Introduction
In April 2004, the world health organization (WHO)
and the world bank released the road traffic report injury
prevention, which predicted that by 2020, road traffic injuries
will become the third global disease burden, among which fatigue
driving is one of the causes (1).
The direct and indirect total loss and social cost caused by
fatigue are extensive (2).
Currently, there is no convenient fatigue detection method, and
several research groups are actively studying occupational fatigue
detection. In Western developed countries, we see a substantial
investment in this field (3).
However, the objective data are very difficult to obtain, and the
measuring methods and the evaluation indexes are very difficult to
quantify (4,5). The sensitivity of existing fatigue
detection methods is high; however, it is invasive and the signal
paste electrode needs to be extracted.
The percentage of eyelid closure over the pupil over
time (PERCLOS) has a high measurement accuracy, and its detection
of behavioral characteristics is straightforward. Nevertheless, the
detection and identification rules are complex, the pupil
measurement information is difficult to extract, and the detection
of viewing direction and mouth state is greatly affected by the
individual, light and physiological conditions. Additionally, the
method has poor reliability and anti-interference performance.
Previous findings showed that the microarray technology developed
in Japan and the vehicle-mounted module system (6) developed in the USA have improved
detection efficiency. However, it is difficult for the method to be
popularized due to the complex design and high-cost performance
(7,8). Recently, Bhatti et al (9,10)
reported that the level of DNA damage repair in individuals who
work at night (work for 8 h at night and change shifts after 6:00
a.m. the next morning) is very low compared with that in
individuals who sleep at night. The former is only 20% of the
latter. The problem associated with this method is the
inconvenience associated with urine sampling and the fact that this
method can be affected by renal function and drinking. Thus far,
there has been no convenient, reliable, non-invasive and highly
cost-effective fatigue detection method, and it is imperative that
one or a group of clinical markers be identified. This is the
present hotspot and difficulty in research worldwide, as well as a
major public health problem faced by developing countries and
developed countries (11).
The study on fatigue involves the physiology,
molecular biomedicine, preventive medicine, computer technology,
imaging and other fields. In this study, based on the previous
disciplinary studies, the saliva that was clinically collected in a
convenient and non-invasive manner was used (8–10).
Compared with the innovated blood detection and urine detection
affected by kidney and other factors, the saliva secreted by the
glands is more stable and easy to be sampled. The prospect of
saliva component analysis used for fatigue detection has attracted
much attention in China as well as other countries (12). The determination of degree of fatigue
via detecting saliva, developed jointly by the University of Toyama
and enterprises, is simpler than the method of measuring brain
waves. Studies have shown that the salivary gangliosides, cortisol
and other small molecules show regular changes in the fatigue
state, but it is difficult to establish a convenient detection
system. The indices obtained in these studies are usually unstable
small molecular substances, which are not conducive to the later
promotion and application of fatigue detection (13). In this study, the mature saliva time
of flight mass spectrometry at present was performed to identify
the different high-molecular-weight peptide segments in the fatigue
and non-fatigue states to provide an important theoretical basis
for the study on more convenient and stable fatigue markers. The
type of fatigue investigated in the current study was the fatigue
caused by continuous work. After volunteers' continuous work,
fatigue Theta waves were monitored, and the changes of the peptide
spectrum was detected in saliva by MALDI-TOF-MS at different
time-points. The fatigue wave of ECG was used as the diagnostic
criterion to identify fatigue bio-markers. The current study
investigated occupational fatigue markers caused by continuous work
based on the diagnostic criteria of chronic fatigue syndrome
previously described (23). Fukuda
et al (14) developed the
diagnostic standard of fatigue that was recognized as a golden
standard by the international medical community, as well as EEG
Theta wave, ECG HF and LF/HF ratio changes.
Materials and methods
Inclusion and exclusion criteria
Inclusion criteria were: normal healthy men and
women aged 30–45 years, who signed the informed consent, and who
were without organic diseases and chronic fatigue symptoms.
Exclusion criteria were according to Breithaupt-Groegler et
al (15): i) individuals with
persistent or recurrent fatigue for >6 months; ii) individuals
with sore throat; iii) individuals with neck or axillary lymph node
swelling and pain; iv) individuals with muscle pain; v) individuals
with multiple non-arthritic pain; vi) individuals with headache;
vii) individuals with sleep disorders; viii) individuals with
discomfort for >24 h after fatigue; ix) individuals with oral
disease; x) individuals not taking drugs and dietary nutritional
supplements in the last 3 months, and smokers and those who
received tooth filling treatment during the week prior to the
sampling date were excluded.
Participants
Only 10 healthy volunteers (5 males and 5 females)
aged 30–45 years were enrolled due to the high experimental cost.
The subjects volunteered to participate in this study and signed
the informed consent. Saliva samples were collected from April 10,
2015 to April 12, 2015. The present study was approved by the
Ethics Committee of Hebei University of Engineering, Affiliated
Hospital, College of Medicine (Handan, China) on March 12, 2014,
and informed consent was signed (Clinical Trial Registration no.:
ChiCTR-DCD-14005746).
