Introduction
Malnutrition due to anorexia is one of the most
common problems associated with cancer and anti-cancer therapies.
Of all hospitalized cancer patients in Korea, >50% have
nutritional problems (1), and the
worldwide prevalence of nutrition-associated problems in cancer
patients displays similar patterns (2,3). For
instance, cachexia, a serious malnutrition condition featuring
significant irreversible weight loss in patients with serious
chronic health conditions, is associated with a poor prognosis,
particularly in terms of functional performance in daily life and
anti-cancer therapy tolerance (4).
Therefore, nutritional support and a proactive treatment strategies
for malnutrition itself and associated symptoms are necessary in
cancer care to improve clinical outcomes for cancer patients
(5).
Cancer-associated nutritional problems may have
various origins, and chemotherapy is one of the potential causes of
this condition, which is due to the negative effects on
gastrointestinal (GI) motility and appetite (4). Chemotherapy-induced nausea and vomiting
(CINV) as well as anorexia, which are important acute complications
during anti-cancer chemotherapy, involve various neurotransmitters
and metabolic peptides of the central nervous system (CNS) and GI
tract. Serotonin receptor antagonists and steroid medications,
which are the most frequently prescribed types of drug for this
condition, have been proven to be effective in decreasing acute
symptoms, but due to their high cost and limited capacity to
prevent late CINV, the use of these drugs is still under debate.
Hence, it is essential to identify and develop novel and diverse
pharmacological agents and non-drug interventions for CINV and
anorexia, which are to be used in future clinical practice
(6).
Acupuncture is a non-drug intervention that has been
used for managing various conditions in cancer patients. For
instance, it is used to reduce hot flush in breast cancer patients
(7), relieve various types of
cancer-associated pain, including post-operative and
malignancy-associated pain (8), as
well as to improve cancer-associated fatigue (9) and leukopenia (10). Regarding nutrition-associated
problems, clinical research has indicated that acupuncture may
reduce the frequency and severity of CINV (11). In a rat model, acupuncture
significantly reduced kaolin uptake, which is the most commonly
used alternative measure for evaluating nausea and vomiting.
Furthermore, acupuncture attenuated the decrease in food intake and
body weight due to cisplatin injection through inhibition of
duodenal serotonin secretion (12).
Although the validity of studies using acupuncture are under debate
due to issues including the suitability of placebo (sham) needles
as a control intervention and small effect sizes that do not simply
exceed non-specific (placebo) effects (13), the clinical effectiveness of
acupuncture for anorexia in cancer patients has been convincingly
demonstrated (14,15). However, the mechanisms underlying the
effects of acupuncture on chemotherapy-induced anorexia have
remained to be fully elucidated in an in vivo study.
Ghrelin and cholecystokinin (CCK) are two
representative GI hormones, which regulate feeding and may serve as
therapeutic targets for anorexia (16). The present study focused on the
changes of these two hormones that are mainly associated with
feeding regulation as opposed to other hormones, e.g. insulin and
leptin, which are involved in metabolic disorders. In the present
study, the anti-anorexigenic effects of acupuncture treatment and
changes in peptide hormone levels associated with anorexia were
assessed as a means of investigating the underlying mechanisms of
the efficacy of acupuncture in a rat model of cisplatin-induced
anorexia. Electroacupuncture (EA) is a specific type of
acupuncture, which stimulates acupuncture points with electric
current and is commonly used due to its ease of operation and
constant stimulation delivery. We adopted EA as the main
intervention for this study.
Materials and methods
Animals
In total, 32 male Wistar rats (age, 7 weeks; weight,
180–200 g) were obtained from Orient Bio Co., Ltd. (Seongnam,
Korea) and used for evaluating the beneficial effect of acupuncture
on chemotherapy-induced anorexia (CIA). Rats were housed at 23±2°C
and 55±5% humidity with a standard 12-h light/dark cycle, and were
given free access to water and a normal diet containing 10% fat for
a period of one week after arrival.
Study procedure
The present study comprised two experiments: In
Experiment 1, the point-specific effect of electroacupuncture (EA)
was assessed to determine the most effective among the potential
acupuncture points, including CV12, PC6 and ST36. In Experiment 2,
changes in the levels of appetite-associated peptides in the serum
and duodenal tissue were evaluated, and changes in c-Fos expression
in the brain were detected, in order to define a possible mechanism
of the effects of acupuncture. For Experiment 1, 20 rats were
randomly allocated into the following five groups according to the
acupuncture points/treatments: Normal saline control group with
acupuncture stimulation (n=4), cisplatin only control group without
acupuncture stimulation (n=4), CV12 EA group (n=4), PC6 EA group
(n=4) and ST36 EA group (n=4). The rats were stimulated daily with
EA four times in total, namely three times prior and once after
cisplatin administration. Rats were housed separately in metabolic
cages during the experimental period, and their body weight was
assessed daily. The changes of food and water intake, as well as
the amount of excreted urine, were also assessed on a daily basis.
According to the results from Experiment 1, EA at CV12 was more
effective compared with the other points in terms of its effect on
food intake and body weight. Thus, CV12 was selected for Experiment
2, which included two experimental groups, cisplatin + CV12 EA
(n=6) and cisplatin only control which received EA at a
non-acupoint, (n=6). Rats were sacrificed on day 3 after cisplatin
administration. The serum and tissue samples were collected and
immediately frozen and stored at −80°C until use.
Induction of anorexia
After adaptation, rats were randomly allocated into
different experimental groups. Animals were injected
intraperitoneally with 6 mg/kg of cisplatin dissolved in saline at
10 AM on day 0, as previously described (12).
Measurement of body weight and food
intake
For Experiment 1, acrylic metabolic cages (cat. no.
JD-C-66, Jeung Do Bio & Plant, Seoul, Korea) were used to
minimize animal stress during the daily assessment of individual
body weight, food intake, water intake and urine excretion
(17,18). The body weight and food intake of
individual rats was measured daily during the 7-day experimental
period. During each 24-h period, food intake was measured by
collecting, drying and weighing the remaining food in the food
container and any spilt in the cage. Body weight, food intake,
water intake and urine excretion measurements and acupuncture
stimulation were performed every day at ~10 am.
EA stimulation
Three acupuncture points, CV12, PC6 and ST36, were
selected based on a previous study and expert opinion (12). All acupuncture points were located
using the acupuncture map for rats based on a commonly followed
text book on acupuncture experiments (19). In the CV12 group, two points (a
center of the mid-line and a point 1 cm below) were selected. In
the PC6 group, the two points were 3 and 5 mm away from the wrist
between the radius and ulnar bones in the left anterior forearm. In
the ST36 group, the two points were 0.7 and 1.4 mm away from the
base of the patella along the tibia in the left anterior lower leg.
Stainless disposable acupuncture needles (0.25×40 mm; DongBang
Acupuncture Inc., Seoul, Korea) were inserted at a depth of 1–2 mm
on each point. Needles were stimulated for 10 min using an EA
system (ES-160; ITO Co. Ltd, Tokyo, Japan) with a low frequency (10
Hz) and low intensity (without muscle contractions). During EA
stimulation, anaesthesia was provided using a rodent inhalation
anaesthesia apparatus (Parkland Scientific, Coral Springs, FL,
USA), which was equipped with interchangeable vaporizers for
isoflurane (Hana Pharm. Co. Ltd., Hwasung, Korea). As carrier gas,
100% oxygen was used at a flow rate of 400 ml/min. The anaesthetic
gas was introduced into the nose mask through a thin tube. The
concentration of anaesthetic gas in the nose mask and in the
induction chamber was 2.8%.
Measurement of plasma ghrelin and
cholecystokinin levels
After sacrification, the collected plasma samples
were promptly centrifuged at 4°C and the supernatant was stored at
−80°C until use. The plasma ghrelin and cholecystokinin (CCK)
levels were determined using commercially available ELISA kits
(cat. nos. EK-069-04 and MM-402, Mitsubishi Kagaku Iatron, Inc.,
Tokyo, Japan), according to the manufacturer's protocol.
