Introduction
Intrahepatic cholestasis of pregnancy (ICP) is a
specific disease during pregnancy, its clinical manifestations
include cutaneous pruritus, formation of jaundice and abnormal
hepatic function (1). The causes of
the disease are not quite clear yet, but this syndrome may severely
threaten fetal growth and development, result in intrauterine fetal
distress and even intrauterine death, and cause premature delivery,
neonatal hypoxia-ischemia and asphyxia (2). Most scholars consider that the
pathogenesis of this disease is notably correlated with maternal
bile acid level, especially the CG level in the body of pregnant
women (3). Long-term high bile acid
level, especially high CG level will cause significant spasm in
vessels on the surface of placental villi (4), and the increased resistance caused by
it will lead to reduced blood flow passing through the area of
placenta intervillous space and decreased oxygenation (4), and further bring about insufficient
fetal perfusion (5), functional
hypoxia-ischemia, difficulty in transshipment of oxygen molecules
and nutrient substance from maternal circulation. This will finally
result in intrauterine fetal distress and affect growth and
development of fetuses (6).
Currently, the treatment for ICP is mainly
symptomatic support measures. Fetal development condition is
closely monitored, and manual intervention is implemented to
terminate pregnancy (7). Its primary
mechanism is that long-term high bile acid level, especially high
CG level, affects the growth and development of fetuses and may
even result in intrauterine fetal distress (8). Although there are many studies on ICP,
there are few systematic correlation studies on effects of ICP on
hepatic function, changes in the inflammatory cytokine level in the
body of pregnant women and fetal outcomes. In order to better
answer the national fundamental policy of better prenatal and
postnatal care, enhance pregnancy outcomes and improve safety of
pregnant and lying-in women and newborns during the perinatal
period, this investigation mainly evaluated the deficiencies
mentioned above, so as to better guide clinical treatment and
improve neonatal outcomes.
Patients and methods
General data
A total of 663 pregnant women admitted to Daqing
Longnan Hospital (Daqing, China) from July 2016 to December 2017
were selected, and patients complicated with inflammatory diseases
of the liver resulted from various factors, gestational diabetes,
gestational hypertension, mental disease or abnormal coagulation
function, those with thyroid dysfunction during pregnancy, those
previously receiving liver- or gall-related operations, or
long-term drinkers were excluded. At the same time, according to
the diagnostic criteria for ICP in the Obstetrics and Gynecology
(7th edition) published in the People's Medical Publishing House
(China) (9), there were 40 patients
diagnosed with ICP, all of whom suffered from no liver-, gall- or
skin-related diseases before pregnancy. After the second and third
trimesters of pregnancy, ICP was manifested as significant and
persistent pruritus, which often occurred in the proximal hands,
feet and limbs, and even mild jaundice was seen. Increased serum
cholic acids were found in the biochemical examination. In
particular, the index of serum cholylglycine (CG) was more than 30
mg/l, and a mild to moderate increase could be found in bilirubin.
The above symptoms and signs returned to normal after
pregnancy.
All the patients and their family members signed the
inclusion consent, and this study was approved by the Ethics
Committee of the Daqing Longnan Hospital. These patients were aged
19–40 years with an average of 29.5±0.3 years. Times of pregnancy:
25 cases of primiparity, 15 cases of re-pregnancy and 10 cases who
had previously undergone cesarean section. Additionally, 40
patients with normal pregnancy aged 19–40 years with a mean age of
29.6±0.3 years were selected as the normal group. Times of
pregnancy: 26 cases of primiparity, 14 cases of re-pregnancy and 9
cases who had previously undergone cesarean section. There were no
statistically significant differences in age, times of pregnancy
and whether they had received previous cesarean section between the
two groups (P>0.05). Comparisons of birth weight, gestational
week at birth, Apgar score at birth, grade II or above amniotic
fluid contamination, intrauterine fetal distress and neonatal
jaundice between the two groups are detailed in Table I. All the included patients were
divided into two groups according to whether their hepatic function
was normal or not. Among them, 40 patients with normal hepatic
function aged 19–40 years with a mean age of 29.4±0.3 years were
included into the normal hepatic function group. Times of
pregnancy: 26 cases of primiparity, 14 cases of re-pregnancy and 11
cases who had previously received cesarean section. Forty patients
with abnormal hepatic function aged 19–40 years with an average age
of 29.4±0.4 years were included into the abnormal hepatic function
group. Times of pregnancy: 27 cases of primiparity, 13 cases of
re-pregnancy and 12 cases who previously had received cesarean
section.
 | Table I.Comparisons of relevant data between
the two groups of newborns (mean ± SD). |
Table I.