Prior to the study, the 10 subjects had good sleep
and underwent an electroencephalogram (EEG) while awake. Since
there was no fatigue wave, the subjects were considered to have no
fatigue or MS/CFS. Subsequently, they began to work continuously to
cause fatigue. Physiological responses were also measured using a
fatigue scale. At different time-points, the fatigue response
gradually increased and the subjects' saliva was collected.
MALDI-TOF-MS peptide peak detection was performed at the end.
Saliva sample collection
i) Saliva sample collection: A Saliva collection
tube (ISO9001 certified; Cryo.s™; Greiner Bio-One GmbH,
Frickenhausen, Germany) was used. The samples were collected by
physicians once prior to shifts commencing and once after at least
18 h. Before sampling, the mouth was rinsed three times with
distilled water (30 ml NS for 1 min) and subjects sat quietly for 5
min in front of a mirror in an upright sitting position; the head
slightly leaned forward and the eyes were kept open for masticatory
movement to stimulate the secretion of saliva. When a certain
amount of saliva was accumulated in the lower jaw, the tongue
reached the upper jaw, the mouth was opened and the tongue was
naturally lifted to form a V shape in the lower lips, allowing the
saliva to flow into the saliva collection tube prepared in advance.
Then 2 ml of saliva was collected and the interval between sampling
and eating and the recipe characteristics were recorded. ii) Saliva
sample storage: The sample was labeled by the science assistant,
and stored at −70°C. iii) Saliva sample detection was conducted by
the National Key Laboratory of Institute of Infectious Diseases
Prevention and Control of Chinese Center for Disease Control and
Prevention (MALDI-TOF MS).
EEG detection and data collection
The EEG was obtained after at least 18 h to verify
the presence of the fatigue wave (the slow wave increased and fast
wave decreased; i.e., the quantities of delta, alpha and beta waves
were compared). When the fatigue wave appeared, the saliva was
immediately collected. EEG was monitored and obtained using the
SOLAR-RTA and SOLAR-BFM brain function monitoring system
(manufactured by SOLAR Electronic Technology Limited) for real-time
analysis and comprehensive analysis of brain function.
Saliva flight mass spectrometry
Reagents and instruments: WCX magnetic beads kit;
α-cyano-4-hydroxycinnamic acid (HCCA) (both from Bruker, Billerica,
MA, USA), mass concentration: 0.3 g/l; ethanol (chromatographic
grade)/acetone (chromatographic grade) = 2/1, prepared freshly;
AutoFlexIII type matrix assisted laser desorption ionization time
of flight (MALDI-TOF) mass spectrometer (Bruker). WCX magnetic bead
processing: WCX magnetic beads kit was taken from the refrigerator
at 4°C and treated according to the protocol. Finally, the eluted
peptide sample solution was transferred into a clean 0.5 ml tube
for mass spectrometric analysis. For sample application and mass
spectrometric analysis, 1 µl peptide sample solution was separated
by magnetic beads and 1 µl saliva was taken and dried at room
temperature, followed by the addition of 1 µl HCCA matrix solution
in the concentration of 3 g/l (dissolved in 50% acetonitrile and 2%
trifluoroacetic acid). The prepared sample panels were placed on
the MALDI-TOF mass spectrometer for analysis. Under the linear
mode, the relative molecular weight between 1,000 and 10,000 Da
with a laser energy of 20% was collected for a total of 400 shots.
Beads enrichment (MB) saliva and saliva supernatant (stock
solution) taken after centrifugation at 12,000 × g for 10 min at
4°C were used for mass spectrometric analysis. A scan was performed
50 times, and the peptide mass fingerprint (PMF) was obtained.
Quality control: Before the test, the relevant group
members were trained. Saliva was collected and the fatigue state
was maintained. The subjects were informed about the details, and
the chest cards were issued to the volunteers. The personnel
responsible for saliva collection guidance and supervision were
assigned. Each member of saliva sampling needed to accept the
strict and unified training. The subjects could collect their own
samples, and a specially-assigned person was responsible for EEG
and supervision of sleep deprivation.
Statistical analysis
The molecular weight of collection ranged from 1 and
15,000 Da, and the standard product was selected for molecular
weight correction. To establish the model and conduct the blind
screening test, the genetic algorithm (GA) model was established
and verified using the Clin Pro Tools (version 3.3.0) software
provided by Bruker.
