaine-taking behaviors in rats.
Addict Biol. 20:663–675. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Abdelwahed OM, Tork OM, Gamal El Din MM,
Rashed L and Zickri M: Effect of glucagon-like peptide-1 analogue;
Exendin-4, on cognitive functions in type 2 diabetes mellitus;
possible modulation of brain derived neurotrophic factor and brain
Visfatin. Brain Res Bull. 139:67–80. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chang G, Liu J, Qin S, Jiang Y, Zhang P,
Yu H, Lu K, Zhang N, Cao L, Wang Y, et al: Cardioprotection by
exenatide: A novel mechanism via improving mitochondrial function
involving the GLP-1 receptor/cAMP/PKA pathway. Int J Mol Med.
41:1693–1703. 2018.PubMed/NCBI
|
|
47
|
Kim S, Jeong J, Jung HS, Kim B, Kim YE,
Lim DS, Kim SD and Song YS: Anti-inflammatory effect of glucagon
like peptide-1 receptor agonist, exendin-4, through modulation of
IB1/JIP1 expression and JNK signaling in stroke. Exp Neurobiol.
26:227–239. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bułdak Ł, Łabuzek K, Bułdak RJ, Machnik G,
Bołdys A and Okopień B: Exenatide (a GLP-1 agonist) improves the
antioxidative potential of in vitro cultured human
monocytes/macrophages. Naunyn Schmiedebergs Arch Pharmacol.
388:905–919. 2015. View Article : Google Scholar : PubMed/NCBI
|