Expression of c-Fos in the nucleus
tractus solitarius (NTS)
Rat brains were freshly dissected and brain stem
tissue corresponding to the NTS cell group was obtained for
analysis (20). For western blot
analysis, tissues were homogenized in non-ionic detergent buffer
(150 mM NaCl, 1% Nonidet-P40, 1 mM EDTA, 5% glycerol and 25 mM
Tris-HCl, pH 7.5) with protease inhibitor cocktail (Roche, Basel,
Switzerland) for 20 min at 4°C. Lysates were centrifuged at 21,130
× g for 40 min at 4°C. Supernatant proteins were collected and
quantified using the Bradford protein assay (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and equal amounts of protein (30 µg/lane)
were separated via SDS-PAGE on a 8% gel. The separated proteins
were transferred onto polyvinylidene difluoride membranes (cat. no.
LC2002; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
blocked with Tris buffered-saline containing Tween™ 20
and 1% bovine serum albumin for 1 h at room temperature. The
membranes were subsequently incubated with c-Fos primary antibody
(1:500; cat. no. 2250) for 2 h at 25°C, followed by incubation with
horseradish peroxide (HRP)-conjugated secondary antibody (1:1,000;
cat. no. 7074; both Cell Signaling Technology, Inc., Danvers, MA,
USA) for 1 h at 25°C. Protein bands were visualized using the
Immobilon Western Chemiluminescent HRP Substrate (EMD Millipore,
Billerica, MA, USA) and analyzed using a luminescent image analyzer
(LAS-4000; GE Healthcare, Little Chalfont, UK). The relative
intensities of each band were measured using ImageJ software
(version 1.51n; National Institutes of Health, Bethesda, MD, USA).
GAPDH (1:1,000; cat. no. 5174; Cell Signaling Technology, Inc.) was
used as a loading control.
Measurement of 5-hydroxytryptamine
(5-HT) levels in duodenal tissue and plasma
Rat duodenal tissue samples were homogenized by
sonication in 0.4 M perchloric acid. The homogenate was kept on ice
for 20 min and then centrifuged at 21,130 × g for 20 min at 4°C.
Blood samples were obtained directly from the heart, added to a
tube containing EDTA and centrifuged at 1,500 × g for 10 min at
4°C. Subsequently, 3.6 µl of 70% perchloric acid was added to 100
µl of a supernatant plasma sample. The mixture was centrifuged at
3,000 × g for 10 min at 4°C. Supernatants were filtered through a
0.22-µm filter and the concentrations of 5-HT were measured using
high-performance liquid chromatography (HPLC) with electrochemical
detection, as described previously (21). The mobile phase consisted of 85 mM
citrate, 100 mM sodium acetate, 0.9 mM sodium octyl sulfate, 0.2 mM
EDTA and 12% methanol, pH 3.7. A total of 20 µl was injected into
an HPLC system fitted with a Nova-Pak C18 column (60Å; 3.9×150 mm;
4 µm; Waters Corporation, Milford, MA, USA) and electrochemical
detector (2465; Waters Corporation), and the 515 HPLC pump (Waters
Corporation) was operated at a constant flow rate of 1.0
ml/min.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the hypothalamus and
gastric tissues using the Tri-RNA reagent (Favorgen, Kaohsiung,
Taiwan), according to the manufacture's protocol. Complementary DNA
(cDNA) was synthesized using the RevertAid First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. qPCR was subsequently performed using the
PowerUp SYBR Green Master Mix (Thermo Fisher Scientific, Inc.),
with primer pairs for each analyte gene (Table I). qPCR was performed using a
Quantstudio 3 Real-Time PCR system (Thermo Fisher Scientific, Inc.)
using the following thermocycling conditions: Initial denaturation
at 95°C for 10 min; 45 cycles of 95°C for 3 sec, and the
appropriate annealing temperature (Table
I) for 30 sec. The relative mRNA expression levels were
