Introduction
Smoking cessation alleviates diseases caused by
smoking (1,2), and the reduction of oxidative damage is
one expected benefit (3). Past
intervention studies reported that smoking cessation yielded
reduced levels of urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) and
8-iso-prostaglandin F2α (8-isoprostane) (3,4). 8-OHdG
reflects the oxidative damage and repair of DNA (5), and 8-isoprostane is a marker of
oxidative stress and lipid peroxidation (6,7). Unlike
selected subjects in experimental studies, general smokers have
varying characteristics; thus, the change in the levels of
oxidative stress in conjunction with the decreasing cotinine level
may be different. In addition, studying interactions between
physical and nutrient factors and smoking cessation against
oxidative stress levels would be of interest to researchers in the
field of cigarette smoking. For instance, an interaction between
cigarette smoking and nutrient supplementation against cancer and
hemorrhagic stroke was previously reported (8). Yet information on the interaction,
including differences in the changes in oxidative stress levels
according to dietary food or nutrient intake during smoking
cessation, is limited. Likewise, not much has been reported about
whether the changes in oxidative stress levels during smoking
cessation differ according to the differences in physical factors
such as sex, age, and BMI. The cross-sectional associations of food
or nutrient intake and physical factors with oxidative stress
levels have been reported (9–12).
However, whether these factors interact with smoking cessation and
influence the changes in oxidative stress levels is unknown. The
aim of the current study was to prospectively record the two-week
change in the levels of oxidative stress for general individuals
who received smoking cessation treatment. We further analyzed
whether physical and nutritional factors influenced the two-week
changes in the levels of oxidative stress.
Patients and methods
Study participants
This was a two-week study that observed patients
starting smoking cessation treatment with varenicline, which has
been approved for use in Japan since 2008 (13). Individuals who met the criteria of
the smoking cessation treatment guidelines were included in the
current study (13), namely, smokers
who were willing to stop smoking immediately, who were diagnosed as
tobacco/nicotine-dependent based on the Tobacco Dependence Screener
(14), whose Brinkman index was 200
or higher, and who agreed to receive smoking cessation treatment.
Individuals who had a history of cancer or cardiovascular disease
were not included in the study. This study was approved by the
ethics committee of the National Institute of Public Health of
Japan (Saitama, Japan). Patients were informed of the purpose and
procedure of the current study, and only those who agreed to
participate signed the consent form. The sample size was determined
based on a previous intervention study (3). Twenty-three individuals whose assays
were available for both the initial visit and a visit two weeks
later and who provided information on their age, sex, and nutrient
intake were analyzed in the current study.
Study design
Participants were treated based on standard
treatment guidelines by a collaboration of the Japanese Circulation
Society, the Japan Lung Cancer Society, the Japanese Cancer
Association, and the Japanese Respiratory Society (13). We used data collected at the initial
visit and a visit two weeks into the course of treatment. At both
visits, casual urine samples were collected and stored at −70°C
until assayed. 8-OHdG levels of the samples were measured using
high performance liquid chromatography with an electrochemical
detector (15). The effect of the
smoking treatment, which was smoking cessation or a reduction of
smoking, was confirmed by a decrease in the level of cotinine. For
the analysis of cotinine, samples were extracted on an Oasis MCX
cartridge and ENVI-Carb™ cartridge. The extracts were analyzed by a
gas chromatography mass spectrometry system (16). For the analysis of 8-isoprostane,
urinary samples were extracted on a C18 solid phase extraction
cartridge and ENVI-Carb™ cartridge. The extracts were analyzed
using a Micromass Quattro LC in MRM mode with an ‘electrospray’
source in negative mode and MassLynx software. For 8-isoprostane,
the limit of detection and limit of quantification were 0.02 and
0.05 ng/ml, respectively, and reproducibility was 2.9%. The
reagents for cotinine (1.0 mg/ml in methanol), 8-OHdG (>98%) and
ENVI-Carb™ cartridge were purchased from Sigma-Aldrich Inc. (Merck
KGaA, Darmstadt, Germany). Cotine-d3 (99.9%) was purchased from CDN
isotopes Inc. (Pointe-Claire, Quebec, Canada). 8-isoprostane
(>99%), 8-isoprostane-d4 (>98%) and OASIS MCX were purchased
from Cayman Chemical (Ann Arbor, MI, USA) and Waters Corporation
(Milford, MA, USA), respectively. All other chemicals and solvents
used were of an analytical grade.
Each subject's height and weight were measured
during the initial visit to the clinic, and BMI was calculated.
Participants also answered a self-administered questionnaire at
this visit. The amount of regular physical activity was estimated
from the validated questionnaire, and this was used to determine
the weekly metabolic equivalents (METs) (17). The intake of food and nutrients in
usual diet was measured with brief-type self-administered diet
history questionnaire (BDHQ) (18).