Comparisons of relevant data between
the two groups of newborns (mean ± SD).
| Variables | Birth weight (g) | Gestational week at
birth (week) | Apgar score at birth
(point) |
|---|
| Observation
group | 2,156.5±26.1 | 34.1±1.1 | 6.2±0.3 |
| Normal group | 3,158.9±33.6 | 38.9±1.2 | 8.2±0.4 |
| t value | 149.009 | 18.649 | 25.298 |
| P-value | <0.001 | <0.001 | <0.001 |
Differences in age, times of pregnancy and a history
of implementation of cesarean section between the two groups were
not statistically significant (P>0.05). In addition, according
to whether inflammatory cytokines [with high-sensitivity C-reactive
protein (hs-CRP) as the standard] were increased, the patients were
divided into two groups. There were 40 patients with normal
inflammatory cytokines aged 19–40 years with an average of 29.5±0.5
years included in the normal inflammatory cytokine group. Times of
pregnancy: 28 cases of primiparity, 12 cases of re-pregnancy and 12
cases who had previously received cesarean section. Forty patients
with abnormal inflammatory cytokines aged 19–40 years with an
average age of 29.6±0.5 years were included into the abnormal
inflammatory cytokine group. Times of pregnancy: 29 cases of
primiparity, 11 cases of re-pregnancy and 13 cases who had
previously received cesarean section. There were no statistically
significant differences in age, times of pregnancy and
implementation rate of cesarean section between the two groups
(P>0.05).
Intervention methods
All the included patients were given low-flow
oxygen, local antipruritic or corticosteroid treatment. Reduction
of the concentration of bile acids (ursodeoxycholic acid), vitamin
K supplement, vascular dilation, liver protection and other
symptomatic support treatments. Regular fetal heart rate monitoring
and amniotic fluid index test were carried out. In addition, the
levels of bile acid and serum CG in pregnant women and hepatic
function of patients were regularly reviewed. Fetal lung maturation
was closely monitored, and if necessary, cesarean section was
implemented to terminate pregnancy based on the patient's CG level
combined with fetal monitoring results. The normal control group
was treated with routine pregnancy and delivery.
Observational indexes
The relevant data of newborns such as birth weight,
gestational week at birth and Apgar score at birth, intrauterine
and postpartum conditions such as the occurrence of grade II or
above amniotic fluid contamination, intrauterine fetal distress and
the proportion of neonatal jaundice, the levels of
inflammation-related cytokines such as hs-CRP, interleukin-6 (IL-6)
and tumor necrosis factor-alpha (TNF-α), and Apgar scores at birth,
and at 1 and 5 min after birth of newborns delivered by patients
with different hepatic functions and different inflammatory
cytokine levels were compared between the observation and control
groups. The correlation of the level of CG in pregnant women with
umbilical artery systolic-to-diastolic (S/D) ratio in the third
trimester of pregnancy during antenatal inspection, the alanine
aminotransferase level, the hs-CRP level, neonatal Apgar score and
gestational week were analyzed.
Evaluation criteria
Apgar score: Apgar score of newborns at birth was
assessed in this study. The evaluation criteria included five
items: skin color, heart rate pulse, respiratory rate, muscular
tone and motor ability, and nerve reflex. The overall score ranged
from 0 to 10 points. The total score below 7 points represented
neonatal asphyxia and that below 4 points severe neonatal asphyxia.
Amniotic fluid contamination was divided into 3 grades: grade I
with light green amniotic fluid, grade II with dark green or
yellow-green amniotic fluid and grade III with brownish yellow and
viscous amniotic fluid. Intrauterine fetal distress was diagnosed
according to the potential of hydrogen (pH) value of preserved
umbilical venous blood during fetal labor to determine the delivery
of infants. The pH value of umbilical venous blood below 7.25
indicated the presence of intrauterine fetal distress. Blood CG
(3.2 µg/ml) in pregnant women was detected via the latex-enhanced
immunoturbidimetry. The normal value of umbilical artery S/D ratio
in the third trimester of pregnancy, i.e., the ratio of
end-systolic peak (S) to end-diastolic peak (D) of umbilical artery
is generally below 3. Alanine aminotransferase (0-40 U/l) was
detected via Lai colorimetry. Inflammation-related cytokines
including hs-CRP (latex-enhanced immunoturbidimetry, normal value
in serum≤10 mg/l, cat. no. M020801; So-Fe Biomedicine, Co., Ltd.,
Shanghai, China), IL-6 (enzyme-linked immunosorbent assay, normal
value in serum: 0.37–0.46 ng/l; cat. no. KE00007; ProteinTech
Group, Inc., Wuhan, China) and TNF-α (spectrophotometry, normal
value in serum: 5–100 ng/l; cat. no. 17590-1-AP; ProteinTech Group,
Inc.) were tested.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
21.0 (IBM Corp., Armonk, NY, USA) was adopted for statistical
analysis. Measurement data were expressed as mean ± standard
deviation (mean ± SD). The mean values, such as birth weight,
gestational week and Apgar score at birth, were compared using the
t-test. The percentages, such as grade II or above, amniotic fluid
contamination, intrauterine fetal distress and neonatal jaundice
percentages were compared between groups using the χ2
test. Spearmans correlation analyses of the serum CG level with
umbilical artery S/D ratio in the third trimester of pregnancy
during antenatal inspection, the level of alanine aminotransferase,
the level of hs-CRP, neonatal Apgar score and gestational week were
performed using the correlation coefficient method. P<0.05 was
set as the statistically significant difference.