Results
Mass spectrograms of samples
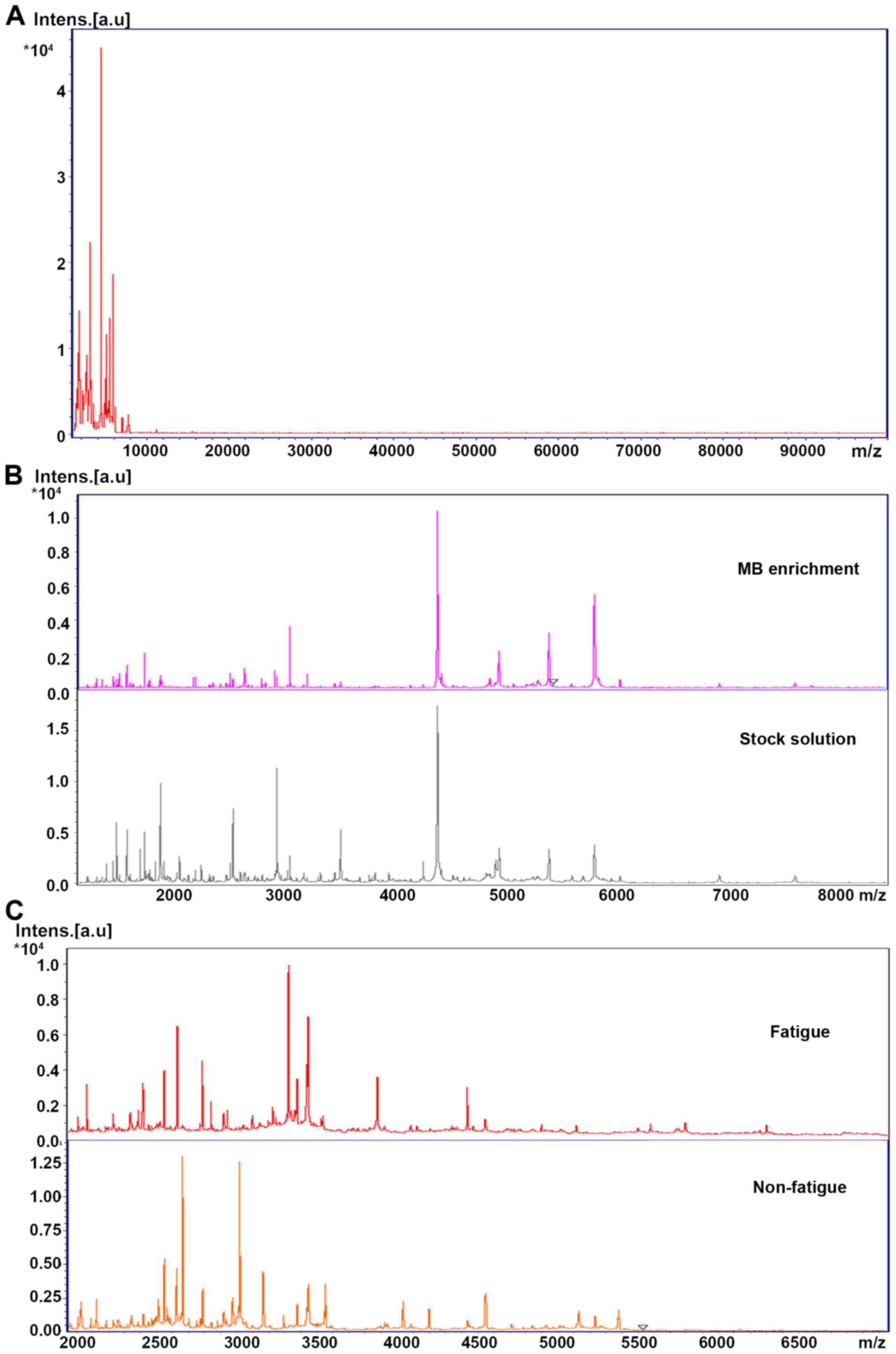
Both MB and direct sampling can detect spectrograms
between 1,000 and 15,000 Da (Fig.
1A), and some peaks which had little effect on the results were
lost in the enrichment (Fig. 1B).
The early and late spectrograms of each person were different
(Fig. 1C), and the cross validation
was not conducted due to the limited sample size, therefore two
spectrograms were taken for each sample. Twenty early and 20 late
spectrograms were used for modeling analysis. Three different
peptides found in the two groups of stock solution samples have
different expression in the fatigue and non-fatigue groups.
With the increase of fatigue, the number of peptides
collected in saliva at different time-points increased
significantly, especially in the range of high molecular weight
(>5,000 Da). Under the same collecting parameters, when the
molecular weight was >3,000, the peptide abundance in the
fatigue population was significantly increased.