Statistical analysis
Values for the levels of cotinine, 8-OHdG, and
8-isoprostane were adjusted for creatinine. These values, as well
as BMI and METs, were logarithmically transformed to approximate a
normal distribution for the analysis. Nutrient and food intakes
were also logarithmically transformed and adjusted for total energy
intake using a residual model for statistical analyses (19). To assess changes in the levels of
cotinine, 8-OHdG, and 8-isoprostane, geometric means with 95%
confidence intervals (CIs) and standard errors were obtained, and
paired t-tests were conducted. To assess the influences of physical
and nutritional factors on changes in the levels of oxidative
stress, age, BMI, physical activity, and the intakes of alcohol,
coffee, and nutrients (total calories, vitamin C, α tocopherol,
vitamin A, iron, carbohydrate, fat, and protein) were categorized
into two groups according to their median value. These traits were
selected from previous studies that reported their association with
oxidative stress levels (11,20–25).
Vitamin A is expressed in retinol equivalents, calculated as a sum
of retinol, β-carotene/12, α-carotene/24, and cryptoxanthin/24. To
investigate the time course of each oxidative stress level, we
performed a two-way repeated ANOVA with the factor of each group
and time. A factor-by-time interaction was assessed for statistical
significance to test whether changes in value over time differ
between the groups of each factor. P<0.05 was considered to
indicate a statistically significant difference.
Results
The characteristics of participants at the initial
visit are summarized in Table I. The
participants ranged between 29 and 82 years of age and 52% of them
were male. Table II summarizes the
geometric means of cotinine, 8-OHdG, and 8-isoprostane levels at
the initial visit and the visit two weeks later. Compared with the
level at the initial visit, the cotinine level was largely and
significantly decreased after two weeks. Levels of 8-OHdG and
8-isoprostane had not changed two weeks after the initiation of
smoking cessation treatment.
 | Table I.Baseline characteristics of 23 smokers
who underwent smoking cessation treatment. |
Table I.
Baseline characteristics of 23 smokers
who underwent smoking cessation treatment.
| Characteristics | Mean (n) | SD (%) | Minimum | Maximum |
|---|
| Age (years) | 46.8 | 15.0 | 29.0 | 82.0 |
| BMI
(kg/m2)a | 23.6 | 5.7 | 16.6 | 42.9 |
| Physical activity
(metabolic equivalents-h/week) | 8.5 | 11.1 | 0.0 | 46.5 |
| Smoking
pack-year | 22.9 | 12.8 | 5.0 | 50.0 |
| The Tobacco
Dependence Screener | 8.0 | 1.6 | 5.0 | 10.0 |
| Alcohol/day (g) | 14.9 | 20.8 | 0.0 | 69.7 |
| coffee/day (g) | 296.7 | 177.5 | 10.0 | 600.0 |
| Vitamin C/day
(mg) | 92.7 | 43.9 | 17.9 | 203.1 |
| α-tocopherol/day
(mg) | 6.4 | 2.4 | 2.0 | 11.3 |
| Vitamin A (retinol
equivalent) (µg/day) | 556.5 | 359.7 | 66.3 | 1553.7 |
| Carbohydrate/day
(g) | 198.8 | 73.0 | 66.0 | 376.5 |
| Fat/day (g) | 44.4 | 17.9 | 19.2 | 84.8 |
| Protein/day
(g) | 56.4 | 25.0 | 27.2 | 134.0 |
| Iron/day (mg) | 6.5 | 2.8 | 2.5 | 12.7 |
| Sex (male, %) | 12 | 52 |
|
|
 | Table II.The geometric means with 95% CI for
urinary cotinine, 8-OHDG and 8-isoprostane before and after the
treatment. |
Table II.
The geometric means with 95% CI for
urinary cotinine, 8-OHDG and 8-isoprostane before and after the
treatment.
|
| Initial visit
n=23 | After 2 weeks
n=23 |
|
|---|
|
|
|
|
|
|---|
| Biomarkers | Meana | 95% CI | Meana | 95% CI | Pb |
|---|
| Cotinine (ng/ml
creatinine) | 1780.60 |
(1373.5–2308.4) | 24.79 | (5.3–117.0) | <.0001 |
| 8-OHdG (ng/mg
creatinine) | 3.02 | (2.24–4.07) | 2.77 | (1.82–4.22) | 0.65 |
| 8-isoprostane
(ng/mg creatinine) | 0.29 | (0.22–0.39) | 0.34 | (0.27–0.43) | 0.32 |
Table III
summarizes the geometric means of 8-OHdG levels at the initial
visit and two-week visit according to each factor, as well as
changes in values over time. The two-way repeated ANOVA showed a
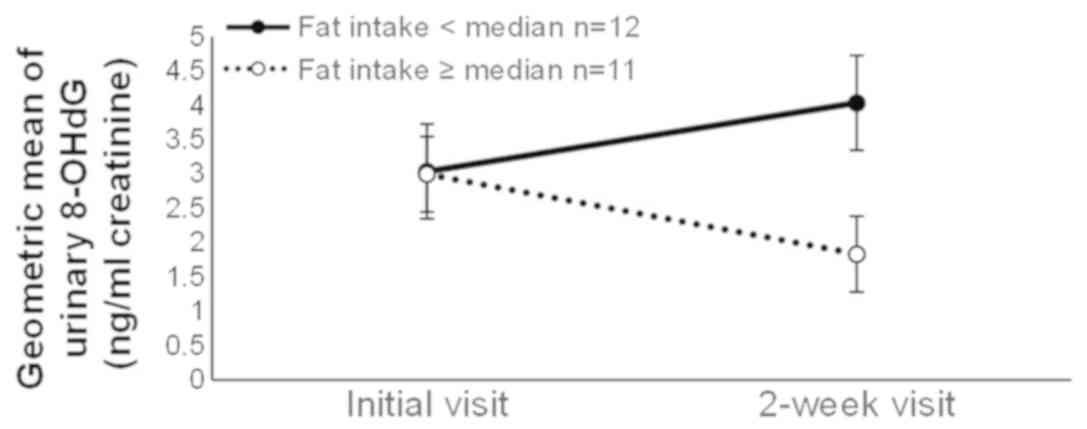
significant factor × time interaction for fat intake, indicating a
decrease in the 8-OHdG level in the group with lower fat intake,
whereas there was an increase in the 8-OHdG level in the group with
higher fat intake (Fig. 1). Other
factor × time interaction terms did not reach statistical
significance.
