Introduction
Gliomas are the most prevalent type of malignant
brain tumor, accounting for 46–70% of all central nervous system
(CNS) tumors, and have a devastating impact on the human health and
life (1). The major characteristics
of gliomas are their invasive growth and high rate of recurrence
after intracranial operation, resulting in a poor prognosis of
affected patients (2,3). Gliomas have been classified by the
World Health Organization into four grades: Grades I and II are for
low-grade tumors, with Grade I tumors being benign tumors and Grade
II tumors having the ability to recur as high-grade gliomas. Grade
III and IV gliomas are high-grade tumors, of which Grade III
gliomas tend to metastasize and recur, and Grade IV tumors are the
most malignant gliomas (4). In spite
of significant advances in surgical techniques and chemotherapeutic
treatments, the median survival rate of glioma patients remains
poor (5–7). It has been reported that the 5-year
survival rate of patients with Grade I and II glioma is 30–70%,
while it is only 9–12 months for patients with Grade III and IV
glioma (8). Therefore, it is of
great importance to explore the molecular mechanisms underlying the
development of gliomas and to identify novel biomarkers for
predicting the progression of gliomas and identifying approaches
for targeted therapies.
MicroRNAs (miRNAs/miRs) are a class of non-coding,
single-stranded, endogenous small RNA molecules of 21–23
nucleotides in length, which regulate gene expression by binding to
the 3′-untranslated region (3′-UTR) of an mRNA target to promote
mRNA degradation and/or translational repression (9,10). It
has been reported that miRNAs are involved in the modulation of a
variety of biological processes, including cell proliferation,
differentiation, apoptosis, migration and tumorigenesis (11,12).
miR-128-3p was identified to speed up cell cycle arrest and
chromosomal instability in mitomycin C-treated lung cancer cells by
suppressing spectrin alpha, non-erythrocytic 1 (13). It was also indicated that miR-128-3p
is intimately associated with hepatocellular carcinoma (14,15) and
acute lymphoblastic leukemia (16).
However, to date, the association between miR-128-3p and gliomas
has remained to be determined in combination with a clinical and
fundamental study.
In the present study, a total of 61 pairs of tumor
tissues and paratumor tissues were collected from glioma patients
and it was identified that the relative expression of miR-218-5p
was downregulated, while neuronal pentraxin 1 (NPTX1) was
upregulated in the tumor tissues. Through a bioinformatics analysis
using Targetscan, NPTX1 was identified as a potential target of
miR-218-5p, which was confirmed using a luciferase reporter assay.
After the human glioma cells were transfected with miR-218-5p
mimics or inhibitor, the insulin receptor substrate 1
(IRS-1)/phosphoinositide-3 kinase (PI3K)/AKT signaling pathway was
suppressed or promoted, respectively. Furthermore, the
IRS-1/PI3K/AKT signaling pathway was inhibited after NPTX1 was
suppressed. Thus, miR-128-3p regulates glioma development via the
IRS-1/PI3K/AKT signaling pathway by targeting NPTX1.
Materials and methods
Patients and tissue samples
Between March 2009 and April 2016, a total of 61
pairs of primary glioma and matched paratumor tissue samples were
collected at the Department of Neurosurgery of the First Hospital
of Lanzhou University (Lanzhou, China). The tumor and adjacent
normal tissues were rapidly frozen in liquid nitrogen and preserved
at −70°C until use. None of the patients had received any blood
transfusion, chemotherapy or radiotherapy prior to the surgery.
Written informed consent was obtained from each patient and
approval of the Ethics Committee of the First Hospital of Lanzhou
University was obtained for using their tissue samples in the
present study.
U251 cell culture and
transfection
The U251 human glioma cell line was purchased from
the cell bank of the Chinese Academy of Sciences (Shanghai, China)
and was cultured in minimum essential medium/Earle's balanced salt
solution (Hyclone; GE Healthcare, Little Chalfont, UK) supplemented
with 10% fetal bovine serum (Hyclone; GE Healthcare), 50 U/ml
penicillin and 50 mg/ml streptomycin at 37°C in a humidified
atmosphere containing 5% CO2. The cells were seeded in a
6-well plate at 5×105 cells/well and the medium was
replaced every 3 days until the confluency reached ~70%. miR-128-3p
mimics (100 nM) or negative control (NC, nonsense miR); Sangon
Biotech Co., Ltd., Shanghai, China) were transfected into U251
cells using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. After 4–6 h of transfection, the medium
was replaced with fresh normal medium and 24 h later, total RNA or
protein was extracted. In U251 cells, 100 nM NPTX1 small
interfering (si)RNA reagent (Sangon Biotech Co., Ltd.) and its
negative control were transfected into the cells. After 6 h, the
medium was replaced with fresh complete medium. Total protein and
RNA were extracted 24 h later. The expression of the NPTX1 protein
was detected by western blot analysis.
Luciferase activity assay
A fragment including the 3′-UTR of human NPTX1 was
amplified from human genomic mRNA (NCBI Reference Sequence:
NM_002522.3) using the following primer pair:
5′-AGCACTGCGCTAGACACTAGGGATAGAGTTGTAAAAAACACA-3′ and
5′-GTGCAAATAACTAGATTTTATATATATATTATATAGAACTGT-3′. pGL3-NPTX1-wild
type (WT) reporter vector was constructed by interception of
polymerase chain reaction (PCR) products of the 3′UTR of NPTX1,
which was cut with XbaI and cloned into the corresponding sites of
the pGL3 vector. The QuikChange II Site-Directed Mutagenesis kit
(Takara Bio Inc., Dalian, China) was used to generate a mutation in
the putative site of miR-128-5p recognition according to the
manufacturer's protocol. A total of 400 ng WT or mutant luciferase
reporter, 100 nM miRNA-128-3p mimics or their NC and 30 ng pRL-TK
Renilla luciferase reporter vector (Promega Corp., Madison, WI,
USA) was added to each well of a 24-well plate containing
4–5×104 cells per well. After transfection for 48 h,
cells were collected and luciferase activity was measured using the
Dual-Luciferase® Reporter Assay system (Promega
Corp.).
Bioinformatics analysis
The Targetscan online platform (http://www.targetscan.org/) was used to predict the
target genes of miR-128-5p with reference to the human gene
sequence. The predicted target genes were denoted and further
analysis was performed to verify them. Furthermore, the specific
binding sequence of the 3′UTR of NPTX1 was also predicted with this
software.
