Introduction
Bronchopulmonary dysplasia (BPD), one of the most
common complications of prematurity, is a lung injury in preterm
infants resulting from oxygen and mechanical ventilation (1,2).
Although progress has been made in neonatal care, the incidence of
BPD is still increasing, mainly because the survival rate of
younger and more premature babies has increased (3). Hence, effective prevention and
treatments for BPD are on urgent need, and their necessary
precondition is a comprehensive understanding of molecular and
pathological mechanisms underlying this disease.
Previous literature has demonstrated that prenatal
factors have important roles in the progression of BPD, such as
surfactant deficiency and maternal infection (4,5).
Recently, genetic variants were discovered in preterm infants of
BPD (6,7). For instance, mutations of the lipid
transporter ATP-binding cassette subfamily A member 3
(ABCA3), which is involved in surfactant synthesis in
alveolar type II cells, have been revealed in BPD premature
newborns (7). Moreover, these
studies have highlighted a potential role for molecular pathways
involved in inflammation and lung development in affected infants.
Cross-talk among gene pathways can be means of regulatory
interaction among different pathways or can express the gene
overlap among pathways (8,9).
In this study, we proposed to reveal hub pathway
cross-talk for premature newborns with BPD based on the integration
of pathway related analyses and Monte Carlo Cross-Validation (MCCV)
algorithm. Ultimately, hub cross-talk was extracted from seed
cross-talk dependent on the MCCV method. The study of cross-talk
involved in BPD could help in defining the molecular mechanisms
leading to the onset and progression of the pathology (10).
Materials and methods
Data collection
Gene expression data with accession number
E-GEOD-8586 (11) was collected from
the ArrayExpress database for premature newborns at risk of BPD
related studies. In E-GEOD-8586, there were 54 newborns with a
gestational age from 23 to 27 weeks. These 54 newborns included 20
infants who later developed BPD (BPD group) and 34 infants who did
not develop BPD (normal controls). There were 23 females and 31
males. Subsequently, in order to control the quality of the data,
background corrections and standard normalizations were
conducted.
Identification of differentially
expressed genes (DEGs)
For investigating the differentially expressed
levels of genes across BPD samples and normal controls, the
empirical bayes (EB) approach was implemented to identify DEGs. In
particular, the EB approach is equivalent to shrinkage of the
estimated sample variances towards a pooled estimate, resulting in
far more stable inference when the number of arrays is small
(12). During this process, P-value
for each gene was adjusted by Benjamini-Hochberg (BH) test
(13). Importantly, only genes that
met the thresholds of false discovery rate (FDR) <0.01 and
|log2FoldChange| >2 simultaneously were considered as
DEGs in BPD and normal control groups.
Determination of differentially
expressed pathways (DEPs)
In the present study, all human biological pathways
were derived from the Ingenuity Pathways Analysis (IPA) tool.
Subsequently, DEGs of BPD were mapped to these biological pathways
with an attempt to make them more correlated with the disease, and
we gained pathways that were responsible for coordinating DEG
activities, named as background pathways. Then we evaluated the
significance between DEGs and genes in background pathways using
Fisher's exact test (14). P-values
were adjusted by BH test (13). In
addition, the cut-off for DEPs across BPD patients and normal
controls was P<0.01.
DEP cross-talk investigation
Based on the DEPs, we further focused on the
relationships among any two of them. If a correlation existed
between two DEPs, the DEP pair would be defined as a cross-talk for
DEPs (10). Hence, we selected the
discriminating score (DS) to access the strength of cross-talk
among DEPs in the present study (10). Supposing that × and y were two DEPs
in cross-talk, the DS was computed as following formula:
DS(x,y)=|ux-uy|σx+σy
Seed cross-talk extraction
With an attempt to evaluate the functional
activities of DEP cross-talk more deeply, a random forest (RF)
classification model (15) was
utilized to access the classified performance of these pairs
between BPD and normal control groups. The method was comprised of
three sections, first, drawing Ntree bootstrap samples dependent on
the DS for each DEP cross-talk (Ntree = 500);
secondly, growing a regression tree for each of the bootstrap
samples; and finally, aggregating the predictions of the
Ntree trees. The top 10 DEP cross-talk in
descending order of AUC were defined as seed cross-talk.