quantified using the 2−ΔΔCq method and normalized to the
reference gene, β-actin (22).
 | Table I.Primer pairs for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer pairs for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′-3′) | Annealing
temperature (°C) |
|---|
| NGF | F:
TGACTCCAAGCACTGGAACTCAT | 60 |
|
| R:
GTTTGTCGTCTGTTGTCAACGC |
|
| BDNF | F:
GATGAGGACCAGAAGGTTCG | 65 |
|
| R:
GATTGGGTAGTTCGGCATTG |
|
| TNF-α | F:
AAATGGGCTCCCTCTCATCAGTTC | 58 |
|
| R:
TCTGCTTGGTGGTTTGCTACGAC |
|
| IL-6 | F:
TCAACTCCATCTGCCCTTCAG | 58 |
|
| R:
AAGGCAACTGGCTGGAAGTCT |
|
| IL-1β | F:
CACCTCTCAAGCAGAGCACAG | 60 |
|
| R:
GGGTTCCATGGTGAAGTCAAC |
|
| NPY | F:
TGTCTCAGGGCTGGATCTCT | 59 |
|
| R:
TACTCCGCTCTGCGACACTA |
|
| POMC | F:
GCTTCATGACCTCCGAGAAG | 59 |
|
| R:
TCTTGATGATGGCGTTCTTG |
|
| Ghrelin | F:
AGCCCAGCAGAGAAAGGAAT | 59 |
|
| R:
GTGGCTGCAGTTTAGCTGGT |
|
| β-actin | F:
AAGTCCCTCACCCTCCCAAAAG | 58 |
|
| R:
AAGCAATGCTGTCACCTTCCC |
|
Statistical analysis
All data, including body weight, food intake, water
intake, urine excretion, levels of serum ghrelin, CCK, 5-HT and
c-Fos expression were expressed as the mean ± standard error of the
mean which were normalized to the control group. Changes in body
weight, food intake, water intake and urine excretion were compared
prior to cisplatin injection and at days 1, 2, 3 and day 4 among
the different acupuncture point stimulation groups, with positive
values implying an increase in these parameters. As the
experimental groups only included a small number of animals, a
non-parametric analysis method was adopted for statistical
analysis. For Experiment 1, the Kruskal-Wallis test was used for
statistical analysis for each variable. Dunn's pairwise test with
Bonferroni adjustment was performed as a post-hoc test. For the
analysis of the results of Experiment 2, the Mann-Whitney U-test
was used for comparison of each variable between the CV12 EA and
control groups. The SPSS statistical package version 21 was used
for all analyses (IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Exploring the best conditions for the
EA stimulation: Selection of the most effective acupuncture
points
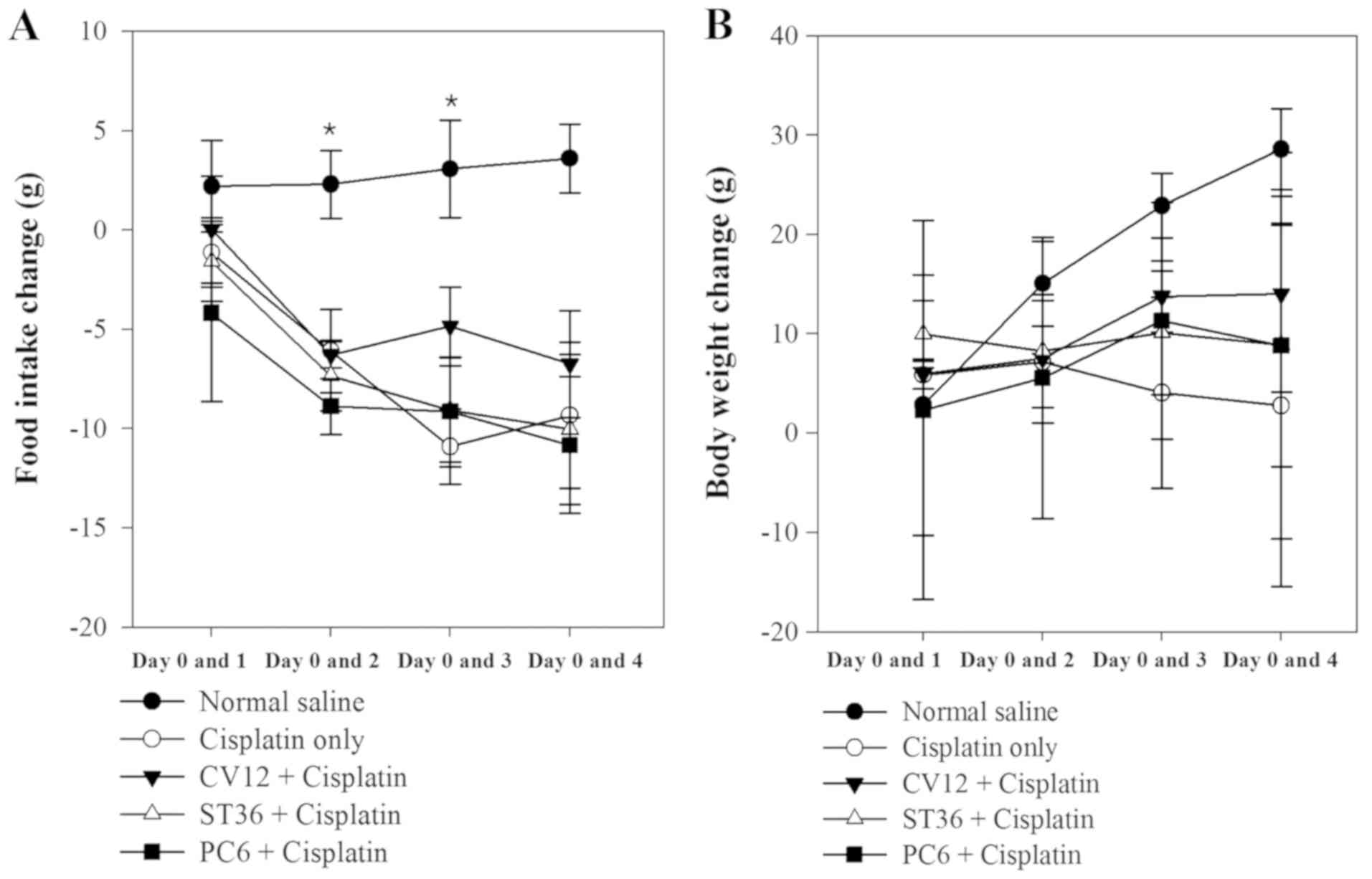
In Experiment 1, different acupuncture points were
revealed to exert different anti-anorexigenic effects, as indicated
by the body weight change and food intake of the animals. In the
normal saline group (untreated control), a progressive increase of
food intake was observed during the study. However, after cisplatin
injection, the food intake decreased rapidly in the EA groups and
the Cisplatin only group of rats. The decrease of food intake was
comparatively lower in the CV12 EA group between days 0 and 1
(Normal control, 2.2±2.3 g; Cisplatin group, −1.1±1.8 g; PC6,
−4.2±4.4 g; ST36, −1.6±2.0 g; CV12, 0.0±2.7; P=0.648). On days 2
and 3, the lowest decrease in food intake among the EA groups was
observed in the CV12 EA group and there was a statistically
significant difference (day 2 vs. day 0: Normal control, 2.3±1.7 g;
Cisplatin group, −6.1±2.1; PC6, −8.9±1.4; ST36, −7.3±1.8 g; CV12,
−6.3±0.7 g; P=0.032. Day 3 vs. day 0: Normal control, 3.1±2.5 g;
Cisplatin group, −10.9±1.9 g; PC6, −9.2±2.7 g; ST36, −9.1±2.6 g;
CV12, −4.9±2.0 g; P=0.019). At the final assessment on day 4, the
CV12 group also exhibited the lowest decrease in food intake
compared with that at day 0 among the EA groups, but there was no
significant difference (Normal control, 3.6±1.7; Cisplatin group,
−9.3±3.7 g; PC6, −10.8±3.4 g; ST36; −10.0±3.8 g; CV12, −6.8±2.7;
P=0.084).
As displayed in Fig.
1, the body weight increased significantly in the Normal