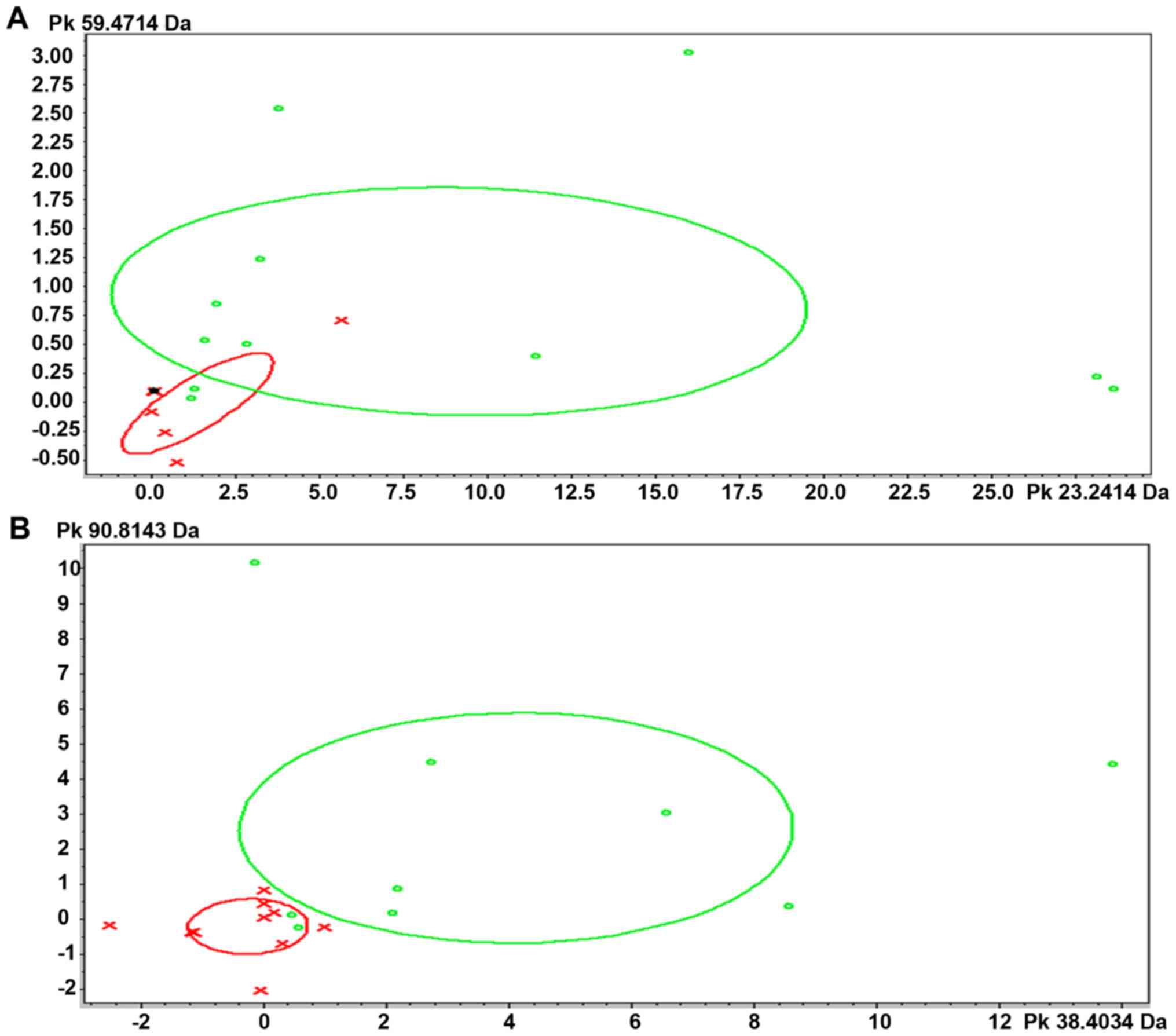
Model identification
The model was established via the mass spectra of MB
and supernatant (stock solution) directly collected after
centrifugation. A GA model was constructed to distinguish the
fatigue and non-fatigue groups. The two parameters of
cross-validation and recognition capability were calculated by
ClinProTools (Bruker). For the GA typing model, the crossvalidation
values, which reflect the model's ability to handle variability
among test spectra, and the recognition capability value, which
reflects the model's ability to correctly identify its component
spectra, were all 100%. The cross validity of the MB model was
92.06%, while that of the stock solution model was 95.49% (Table I). The strain distribution map based
on the GA classification model also showed that the fatigue and
non-fatigue groups could be divided into one of two categories
based on their PMFs (Fig. 2).
 | Table I.Basic information, cross validity (%)
and acceptability (%) of the two models. |
Table I.
Basic information, cross validity (%)
and acceptability (%) of the two models.
| Model name | Algorithm | Cross
validation | Recognition
capability |
|---|
| MB | GA | 92.06% | 100% |
| Stock solution | GA | 95.49% | 100% |
Discussion
The damage caused by fatigue every year accounts for
21.7% in the global occupational injuries, and 57% of traffic
associated deaths can be linked to fatigue (16). In China, approximately 600,000
individuals lose their lives annually due to this type of accident
(17). However, there is no
non-invasive and convenient fatigue testing technology, similar to
that for detecting drivers' alcohol level. In the Center for Health
Studies of University of Sydney, the EEG signals collected were
treated with artificial neural network and it was found that Theta
wave was increased significantly under fatigue state, which
provided the basis for the detection of fatigue (18). EEG is also known as the golden
standard for testing fatigue, which can be used as the reference of
diagnostic test (19). However, the
signal must be extracted using the electrode in contact with the
human body (20). At present, there
is no golden standard for the diagnosis of fatigue, and most of the
tools for diagnosing fatigue are fatigue scale, such as in
literature (21–23). Fukuda et al (14) developed the diagnostic standard of
fatigue that was recognized as a golden standard by the
international medical community, as well as EEG Theta wave, ECG HF
and LF/HF ratio changes. The fatigue diagnostic devices in the past
were mainly the driving anti-sleep device (24), eye movement measurement system
(25), automobile driver fatigue
evaluation method (26) and
high-speed image processing chip TMS320DM642 fatigue detection and
early warning system (TI). The cost associated with these methods
is high and its popularity is also limited (27). In the present study, the
time-of-flight mass spectrometer was used for analysis of saliva
samples obtained from a number of individuals under the limited
fatigue state. Significant differences were found in the detected
spectrograms, and these spectrograms were obtained within
2,000-15,000 Da by the MB and direct sampling. Several differential
peptides were found and the saliva peptide mapping showed
significant changes with a certain rule with the appearance of
fatigue. These results proved the feasibility of fatigue
identification via the analysis of saliva marker peptide. The model
was established using the mass spectrums of MB and supernatant
(stock solution) directly collected after centrifugation. The cross
validity of MB model was 92.06%, while that of stock solution model
was 95.49%.