 | Table III.Geometric means of 8-OHdG levels at
the initial visit and 2-week visit according to the physical and
nutritional factors. |
Table III.
Geometric means of 8-OHdG levels at
the initial visit and 2-week visit according to the physical and
nutritional factors.
|
|
| < Median
value |
| ≥ Median value |
|
|---|
|
|
|
|
|
|
|
|---|
|
|
| Initial visit | 2-week visit |
| Initial visit | 2-week visit |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
|
Characteristics | n | Geometric mean | 95% CI | Geometric mean | 95% CI | n | Geometric mean | 95% CI | Geometric mean | 95% CI | P-value for group
by time interactiona |
|---|
| Age (years) | 12 | 2.95 | (1.79–4.84) | 2.66 | (1.33–5.30) | 11 | 3.10 | (2.05–4.68) | 2.90 | (1.60–5.26) | 0.92 |
| BMI | 10 | 3.41 | (2.16–5.38) | 2.47 | (0.94–6.50) | 10 | 3.22 | (2.01–5.15) | 3.05 | (1.88–4.95) | 0.51 |
| Alcohol
(g/day) | 12 | 2.34 | (1.44–3.80) | 1.90 | (1.04–3.47) | 11 | 3.98 | (2.83–5.58) | 4.18 | (2.35–7.46) | 0.49 |
| Coffee (g/day) | 12 | 3.12 | (2.03–4.78) | 3.37 | (2.35–4.85) | 11 | 2.91 | (1.76–4.80) | 2.23 | (0.94–5.27) | 0.36 |
| Total calories
(kcal/day) | 12 | 3.07 | (1.86–5.07) | 3.31 | (2.17–5.06) | 11 | 2.96 | (1.96–4.45) | 2.28 | (0.99–5.21) | 0.38 |
| Vitamin C
(mg/day) | 12 | 2.20 | (1.44–3.36) | 2.19 | (1.11–4.36) | 11 | 4.25 | (2.90–6.22) | 3.57 | (2.05–6.20) | 0.66 |
| α-tocopherol
(mg/day) | 12 | 3.17 | (2.17–4.64) | 3.34 | (2.13–5.26) | 11 | 2.85 | (1.01–5.05) | 2.25 | (1.01–5.05) | 0.45 |
| Vitamin A (retinol
equivalent) |
| (µg/day) | 12 | 2.55 | (1.63–3.99) | 2.64 | (1.41–4.93) | 11 | 3.62 | (2.32–5.66) | 2.92 | (1.48–5.75) | 0.51 |
| Iron (mg/day) | 12 | 2.76 | (1.74–4.39) | 2.87 | (1.75–4.72) | 11 | 3.32 | (2.12–5.21) | 2.66 | (1.19–5.92) | 0.49 |
| Carbohydrate
(g/day) | 12 | 3.17 | (2.31–4.36) | 2.10 | (0.97–4.54) | 11 | 2.86 | (1.58–5.16) | 3.75 | (2.64–5.31) | 0.06 |
| Fat (g/day) | 12 | 3.04 | (1.84–5.01) | 4.04 | (2.98–5.47) | 11 | 3.00 | (1.99–4.51) | 1.83 | (0.81–4.16) | 0.03 |
| Protein
(g/day) | 12 | 3.05 | (1.94–4.81) | 3.73 | (2.61–5.32) | 11 | 2.98 | (1.86–4.77) | 2.00 | (0.87–4.59) | 0.11 |
| α-tocopherol and
vitamin A | α-tocopherol <
Median or/and vitamin A < Median | α-tocopherol ≥
Median and vitamin A ≥ Median |
|
| 16 | 2.94 | (2.05–4.22) | 2.66 | (1.59–4.47) | 7 | 3.20 | (1.56–6.56) | 3.03 | (1.14–8.02) | 0.92 |
| Sex | Male | Female |
|
| 12 | 3.05 | (2.01–4.63) | 2.09 | (1.01–4.36) | 11 | 2.98 | (1.79–4.97) | 3.75 | (2.42–5.83) | 0.10 |
Table IV summarizes
the geometric means of 8-isoprostane levels at the initial visit