control group between Day 0 and 2 and Day 0 and 3, however, there
was an overall decrease in body weight following treatment with
Cisplatin. In all of the EA groups, the body weight increased
gradually, but the CV12 group exhibited the highest increase in
most assessments (day 1 vs. 0: Normal control, 2.9±6.6 g; Cisplatin
group, 5.9±0.7; PC6, 2.4±9.5 g; ST36, 10.0±1.7 g; CV12, 5.9±0.8;
P=0.389. Day 2 vs. 0: Normal control, 15.1±2.1 g; Cisplatin group,
7.2±3.1 g; PC6, 5.6±7.1 g; ST36, 8.3 vs. 2.9; CV12, 7.5±0.23 g
P=0.223. Day 3 vs. 0: Normal control, 22.9±1.6 g; Cisplatin group,
4.1±4.8 g; PC6, 11.3± 6.0 g; ST36, 10.1±3.1 g; CV12, 13.8±1.8 g;
P=0.63. Day 4 vs. 0: Normal control, 28.6±2.0 g; Cisplatin group,
2.8±9.1 g; PC6, 8.8±9.7 g; ST36, 8.9±6.1 g; CV12, 14.0±4.9;
P=0.156). Regarding the water intake and urine excretion, no
significant differences were identified between any of the groups
(Table II).
 | Table II.Changes in food intake, body weight,
water intake and urine excretion in Experiment 1 compared with day
0. |
Table II.
Changes in food intake, body weight,
water intake and urine excretion in Experiment 1 compared with day
0.
|
Parameter/group | Day 1 | Day 2 | Day 3 | Day 4 |
|---|
| Changes in food
intake (g) |
|
|
|
|
| Normal
control | 2.2±2.3 | 2.3±1.7 | 3.1±2.5 | 3.6±1.7 |
|
Cisplatin | −1.1±1.8 | −6.1±2.1 |
−10.9±1.9a | −9.3±3.7 |
| CV12
EA | 0±2.7 | −6.3±0.7 | −4.9±2.0 | −6.8±2.7 |
| ST36
EA | −1.6±2.0 | −7.3±1.8 | −9.1±2.6 | −10.0±3.8 |
| PC6
EA | −4.2±4.4 | −8.9±1.4 | −9.2±5.5 | −10.8±3.4 |
|
P-value | 0.648 | 0.032 | 0.019 | 0.084 |
| Changes in body
weight (g) |
|
|
|
|
| Normal
control | 2.8±6.6 | 15.0±2.1 | 22.9±1.6 | 28.6±2.0 |
|
Cisplatin | 5.9±6.9 | 7.2±3.1 | 4.1±4.8 | 2.8±9.1 |
| CV12
EA | 5.6±0.8 | 7.5±0.2 | 13.8±1.8 | 14.0±4.9 |
| ST36
EA | 10.0±1.7 | 8.3±2.9 | 10.1±3.1 | 8.9±6.1 |
| PC6
EA | 2.4±9.5 | 5.6±7.1 | 11.3±6.0 | 8.8±9.7 |
|
P-value | 0.389 | 0.223 | 0.063 | 0.156 |
| Changes in water
intake (ml) |
|
|
|
|
| Normal
control | −9.8±8.1 | 12.0±6.7 | −3.5±7.1 | −5.5±5.2 |
|
Cisplatin | 3.0±4.0 | −6.5±6.7 | −11.5±3.4 | −1.5±5.9 |
| CV12
EA | 3.8±6.8 | 4.0±4.8 | 3.0±5.6 | 3.5±5.6 |
| ST36
EA | −0.3±5.0 | −2.5±10.3 | −7.0±1.7 | −8.5±2.8 |
| PC6
EA | 1.0±9.4 | −10.0±4.5 | 1.5±7.6 | −9.0±4.0 |
|
P-value | 0.797 | 0.154 | 0.247 | 0.289 |
| Changes in urine
excretion (ml) |
|
|
|
|
| Normal
control | −2.6±1.2 | −2.6±1.2 | −2.9±1.1 | −1.1±1.4 |
|
Cisplatin | −2.0±1.8 | −2.0±1.8 | −3.4±1.5 | −1.1±1.7 |
| CV12
EA | 0.0±0.0 | 0.0±0.0 | 0.5±2.8 | 0.8±3.4 |
| ST36
EA | −0.6±0.4 | −0.6±0.4 | −1.2±2.1 | 3.5±2.5 |
| PC6
EA | −2.4±0.7 | −2.4±0.7 | 3.0±2.8 | 3.1±1.9 |
|
P-value | 0.213 | 0.213 | 0.316 | 0.363 |
Experiment 2: Effect of EA at CV12 on
the serum ghrelin and CCK levels in rats with cisplatin-induced
anorexia
According to Experiment 1, food intake decreased the
least in rats treated by EA at CV12 with an inhibition of normal
weight increase in cisplatin-induced anorexia compared with the
other acupuncture point stimulation groups (Table II). Therefore, the CV12 acupoint was
used to assess the effect of EA on the regulation of peptides that
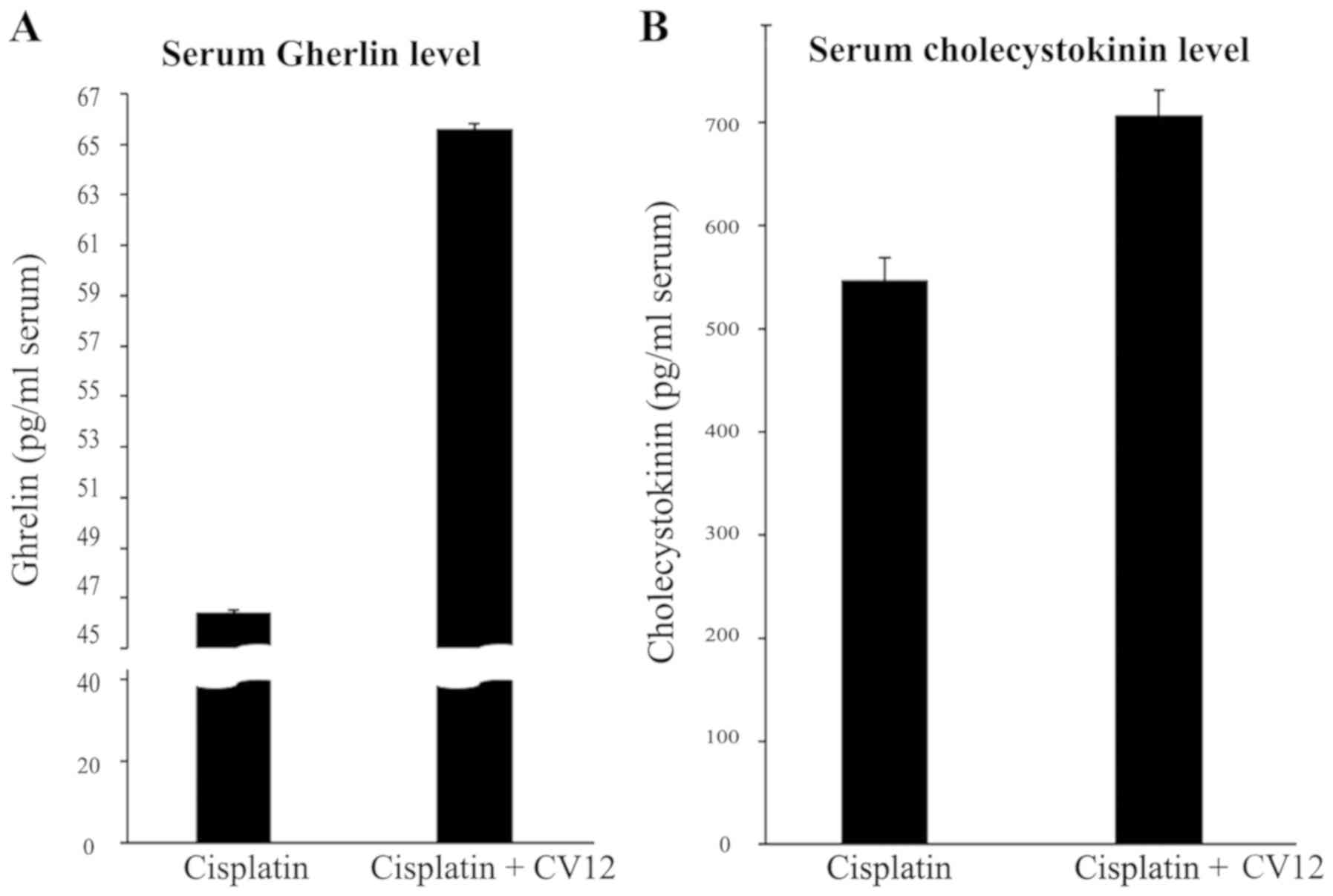
are closely associated with appetite. An increased level of serum
ghrelin was observed in the CV12 EA group compared with that in the
control group at day 3 (65.6±0.2 vs. 46.4±0.1 pg/ml; P=0.057), and
CCK exhibited a similar pattern (706.2±24.7 vs. 547.6±21.4 pg/ml;
P=0.057; Fig. 2). In addition,
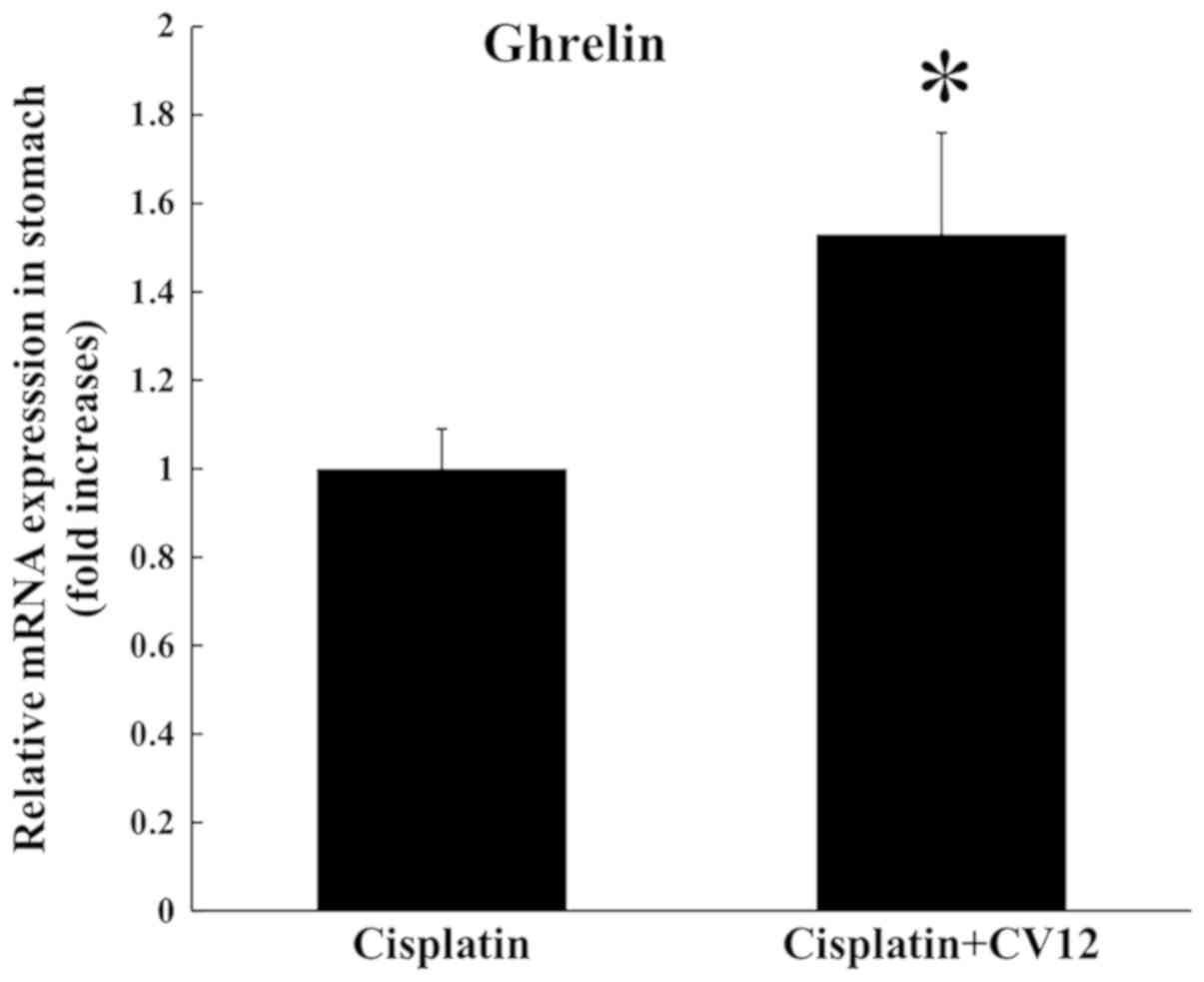
RT-qPCR analysis indicated that the expression of ghrelin mRNA in
the stomach was significantly increased in the CV12 EA group
compared with that in the control group (Fig. 3).