Reverse transcription-quantitative
(RT-q) PCR analysis
Total RNA was extracted from tissues of glioma or
transfected cells using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), and l µg total RNA was reverse transcribed using
a PrimeScript RT Reagent kit with a gDNA Eraser (cat. nos. RR047A
and RR047B; each, Takara Bio, Inc., Dalian, China) according to the
manufacturer's protocol. Subsequently, PCR amplification was
performed in a total reaction volume of 20 µl comprising 1 µl NPTX1
primers (0.2 µmol/l, Sangon Biotech Co., Ltd.), 10 µl SYBR green
(Takara Bio, Inc.), 6 µl diethyl pyrocarbonate-treated water
(Sangon Biotech Co., Ltd.) and 2 µl complementary DNA product, in a
Real-Time LightCycler® 96 System (Roche Diagnostics,
Basel, Switzerland). The sequences of the primers were as follows:
miR-128-3p forward, 5′-GACTGCCGAGCGAGCG-3′ and reverse,
5′-GACGCCGAGGCACTCTCTCCT-3′; U6 forward,
5′-CCATCGGAAGCTCGTATACGAAATT-3′ and reverse,
5′-GGCCTCTCGAACTTGCGTGTCAG-3′; NPTX1 forward,
5′-TTGACAGTTGCATCACAACG-3′ and reverse, 5′-CCAGCTATGGCCTGCGACCG-3′;
β-actin forward, GCTGTCCCTGTATGCCTCT-3′ and reverse,
TGTCACGCACGATTTCC-3′. The following thermocycling conditions were
applied: 5 sec at 95°C, followed by 40 cycles of 1 sec at 95°C and
20 sec at 65°C, followed by 5 sec at 72°C. The comparative
2−ΔΔCq method was used for relative quantification of
gene expression (17). Each sample
utilized in reverse transcription and the PCR amplification of cDNA
were analyzed three times.
Western blot analysis
Total protein was extracted from collected tissues
and cells using radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China) supplemented
with 100X proteinase inhibitor and phosphatase inhibitor, and then
the proteins (25 µg) were separated by 10% SDS-PAGE following
protein determination using the BCA method. Subsequently, the
proteins were transferred to a polyvinylidene difluoride membrane
(EMD Millipore, Billerica, MA, USA), which was then blocked with 5%
non-fat milk for 1 h at room temperature. The primary antibodies
were anti-NPTX1 (cat. no. 3725; 1:1,000 dilution), anti-IRS-1 (cat.
no. 2382; 1:500 dilution), anti-phosphorylated (p)-IRS-1 (Tyr895)
(cat. no. 3070; 1:500 dilution), anti-PI3K (cat. no. 4292; 1:1,000
dilution) anti-p-AKT (ser473; cat. no. 12694; 1:1,000 dilution),
anti-AKT (cat. no. 2938; 1:1,000 dilution) and anti-β-actin (cat.
no. 4970; 1:1,000 dilution) and were all rabbit monoclonal
antibodies (Cell Signaling Technology, Inc., Danvers, MA, USA) in
4°C overnight. The secondary antibody (cat. no. A0208; Beyotime
Institute of Biotechnology) was used at the dilution of 1:5,000 and
incubated at room temperature for 90 min. Blots were visualized
with an enhanced chemiluminescence kit (cat. no. 15159; Invitrogen;
Thermo Fisher Scientific, Inc.). The gray bands were analyzed with
Image J software 10.8 (National Institutes of Health, Bethesda, MD,
USA) to compare the expression between targeted proteins and
internal controls.
U251 proliferation assay
To detect the proliferation activity of transfected
U251 cells, the MTT assay was performed using a Cell Proliferation
Kit I (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in a 96-well
plate at a density of 4×103 per well according to the
manufacturer's instructions. After 24, 48 and 72 h of incubation,
MTT-formazan production was estimated by a VersaMax (Molecular
Devices, LLC, Sunnyvale, CA, USA) at 570 nm to evaluate the
proliferation rate. The index was determined at 48 and 72 h and
normalized to that at 24 h.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. All statistical analyses were performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). The differences between
two groups were compared using a paired Student's t-test and the
differences between three or more groups using one-way analysis of
variance followed by a Tukey's post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-128-3p and NPTX1 are
differentially expressed in glioma tissues
The Targetscan search indicated that NPTX1
was a target gene of miR-128-3p. Thus, miR-128-3p and NPTX1
was assessed in glioma tissues. Between March 2009 and April 2016,
a total of 61 pairs of glioma tumor tissues and the adjacent normal
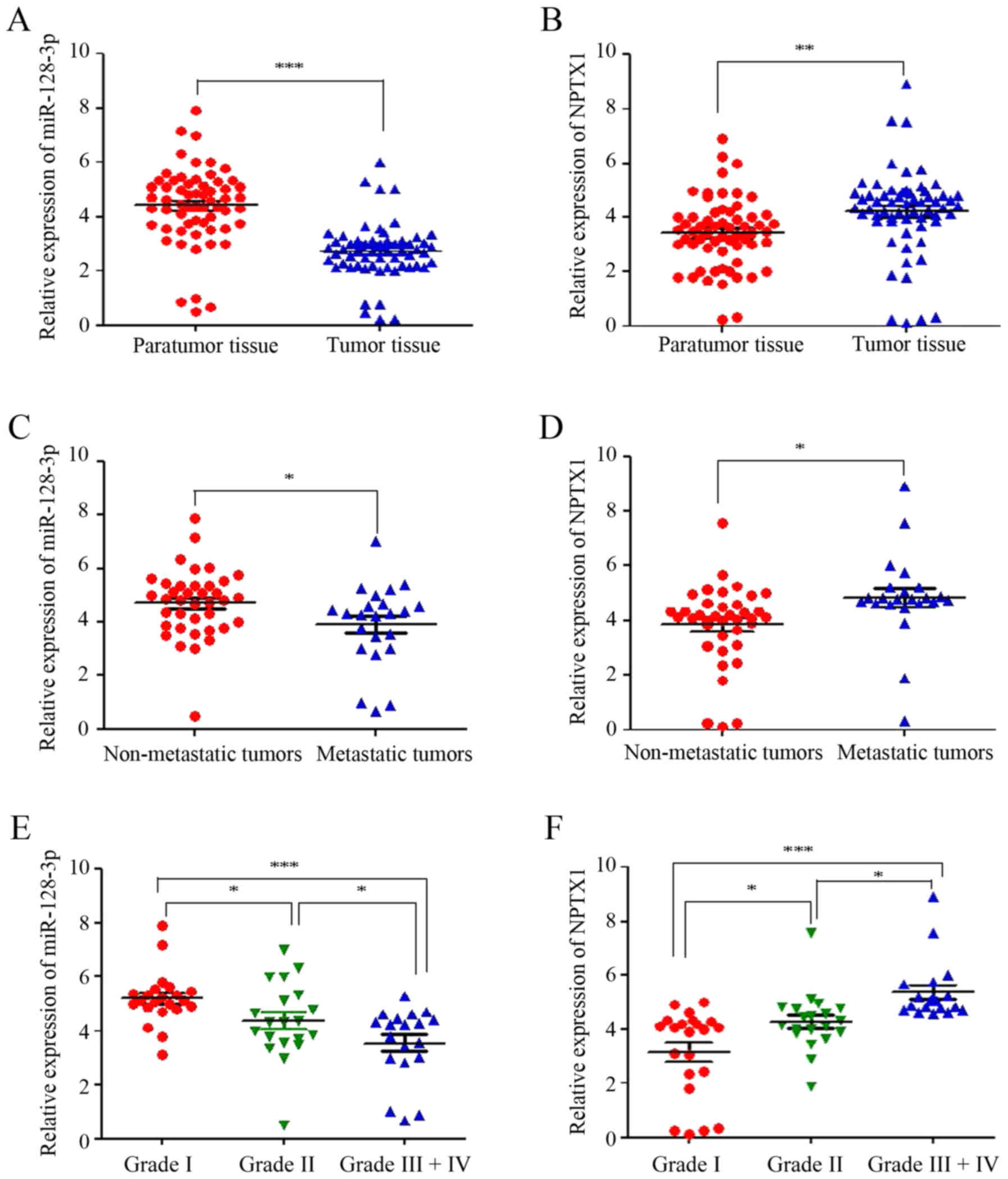
tissues were collected. RT-qPCR analysis indicated that the
relative expression of miR-128-3p was significantly decreased,
while NPTX1 was elevated in the tumor tissues compared with that in
the normal tissues (Fig. 1A and B).