and two-week visit according to each factor, as well as the change
in values over time. A significant factor × time interaction was
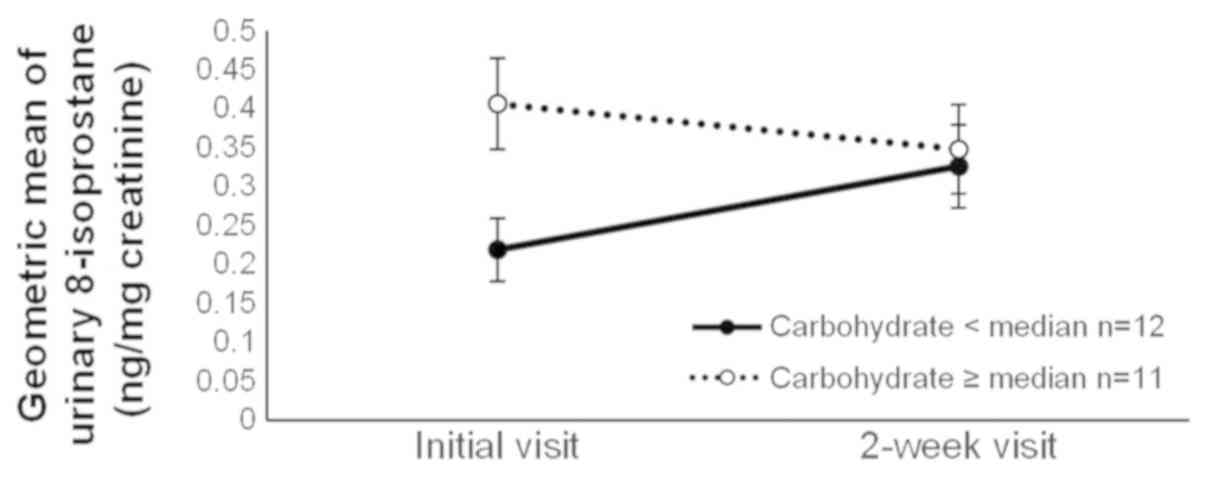
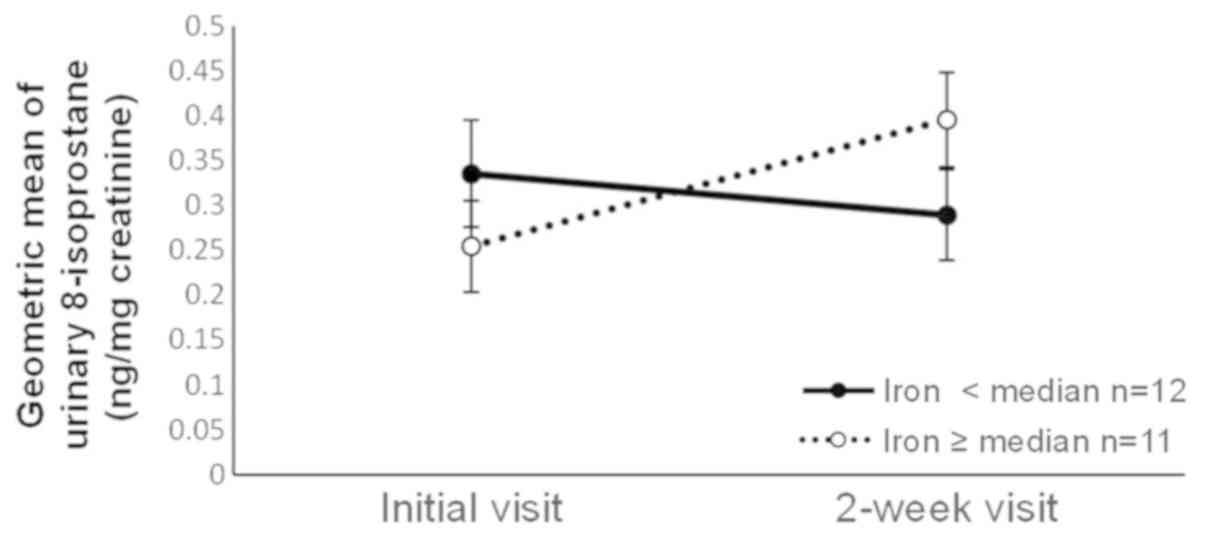
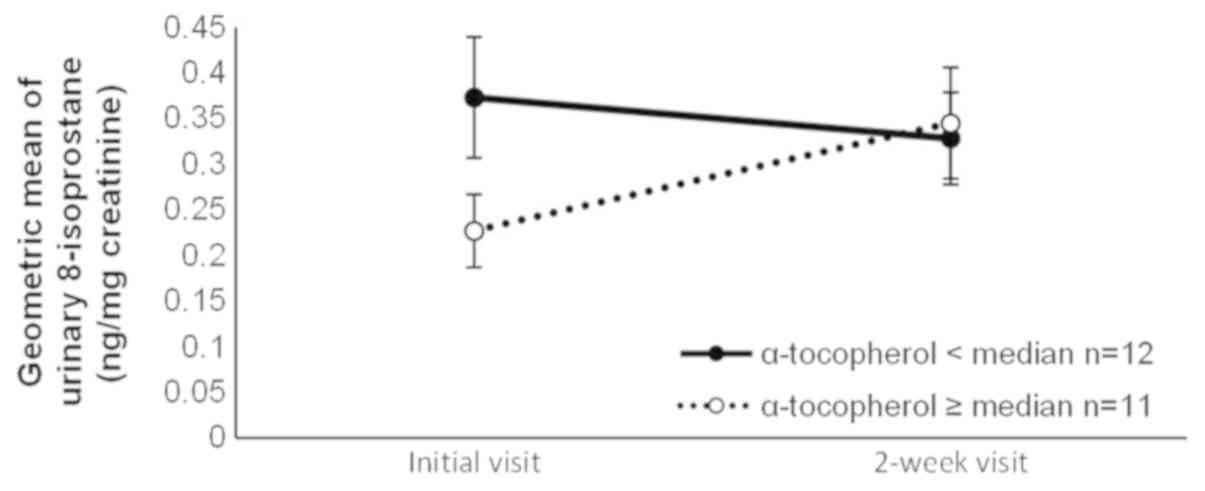
observed for α-tocopherol, iron, and carbohydrate intakes. The
level of 8-isoprostane increased in the group with low carbohydrate
intake, whereas it decreased in the group with high carbohydrate
intake (Fig. 2). The 8-isoprostane
level decreased in the group with low iron intake, whereas it
increased in the group with high iron intake (Fig. 3). The 8-isoprostane level decreased
slightly in the group with low α-tocopherol intake but increased in
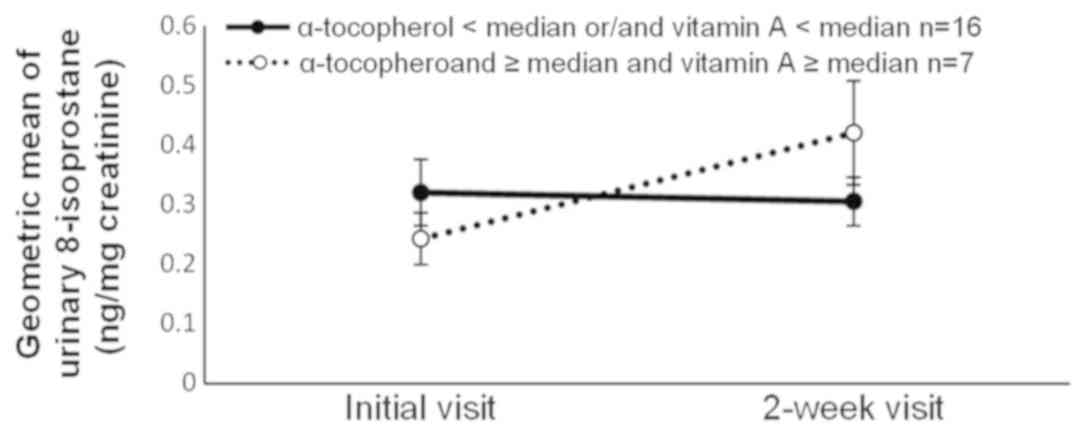
the group with high α-tocopherol intake (Fig. 4). We performed an ad hoc analysis of
the intake of α-tocopherol and vitamin A combined. The methods of
statistical analysis was the same as those for each nutrient
intake. The 8-isoprostane level increased in the group with high
intakes of both α-tocopherol and vitamin A, whereas it slightly
decreased in the group with low intakes of either or both nutrients
(Fig. 5).
 | Table IV.Geometric means of 8-isoprostane
levels at the initial visit and 2-week visit according to the
demographic, physical and nutritional factors. |
Table IV.
Geometric means of 8-isoprostane
levels at the initial visit and 2-week visit according to the
demographic, physical and nutritional factors.
|
|
| < Median
value |
| ≥ Median value |
|
|---|
|
|
|
|
|
|
|
|---|
|
|
| Initial visit | 2-week visit |
| Initial visit | 2-week visit |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
|
Characteristics | n | Geometric mean | 95% CI | Geometric mean | 95% CI | n | Geometric mean | 95% CI | Geometric mean | 95% CI | P-value for group
by time interactiona |
|---|
| Age (years) | 12 | 0.35 | (0.23–0.55) | 0.41 | (0.32–0.52) | 11 | 0.24 | (0.17–0.34) | 0.27 | (0.18–0.42) | 0.93 |
| BMI | 10 | 0.25 | (0.14–0.46) | 0.40 | (0.28–0.56) | 10 | 0.36 | (0.26–0.50) | 0.33 | (0.21–0.51) | 0.06 |
| Alcohol
(g/day) | 12 | 0.27 | (0.18–0.41) | 0.28 | (0.19–0.41) | 11 | 0.32 | (0.21–0.49) | 0.41 | (0.31–0.54) | 0.45 |
| Coffee (g/day) | 12 | 0.31 | (0.20–0.48) | 0.37 | (0.28–0.49) | 11 | 0.28 | (0.18–0.42) | 0.30 | (0.19–0.46) | 0.69 |
| Total calories
(kcal/day) | 12 | 0.26 | (0.18–0.37) | 0.29 | (0.21–0.38) | 11 | 0.33 | (0.20–0.54) | 0.40 | (0.27–0.60) | 0.71 |
| Vitamin C
(mg/day) | 12 | 0.33 | (0.21–0.52) | 0.34 | (0.24–0.47) | 11 | 0.26 | (0.18–0.37) | 0.34 | (0.22–0.50) | 0.35 |