MCCV for seed cross-talks
As mentioned above, seed cross-talk was identified,
and then the MCCV method was adopted to validate the biological
functions of seed cross-talk in BPD samples (16). The 54 samples were randomly split
into two sets, the calibration set (Sc), contained nc samples for
fitting the models; and the validation set (Sv), included nv
samples for validating the model.
MCCVnv=1Rnv∑k=1R‖E‖2
Of which E represents the squared prediction error,
R represents the procedure repeated times (R=50). Theoretically,
the fewer samples used in model calibration, the more repeat times
were needed. For each bootstrap, the DEGs, DEPs, cross-talk and
their DS values were carried out. The repeat times of DEP
cross-talk were statistically calculated. More repeat times might
imply more significance of this cross-talk.
Results
DEGs
In the present study, there were 20,514 genes in the
pretreated E-GEOD-8586. After performing EB algorithm, a total of
132 DEGs which satisfied the cut-off (P<0.01 and
|log2FoldChange| >2) between BPD premature newborns
and normal controls were obtained. All DEGs were ranked in
descending order of their P-values (Table I). The smaller the P-value, the more
significant the expression of the DEG was. The most significant
five DEGs across BPD group and normal controls were EMILIN1
(P=4.29Ex10−6), MFAP51 (P=8.77Ex10−6),
SNCA1 (P=9.23Ex10−6), interleukin-62
(IL-62) (P=3.14Ex10−5) and STRADB1
(P=7.56Ex10−5).
 | Table I.Differentially expressed genes
(DEGs). |
Table I.
Differentially expressed genes
(DEGs).
| Rank | Genes | Rank | Genes | Rank | Genes | Rank | Genes |
|---|
| 1 | EMILIN1 | 34 | LINC00702 | 67 | TRIM58 | 100 | CXCL3 |
| 2 | MFAP51 | 35 | SLC25A391 | 68 | BPGM | 101 | MORC4 |
| 3 | SNCA1 | 36 | STRADB2 | 69 | SLC19A2 | 102 | C3 |
| 4 | IL-62 | 37 | XK1 | 70 | ZBTB16 | 103 | SERPINE11 |
| 5 | STRADB1 | 38 | EPB422 | 71 | SNCA | 104 | NPTN-IT1 |
| 6 | CXCL12 | 39 | AHSP1 | 72 | DMTN | 105 | PTGS2 |
| 7 | BPGM1 | 40 | KRT13 | 73 | EPB42 | 106 | CXCL6 |
| 8 | CXCL32 | 41 | S100A91 | 74 | PTX31 | 107 | SGK1 |
| 9 |
TNFAIP32 | 42 | SOD22 | 75 | IL-61 | 108 | CXCL8 |
| 10 | SOD21 | 43 | BOK | 76 | HEMGN | 109 | S100A8 |
| 11 | EPB421 | 44 | GYS1 | 77 | KRT1 | 110 | TNFAIP3 |
| 12 | PTX32 | 45 | CCL22 | 78 | BLVRB | 111 | LIF |
| 13 | CCL21 | 46 | S100A81 | 79 | STRADB | 112 | CXCL1 |