In addition, the relative expression of miR-128-3p was also lower
in metastatic tissues than that in non-metastatic tissues, but the
NPTX1 expression was higher in metastatic tissues (Fig. 1C and D). According to the diagnostic
criteria of the world health organization, the glioma patients were
stratified according to their grade [grade I (n=22), II (n=20) and
III+IV (n=19)]. The miR-128-3p expression was lowest in the grade
III+IV group but highest in the grade I group. Conversely, NPTX1
expression was the highest in the grade III+IV group, while it was
lowest in the grade I group (Fig. 1E and
F). These results illustrated that miR-128-3p and NPTX1 are
closely associated with gliomas.
NPTX1 is targeted by miR-128-3p
In order to further clarify the association between
miR-128-3p and NPTX1, a Targetscan software analysis was performed
to identify the miR-128-3p binding sequence in the 3′-UTR of NPTX1
and the core of the binding sequence was deleted to generate a
mutant sequence (Fig. 2A and B).
Subsequently, U251 cells were co-transfected with miR-128-3p mimics
and luciferase reporter vectors containing the WT or mutant
miR-128-3p binding sequence of the 3′-UTR of NPTX1. It was revealed
that the luciferase activity was significantly decreased in the WT
group (NPTX1 WT + miR-128-3p mimic group) in comparison with
the mutant (NPTX1 mutant + miR-128-3p mimic group) and blank
group (Blank + miR-128-3p mimic group), while no difference was
observed between the mutant and blank groups (Fig. 2C). Of note, the luciferase activity
was upregulated after the reporter vector driven by the binding
sequence in the 3′-UTR of NPTX1 and miR-128-3p inhibitor
(NPTX1 3′-UTR + miR-128-3p inhibitor group) were
co-transfected into the U251 cells compared with the blank group
(NPTX1 3′-UTR + Blank). Furthermore, the opposite result was
obtained after the reporter vector driven by the binding sequence
in the 3′-UTR of NPTX1 and miR-128-3p mimics (NPTX1 3′-UTR +