Duodenal and plasma 5-HT
concentration
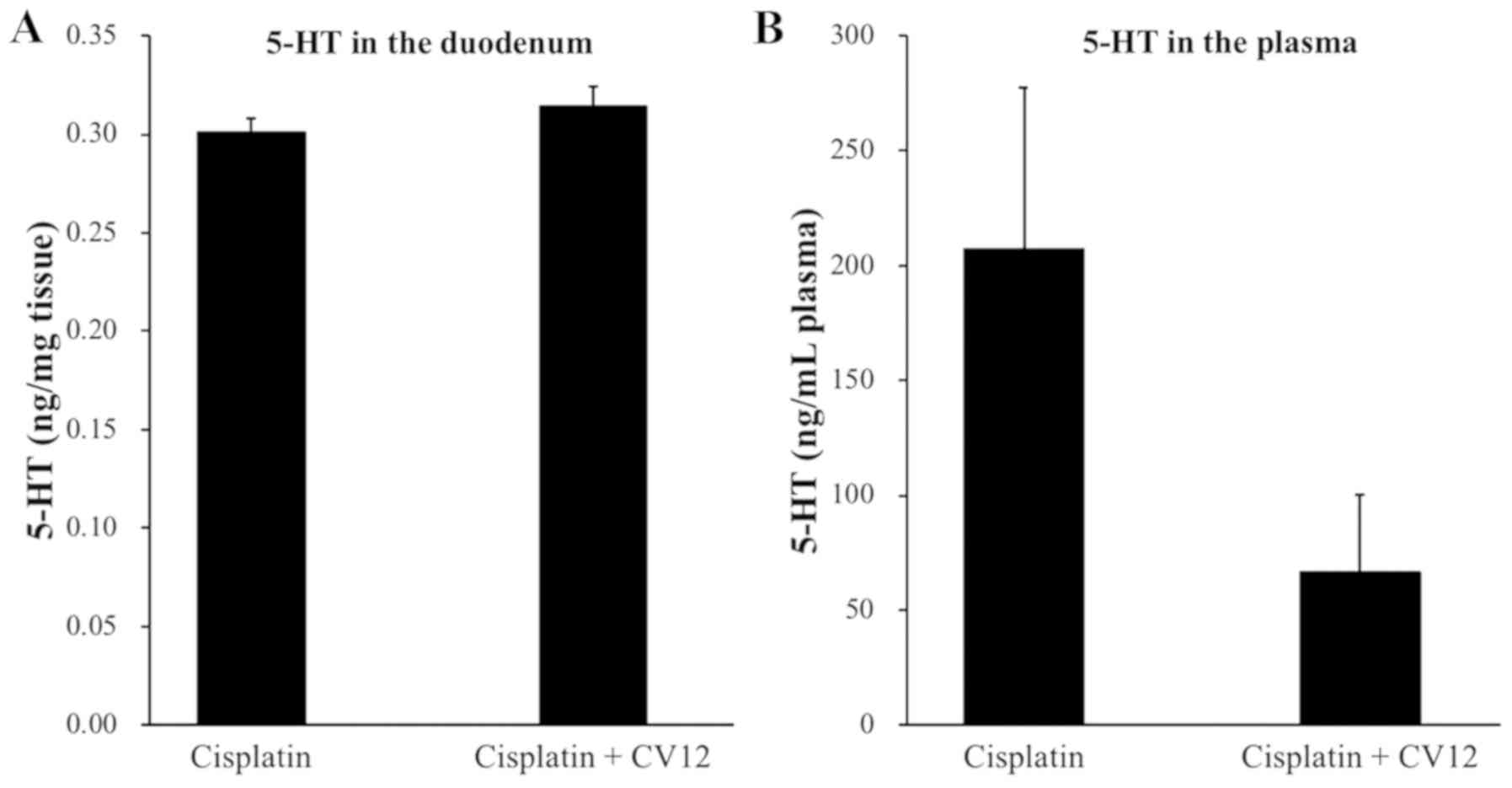
Regarding the secretion of duodenal 5-HT in rats,
the levels were undetectably low in the duodenum, which suggests
that the 5-HT concentration in the duodenum was not influenced by
the CV12 EA treatment. In addition, the plasma levels of 5-HT were
higher in the control group than that in the CV12 EA group, but the
difference was not statistically significant (207.3±70.1 vs.
66.3±33.4 ng/ml; P=0.132; Fig.
4).
mRNA expression of neurotropic factors
and cytokines in hypothalamus
Although there was no statistical significance, the
expression of NPY mRNA in the hypothalamus was increased in the
cisplatin + CV12 EA group compared with that in the cisplatin
group. However, the expression of POMC mRNA in the hypothalamus was
not significantly affected by CV12 EA compared with that in the
cisplatin group. Furthermore, the results indicated that the mRNA
expression levels of BDNF in the CV12 EA group were significantly
higher compared with those in the cisplatin only group, whereas
there were no changes in the mRNA expression levels of NGF.
Furthermore, the mRNA expression levels of cytokines, including
TNF-α, IL-1β and IL-6, were not significantly different between the
two groups (Fig. 5).
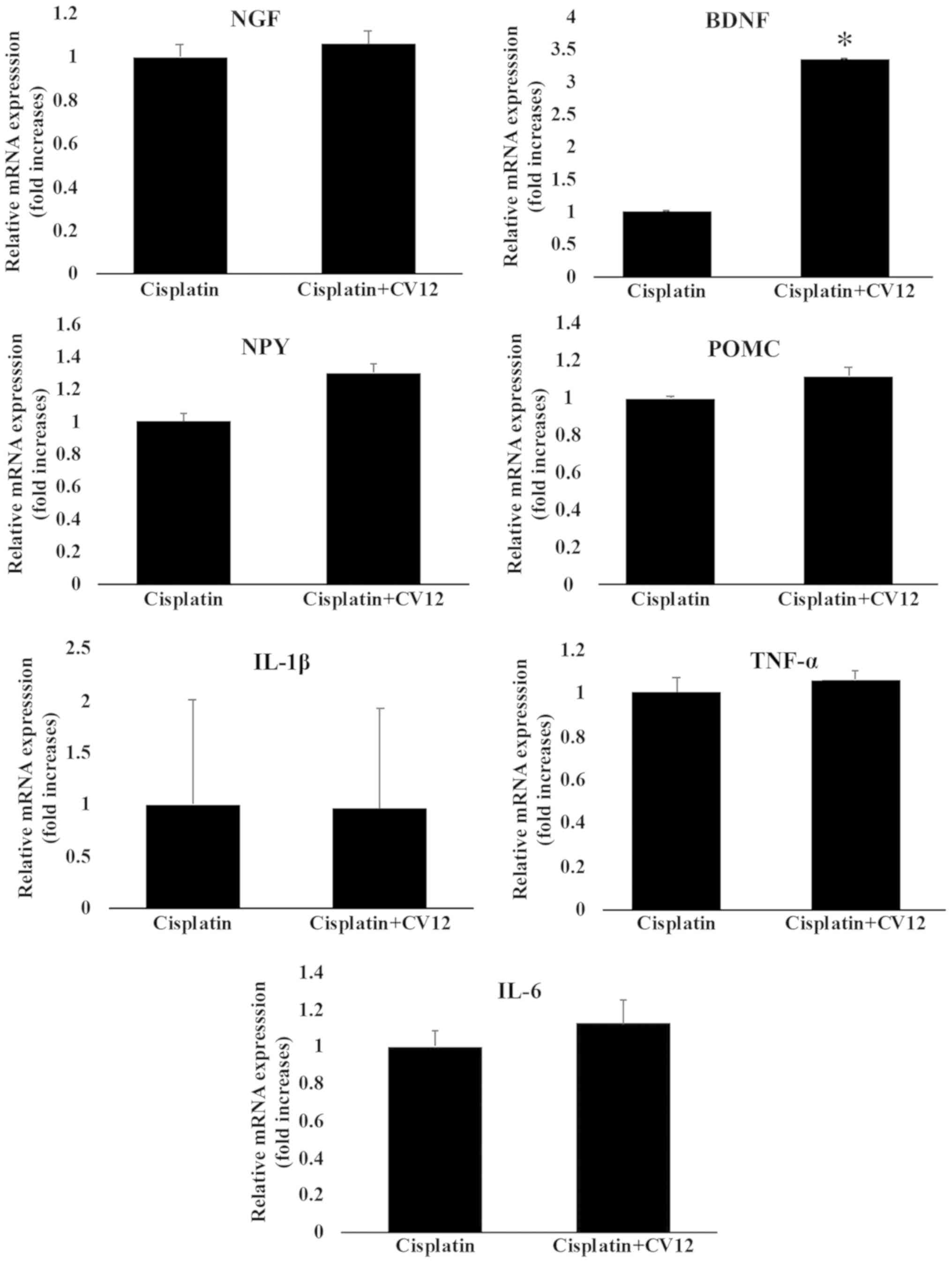 | Figure 5.mRNA expression of neurotropic
factors and cytokines in the hypothalamus. The mRNA expression of
neurotropic factors and cytokines in the hypothalamus, including
NGF, BDNF, TNF-α, IL-6, IL-1β, NPY and POMC were compared between
the Cisplatin + CV12 group and the Cisplatin group. The
Mann-Whitney U-test was used for comparison. Groups: Cisplatin,
control group in the cisplatin injection-induced anorexia rat
model; Cisplatin + CV12, CV12 electroacupuncture group in the
cisplatin injection-induced anorexia rat model. NGF, nerve growth
factor; BDNF, brain-derived neurotrophic factor; TNF-α, tumor
necrosis factor α; IL-6, interleukin 6; NPY, neuropeptide Y; POMC,
pro-opiomelanocortin. *P<0.05. |
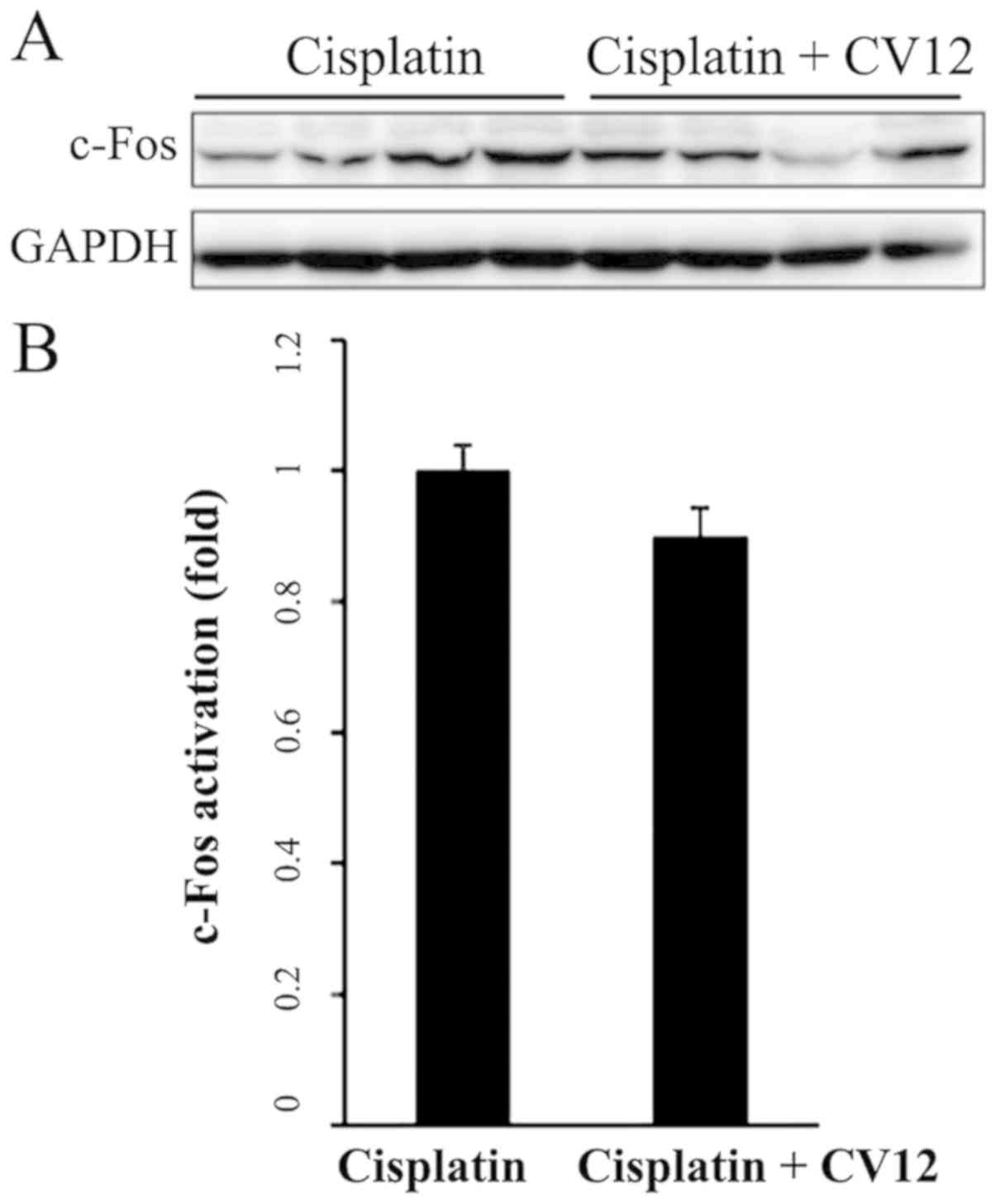
c-Fos expression in the NTS
Western blot analysis indicated that in the CV12 EA
group, the protein expression levels of c-Fos in the NTS were
downregulated compared with those in the control group; however,
this reduction was not statistically significant (Fig. 6).
Discussion
In the present study, the anti-anorexigenic effect
of EA was evaluated in a rat model of cisplatin-induced anorexia.
Among the potential acupuncture points, including CV12, PC6, and
ST36, CV12 was observed to be the most effective in preventing the
cisplatin-associated decrease in food intake and inhibition of body
weight increase. Assessment of the serum levels of ghrelin and CCK
revealed that compared with the controls, EA at CV12 enhanced the
secretion of ghrelin and CCK into the serum. Consistent with these
results, the mRNA expression of ghrelin in the stomach was also
increased by EA at CV12. These results suggest that EA treatment at
CV12 may reduce CIA through increasing the secretion of
anti-anorexigenic peptides, including ghrelin and CCK.
The present study has several advantages. First, the
point-specific effects of acupuncture were compared to reveal the