In recent years, saliva proteomics research has
attracted wide attention (28), and
309 different proteins have been identified successfully using the
proteomics technique from normal human whole saliva. By dividing
the functions of these proteins, proteins with unknown functions
account for the most (28.7%), the immune-related proteins account
for 21%, whereas proteins with functions linked to replication and
repair accounted for 1.6%. In addition, proteins involved in the
cell dynamics and those secretion-related account for 4.8%,
transcription and ribose-related proteins account for 2.3%, the
cell proliferation and cell cycle-related proteins account for
4.2%, signal transduction proteins for 9.7%, metabolism-related
protein for 5.2%, and cytoskeleton and intima-related proteins for
7.1% (29). The unique and rich
proteins in saliva, as a body fluid with a complex ingredient and a
variety of biological functions, are undoubtedly the potential
source for ideal biomarkers for tumors and other diseases. For a
long time, the original technology limited the flux analysis and
accurate determination of oral saliva protein; thus, the biological
functions of most saliva proteins are unknown, and the potential
role of saliva in the diagnosis and prognosis of human diseases is
not apparent. With the application of high-throughput and
high-precision proteomics, it has been possible to apply biomarkers
of saliva proteins in the early diagnosis and prevention of
different illnesses, bio-protein-targeted therapy and prognostic
monitoring (30). This also served
as the theoretical basis for selecting saliva in the present
study.
Fatigue is a complex biochemical process, and a
single biochemical index cannot accurately determine whether the
body suffers from fatigue or not. The proteomics study of saliva
provides a new direction for the detection of fatigue through
saliva. Saliva contains a variety of bioactive ingredients,
including some biomarkers that can be used for disease diagnosis
(31). Compared with blood and
urine, the collection process of saliva is simpler and it is
completely non-invasive. The role of saliva, instead of blood and
urine, in the non-invasive diagnosis of disease, has become
increasingly apparent. Studies have shown that small molecules,
such as salivary gangliosides (molecular weight: 1,563.85 Da) and
cortisol (362.47 Da), are likely to show regular changes under
fatigue state, but it is difficult to establish a convenient
detection system (32). The prospect
of saliva component analysis used for fatigue detection has begun
to attract much attention in China as well as other countries.
Compared with the method that uses brain waves for the
determination of fatigue degree, saliva is simpler and less
invasive. This method was originally developed by University of
Toyama and enterprises.
The mechanism of fatigue is very complex. It has
been argued that fatigue was the result of neuro-endocrine-immune
network dysfunction caused by a variety of infections and stress,
while recent studies have further found that the genetic and
metabolic factors may also be involved (33). Many studies also investigated the
changes in blood chemical composition during the long-term
exercise, however the exact pathogenesis is unclear and biomarkers
to be used for diagnosis, prevention and treatment has not been
found yet. Currently, there is no universally accepted assessment
criterion for fatigue. An ideal assessment method for fatigue
degree determination can be used for real-time non-invasive
assessment with high sensitivity and stability, and can effectively
prevent and reduce risks associated with fatigue. Saliva can be
obtained in a non-invasive manner. There are several reports on
changes in saliva composition (34),
most of which have been focused on the changes in the levels of
metabolic products in the saliva of athletes and soldiers. Besides,
the study on fatigue in the medical field is also a marginalized
problem (35), and the emphasis lies
on the analysis of small molecular substances with the molecular
weights below 1,000 Da (36–39). The fatigue-related indexes found in
previous studies included cortisol, testosterone and
dehydroepiandrosterone (40).
Indexes obtained in those studies are small and unstable molecules,
which were not conducive to the promotion and application of
fatigue testing. For example, Kume et al (41) established the fatigue rat model and
found that valine (molecular weight: 117.15 Da), leucine (131.18
Da) and isoleucine (131.17 Da) were significantly increased in the
fatigue group compared with those in controlled feeding group, but
the levels of citrulline and hydroxyproline were significantly
reduced. The level of plasma total nitric oxide in fatigue group
was increased, suggesting systemic oxidative stress. In addition,
the plasma metabolites in fatigue group were involved in citric
acid cycle, such as cis-aconitic acid and isocitrate (42). Mathematical model analysis was
performed in several studies to screen the different indexes from
the healthy control group from dozens of metabolites, such as
cis-aconitate, socitrate, citrate and malate (43). Michael et al (44) found through metabolomics analysis
that there were metabolites used as the fatigue markers in the
saliva of athletes after three-day soccer game, namely
3-methylhistidine (short form: 3M-His), glucose-1-phosphate (short
form: G-1-P), glucose 6-phosphate (short form: G-6-P) and some
amino acid compound. However, the diagnostic efficacy of these
markers was not further analyzed. Recently, Kataoka et al
(45) measured the levels of
testosterone (TES), cortisol (CRT) and dehydroepiandrosterone
(DHEA) in saliva using the automatic in-tube solid-phase
micro-extraction (SPME) and liquid chromatography-tandem mass
spectrometry (LC-MS/MS) and found that SPME and LC-MS/MS had a good
linear relation (R≥0.9998), precision (intra-day and inter-day
precision was 4.9 and 8.5%) and detection sensitivity (limit of
quantitation was ~0.01, 0.03 and 0.29 ng/ml saliva) after the
treatment of 40 µl samples. Furthermore, this method was also used
to analyze the changes in the levels of TES, CRT and DHEA in saliva
under pressure and fatigue, showing the advantages of
mass-spectrometric technique.