| 14 | HBM1 | 47 | KRT141 | 80 | FAM46C | 113 | FOSB1 |
| 15 | CXCL62 | 48 | MFAP52 | 81 | UCP2 | 114 | CCL20 |
| 16 | KRT14 | 49 | ZBTB163 | 82 |
SERPINE12 | 115 | IL-6 |
| 17 | PLEKHO1 | 50 | CCL202 | 83 | XK | 116 | SOD2 |
| 18 | UCP21 | 51 | CXCL33 | 84 | AHSP | 117 | TNFAIP6 |
| 19 | KAT2B1 | 52 | HBM4 | 85 | GCH1 | 118 | PTX3 |
| 20 | POC1B | 53 | CXCL63 | 86 | CHRDL2 | 119 | S100A9 |
| 21 | ADIPOR1 | 54 | PTX33 | 87 | LIF1 | 120 | CCL2 |
| 22 | ID1 | 55 | CXCL82 | 88 | CXCL31 | 121 | S100A12 |
| 23 | HBQ1 | 56 | CXCL13 | 89 |
TNFAIP61 | 122 | MFAP5 |
| 24 | FAM46C1 | 57 | SQRDL | 90 | CCL201 | 123 | EGR1 |
| 25 | DCAF12 | 58 | MAP3K8 | 91 |
SLC25A39 | 124 | FOS |
| 26 | BPGM2 | 59 | DCK | 92 | HBM | 125 | FOSB |
| 27 |
C15orf48 | 60 | SUSD6 | 93 | CSF3 | 126 |
SERPINE1 |
| 28 |
PLA2G2A1 | 61 | KAT2B | 94 |
TNFAIP31 | 127 | FOSB2 |
| 29 |
SERPINB2 | 62 | GYPA | 95 | CXCL11 | 128 | EGR11 |
| 30 | ZBTB162 | 63 | SGK11 | 96 | CXCL61 | 129 | FOS1 |
| 31 | HBM3 | 64 | KRT5 | 97 | CXCL81 | 130 | ZBTB161 |
| 32 | HEMGN1 | 65 | YOD1 | 98 | PLA2G2A | 131 | HBM2 |
| 33 | SNCA2 | 66 | PIP4K2A | 99 | SELE | 132 | RPS4Y1 |
DEPs
As described above, the IPA tool with 589 biological
pathways (covering 5,169 genes) was implemented to execute pathway
enrichment analysis for 132 DEGs of BPD infants, and the Fisher's
exact test was used to evaluate significance of the pathways.
Consequently, a total of 137 DEPs with P<0.01 were identified
between BPD samples and normal controls, of which role of IL-17A in
psoriasis (P=5.96Ex10−13) was the most significant one.
Furthermore, DEPs with larger number of DEGs might be more
important than that of the smaller ones. The DEPs with DEG number
≥4 are listed in Table II. Among
these DEPs, flucocorticoid receptor signaling
(P=7.11Ex10−7) contains 6 DEGs. Interestingly, the
number of DEGs enriched in 6 DEPs was 5, including granulocyte
adhesion and diapedesis (P=1.55Ex10−6), role of IL-17A
in psoriasis (P=5.96Ex10−13), glucocorticoid receptor
signaling (P=5.32Ex10−6), role of IL-17A in arthritis
(P=2.24E-10−8), IL-17A signaling in airway cells
(P=4.54Ex10−8) and flucocorticoid receptor signaling
(P=4.70Ex10×10−5).
 | Table II.Differentially expressed pathways
(DEPs) with DEG number ≥4. |
Table II.
Differentially expressed pathways
(DEPs) with DEG number ≥4.