miR-128-3p mimic group) were transfected into the cells in
comparison with the blank group (Fig.
2D). In U251 cells transfected with miR-128-3p mimics, the
expression of NPTX1 was decreased at the protein level (Fig. 2E), while the opposite effect was
obtained after miR-128-3p inhibitor was transfected into the cells
(Fig. 2F).
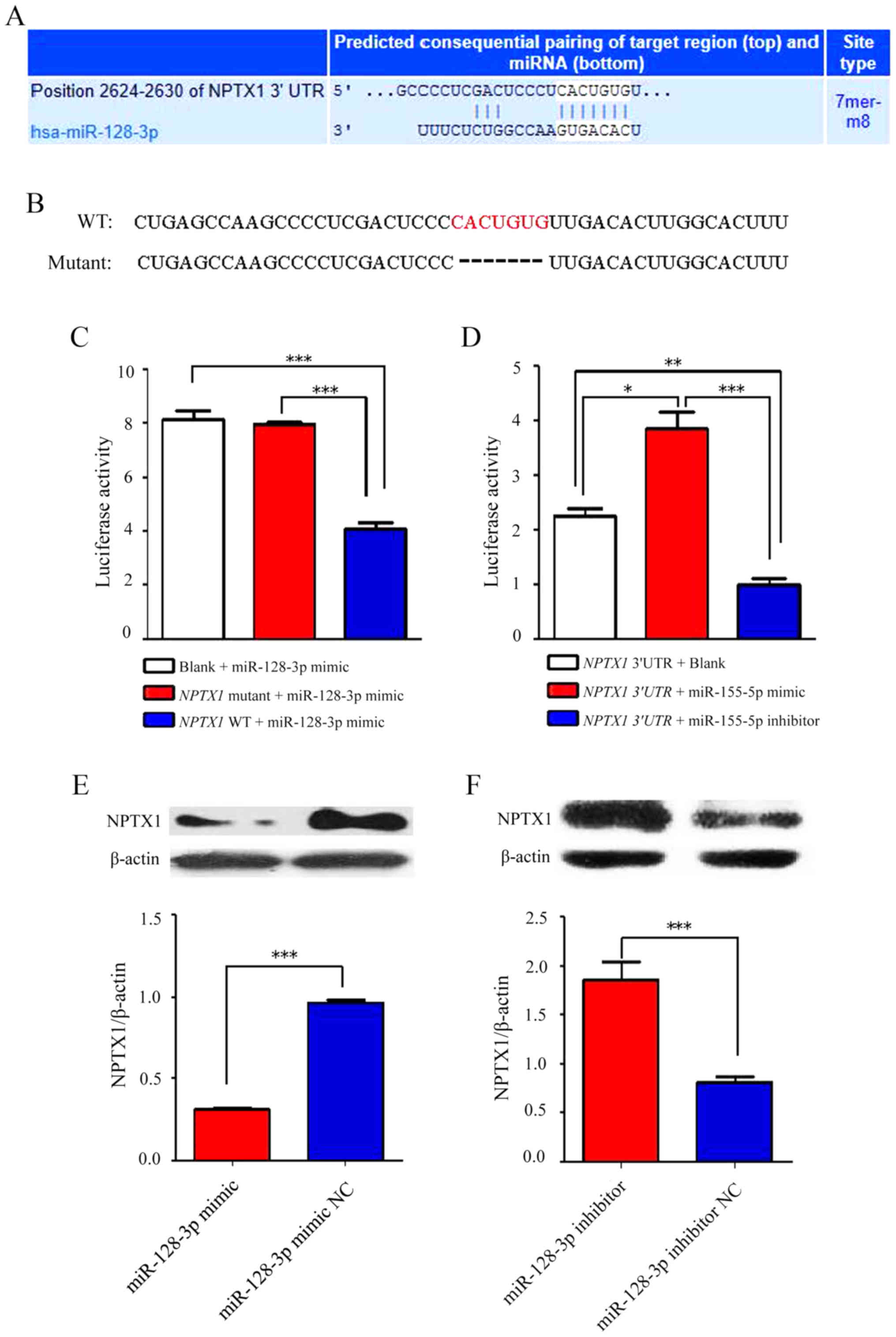 | Figure 2.NFTX-1 is a direct target of
miR-128-3p. (A) The predicted binding sequences of the 3′-UTR of
NPTX1 and miR-128-3p. (B) The sequences on the top refer to the
complete binding sequences of the 3′-UTR of NPTX1 and miR-128-3p,
while the ones at the bottom refer to the mutation sequences
without core sequences. (C) After the binding sequences of 3′-UTR
of NPTX1 and miR-128-3p mimics were transfected into U251 cells,
the luciferase activity was obviously decreased; however, no
statistically significant difference was identified after their
mutated sequences were transfected into the cells. (D) The
luciferase activity was significantly promoted after the binding
sequences of NPTX1 3′-UTR and miR-128-3p inhibitor were transfected
into U251 cells, but the results were opposite after the binding
sequences of the NPTX1 3′-UTR and miR-128-3p mimics were
transfected into the cells. (E and F) After U251 cells were
transfected with miR-128-3p mimics, the protein expression of NPTX1
was decreased compared with that in the NC group, while it was
increased after transfection with miR-128-3p inhibitor. *P<0.05,
**P<0.01, ***P<0.001. NPTX1, neuronal pentraxin 1; miR,
microRNA; UTR, untranslated region; WT, wild-type; NC, negative
control; hsa, Homo sapiens. |
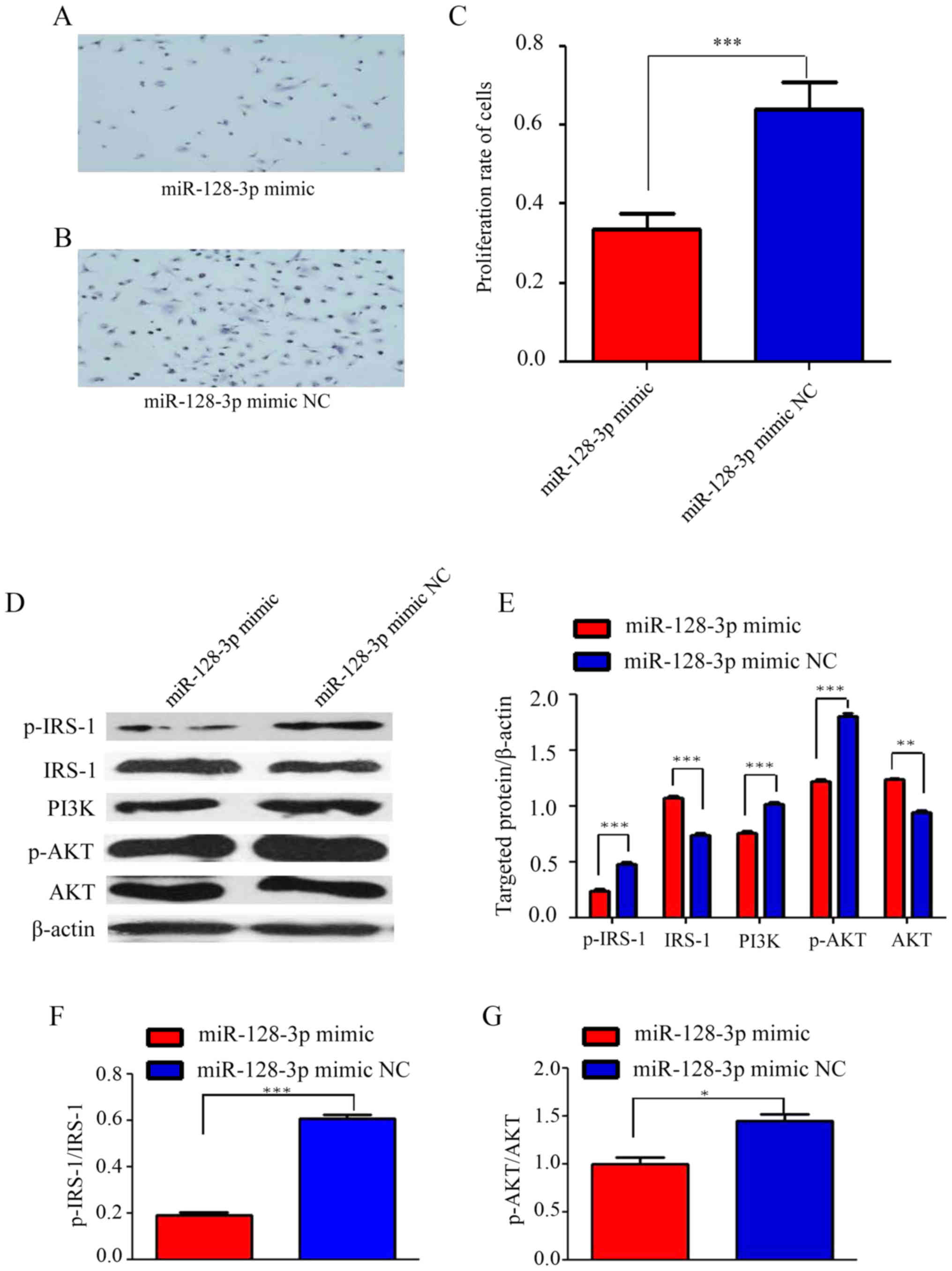
miR-128-3p mimics suppress
IRS-1/PI3K/AKT signaling in U251 cells
Cultured U251 cells were transfected with miR-128-3p
mimics or NC. Through visual observation, it was determined that
the proliferation rate of the cells was reduced in the miR-128-3p
mimics group (Fig. 3A) in comparison
with that in the NC group (Fig. 3B).