| α-tocopherol
(mg/day) | 12 | 0.37 | (0.25–0.55) | 0.33 | (0.23–0.46) | 11 | 0.23 | (0.15–0.34) | 0.35 | (0.23–0.51) | 0.03 |
| Vitamin A (retinol
equivalent) (µg/day) | 12 | 0.29 | (0.18–0.47) | 0.29 | (0.20–0.43) | 11 | 0.30 | (0.22–0.42) | 0.39 | (0.29–0.53) | 0.33 |
| Iron (mg/day) | 12 | 0.34 | (0.23–0.50) | 0.29 | (0.20–0.43) | 11 | 0.25 | (0.16–0.40) | 0.40 | (0.29–0.53) | 0.02 |
| Carbohydrate
(g/day) | 12 | 0.22 | (0.15–0.33) | 0.33 | (0.23–0.47) | 11 | 0.41 | (0.29–0.56) | 0.35 | (0.24–0.50) | 0.03 |
| Fat (g/day) | 12 | 0.36 | (0.24–0.54) | 0.34 | (0.25–0.48) | 11 | 0.24 | (0.16–0.35) | 0.33 | (0.22–0.49) | 0.14 |
| Protein
(g/day) | 12 | 0.35 | (0.23–0.51) | 0.37 | (0.27–0.51) | 11 | 0.25 | (0.16–0.38) | 0.31 | (0.21–0.46) | 0.56 |
| α-tocopherol and
vitamin A | α-tocopherol
<Median or/and vitamin A <Median | α-tocopherol
≥Median and vitamin A ≥Median |
|
| 16 | 0.32 | (0.22–0.46) | 0.30 | (0.23–0.40) | 7 | 0.24 | (0.16–0.38) | 0.42 | (0.25–0.70) | 0.03 |
| Sex | Male | Female |
|
| 12 | 0.29 | (0.19–0.46) | 0.33 | (0.26–0.43) | 11 | 0.30 | (1.79–4.97) | 0.34 | (0.21–0.54) | 0.996 |
Discussion
We did not observe a decrease in the levels of
oxidative DNA and lipid peroxidation, which were estimated from
urinary 8-OHdG and 8-isoprostane, respectively, two weeks after
starting smoking cessation treatment. This was despite the fact
that subjects quit smoking or largely reduced the number of
cigarettes smoked, which was estimated from the cotinine levels of
the same urine samples. This study was first to assess changes in
oxidative stress levels among smokers with greater demographic
variability in an actual clinical setting. The result contradicts a
previous finding from an intervention study, which reported a
reduction of 8-OHdG and 8-isoprostane levels after two weeks of
smoking cessation (3). We did not
find this surprising; our data were collected in a clinical
setting, and study participants had wide demographic variability,
whereas the previous study was conducted among less varied
subjects-young healthy male medical students (3).
In the current study, the comparison by dietary
intake implied that changes in oxidative stress levels were
modified by the regular intake of certain nutrients. Although not
many studies have reported changes in oxidative stress levels
during smoking cessation, previous findings with different study
designs may reasonably support our findings. Previous
cross-sectional studies reported an inverse association between fat