| DEPs | P-value | Number of genes in
pathway | Number of DEGs |
|---|
| Glucocorticoid
receptor signaling |
7.11×10−7 | 255 | 6 |
| Granulocyte
adhesion and diapedesis |
1.55×10−6 | 163 | 5 |
| Role of IL-17A in
psoriasis |
5.96×10−13 | 13 | 5 |
| Glucocorticoid
receptor signaling |
5.32×10−6 | 255 | 5 |
| Role of IL-17A in
arthritis |
2.24×10−8 | 54 | 5 |
| IL-17A signaling in
airway cells |
4.54×10−8 | 63 | 5 |
| Glucocorticoid
receptor signaling |
4.70×10−5 | 255 | 5 |
| Role of IL-17A in
arthritis |
6.74×10−7 | 54 | 4 |
| IL-17A signaling in
airway cells |
1.18×10−6 | 63 | 4 |
| Agranulocyte
adhesion and diapedesis |
5.89×10−5 | 173 | 4 |
| Role of IL-17A in
arthritis |
2.72×10−7 | 54 | 4 |
| IL-17A signaling in
airway cells |
4.78×10−7 | 63 | 4 |
| Glucocorticoid
receptor signaling |
2.25×10−5 | 255 | 4 |
| Atherosclerosis
signaling |
6.60×10−8 | 119 | 4 |
| Role of IL-17A in
psoriasis |
2.63×10−9 | 13 | 4 |
| Granulocyte
adhesion and diapedesis |
1.07×10−4 | 163 | 4 |
| Agranulocyte
adhesion and diapedesis |
1.36×10−4 | 173 | 4 |
Seed cross-talk
In this section, first of all, DS was assigned to
any pair of DEPs or cross-talk by comparing their gene expression
levels. In consequence, 609 cross-talk among 137 DEPs were
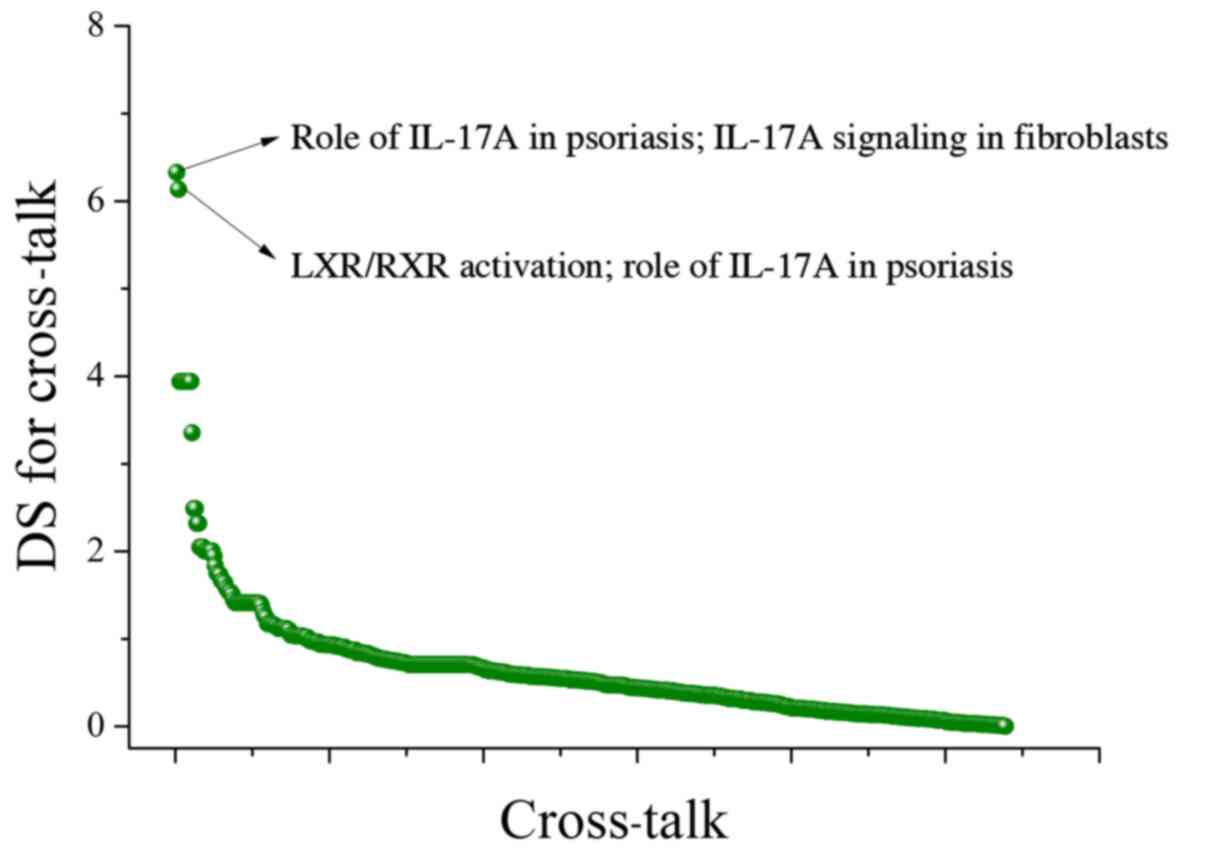
identified, and the DS distribution is illustrated in Fig. 1. Two of the cross-talk had higher DS
than the others: Role of IL-17A in psoriasis; IL-17A signaling in
fibroblasts (DS=6.328) and LXR/RXR activation; role of IL-17A in
psoriasis (DS=6.134). Subsequently, we applied the RF
classification of cross-talk for each sample based on the DS, and
chose the AUC to evaluate the classification performance of each
cross-talk. The cross-talk was ranked according to AUC in a
descending order and the top 10 cross-talk were denoted as seed
cross-talks (Table III). The best
cross-talk was the pair of pathways acute phase response signaling
and gepatic fibrosis/hepatic stellate cell activation (AUC=0.915).
Besides, the AUC for the following two cross-talks were ranged from
0.8 to 0.9: The pair of pathways role of IL-17F in allergic