An MTT assay confirmed that the proliferation rate of the cells in
the miR-128-3p mimics group was significantly lower than that in
the NC group (Fig. 3C). In addition,
western blot analysis clearly proved that compared with those in
the NC group, the protein levels of p-IRS-1, PI3K and p-AKT were
significantly decreased in the miR-128-3p mimics group, while the
expression of IRS-1 and AKT was significantly increased (Fig. 3D and E). This result indicated that
the IRS-1/PI3K/AKT signaling pathway was suppressed by the
miR-128-3p mimics.
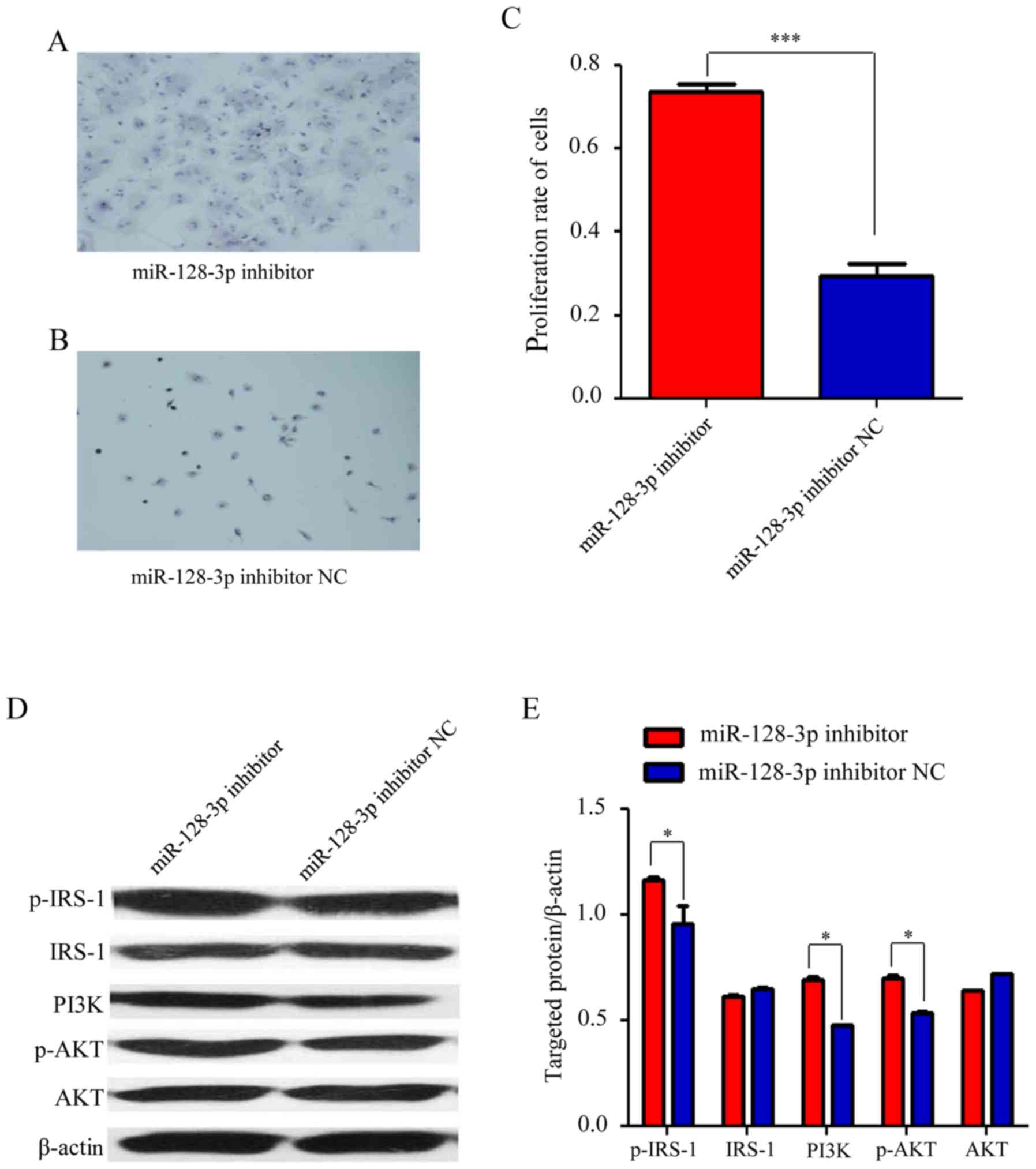
miR-128-3p inhibitor increases
IRS-1/PI3K/AKT signaling in U251 cells
miR-128-3p inhibitor and its NC were transfected
into the cultured U251 cells, and visual observation revealed that
the proliferation rate of the cells was increased in the miR-128-3p
inhibitor group (Fig. 4A) in
comparison with that in the NC group (Fig. 4B). An MTT assay confirmed that the
proliferation rate of the cells in the miR-128-3p inhibitor group
was higher than that in the NC group (Fig. 4C). Furthermore, compared with those
in the NC group, the levels of p-IRS-1, PI3K and p-AKT were
significantly increased in the miR-128-3p inhibitor group, while
the expression of IRS-1 and AKT was not significantly different
(Fig. 4D and E). This result
indicated that IRS-1/PI3K/AKT signaling was enhanced after
miR-128-3p was inhibited.
Suppression of NPTX1 inhibits
IRS-1/PI3K/AKT signaling
In order to further demonstrate the association
between NPTX1 and the IRS-1/PI3K/AKT signaling pathway, NPTX1 siRNA
was transfected into the cultured U251 cells, which caused a
significant decrease in the levels of p-IRS-1, PI3K and p-AKT
compared with that in the NC group (Fig.
5A and B). However, the expression of total IRS-1 and AKT was
increased in the NPTX1 siRNA group. This meant that compared with
the protein levels normalized to β-actin, the p-IRS-1 and p-AKT
levels relative to IRS-1 and AKT, respectively, were even higher.
However, after the expression of NPTX1 was inhibited with siRNA,
the expression of miR-128-3p was not significantly different
(Fig. 5C).