Changes in such small molecules may be associated
with fatigue, but these molecules are also susceptible to diets and
other health conditions. At the same time, these small molecule
markers often lack good antigenicity, and are not easy to detect
using the simple methods, such as immunology or biosensor. In this
study, the time-of-flight mass spectrometer was used and the
results showed that there were significant differences in the
spectrograms obtained by the MB and direct sampling within
2,000-15,000 Da. Several differential peptides were detected
between groups and the saliva peptide mapping showed significant
changes. These results proved the feasibility of fatigue
identification via the analysis of saliva marker peptide. The
molecular weight range of the markers obtained in this study laid a
good foundation for finding the large molecular weight proteome in
the later stage. Moreover, the large molecular weight indexes are
stable, providing a theoretical basis for finding the fatigue
detection indexes similar to the markers used in alcohol
detection.
According to the ‘Nihon Keizai Shimbun’ (46), the number of special glucocorticoids
in blood may increase during the fatigue, and saliva will secrete
α-amylase. The so-called ‘heart instrument’ is equipped with a
chip: The human saliva is placed on the chip, and then the chip is
pasted to the instrument to detect the degree of fatigue. The
saliva samples of Wiewelhove et al (47) were obtained from 9 cyclists who
participated in a cross-study study aiming at simulating the
long-term manual labor, military action and fire rescue during the
rest period. The results of this study showed that the distribution
difference in fatigue biomarker index (FBI) of subjects was
decreased with the increase of the time of physical activity. The
self-reported fatigue degree had a significantly positive
correlation with the participants' CFS and serum leptin (16,000 Da)
in healthy control, supporting our main hypothesis. The machine
learning algorithm can distinguish the 78.3% low fatigue days in
the high CFS group (48). Related
studies found through the conventional evaluation of fatigue and
recovery during the high-intensity interval training that creatine
kinase (42,000 Da) had statistically significant difference during
the fatigue and rest periods (47).
The above research confirmed the correctness of results in this
study indirectly.
Due to the high cost of this study, the sample size
was not large. After receiving fund support, we aim to expand the
sample size and carry out further study in different groups of
individuals.
We concluded that there were different peaks within
the molecular weight of 2,000-15,000 Da, which laid a good
foundation for the later study on proteomics-based fatigue markers.
The idea of fatigue-related biomarkers within 2,000-15,000 Da in
this study was also characterized by the stable composition under
test, less interference factors in vivo and easy conversion
and popularization of the detection system. The exploration of
fatigue-related biomarkers has theoretical significance and
application prospects. In the future, the fatigue queue will be
built to find the stable and specific biomarkers and its
combinations under the fatigue state and establish the
fatigue-related biomarker evaluation model using the mature mass
spectrometry high-throughput sample analysis technique. Compared
with the previous studies on saliva metabolomics, proteomics
results are more stable. In further research, it is expected to
systematically establish the saliva identification spectrum that
can be used for fatigue identification, so as to provide a
scientific basis for the further realization of convenient fatigue
detection methods based on the biosensor technique, which has both
important theoretical and practical significance.
Acknowledgements
Not applicable.
Funding
This research was supported by the National Natural
Science Foundation of China (Project no. 81373095. ‘Selection and
Valuation of Fatigue Related Biological Marker in the Human
Saliva’.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YX and DX drafted the manuscript. YX, DX, HZ and LH
were mainly devoted to collecting and interpreting the data. YG and
XP helped with electroencephalogram detection. XG, ZL and JZ were
responsible for the saliva flight mass spectrometry analysis. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Hebei University of Engineering, Affiliated Hospital, College of
Medicine (Handan, China). Signed written informed consents were
obtained from the volunteers.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Peden M, Scurfield R, Sleet D, Mohan D,
Hyder AA, Jarawan E and Mathers C: World report on road traffic
injury prevention. World Health Organisation; Geneva: pp.
2172004
|
|
2
|
Sansó N, Galiana L, Oliver A, Pascual A,
Sinclair S and Benito E: Palliative care professionals' inner life:
Exploring the relationships among awareness, self-care, and
compassion satisfaction and fatigue, burnout, and coping with
death. J Pain Symptom Manage. 50:200–207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Keltai M: Johnson JA, Kowey PR, Ried LD
and Tueth M: INVEST substudies: Design and patient characteristics.
Clin Cardiol. 24 (Suppl 11):V9–V11. 2001.PubMed/NCBI
|
|
4
|
Yang G, Lin Y and Bhattacharya P: A driver
fatigue recognition model using fusion of multiple features. 2005
IEEE International Conference on Systems, Man and Cybernetics. 2.