inflammatory airway diseases and role of IL-17A in psoriasis
(AUC=0.857), and the pair of pathways LXR/RXR activation and
hepatic fibrosis/hepatic stellate cell activation (AUC=0.812).
 | Table III.Seed cross-talk with AUC value for
random forest (RF) classification. |
Table III.
Seed cross-talk with AUC value for
random forest (RF) classification.
| ID | Cross-talk | AUC |
|---|
| 1 | Acute phase
response signaling; hepatic fibrosis/hepatic stellate cell
activation | 0.915 |
| 2 | Role of IL-17F in
allergic inflammatory airway diseases; role of IL-17A in
psoriasis | 0.857 |
| 3 | LXR/RXR activation;
hepatic fibrosis/hepatic stellate cell activation | 0.812 |
| 4 | LXR/RXR activation;
acute phase response signaling | 0.799 |
| 5 | Role of IL-17A in
psoriasis; IL-17A signaling in fibroblasts | 0.790 |
| 6 | IL-17A signaling in
airway cells; role of hypercytokinemia/hyperchemokinemia in the
pathogenesis of influenza | 0.786 |
| 7 | IL-17 signaling;
differential regulation of cytokine production in intestinal
epithelial cells by IL-17A and IL-17F | 0.786 |
| 8 | Role of IL-17F in
allergic inflammatory airway diseases; atherosclerosis
signaling | 0.782 |
| 9 | LXR/RXR activation;
glucocorticoid receptor signaling | 0.782 |
| 10 | IL-17A signaling in
gastric cells; role of hypercytokinemia/hyperchemokinemia in the
pathogenesis of influenza | 0.778 |
Hub cross-talk
Herein, we divided 20 BPD samples and 30 normal
controls of total 54 samples in the gene expression data into two
sets according to the ratio of 6:4. Among the 10 seed cross-talk,
we uncovered that 3 cross-talk were repeated many times. The pair
of pathways role of IL-17F in allergic inflammatory airway diseases
and role of IL-17A in psoriasis was repeated 29 times. The pair of
pathways role of IL-17A in psoriasis and IL-17A signaling in
fibroblasts was repeated 28 times. The pair of pathways IL-17A
signaling in airway cells and role of
hypercytokinemia/hyperchemokinemia in the pathogenesis of influenza
was repeated 28 times. Hence we defined these three cross-talk as
hub cross-talk for BPD patients, which might play more crucial
roles in the progression of BPD samples than the others.