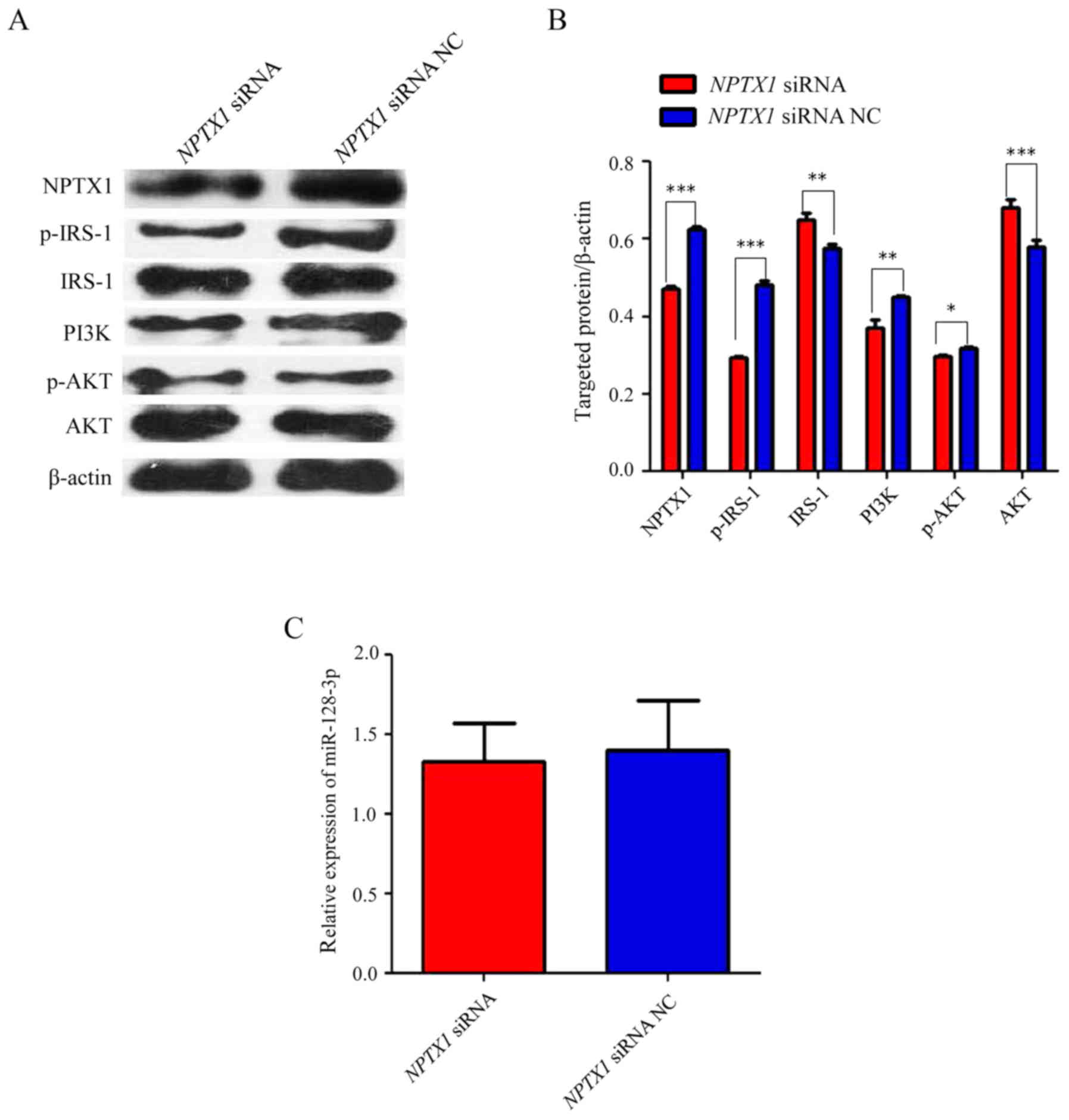 | Figure 5.IRS-1/PI3K/AKT signaling pathway is
suppressed by inhibition of NPTX1. (A and B) The expression of
p-IRS-1, PI3K and p-AKT was significantly suppressed after
transfection with NPTX1 siRNA, which inhibited the expression of
NPTX1 at the translational level. However, the expression of IRS-1
and AKT was significantly increased in the NPTX1 siRNA group in
comparison with that in the NC group. (C) After NPTX1 siRNA was
transfected into the cultured U251 cells, the expression of
miR-128-3p in the NPTX1 siRNA group was not significantly different
from that in the NC group. *P<0.05, **P<0.01, ***P<0.001.
NPTX1, neuronal pentraxin 1; miR, microRNA; siRNA, small hairpin
RNA; NC, negative control. |
Discussion
Glioma is the most prevalent type of malignant
carcinoma of the CNS and has a yearly incidence of almost 6 per
100,000 individuals in the US (18).
Gliomas represent a severe treat to human health and life, and are
therefore intensively studied. During glioma cell migration and
invasion, transforming growth factor-β receptor type 2 exerts a key
role and it may serve as a prognostic predictor and a therapeutic
target for glioma (19). It has been
indicated that sea buckthorn leaf extract suppresses the
proliferation of rat glioma cells via inducing early events of
apoptosis and it may therefore serve as a potential therapeutic for
the treatment of glioma (20). It
has been reported that atorvastatin has cytotoxic effects and
induces autophagy, and simultaneously reduces the migration and
proliferation of human A172 glioma cells (21). However, in spite of the abundancy of
studies on gliomas, the exact molecular mechanisms associated with
gliomas and therapeutic targets leading to an efficient treatment
approach remain to be elucidated, and additional study is therefore
required.
Certain miRNAs are known to be associated with tumor
development and progression through regulating the translation or
causing degradation of their target mRNAs (22,23).
Regarding the elucidation of the exact mechanisms underlying the
pathological processes of various diseases, particularly cancers,
miRNAs may provide a novel approach for their diagnosis and
treatment (24). A variety of
studies revealed that miRNAs are involved in the development and
progression of gliomas. A previous study suggested that low
expression of miR-508-5p was associated with glioma patient
survival; it may therefore serve as a novel prognostic factor and
also lead to novel treatment strategies for glioma (25). It has been proved that overexpression
of miR-497 is associated with resistance to temozolomide in human
glioma cells by targeting mammalian target of rapamycin/B-cell
lymphoma 2 (26). It has also been
reported that miR-302a is an important tumor suppressor of glioma
progression by directly targeting GRB2-associated binding protein,
thus providing novel insight into the molecular mechanisms
underlying the genesis, development and progression of glioma
(27). Furthermore, miR-128-3p was
reported to be closely associated with lung cancer (13), hepatocellular carcinoma (14,15) and
gastric cancer (28). However, the
association between miR-128-3p and glioma has remained to be
determined.