Results
Comparison of relevant data between
the groups of newborns
The birth weight of the observation group was
lighter than that of the normal group (P<0.05), the gestational
week at birth was earlier than that of the normal group
(P<0.05), and Apgar score at birth was lower than that of the
normal group (P<0.05) (Table
I).
Comparison of intrauterine and
postpartum conditions between the groups
The proportions of grade II or above amniotic fluid
contamination, intrauterine fetal distress and neonatal jaundice in
the observation group were significantly higher than those in the
normal group (P<0.05) (Table
II).
 | Table II.Comparison of intrauterine and
postpartum conditions between the groups (n). |
Table II.
Comparison of intrauterine and
postpartum conditions between the groups (n).
| Variables | Grade II or above
amniotic fluid contamination | Intrauterine fetal
distress | Neonatal
jaundice |
|---|
| Observation
group | 29 | 30 | 31 |
| Normal group | 1 | 2 | 1 |
| t value | 38.880 | 37.969 | 43.802 |
| P-value | <0.001 | <0.001 | <0.001 |
Comparison of the levels of
inflammation-related cytokines between the groups
The levels of hs-CRP, IL-6 and TNF-α among the
inflammation-related cytokines in the observation group detected at
the time of inclusion were notably higher than those in the control
group (P<0.05) (Table III).
 | Table III.Comparison of the levels of
inflammation-related cytokines between the groups (mean ± SD). |
Table III.
Comparison of the levels of
inflammation-related cytokines between the groups (mean ± SD).
| Variables | hs-CRP (mg/l) | IL-6 (ng/l) | TNF-α (ng/l) |
|---|
| Observation
group | 13.6±0.7 | 0.68±0.1 | 146.5±8.3 |
| Normal group |
8.5±0.4 | 0.43±0.1 |
83.2±6.6 |
| t value | 40.008 | 11.180 | 37.753 |
| P-value | <0.001 | <0.001 | <0.001 |
Changes in Apgar scores of newborns
with different hepatic functions
Apgar scores of newborns at birth and at 1 and 5 min
after birth in the normal hepatic function group were evidently
higher than those in the abnormal hepatic function group
(P<0.05) (Table IV).
 | Table IV.Changes in Apgar scores of newborns
with different hepatic functions (point, mean ± SD). |
Table IV.
Changes in Apgar scores of newborns
with different hepatic functions (point, mean ± SD).
| Variables | At birth | At 1 min after
birth | At 5 min after
birth |
|---|
| Normal hepatic
function group | 8.2±0.4 | 8.5±0.5 | 9.1±0.3 |
| Abnormal hepatic
function group | 6.2±0.3 | 6.6±0.4 | 7.1±0.2 |
| t value | 40.008 | 18.767 | 35.082 |
| P-value | <0.001 | <0.001 | <0.001 |
Changes in Apgar scores of newborns
with different levels of inflammatory cytokines
Apgar scores of newborns at birth and at 1 and 5 min
after birth in the normal inflammatory cytokine group were higher
than those in the abnormal inflammatory cytokine group (P<0.05)
(Table V).
 | Table V.Changes in Apgar scores of newborns
with different levels of inflammatory cytokines (point, mean ±
SD). |
Table V.