Discussion
In the present study, we investigated hub cross-talk
of DEPs for BPD samples by integrating a series of bioinformatics
analyses, identification of DEGs, extraction of DEPs, exploration
of seed cross-talk and investigation of hub cross-talk. Although
the scenario might be intuitive, the biological functions and
activities of DEPs have not been well studies (17,18). In
order to increase the confidence and feasibility of our study, we
implemented the MCCV to screen the hub cross-talk (10,19).
Consequently, a total of 3 hub cross-talk for
premature newborns with BPD were gained. IL-17F is a cytokine that
shares sequence similarity with IL-17 and is expressed by activated
T cells (20). It inhibits
angiogenesis of endothelial cells and induces endothelial cells to
produce IL-2, TGFB1/TGFB, and signaling and function in
inflammation (21). The involvement
of IL-17A/IL-17F in inflammatory immune responses and host defense
mechanisms, and the correlation between IL-17A/IL-17F with other
cytokines has been reviewed previously (22,23). The
review indicated that IL-17A and IL-17F possibly work together in
human disease. Coincidently, in our results, the hub cross-talk
(role of IL-17F in allergic inflammatory airway diseases and role
of IL-17A in psoriasis) also revealed this phenomenon in BPD
development.
IL-17A has been demonstrated to take vital part in
allergic airway inflammation, and promote inflammation via inducing
various proinflammatory cytokines and chemokines, recruiting
neutrophils, increasing antibody production, and activating T cells
(24). We found that IL-17A was
correlated to all of the three hub cross-talk of BPD infants,
including the pair of pathways role of IL-17F in allergic
inflammatory airway diseases and role of IL-17A in psoriasis; the
pair of pathways role of IL-17A in psoriasis and IL-17A signaling
in fibroblasts; the pair of pathways IL-17A signaling in airway
cells and role of hypercytokinemia/hyperchemokinemia in the
pathogenesis of influenza. Importantly, IL-17A has been reported to
exhibit a vital role in the development of pulmonary fibrosis,
suggesting it is a potential treatment target for
inflammation-induced fibrosis (25).
Taken together, we inferred that the three hub cross-talk closely
correlated to BPD.
In summary, 3 hub cross-talk among DEPs were
identified in our study, which might give insight into revealing
the molecular mechanism underlying the premature newborns with
BPD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used in this study are available from
the corresponding author on reasonable request.
Authors' contributions
CW, RP and BZ conceived the study. MC and GC
acquired, and analyzed the data. CW wrote the manuscript. RP, GC
and MX explained the results and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bancalari E and Claure N: Bronchopulmonary
dysplasia: Definitions and epidemiology. In: Bronchopulmonary
Dysplasia. Bhandari V: Springer International Publishing.
(Switzerland). 167–182. 2016.
|
|
2
|
Smith VC, Zupancic JA, McCormick MC, Croen
LA, Greene J, Escobar GJ and Richardson DK: Rehospitalization in
the first year of life among infants with bronchopulmonary
dysplasia. J Pediatr. 144:799–803. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bhandari A and Panitch H: An update on the
post-NICU discharge management of bronchopulmonary dysplasia. Semin
Perinatol. 42:471–477. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Watterberg KL, Demers LM, Scott SM and
Murphy S: Chorioamnionitis and early lung inflammation in infants
in whom bronchopulmonary dysplasia develops. Pediatrics.