intake and the urinary 8-OHdG level, although statistical
significance was not achieved (5,26). These
studies correspond with our finding that the 8-OHdG level decreased
during smoking cessation among smokers with high fat intake but
increased among those with low fat intake, although no mechanism
was suggested.
The 8-isoprostane level decreased after two weeks of
smoking cessation treatment among smokers with high carbohydrate
intake, whereas the level increased among those with low
carbohydrate intake. Studies of the association between the
8-isoprostane level and carbohydrate intake have been limited;
however, one study reported a significant inverse correlation
between carbohydrate intake and serum total antioxidant capacity
among breast cancer patients and controls (27). A study of cyclists reported that the
increase in F2-isoprostanes was significantly lower
after the ingestion of carbohydrate beverages as compared to
placebo beverages (28). This study
speculated that this was induced by the suppression of the cortisol
and epinephrine levels after the intake of carbohydrate. The level
of epinephrine also increases with smoking (29), which may explain a mechanism for the
observed interaction between carbohydrate intake and smoking
cessation.
We did not expect that a high intake of dietary
α-tocopherol would be associated with an increase in the level of
8-isoprostane during the smoking cessation period. The antioxidant
activity of α-tocopherol or vitamin E in diets or from supplements
has been frequently reported (30–32).
However, a study of patients with histories of colorectal adenoma
reported an interesting result: A supplementary cocktail that
included α-tocopherol and β-carotene increased
F2-isoprostane levels among smokers, whereas it
decreased them among non-smokers (33). Our additional analysis indicated that
individuals who had high intakes of both α-tocopherol and vitamin A
had an increasing change in their levels of 8-isoprostane. A study
in rats may explain the mechanism, which is a pro-lipid
peroxidation activity of β-carotene (34). However, no evidence has suggested
that vitamin E by itself acted as a pro-oxidant among smokers.
Another explanation may be that the sufficient intake of dietary
antioxidant nutrients may have suppressed the oxidative stress
level before the initiation of smoking cessation treatment. There
may be certain threshold levels of oxidative stress level to make
smoking cessation be effective. Further analysis exploring the
interaction between these nutrients is difficult with our data.