IEEE Xplore, Waikoloa; pp. 1777–1784. 2006
|
|
5
|
Ting PH, Hwang JR, Doong JL and Jeng MC:
Driver fatigue and highway driving: A simulator study. Physiol
Behav. 94:448–453. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wishaw KJ, Munford BJ and Roby HP: The
CareFlight Stretcher Bridge: A compact mobile intensive care unit.
Anaesth Intensive Care. 18:234–238. 1990.PubMed/NCBI
|
|
7
|
Sommer D and Golz M: Evaluation of PERCLOS
based current fatigue monitoring technologies. Conf Proc IEEE Eng
Med Biol Soc. 2010:4456–4459. 2010.PubMed/NCBI
|
|
8
|
Daza IG, Bergasa LM, Bronte S, Yebes JJ,
Almazán J and Arroyo R: Fusion of optimized indicators from
Advanced Driver Assistance Systems (ADAS) for driver drowsiness
detection. Sensors (Basel). 14:1106–1131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhatti P, Cushing-Haugen KL, Wicklund KG,
Doherty JA and Rossing MA: Nightshift work and risk of ovarian
cancer. Occup Environ Med. 70:231–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhatti P, Mirick DK, Randolph TW, Gong J,
Buchanan DT, Zhang JJ and Davis S: Oxidative DNA damage during
night shift work. Occup Environ Med. 74:680–683. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Malmir K, Olyaei GR, Talebian S, Jamshidi
AA and Ganguie MA: Effects of peroneal muscles fatigue on dynamic
stability following lateral hop landing: Time to stabilization vs.
dynamic postural stability index. J Sport Rehabil. Sep
27–2018.(Epub ahead of print). PubMed/NCBI
|
|
12
|
Zanotti L, Paderno A, Piazza C, Pagan E,
Bignotti E, Romani C, Bandiera E, Calza S, Del Bon F, Nicolai P, et
al: Epidermal growth factor receptor detection in serum and saliva
as a diagnostic and prognostic tool in oral cancer. Laryngoscope.
127:E408–E414. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Q and Xiong ZY: The biologic
mechanism of the central fatigue. J Phys Educ. 10:42–44. 2003.(In
Chinese).
|
|
14
|
Fukuda K, Straus SE, Hickie I, Sharpe MC,
Dobbins JG and Komaroff A; International Chronic Fatigue Syndrome
Study Group, : The chronic fatigue syndrome: A comprehensive
approach to its definition and study. Ann Intern Med. 121:953–959.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Breithaupt-Groegler K, Coch C, Coenen M,
Donath F, Erb-Zohar K, Francke K, Goehler K, Iovino M, Kammerer KP,
Mikus G, et al: Who is a ‘healthy subject’? - consensus results on
pivotal eligibility criteria for clinical trials. Eur J Clin
Pharmacol. 73:409–416. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dasari D, Shou G and Ding L: ICA-derived
EEG correlates to mental fatigue, effort, and workload in a
realistically simulated air traffic control task. Front Neurosci.
11:2972017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Polo-Kantola P, Laine A, Aromaa M, Rautava
P, Markkula J, Vahlberg T and Sillanpää M: A population-based
survey of sleep disturbances in middle-aged women-associations with
health, health related quality of life and health behavior.
Maturitas. 77:255–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu W, Li Y, Sun Y and Mosleh A: A model of
BGA thermal fatigue life prediction considering load sequence
effects. Materials (Basel). 9:E8602016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kappel SL, Looney D, Mandic DP and Kidmose
P: Physiological artifacts in scalp EEG and ear-EEG. Biomed Eng
Online. 16:1032017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rottoli M, La Gioia S, Frigeni B and
Barcella V: Pathophysiology, assessment and management of multiple
sclerosis fatigue: An update. Expert Rev Neurother. 17:373–379.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De Vries J, Michielsen H, Van Heck GL and
Drent M: Measuring fatigue in sarcoidosis: the Fatigue Assessment
Scale (FAS). Br J Health Psychol. 9:279–291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miao Y, Liu X, Liu W, Xie H and Deng G:
Initial revision of the Chinese version of multidimensional fatigue
inventory-20 in medical staff of military basic level. Chin Ment
Health J. 22:658–660, 668. 2008.(In Chinese).
|
|
23
|
Schwartz JE, Jandorf L and Krupp LB: The
measurement of fatigue: A new instrument. J Psychosom Res.
37:753–762. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
King LM, Nguyen HT and Lal SK: Early
driver fatigue detection from electroencephalography signals using
artificial neural networks. Conf Proc IEEE Eng Med Biol Soc.
1:2187–2190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu R and Wang H: Detection of driving
fatigue by using noncontact EMG and ECG signals measurement system.