The present study indicated that the expression of
miR-128-3p was significantly lower in glioma tissues than in
paratumor tissues. In addition, in metastatic tissues, the relative
expression of miR-128-3p was also decreased in comparison with that
in non-metastatic tumor tissues. Furthermore, the relative
expression of miR-128-3p was lowest in grade III+IV glioma,
followed by in that in grade II and grade I glioma. These results
demonstrated that miR-128-3p was intimately associated with
gliomas. It has been demonstrated that a synergistic action of
metformin and aspirin regulates the transcriptional profile of
pancreatic cancer cells and NPTX1 was upregulated by >10-fold
(29). Specific gene expression
signatures have been identified in individuals and NPTX1 expression
was decreased in mouse strains susceptible to pulmonary adenomas
(30). NPTX1 was reported to be
intimately associated with colorectal cancer (31), high-grade cervical intraepithelial
neoplasia and cervical cancer (32)
as biomarkers involved in methylation. These previous studies
demonstrated that NPTX1 is closely associated with a variety of
tumor types, but the association between NPTX1 and glioma has
remained to be elucidated. In the present study, the expression of
NPTX1 was significantly elevated in tumor tissues compared with
that in normal tissues. In metastatic tissues, the relative
expression of NPTX1 was also elevated in comparison with that in
non-metastatic tumor tissues. In addition, the relative expression
of NPTX1 was the highest in grade III+IV, followed by that in grade
II and grade I glioma. These results clearly illustrated that NPTX1
was positively associated with glioma and inversely associated with
miR-128-3p expression. A Targetscan analysis, which initially
confirmed that miR-128-3p could target NPTX1, followed by a
luciferase reporter assay and transfection experiments further
confirmed this result. It has been demonstrated that leptin
stimulates the migration of human prostate cancer cells, and that
one of the underlying mechanisms was transcriptional upregulation
of αvβ3 integrin expression through the [long form of the obese
gene (leptin) receptor] OBRl/IRS-1/PI3K/Akt/nuclear factor (NF)-κB
signal transduction pathway (33).
In addition, leptin promotes the migration of chondrosarcoma cells
by increasing αvβ3 integrin expression through the
OBR1/IRS-1/PI3K/Akt/NF-κB signal transduction pathway (34). A previous study suggested that the
IRS-1/PI3K/AKT signaling pathway was positively correlated with
tumor growth, proliferation and migration. In the present study,
after the glioma cells were transfected with miR-128-3p mimics, the
IRS-1/PI3K/AKT signaling pathway was inhibited, while it was
activated by transfection with miR-128-3p inhibitor. In addition,
after NPTX1 was suppressed with specific siRNA, the IRS-1/PI3K/AKT
signaling pathway was inhibited; however, the expression of
miR-128-3p was not affected. These results demonstrated that in the
growth and differentiation of glioma cells, miR-128-3p regulates
the activity of the NPTX1/IRS-1/PI3K/AKT signaling pathway.
Furthermore, the IRS-1/PI3K/AKT signaling pathway was situated
downstream of NPTX1, which was able to modulate IRS-1/PI3K/AKT
signaling pathway activity. However, miR-128-3p could not be
reversely regulated by NPTX1 as expected, which was directly
targeted by miR-128-3p.
The present study provided novel biomarkers,
miR-128-3p and NPTX1, for the diagnosis of glioma, which may also
serve as therapeutic targets in the treatment of glioma. miR-128-3p
was revealed to have a vital role in the regulation of glioma
growth, proliferation through activating the IRS-1/PI3K/AKT
signaling pathway by targeting NPTX1.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QLG was fully responsible for the study design,
experimental adjustment and drafting and finalizing the manuscript.
LMH and BW performed the majority of the experiments. MHZ and YHZ
performed the transfection assays and certain protein measurements
by western blotting and statistical analysis. JGX and GY conducted
the densitometry, statistical analysis and participated in the
coordination of the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the First Hospital of Lanzhou University (Lanzhou, China) and
written informed consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Qaddoumi I, Sultan I and Gajjar A: Outcome
and prognostic features in pediatric gliomas: A review of 6212
cases from the surveillance, epidemiology, and end results
database. Cancer. 115:5761–5770. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McCarthy BJ, Shibui S, Kayama T, Miyaoka
E, Narita Y, Murakami M, Matsuda A, Matsuda T, Sobue T, Palis BE,
et al: Primary CNS germ cell tumors in Japan and the United States:
An analysis of 4 tumor registries. Neurol Oncol. 14:1194–1200.
2012. View Article : Google Scholar
|
|
3
|
Ye ZN, Liu JP, Wu LY, Zhang XS, Zhuang Z,
Chen Q, Lu Y, Liu CG, Zhang ZH, Zhang HS, et al: Retraction notice
to ‘Downregulation of miR-204 expression correlates with poor
clinical outcome of glioma patients’ [Hum Pathol 2017; 63: 46–52].
Hum Pathol. 63:R12017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Keller M, Rüegg A, Werner S and Beer HD:
Active caspase-1 is a regulator of unconventional protein
secretion. Cell. 132:818–831. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Berges R, Balzeau J, Peterson AC and Eyer
J: A tubulin binding peptide targets glioma cells disrupting their
microtubules, blocking migration, and inducing apoptosis. Mol Ther.
20:1367–1377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li B, Huang MZ, Wang XQ, Tao BB, Zhong J,
Wang XH, Zhang WC and Li ST: TMEM140 is associated with the
prognosis of glioma by promoting cell viability and invasion. J
Hematol Oncol. 8:892015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Z, Guo Q, Wang R, Xu G, Li P, Sun Y,
She X, Liu Q, Chen Q, Yu Z, et al: The D domain of LRRC4 anchors
ERK1/2 in the cytoplasm and competitively inhibits MEK/ERK
activation in glioma cells. J Hematol Oncol. 9:1302016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Yang H, Gong B, Jiang C and Yang
L: Combined gene expression and protein interaction analysis of
dynamic modularity in glioma prognosis. J Neurooncol. 107:281–288.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fabian MR and Sonenberg N: The mechanics
of miRNA-mediated gene silencing: A look under the hood of miRISC.
Nat Struct Mol Biol. 6:586–593. 2012. View Article : Google Scholar
|
|
10
|
Samir M, Vaas LA and Pessler F: MicroRNAs
in the host response to viral infections of veterinary importance.