Changes in Apgar scores of newborns
with different levels of inflammatory cytokines (point, mean ±
SD).
| Variables | At birth | At 1 min after
birth | At 5 min after
birth |
|---|
| Normal inflammatory
cytokine group | 8.3±0.4 | 8.5±0.5 | 9.2±0.3 |
| Abnormal inflammatory
cytokine group | 6.4±0.3 | 6.7±0.4 | 7.0±0.2 |
| t value | 24.033 | 17.779 | 38.591 |
| P-value | <0.001 | <0.001 | <0.001 |
Correlation analyses of the serum CG
level with umbilical artery S/D ratio values in the third trimester
of pregnancy during antenatal inspection, the level of alanine
aminotransferase, the level of hs-CRP, neonatal Apgar score and
gestational week
The level of serum CG in pregnant women with ICP was
positively correlated with umbilical artery S/D ratio in the third
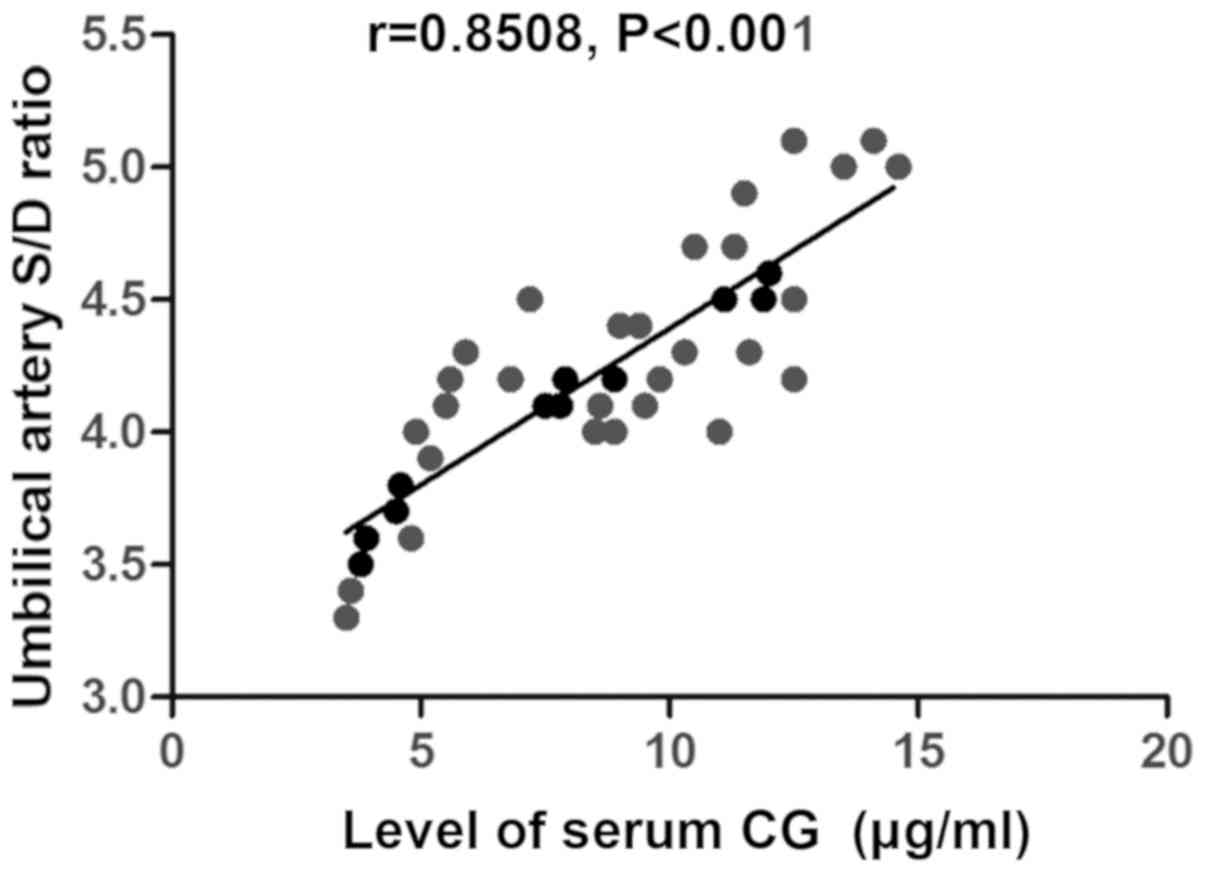
trimester of pregnancy during antenatal inspection (r=0.8508,
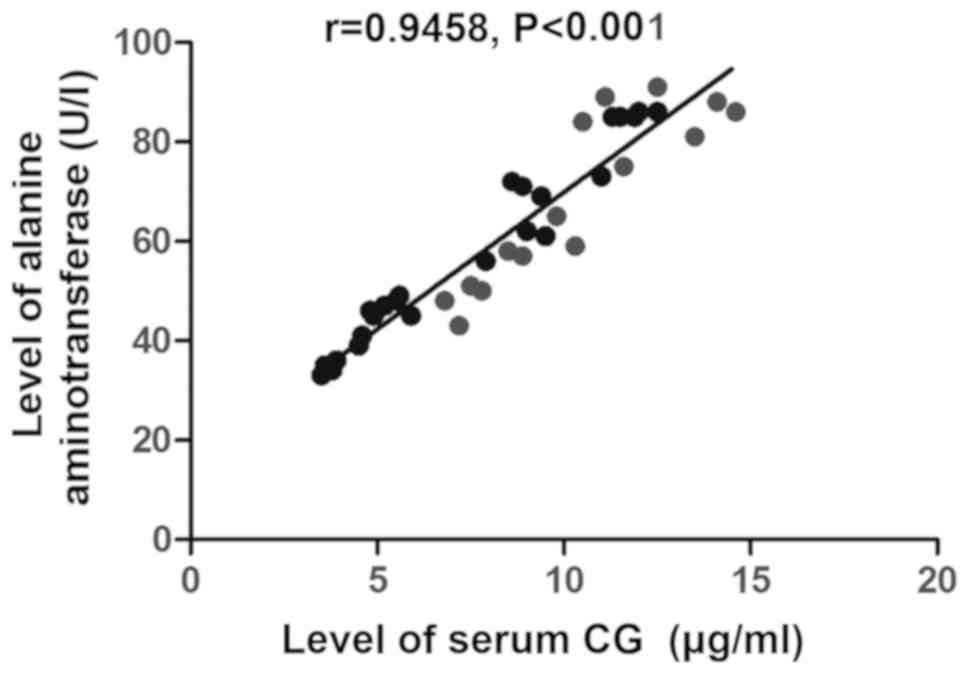
P<0.001) (Fig. 1), the level of
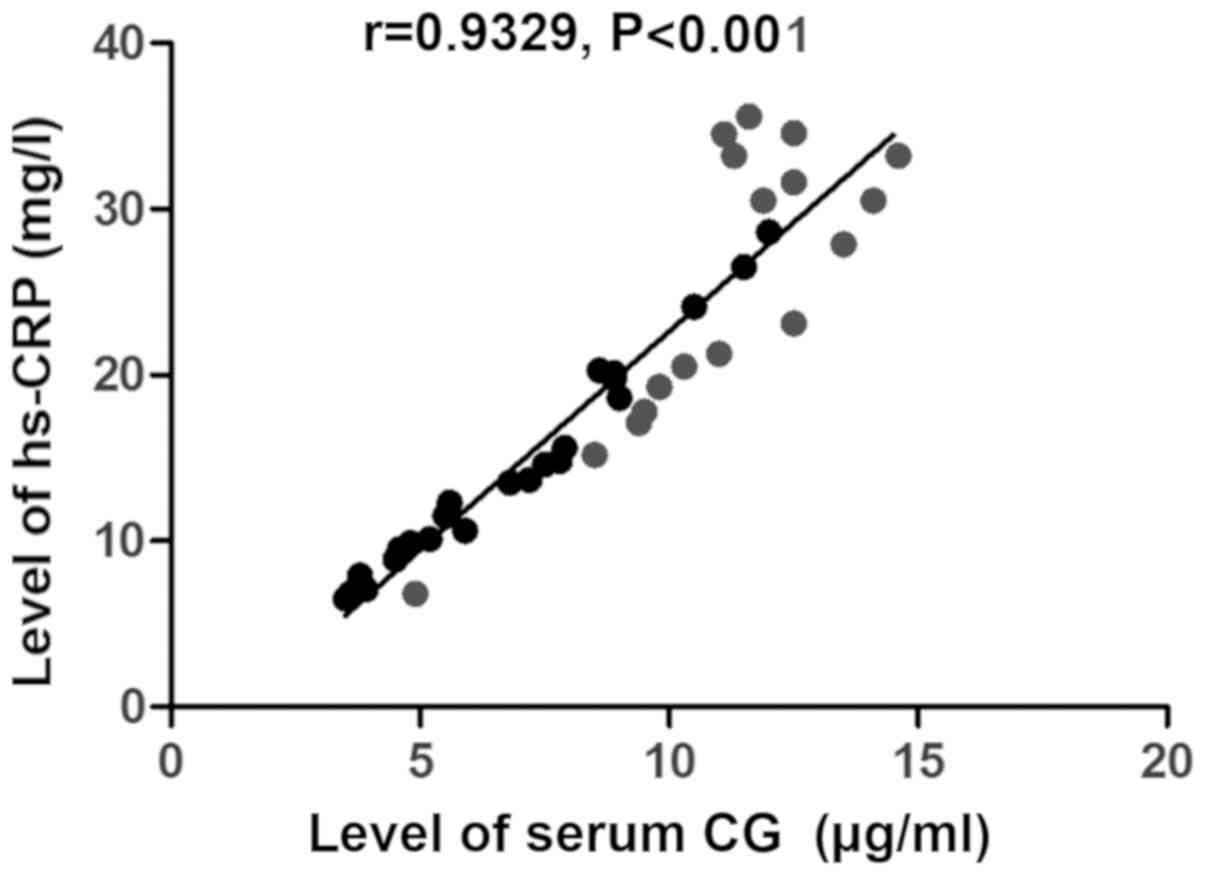
alanine aminotransferase (r=0.9458, P<0.001) (Fig. 2) and the level of hs-CRP (r=0.9329,
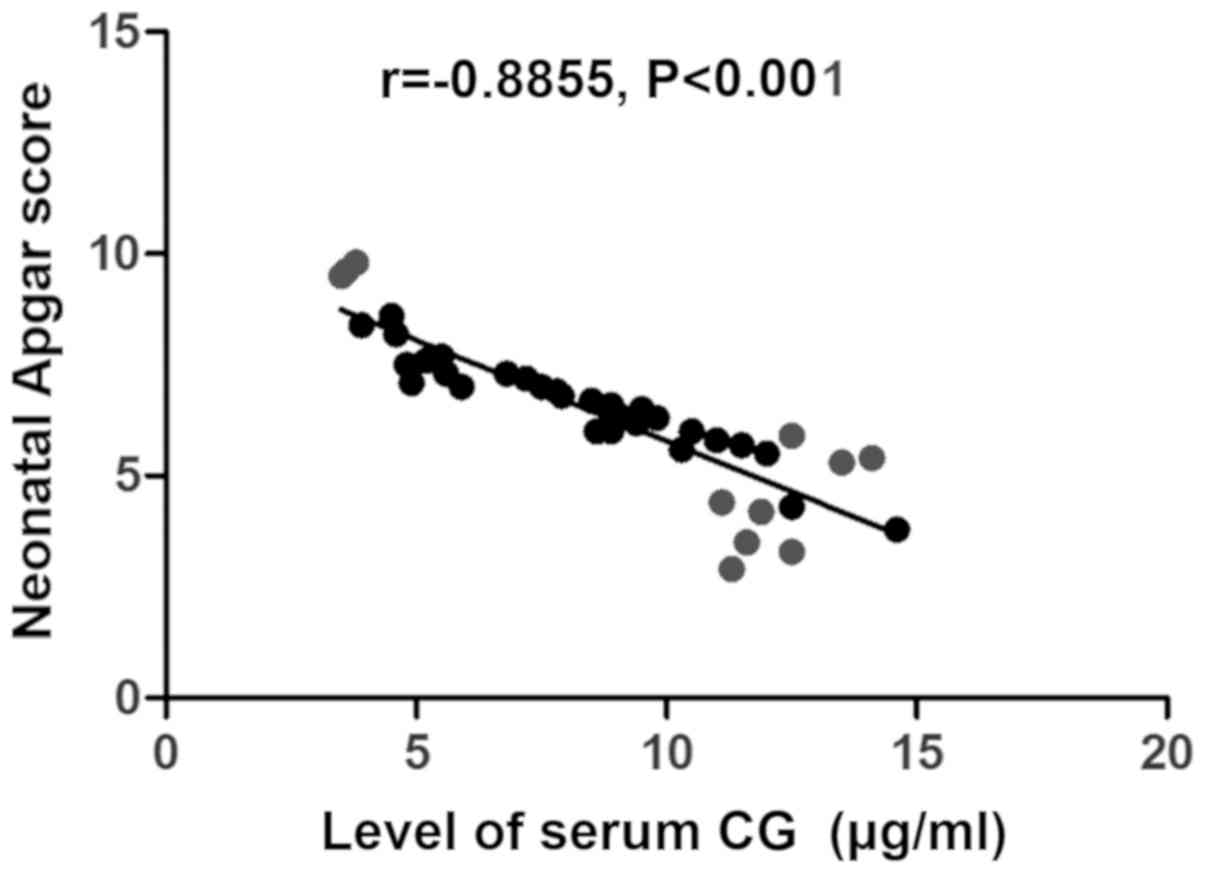
P<0.001) (Fig. 3), but negatively
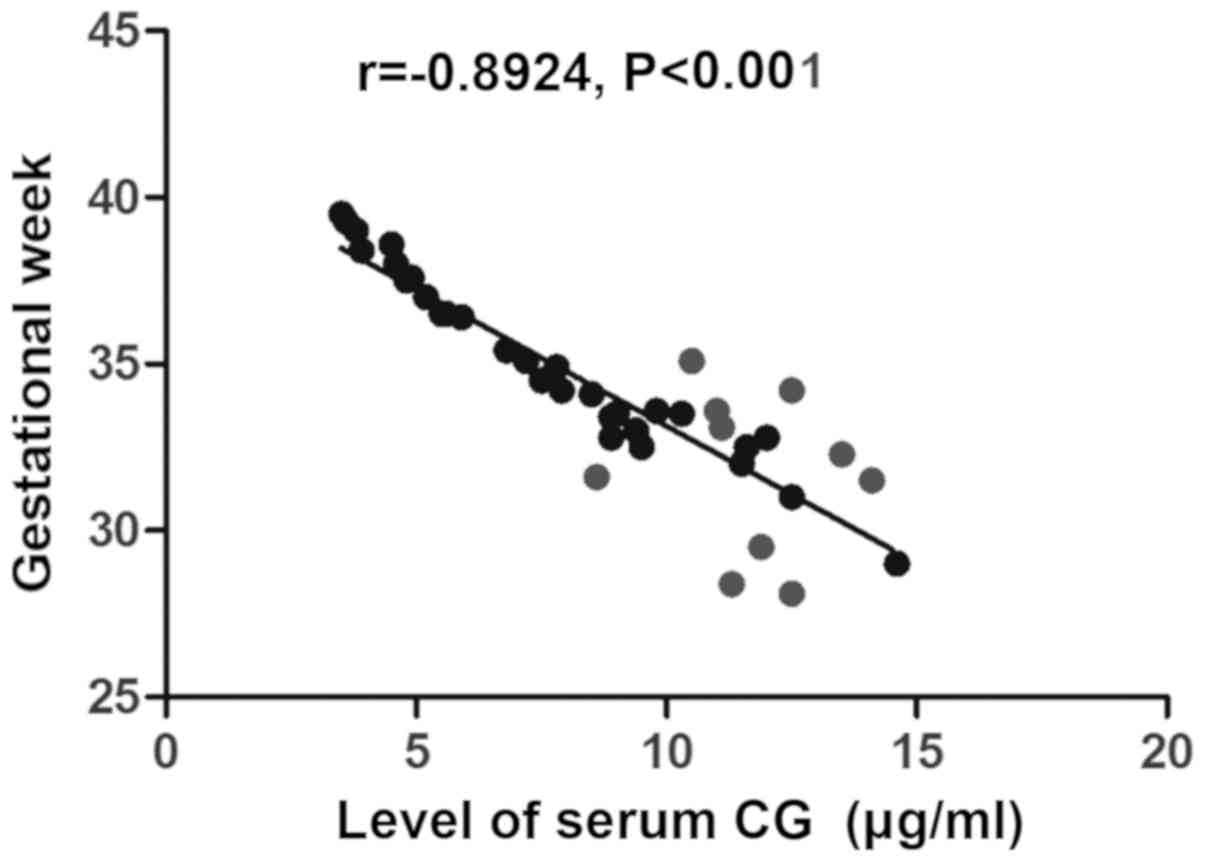
related to neonatal Apgar score (r=−0.8855, P<0.001) (Fig. 4) and gestational week (r=−0.8924,
P<0.001) (Fig. 5).
Discussion
ICP is a distinctive disease in the second and third
trimesters of pregnancy, mainly manifested as pruritus, jaundice
and abnormal hepatic function (10).
A previous study (11) indicated
that the high-level estrogen for a long term during pregnancy leads
to excessive bile formation and acatharsia (12). Besides, selenium deficiency during
pregnancy, ATP8B1 gene defects in pregnant women and positive
cytochrome P450 1A2 (CYPIA2), an estrogen metabolism gene, are
related factors contributing to ICP (13). ICP is harmful to the fetus and has a
great adverse effect on the perinatal prognosis. The maternal
cholestasis leads to abnormal liver function, which in turn leads
to the decrease of normal endocrine function and toxin degradation
ability of the liver (14). Over
time, maternal bile acids and toxins are transferred to the fetus
via placental circulation, leading to premature delivery,
intrauterine distress and even fetal asphyxia (15). For the diagnosis and treatment of ICP
during pregnancy, serum CG has the highest specificity, which can
provide a better guidance for the clinical treatment. This study
explored the effects of the level of serum CG in patients with ICP
on hepatic function, inflammatory cytokines and fetal outcomes.
In the present study, all patients with cholestasis
of pregnancy were treated with general prescription for ICP.