most effective stimulation points of EA. A total of 361 standard
acupuncture points and numerous Ashi-points are currently used in
clinical practice (23), and each
point is selected based on the location and point-specific effect
of acupuncture (24). CV12, PC6 and
ST36 are generally regarded as effective acupuncture points for
treating GI symptoms and diseases (25,26). In
addition to being used for treating various gastric symptoms, these
points are used to improve appetite in various populations
(27,28). In the present study, these possible
acupuncture points were tested and the effect of EA at these points
on promoting appetite and inhibiting weight loss were compared in a
rat model of cisplatin-induced anorexia. The present results were
consistent with those of a previous study (12). Furthermore, the underlying mechanisms
of the effects of EA on CIA were assessed. In common clinical
practice, 5-HT receptor antagonists are the most commonly
prescribed drugs for the prevention of CINV in cancer patients
(29). Previous clinical (30) and experimental studies (12) have typically focused on the
anti-emetic effect of acupuncture during chemotherapy in subjects
with cancer. 5-HT secretion and 5-HT receptor regulation, which are
key factors in nausea and vomiting, have also been a major focus of
acupuncture research (12). In the
present study, the serum levels of ghrelin and CCK, which are
closely associated with appetite, were evaluated, and it was
revealed that EA may increase the secretion of ghrelin and CCK in
rats with cisplatin-induced anorexia. EA also increased the
expression of ghrelin mRNA in stomach tissue of cisplatin-induced
anorexic rats. It may be hypothesized that EA exerts its effects to
reduce the problems in cancer patients undergoing chemotherapy
associated with intake of nutrition via multiple pathways. Finally,
the long-term effect of acupuncture (>2 days) was assessed,
which was not performed in the previous study (12). The present study indicated that
cisplatin significantly reduced the food intake and the increase in
body weight at 72 h after administration. To assess the subacute or
chronic anorexia associated with chemotherapy, longer-term studies
of >1 week may be necessary.
The present study also has several disadvantages
that should be addressed. First, short evaluation periods led to
significant setbacks due to lack of information on the long-term
effects of treatments, and in addition, the number of rats was
small. Furthermore, CINV has been a major focus of research on
chemotherapy, and thus, numerous studies have evaluated nausea and
vomiting in animal models. Pica behavior is observed in animal
models that experience environment- or therapy-triggered nausea
(31). Taking notice of this
phenomenon, researchers have begun to evaluate kaolin intake during
chemotherapy instead of nausea and vomiting in rats (32). In the present study, kaolin intake
was not assessed for evaluating nausea and vomiting in rats, as
this may rather be considered to be an alternative approach to
directly assess nausea and vomiting. In addition, the assessment of
kaolin intake may prohibit the precise measurement of food intake
due to the consumption of kaolin at the same time as food.