Int J Neural Syst. 24:14500062014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hostens I and Ramon H: Assessment of
muscle fatigue in low level monotonous task performance during car
driving. J Electromyogr Kinesiol. 15:266–274. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jarrett MA, Gable PA, Rondon AT, Neal LB,
Price HF and Hilton DC: An EEG Study of Children With and Without
ADHD Symptoms: Between-group differences and associations with
sluggish cognitive tempo symptoms. J Atten Disord. Aug 1–2017.(Epub
ahead of print). View Article : Google Scholar
|
|
28
|
Philip P, Sagaspe P, Taillard J, Moore N,
Guilleminault C, Sanchez-Ortuno M, Akerstedt T and Bioulac B:
Fatigue, sleep restriction, and performance in automobile drivers:
A controlled study in a natural environment. Sleep. 26:277–280.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu YL, Gong YN, Xiao D, Zhao CX, Gao XH,
Peng XH, Xi AP, He LH, Lu LP, Ding M, et al: Discovery and
identification of fatigue-related biomarkers in human saliva. Eur
Rev Med Pharmacol Sci. 22:8519–8536. 2018.PubMed/NCBI
|
|
30
|
Lim SM and Chia SE: The prevalence of
fatigue and associated health and safety risk factors among taxi
drivers in Singapore. Singapore Med J. 56:92–97. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Korostoff A, Reder L, Masood R and Sinha
UK: The role of salivary cytokine biomarkers in tongue cancer
invasion and mortality. Oral Oncol. 47:282–287. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Francischetti IM, Ma D, Andersen JF and
Ribeiro JM: Evidence for a lectin specific for sulfated glycans in
the salivary gland of the malaria vector, Anopheles gambiae. PLoS
One. 9:e1072952014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Singh VP, Khandelwal B and Sherpa NT:
Psycho-neuro-endocrine-immune mechanisms of action of yoga in type
II diabetes. Anc Sci Life. 35:12–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun W, Zhang X, Peeta S, He X, Li Y and
Zhu S: A self-adaptive dynamic recognition model for fatigue
driving based on multi-source information and two levels of fusion.
Sensors (Basel). 15:24191–24213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu S, Loo JA and Wong DT: Human saliva
proteome analysis and disease biomarker discovery. Expert Rev
Proteomics. 4:531–538. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Castro M, Elias PC, Martinelli CE Jr,
Antonini SR, Santiago L and Moreira AC: Salivary cortisol as a tool
for physiological studies and diagnostic strategies. Braz J Med
Biol Res. 33:1171–1175. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Makary MA and Daniel M: Medical error -
the third leading cause of death in the US. BMJ. 353:i21392016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
You L, Ren J, Yang B, Regenstein J and
Zhao M: Antifatigue activities of loach protein hydrolysates with
different antioxidant activities. J Agric Food Chem.
60:12324–12331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu CC, Liao MH, Chen SJ and Yen MH:
Tetramethylpyradizine prevents inducible NO synthase expression and
improves survival in rodent models of endotoxic shock. Naunyn
Schmiedebergs Arch Pharmacol. 360:435–444. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Al-Turki HA: Dehydroepiandrosterone
supplementation in women undergoing assisted reproductive
technology with poor ovarian response. A prospective case-control
study. J Int Med Res. 46:143–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kume S, Yamato M, Tamura Y, Jin G, Nakano
M, Miyashige Y, Eguchi A, Ogata Y, Goda N, Iwai K, et al: Potential
biomarkers of fatigue identified by plasma metabolome analysis in
rats. PLoS One. 10:e01201062015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jones RL, Owen LJ, Adaway JE and Keevil
BG: Simultaneous analysis of cortisol and cortisone in saliva using
XLC-MS/MS for fully automated online solid phase extraction. J
Chromatogr B Analyt Technol Biomed Life Sci 881–882. 42–48. 2012.
View Article : Google Scholar
|
|
43
|
Ra SG, Maeda S, Higashino R, Imai T and
Miyakawa S: Metabolomics of salivary fatigue markers in soccer
players after consecutive games. Appl Physiol Nutr Metab.
39:1120–1126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Michael DJ, Valle B, Cox J, Kalns JE and
Fogt DL: Salivary biomarkers of physical fatigue as markers of
sleep deprivation. J Clin Sleep Med. 9:1325–1331. 2013.PubMed/NCBI
|
|
45
|
Kataoka H, Ehara K, Yasuhara R and Saito
K: Simultaneous determination of testosterone, cortisol, and
dehydroepiandrosterone in saliva by stable isotope dilution on-line
in-tube solid-phase microextraction coupled with liquid
chromatography-tandem mass spectrometry. Anal Bioanal Chem.
405:331–340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Michael DJ, Daugherty S, Santos A, Ruby BC
and Kalns JE: Fatigue biomarker index: An objective salivary
measure of fatigue level. Accid Anal Prev. 45 (Suppl):68–73. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wiewelhove T, Raeder C, Meyer T, Kellmann
M, Pfeiffer M and Ferrauti A: Markers for routine assessment of
fatigue and recovery in male and female team sport athletes during
high-intensity interval training. PLoS One. 10:e01398012015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Stringer EA, Baker KS, Carroll IR, Montoya
JG, Chu L, Maecker HT and Younger JW: Daily cytokine fluctuations,
driven by leptin, are associated with fatigue severity in chronic
fatigue syndrome: evidence of inflammatory pathology. J Transl Med.
11:932013. View Article : Google Scholar : PubMed/NCBI
|