97:210–215. 1996.PubMed/NCBI
|
|
5
|
Glaser K and Speer CP: Pre and postnatal
inflammation in the pathogenesis of bronchopulmonary dysplasia. In:
Bronchopulmonary Dysplasia. Bhandari V: Springer International
Publishing. (Switzerland). 55–77. 2016.PubMed/NCBI
|
|
6
|
Gadhia MM, Cutter GR, Abman SH and
Kinsella JP: Effects of early inhaled nitric oxide therapy and
vitamin A supplementation on the risk for bronchopulmonary
dysplasia in premature newborns with respiratory failure. J
Pediatr. 164:744–748. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lavoie PM: Genetics of bronchopulmonary
dysplasia. In: Bronchopulmonary Dysplasia. Bhandari V: Springer
International Publishing. (Switzerland). 109–127. 2016.PubMed/NCBI
|
|
8
|
Aksamitiene E, Kiyatkin AB and Kholodenko
BN: Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt
pathways: A fine balance. Biochem Soc Trans. 40:139–146. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bernards R: A missing link in
genotype-directed cancer therapy. Cell. 151:465–468. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Colaprico A, Cava C, Bertoli G, Bontempi G
and Castiglioni I: Integrative analysis with Monte Carlo
cross-validation reveals miRNAs regulating pathways cross-talk in
aggressive breast cancer. Biomed Res Int. 2015:8313142015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cohen J, Van Marter LJ, Sun Y, Allred E,
Leviton A and Kohane IS: Perturbation of gene expression of the
chromatin remodeling pathway in premature newborns at risk for
bronchopulmonary dysplasia. Genome Biol. 8:R2102007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smyth GK: Linear models and empirical
bayes methods for assessing differential expression in microarray
experiments. Stat Appl Genet Mol Biol. 3:Article32004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bogdan M, Ghosh JK and Tokdar ST: A
comparison of the Benjamini-Hochberg procedure with some Bayesian
rules for multiple testing. In: Beyond Parametrics in
Interdisciplinary Research. Festschrift in Honor of Professor.
Pranab K. Sen.: 1:Institute of Mathematical Statistics. (Beachwood,
OΗ). 211–230. 2008.
|
|
14
|
Routledge R: Fisher's exact test. In:
Encyclopedia of Biostatistics. John Wiley Publishing. (New York,
NY). 2005.
|
|
15
|
Liaw A and Wiener M: Classification and
regression by randomForest. R News. 2:18–22. 2002.
|
|
16
|
Xu QS and Liang YZ: Monte Carlo cross
validation. Chemom Intell Lab Syst. 56:1–11. 2001. View Article : Google Scholar
|
|
17
|
Donato M, Xu Z, Tomoiaga A, Granneman JG,
Mackenzie RG, Bao R, Than NG, Westfall PH, Romero R and Draghici S:
Analysis and correction of cross-talk effects in pathway analysis.
Genome Res. 23:1885–1893. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang H, Cheng C and Zhang W: Average
rank-based score to measure deregulation of molecular pathway gene
sets. PLoS One. 6:e275792011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu QS, Liang YZ and Du YP: Monte Carlo
cross-validation for selecting a model and estimating the
prediction error in multivariate calibration. J Chemometr.
18:112–120. 2004. View
Article : Google Scholar
|
|
20
|
Besnard AG, Sabat R, Dumoutier L, Renauld
JC, Willart M, Lambrecht B, Teixeira MM, Charron S, Fick L, Erard
F, et al: Dual role of IL-22 in allergic airway inflammation and
its cross-talk with IL-17A. Am J Respir Crit Care Med.
183:1153–1163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang SH and Dong C: IL-17F: Regulation,
signaling and function in inflammation. Cytokine. 46:7–11. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jin W and Dong C: IL-17 cytokines in
immunity and inflammation. Emerg Microbes Infect. 2:e602013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Puel A, Döffinger R, Natividad A, Chrabieh
M, Barcenas-Morales G, Picard C, Cobat A, Ouachée-Chardin M, Toulon
A, Bustamante J, et al: Autoantibodies against IL-17A, IL-17F, and
IL-22 in patients with chronic mucocutaneous candidiasis and
autoimmune polyendocrine syndrome type I. J Exp Med. 207:291–297.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iwakura Y, Nakae S, Saijo S and Ishigame
H: The roles of IL-17A in inflammatory immune responses and host
defense against pathogens. Immunol Rev. 226:57–79. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wilson MS, Madala SK, Ramalingam TR,
Gochuico BR, Rosas IO, Cheever AW and Wynn TA: Bleomycin and
IL-1β-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med.
207:535–552. 2010. View Article : Google Scholar : PubMed/NCBI
|