Furthermore, while a previous study assessed the effect of
multi-point stimulation (12), this
was not assessed in the present study. Acupuncture is generally
performed on multiple points at the same time. There are numerous
significant differences between single-point and multi-point
stimulation, including the aspect of the location of the
stimulation and the intensity; this may affect the possible
mechanism of the effects of acupuncture. To better reflect the
situation in clinical practice, a combination of different
acupuncture stimulation points should be evaluated in future
studies. In addition, the present study did not include any group
where rats were only kept without injection of normal saline and
acupuncture stimulation in order to evaluate whether single
acupuncture may influence food consumption or weight changes.
Instead, we included groups treated with cisplatin only and sham
acupuncture at non-acupuncture points as control groups to show the
potential effects of acupuncture. Future study is required with
inclusion of such a group for assessing the effect of acupuncture
stimulation on the feeding behaviour. In addition to this, EA at a
non-acupuncture point is also necessary to evaluate whether there
is an acupuncture point specific effect or not. Finally, most
comparisons of the present study did not indicate any statistically
significant differences between the groups, which may originate
from the small sample size per each group and the inappropriate
experimental conditions, including the frequency or intensity of EA
stimulation; indeed, these remain unsolved issues urging future
research.
The present study suggests that the mechanisms by
which acupuncture ameliorates CIA and CINV include the regulation
of 5-HT and CNS activation. Cisplatin and other chemotherapeutic
agents induce a mucosal injury of the intestines that includes
serotonin-producing cells (33), and
induce nausea and vomiting through signal transmission to the brain
emetic center via the vagus nerve to abruptly release excessive
5-HT (34). Based on the results of
the present and a previous study (12), EA at CV12 may affect 5-HT secretion
in the duodenum and plasma, and deactivate neuronal transmission in
the NTS, which may be evaluated through c-Fos protein expression as
a metabolic marker of neuronal activation (35,36).
These results suggest that acupuncture may exert an anti-emetic
effect through regulating the secretion of the nausea-triggering
peptide 5-HT and controlling the emetic center in the NTS through
afferent vagal stimulation.
NPY and POMC, which are abundant in hypothalamic
arcuate nucleus, have been reported to engage in the promotion or
inhibition of feeding, respectively (37). In the present study, the mRNA
expression of these two genes in the hypothalamus was evaluated. It
was indicated that EA-mediated appetite regulation is not
associated with the mRNA expression of NPY and POMC. BDNF has been
reported to be highly expressed in the ventromedial hypothalamus,
where it regulates energy homeostasis (37,38). In
the present study, the mRNA expression of BDNF exhibited a
significant increase compared with that in the control group.
Previously, glucose administration under fasting conditions
increased in the mRNA expression levels of BDNF consistently with
its role in satiety (38).
Furthermore, no changes in the mRNA expression levels of cytokines,
including TNF-α, IL-6 and IL-1β, were detected in the hypothalamus.
Therefore, the present results suggest that the EA-mediated
increase of BDNF mRNA expression in the hypothalamus may be a
crucial mechanism for the regulation of CIA, but the exact
mechanism involving NPY, POMC, NGF and BDNF should be further
evaluated in the future.
In the present study, chemotherapy-induced eating
anorexia and loss of appetite were examined. It was indicated that
acupuncture stimulation increased weight gain and food intake in an
animal model of CIA, and the present study reproduced the results
observed in a previous study (12).
Ghrelin is a multi-functional endogenous peptide that is important
for maintaining energy metabolism, and that affects the eating
behavior (36). CCK is secreted in
the duodenum for fat and protein digestion, and typically acts as a
hunger suppressor through inducing satiety and reducing food intake
(39). Plasma ghrelin levels are
decreased after cisplatin injection, which may account for the loss
of appetite in chemotherapy patients (40). However, little is known about the
effect of cisplatin injection on CCK levels. Serum ghrelin levels
were increased after CV12 EA treatment (12), and a similar increase in CCK levels
was also observed in the present study. These results suggest that
the beneficial effect of CV12 EA on cisplatin-induced anorexia is
mediated via humoral appetite regulation through central mechanisms
involving POMC and NPY as well as peripheral mechanisms involving
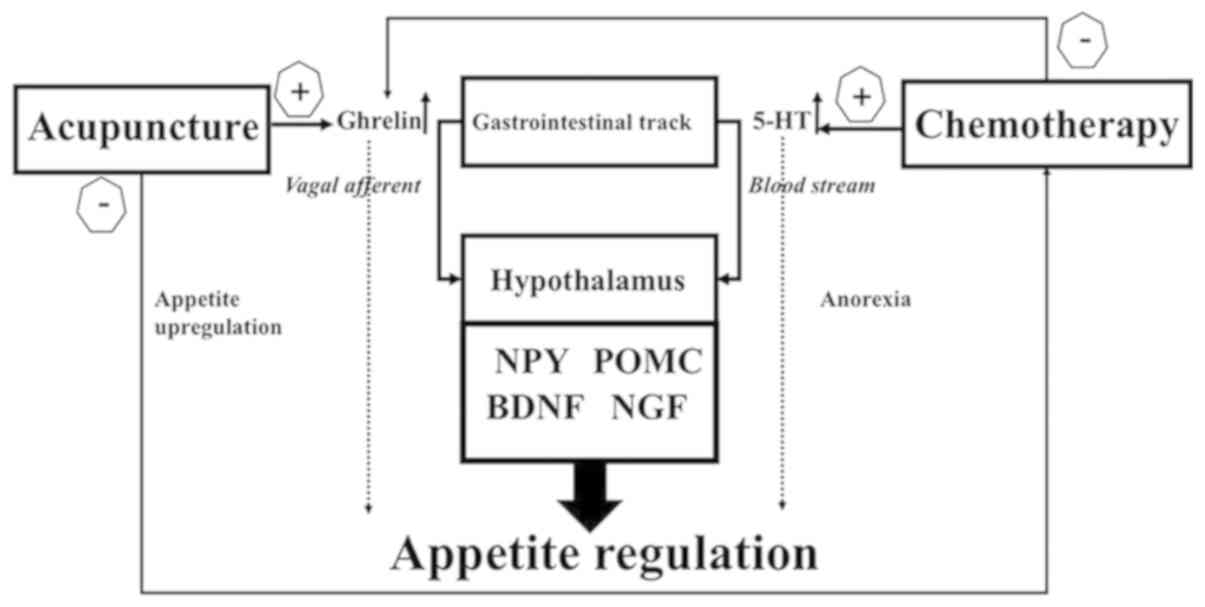
5-HT, ghrelin and CCK as illustrated in Fig. 7. In the future, more research is
required to elucidate the mechanisms underlying the effect of
acupuncture.
In conclusion, CV12 EA may improve the appetite by
increasing the secretion of serum ghrelin and CCK, in addition to
exerting an anti-emetic effect through regulation of 5-HT secretion
and vagal nerve activation in cisplatin-induced anorexic rats.
Nutrition-associated problems caused by chemotherapy require to be
addressed using a multidirectional approach, and acupuncture may be
considered as one of the potential complementary therapies for this
condition.
Acknowledgements
Not applicable.
Funding
The current study was supported by a grant from the
Korea Health Technology R&D Project through the Korea Health
Industry Development Institute, funded by the Ministry of Health
& Welfare, Republic of Korea (grant no. HI15C0089).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KSK, JWK and THK contributed to the concept and
design of the study. KSK, WH, YB, HJC, JYB, JHS, JWK and THK
performed the experiments. KSK, JWK and THK analyzed the data. KSK
and THK prepared the first draft and revised the manuscript. All
authors read and approved the final manuscript.
Ethical approval and consent to
participate
All procedures involving the use of live animals
described in the present study were approved by the Institutional
Animal Care and Use Committee of Gachon University (Seongnam,
Korea; approval no. GIACUC-R2015011) in December 2015. In addition,
animal experimentation was performed in strict accordance with the
National Institutes of Health Guide for the Care and Use of
Experimental Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wie GA, Cho YA, Kim SY, Kim SM, Bae JM and
Joung H: Prevalence and risk factors of malnutrition among cancer
patients according to tumor location and stage in the national
cancer center in Korea. Nutrition. 26:263–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pressoir M, Desné S, Berchery D, Rossignol
G, Poiree B, Meslier M, Traversier S, Vittot M, Simon M, Gekiere
JP, et al: Prevalence, risk factors and clinical implications of
malnutrition in french comprehensive cancer centres. Br J Cancer.