The current study has several limitations. The
limited number of participants caused a lack of statistical power,
which would have caused our failure to observe a decrease in
oxidative stress during smoking cessation treatment. The accurate
measures utilized in the current study may have diminished this
limitation. Another major limitation is that there is no control
group, and all participants underwent smoking cessation treatment.
The main objective of this study was to observe general individuals
who were willing to undergo smoking cessation treatment, and
changes in oxidative stress levels were evaluated according to the
levels of several key factors. The study period was limited to two
weeks, and further follow-up was not available. Achievement of
smoking cessation during the two weeks was confirmed by measuring
cotinine level; however, the final outcome of the smoking cessation
treatment was not evaluated. Despite these limitations, important
information can be drawn from the current results, especially since
limited data exist on the change in oxidative stress levels during
a smoking cessation period.
In conclusion, we observed a decrease in cotinine
levels but not in oxidative stress levels among Japanese
participants who underwent smoking cessation treatment. The change
in oxidative stress levels two weeks from the beginning of the
smoking cessation treatment may vary based upon several factors.
Levels of 8-OHdG decreased among smokers with high fat intake,
whereas they increased among smokers with low fat intake. The level
of 8-isoprostane increased among smokers with low carbohydrate
intake but decreased among those with high carbohydrate intake,
decreased among those with low iron intake but increased among
those with high iron intake, and decreased among smokers with low
α-tocopherol intake whereas it increased among those with high
α-tocopherol intake. Further research on changes in oxidative
stress levels and various factors among smokers undergoing smoking
cessation treatment with a greater number of subjects, as well as
research on estimating the changes among such smokers in general
populations may confirm our current findings.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by grants
from the All Japan Coffee Association, the Ministry of Education,
Culture, Sports, Science and Technology, Japan, JSPS KAKENHI
[Grant-in-Aid for Scientific Research (C); grant nos. JP23590832
and JP26460758] and the Ministry of Health, Labour and Welfare
Sciences Research grants (Tokyo, Japan).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
SO and TI conceptualized and designed the study. YI
and NK measured the oxidative stress levels. TS, JO, KS, TK, and TI
acquired data. SO drafted the manuscript, and YI, TS, JO, KS, TK,
NK, and TI revised it critically. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of the National Institute of Public Health of Japan
(Saitama, Japan). All participants were informed of the purpose and
procedures of the current study and signed the consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Harris KK, Zopey M and Friedman TC:
Metabolic effects of smoking cessation. Nat Rev Endocrinol.
12:299–308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ikeda F, Ninomiya T, Doi Y, Hata J,
Fukuhara M, Matsumoto T and Kiyohara Y: Smoking cessation improves
mortality in Japanese men: The Hisayama study. Tob Control.
21:416–421. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morita H, Ikeda H, Haramaki N, Eguchi H
and Imaizumi T: Only two-week smoking cessation improves platelet
aggregability and intraplatelet redox imbalance of long-term
smokers. J Am Coll Cardiol. 45:589–594. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Priemé H, Loft S, Klarlund M, Grønbaek K,
Tønnesen P and Poulsen HE: Effect of smoking cessation on oxidative
DNA modification estimated by 8-oxo-7,8-dihydro-2′-deoxyguanosine
excretion. Carcinogenesis. 19:347–351. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Loft S, Vistisen K, Ewertz M, Tjønneland
A, Overvad K and Poulsen HE: Oxidative DNA damage estimated by
8-hydroxydeoxyguanosine excretion in humans: Influence of smoking,
gender and body mass index. Carcinogenesis. 13:2241–2247. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roberts LJ II and Morrow JD: Products of
the isoprostane pathway: Unique bioactive compounds and markers of
lipid peroxidation. Cell Mol Life Sci. 59:808–820. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davi G, Falco A and Patrono C:
Determinants of F2-isoprostane biosynthesis and
inhibition in man. Chem Phys Lipids. 128:149–163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alpha-Tocopherol, Beta Carotene Cancer
Prevention Study Group. The effect of vitamin E and beta carotene
on the incidence of lung cancer and other cancers in male smokers.
N Engl J Med. 330:1029–1035. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cocate PG, Natali AJ, Oliveira Ad, Longo
GZ, Alfenas Rde C, Peluzio Mdo C, Santos EC, Buthers JM, Oliveira
LL and Hermsdorff HH: Fruit and vegetable intake and related
nutrients are associated with oxidative stress markers in
middle-aged men. Nutrition. 30:660–665. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Block G, Dietrich M, Norkus EP, Morrow JD,
Hudes M, Caan B and Packer L: Factors associated with oxidative
stress in human populations. Am J Epidemiol. 156:274–285. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sakano N, Wang DH, Takahashi N, Wang B,
Sauriasari R, Kanbara S, Sato Y, Takigawa T, Takaki J and Ogino K:
Oxidative stress biomarkers and lifestyles in Japanese healthy
people. J Clin Biochem Nutr. 44:185–195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mizoue T, Kasai H, Kubo T and Tokunaga S:
Leanness, smoking, and enhanced oxidative DNA damage. Cancer
Epidemiol Biomarkers Prev. 15:582–585. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
The Japanese Circulation Society, the
Japan Lung Cancer Society, Cancer Association the Japanese
Respiratory Society: Smoking Cessation Treatment Guidelines. (6th).