Front Vet Sci. 3:862016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou W, Zou B, Liu L, Cui K, Gao J, Yuan S
and Cong N: MicroRNA-98 acts as a tumor suppressor in
hepatocellular carcinoma via targeting SALL4. Oncotarget.
7:74059–74073. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi E, Choi E and Hwang KC: MicroRNAs as
novel regulators of stem cell fate. World J Stem Cells. 5:172–187.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang R, Liu C, Niu Y, Jing Y, Zhang H,
Wang J, Yang J, Zen K, Zhang J, Zhang CY and Li D: MicroRNA-128-3p
regulates mitomycin C-induced DNA damage response in lung cancer
cells through repressing SPTAN1. Oncotarget. 8:58098–58107.
2016.PubMed/NCBI
|
|
14
|
Huang CY, Huang XP, Zhu JY, Chen ZG, Li
XJ, Zhang XH, Huang S, He JB, Lian F, Zhao YN and Wu GB: miR-128-3p
suppresses hepatocellular carcinoma proliferation by regulating
PIK3R1 and is correlated with the prognosis of HCC patients. Oncol
Rep. 33:2889–2898. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu D, Green B, Marrone A, Guo Y, Kadlubar
S, Lin D, Fuscoe J, Pogribny I and Ning B: Suppression of CYP2C9 by
microRNA hsa-miR-128-3p in human liver cells and association with
hepatocellular carcinoma. Sci Rep. 5:85342015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mets E, Van Peer G, Van der Meulen J,
Boice M, Taghon T, Goossens S, Mestdagh P, Benoit Y, De Moerloose
B, Van Roy N, et al: MicroRNA-128-3p is a novel oncomiR targeting
PHF6 in T-cell acute lymphoblastic leukemia. Haematologica.
99:1326–1333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Easwaran H, Tsai HC and Baylin SB: Cancer
epigenetics: Tumor heterogeneity, plasticity of stem-like states,
and drug resistance. Mol Cell. 54:716–727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu S, Chen H, Zhang Y, Wang C, Liu K, Wang
H and Luo J: MicroRNA-520c inhibits glioma cell migration and
invasion by the suppression of transforming growth factor-β
receptor type 2. Oncol Rep. 37:1691–1697. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim SJ, Hwang E, Yi SS, Song KD, Lee HK,
Heo TH, Park SK, Jung YJ and Jun HS: Sea buckthorn leaf extract
inhibits glioma cell growth by reducing reactive oxygen species and
promoting apoptosis. Appl Biochem Biotechnol. 182:1663–1674. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oliveira KA, Dal-Cim T, Lopes FG, Ludka
FK, Nedel CB and Tasca CI: Atorvastatin promotes cytotoxicity and
reduces migration and proliferation of human A172Glioma cells. Mol
Neurobiol. 55:1509–1523. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dang X, Ma A, Yang L, Hu H, Zhu B, Shang
D, Chen T and Luo Y: MicroRNA-26a regulates tumorigenic properties
of EZH2 in human lung carcinoma cells. Cancer Genet. 205:113–123.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qi P, Cheng SQ, Wang H, Li N, Chen YF and
Gao CF: Serum microRNAs as biomarkers for hepatocellular carcinoma
in Chinese patients with chronic hepatitis B virus infection. PLoS
One. 6:e284862011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shao XJ, Miao MH, Xue J, Xue J, Ji XQ and
Zhu H: The down-regulation of microrna-497 contributes to cell
growth and cisplatin resistance through PI3K/akt pathway in
osteosarcoma. Cell Physiol Biochem. 36:2051–2062. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu YH, Li B, Meng FG and Qiu L:
MiR-508-5p is a prognostic marker and inhibits cell proliferation
and migration in glioma. Eur Rev Med Pharmacol Sci. 21:76–81.
2017.PubMed/NCBI
|
|
26
|
Zhu D, Tu M, Zeng B, Cai L, Zheng W, Su Z
and Yu Z: Up-regulation of miR-497 confers resistance to
temozolomide in human glioma cells by targeting mTOR/Bcl-2. Cancer
Med. 6:452–462. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma J, Yu J, Liu J, Yang X, Lou M, Liu J,
Feng F, Ji P and Wang L: MicroRNA-302a targets GAB2 to suppress
cell proliferation, migration and invasion of glioma. Oncol Rep.
37:1159–1167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ibarrola-Villava M, Llorca-Cardeñosa MJ,
Tarazona N, Mongort C, Fleitas T, Perez-Fidalgo JA, Roselló S,
Navarro S, Ribas G and Cervantes A: Deregulation of ARID1A, CDH1,
cMET and PIK3CA and target-related microRNA expression in gastric
cancer. Oncotarget. 6:26935–26945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yue W, Wang T, Zachariah E, Lin Y, Yang
CS, Xu Q, DiPaola RS and Tan XL: Transcriptomic analysis of
pancreatic cancer cells in response to metformin and aspirin: An
implication of synergy. Sci Rep. 5:133902015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stearns TM, Cario CL, Savage HS, Sundberg
JP, Paigen B and Berndt A: Early gene expression differences in
inbred mouse strains with susceptibility to pulmonary adenomas. Exp
Mol Pathol. 93:455–461. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mori Y, Olaru AV, Cheng Y, Agarwal R, Yang
J, Luvsanjav D, Yu W, Selaru FM, Hutfless S, Lazarev M, et al:
Novel candidate colorectal cancer biomarkers identified by
methylation microarray-based scanning. Endocr Relat Cancer.
18:465–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang N, Eijsink JJ, Lendvai A, Volders HH,
Klip H, Buikema HJ, van Hemel BM, Schuuring E, van der Zee AG and
Wisman GB: Methylation markers for CCNA1 and C13ORF18 are strongly
associated with high-grade cervical intraepithelial neoplasia and
cervical cancer in cervical scrapings. Cancer Epidemiol Biomarkers
Prev. 18:3000–3007. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang CY, Yu HS, Lai TY, Yeh YL, Su CC,
Hsu HH, Tsai FJ, Tsai CH, Wu HC and Tang CH: Leptin increases
motility and integrin up-regulation in human prostate cancer cells.
J Cell Physiol. 226:1274–1282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang SN, Chen HT, Tsou HK, Huang CY, Yang
WH, Su CM, Fong YC, Tseng WP and Tang CH: Leptin enhances cell
migration in human chondrosarcoma cells through OBRl leptin
receptor. Carcinogenesis. 30:566–574. 2009. View Article : Google Scholar : PubMed/NCBI
|