102:966–971. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Segura A, Pardo J, Jara C, Zugazabeitia L,
Carulla J, de Las Peñas R, García-Cabrera E, Luz Azuara M, Casadó J
and Gómez-Candela C: An epidemiological evaluation of the
prevalence of malnutrition in Spanish patients with locally
advanced or metastatic cancer. Clin Nutr. 24:801–814. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pender A, Moocraft SY and Lee DLY: Side
effects and complications of cancer and its treatment. In: Clinical
Problems in Oncology: A Practical Guide to Management. (1st).
Wiley-Blackwell. (Chichester, UK). 277–286. 2014.
|
|
5
|
von Meyenfeldt M: Cancer-associated
malnutrition: An introduction. Eur J Oncol Nurs. 9 (Suppl
9):S35–S38. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Navari RM: Pharmacological management of
chemotherapy- induced nausea and vomiting. Focus on recent
developments. Drugs. 69:515–533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen YP, Liu T, Peng YY, Wang YP, Chen H,
Fan YF and Zhang L: Acupuncture for hot flashes in women with
breast cancer: A systematic review. J Cancer Res Ther. 12:535–542.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiu HY, Hsieh YJ and Tsai PS: Systematic
review and meta-analysis of acupuncture to reduce cancer-related
pain. Eur J Cancer Care (Engl). 26:124572017. View Article : Google Scholar
|
|
9
|
Zeng Y, Luo T, Finnegan-John J and Cheng
AS: Meta-analysis of randomized controlled trials of acupuncture
for cancer-related fatigue. Integr Cancer Ther. 13:193–200. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu W, Hu D, Dean-Clower E, Doherty-Gilman
A, Legedza AT, Lee H, Matulonis U and Rosenthal DS: Acupuncture for
chemotherapy-induced leukopenia: Exploratory meta-analysis of
randomized controlled trials. J Soc Integr Oncol. 5:1–10. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu X, Chung VC, Hui EP, Ziea ET, Ng BF, Ho
RS, Tsoi KK, Wong SY and Wu JC: Effectiveness of acupuncture and
related therapies for palliative care of cancer: Overview of
systematic reviews. Sci Rep. 5:167762015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cui Y, Wang L, Shi G, Liu L, Pei P and Guo
J: Electroacupuncture alleviates cisplatin-induced nausea in rats.
Acupunct Med. 34:120–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim TH, Kang JW and Lee MS: What is lost
in the acupuncture trial when using a sham intervention? Acupunct
Med. 35:384–386. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeon JH, Yoon J, Cho CK, Jung IC, Kim S,
Lee SH and Yoo HS: Effect of acupuncture for
radioactive-iodine-induced anorexia in thyroid cancer patients: A
randomized, double-blinded, sham-controlled pilot study. Integr
Cancer Ther. 14:221–230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoon SL, Grundmann O, Williams JJ and
Carriere G: Novel intervention with acupuncture for anorexia and
cachexia in patients with gastrointestinal tract cancers: A
feasibility study. Oncol Nurs Forum. 42:E102–E109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Date Y, Toshinai K, Koda S, Miyazato M,
Shimbara T, Tsuruta T, Niijima A, Kangawa K and Nakazato M:
Peripheral interaction of ghrelin with cholecystokinin on feeding
regulation. Endocrinology. 146:3518–3525. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ecelbarger CA, Sands JM, Doran JJ, Cacini
W and Kishore BK: Expression of salt and urea transporters in rat
kidney during cisplatin-induced polyuria. Kidney Int. 60:2274–2282.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Borner T, Loi L, Pietra C, Giuliano C,
Lutz TA and Riediger T: The ghrelin receptor agonist HM01 mimics
the neuronal effects of ghrelin in the arcuate nucleus and
attenuates anorexia-cachexia syndrome in tumor-bearing rats. Am J
Physiol Regul Integr Comp Physiol. 311:R89–R96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Z: Experimental acupuncture science.
Beijing: China Press of Traditional Chinese Medicine.
327:1462003.
|
|
20
|
Zhou D, Wan Y, Xie D, Wang Y, Wei J, Yan
Q, Lu P, Mo L, Xie J, Yang S and Qi X: DNMT1 mediates
chemosensitivity by reducing methylation of miRNA-20a promoter in
glioma cells. Exp Mol Med. 47:e1822015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tianzhu Z, Shihai Y and Juan D:
Antidepressant-like effects of cordycepin in a mice model of
chronic unpredictable mild stress. Evid Based Complement Alternat
Med. 2014:438562014. View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
WHO standard acupuncture point locations
in the Western Pacific 106 Region. World Health Organization,
Geneva. 67158, 229. 2008.
|
|
24
|
Choi EM, Jiang F and Longhurst JC: Point
specificity in acupuncture. Chin Med. 7:42012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takahashi T: Acupuncture for functional
gastrointestinal disorders. J Gastroenterol. 41:408–417. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jinsheng H: Acupuncture treatment of
vomiting. J Traditional Chin Med. 28:75–78. 2008.(In Chinese).
View Article : Google Scholar
|
|
27
|
Kim HY, Seong WY and Kim KB: A literature
study on treatment of infantile anorexia based on Chinese medical
journals. J Korean Oriental Pediatrics. 27:87–98. 2013. View Article : Google Scholar
|
|
28
|
Yoveline A, Abdullah M, Darmawan G,
Mihardja H and Sungkar S: Acupuncture in the management of
functional dyspepsia. The Indonesian Journal of Gastroenterology,
Hepatology, and Digestive Endoscopy. 13:pp. 49–55. 2012, https://media.neliti.com/media/publications/67584-ID-none.pdf
|
|
29
|
Billio A, Morello E and Clarke MJ:
Serotonin receptor antagonists for highly emetogenic chemotherapy
in adults. Cochrane Database Syst Rev. 20:CD0062722010.
|
|
30
|
Ezzo JM, Richardson MA, Vickers A, Allen
C, Dibble SL, Issell BF, Lao L, Pearl M, Ramirez G, Roscoe J, et
al: Acupuncture-point stimulation for chemotherapy-induced nausea
or vomiting. Cochrane Database Syst Rev. 19:CD0022852006.
|
|
31
|
Nakajima S and Katayama T: Running-based
pica in rats. Evidence for the gastrointestinal discomfort
hypothesis of running-based taste aversion. Appetite. 83:178–184.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saito R and Takano Y: Easy method for
emesis using rats. Nihon Yakurigaku Zasshi. 127:461–466. 2006.(In
Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Scarantino CW, Ornitz RD, Hoffman LG and
Anderson RF Jr: On the mechanism of radiation-induced emesis: The
role of serotonin. Int J Radiat Oncol Biol Physics. 30:825–830.
1994. View Article : Google Scholar
|
|
34
|
Andrews PL and Sanger GJ: Abdominal vagal
afferent neurones: An important target for the treatment of
gastrointestinal dysfunction. Curr Opin Pharmacol. 2:650–656. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yuan SY, Vilimas P, Zagorodnyuk VP and
Gibbins IL: Novel spinal pathways identified by neuronal c-Fos
expression after urethrogenital reflex activation in female guinea
pigs. Neuroscience. 288:37–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Holland RA, Leonard JJ, Kensey NA,
Hannikainen PA and De Jonghe BC: Cisplatin induces neuronal
activation and increases central AMPA and NMDA receptor subunit
gene expression in mice. Physiol Behav. 136:79–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rios M: BDNF and the central control of
feeding: Accidental bystander or essential player? Trends Neurosci.
36:83–90. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Unger TJ, Calderon GA, Bradley LC,
Sena-Esteves M and Rios M: Selective deletion of Bdnf in the
ventromedial and dorsomedial hypothalamus of adult mice results in
hyperphagic behavior and obesity. J Neurosci. 27:14265–14274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Moran TH, Norgren R, Crosby RJ and McHugh
PR: Central and peripheral vagal transport of cholecystokinin
binding sites occurs in afferent fibers. Brain Res. 526:95–102.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Takeda H, Sadakane C, Hattori T, Katsurada
T, Ohkawara T, Nagai K and Asaka M: Rikkunshito, an herbal
medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2
receptor antagonism. Gastroenterology. 134:2004–2013. 2008.
View Article : Google Scholar : PubMed/NCBI
|