Tokyo. 2014.(In Japanese).
|
|
14
|
Kawakami N, Takatsuka N, Inaba S and
Shimizu H: Development of a screening questionnaire for
tobacco/nicotine dependence according to ICD-10, DSM-III-R, and
DSM-IV. Addict Behav. 24:155–166. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Inaba Y, Koide S, Yokoyama K and Karube I:
Development of urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG)
measurement method combined with SPE. J Chromatogr Sci. 49:303–309.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim I, Darwin WD and Huestis MA:
Simultaneous determination of nicotine, cotinine, norcotinine, and
trans-3′-hydroxycotinine in human oral fluid using solid phase
extraction and gas chromatography-mass spectrometry. J Chromatogr B
Analyt Technol Biomed Life Sci. 814:233–240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suzuki I, Kawakami N and Shimizu H:
Reliability and validity of a questionnaire for assessment of
energy expenditure and physical activity in epidemiological
studies. J Epidemiol. 8:152–159. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kobayashi S, Murakami K, Sasaki S, Okubo
H, Hirota N, Notsu A, Fukui M and Date C: Comparison of relative
validity of food group intakes estimated by comprehensive and
brief-type self-administered diet history questionnaires against 16
d dietary records in Japanese adults. Public Health Nutr.
14:1200–1211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Willett W and Stampfer MJ: Total energy
intake: Implications for epidemiologic analyses. Am J Epidemiol.
124:17–27. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tamae K, Kawai K, Yamasaki S, Kawanami K,
Ikeda M, Takahashi K, Miyamoto T, Kato N and Kasai H: Effect of
age, smoking and other lifestyle factors on urinary 7-methylguanine
and 8-hydroxydeoxyguanosine. Cancer Sci. 100:715–721. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nordin TC, Done AJ and Traustadottir T:
Acute exercise increases resistance to oxidative stress in young
but not older adults. Age (Dordr). 36:97272014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hori A, Kasai H, Kawai K, Nanri A, Sato M,
Ohta M and Mizoue T: Coffee intake is associated with lower levels
of oxidative DNA damage and decreasing body iron storage in healthy
women. Nutr Cancer. 66:964–969. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thompson HJ, Heimendinger J, Sedlacek S,
Haegele A, Diker A, O'Neill C, Meinecke B, Wolfe P, Zhu Z and Jiang
W: 8-Isoprostane F2alpha excretion is reduced in women
by increased vegetable and fruit intake. Am J Clin Nutr.
82:768–776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hodgson JM, Ward NC, Burke V, Beilin LJ
and Puddey IB: Increased lean red meat intake does not elevate
markers of oxidative stress and inflammation in humans. J Nutr.
137:363–367. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hori A, Mizoue T, Kasai H, Kawai K,
Matsushita Y, Nanri A, Sato M and Ohta M: Body iron store as a
predictor of oxidative DNA damage in healthy men and women. Cancer
Sci. 101:517–522. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Irie M, Tamae K, Iwamoto-Tanaka N and
Kasai H: Occupational and lifestyle factors and urinary
8-hydroxydeoxyguanosine. Cancer Sci. 96:600–606. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yeon JY, Suh YJ, Kim SW, Baik HW, Sung CJ,
Kim HS and Sung MK: Evaluation of dietary factors in relation to
the biomarkers of oxidative stress and inflammation in breast
cancer risk. Nutrition. 27:912–918. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McAnulty S, McAnulty L, Nieman D, Morrow
J, Dumke C and Utter A: Carbohydrate effect: Hormone and oxidative
changes. Int J Sports Med. 28:921–927. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grassi G, Seravalle G, Calhoun DA, Bolla G
and Mancia G: Cigarette smoking and the adrenergic nervous system.
Clin Exp Hypertens A. 14:251–260. 1992.PubMed/NCBI
|
|
30
|
Davi G, Ciabattoni G, Consoli A, Mezzetti
A, Falco A, Santarone S, Pennese E, Vitacolonna E, Bucciarelli T,
Costantini F, et al: In vivo formation of 8-iso-prostaglandin
f2alpha and platelet activation in diabetes mellitus: Effects of
improved metabolic control and vitamin E supplementation.
Circulation. 99:224–229. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Helmersson J, Arnlöv J, Larsson A and Basu
S: Low dietary intake of beta-carotene, alpha-tocopherol and
ascorbic acid is associated with increased inflammatory and
oxidative stress status in a Swedish cohort. Br J Nutr.
101:1775–1782. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Anderson C, Milne GL, Sandler DP and
Nichols HB: Oxidative stress in relation to diet and physical
activity among premenopausal women. Br J Nutr. 116:1416–1424. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hopkins MH, Fedirko V, Jones DP, Terry PD
and Bostick RM: Antioxidant micronutrients and biomarkers of
oxidative stress and inflammation in colorectal adenoma patients:
Results from a randomized, controlled clinical trial. Cancer
Epidemiol Biomarkers Prev. 19:850–858. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Palozza P, Serini S, Trombino S, Lauriola
L, Ranelletti FO and Calviello G: Dual role of beta-carotene in
combination with cigarette smoke aqueous extract on the formation
of mutagenic lipid peroxidation products in lung membranes:
Dependence on pO2. Carcinogenesis. 27:2383–2391. 2006. View Article : Google Scholar : PubMed/NCBI
|