Related conditions of newborns were compared between the two
groups, and it was found, respectively, that birth weight,
gestational week at birth and Apgar score at birth in the
observation group were lighter, earlier and lower than those in the
normal group, indicating that newborns delivered by these patients
with ICP have lighter weight, earlier gestational week and lower
birth Apgar scores. Additionally, comparisons of intrauterine and
postpartum conditions between the two groups revealed that the
incidence rates of grade II or above amniotic fluid contamination,
intrauterine fetal distress and neonatal jaundice in the
observation group were significantly higher than those in the
normal group, further suggesting that the incidence rates of
amniotic fluid contamination, intrauterine fetal distress and
neonatal jaundice are obviously increased in patients with ICP.
Comparison of the levels of inflammation-related cytokines between
the two groups manifested that the levels of hs-CRP, IL-6, and
TNF-α among the inflammation-related cytokines in the observation
group detected at the time of inclusion were markedly higher than
those in the control group, indicating that the levels of
inflammatory cytokines in patients with normal hepatic function are
significantly lower than those in patients with abnormal hepatic
function. In addition, the study on changes in Apgar scores of
newborns delivered by patients with different hepatic functions and
different inflammatory cytokine levels demonstrated that Apgar
scores of newborns at birth and at 1 min and 5 min after birth in
the normal hepatic function and normal inflammatory cytokine groups
were higher than those in the abnormal hepatic function group. This
suggests that the hepatic function and the in vivo
inflammatory cytokine level of pregnant women are associated with
neonatal Apgar score to some extent. Finally, the correlation
analyses of the serum CG level in pregnant women with umbilical
artery S/D ratio in the third trimester of pregnancy during
antenatal inspection, the level of alanine aminotransferase, the
level of hs-CRP, neonatal Apgar score and gestational week
illustrated that the serum CG level in pregnant women was
positively correlated with umbilical artery S/D ratio in the third
trimester of pregnancy during antenatal inspection, the level of
alanine aminotransferase and the level of hs-CRP, but negatively
related to neonatal Apgar score and gestational week.
The high-level cholic acid, especially high CG, in
pregnant women with ICP, may cause the shrinkage of the placental
villus (16), thus, resulting in
edema of the placental trophocytes. Despite the increased number,
edema occurs in the basal villus, and the villous cavity remarkably
declines (17), thus causing a
significant reduction of maternal blood flow in the placental
villus cavity, fetal hypoxia and intrauterine distress (18). ICP in the body evidently stimulates
fetal intestinal peristalsis to be enhanced (19), resulting in meconium-stained amniotic
fluid, neonatal asphyxia and lowered neonatal Apgar score, so the
proportions of contaminated amniotic fluid and intrauterine fetal
distress in patients with ICP are increased. It was also verified
in this study that the serum CG level in pregnant women was
positively correlated with the level of alanine aminotransferase in
hepatic function of pregnant women with ICP, umbilical artery S/D
ratio in the third trimester of pregnancy and the level of hs-CRP,
but negatively related to neonatal Apgar score and gestational
week. With the development of pregnancy, the placental circulatory
resistance decreased, the end-diastolic blood flow rate
accelerated, the umbilical blood flow resistance decreased, and the
umbilical blood flow increased. However, in pregnant women with
ICP, the number of arterioles in the third grade villous trunk of
placenta decreased significantly, resulting in the obstruction of
material exchange between fetus and mother through the umbilical
cord, and the increase of S/D value. Then the placental dysfunction
affects the development of the fetus. In addition, neonatal
jaundice mainly caused hypermetabolism of fetal hemoglobin
catabolism due to intrauterine hypercholidemia and liver cell
injury. After birth, microsomal and glucuronic acid binding
disorder occurred in hepatocytes. Thus, the ability of bilirubin to
decompose is reduced, and jaundice occurs in newborns (20). There was a positive correlation
between serum cholic acid level and hs-CRP level in pregnant women
with ICP. The increase of inflammatory cytokines in the body may
lead to the increase of mitochondria and neutrophils, the increase
of oxygen free radicals, the damage of lipid peroxidation and the
increase of the level of immunological cytokines, which further
aggravates the damage of liver function. It may also promote the
production of a large amount of estrogen in the placenta, leading
to the imbalance of estrogen and progesterone levels in the mother
and placenta, further exacerbating ICP and increasing the level of
serum glycine cholic acid (21).
In conclusion, in patients with ICP, as the level of
CG in the body is increased, the levels of alanine aminotransferase
and hs-CRP in pregnant women and umbilical artery S/D ratio are
significantly increased. Moreover, this leads to lower Apgar score
of newborns, neonatal asphyxia, shortened gestational week and
premature birth.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW and ZL collected the general data of patients and
were responsible for patient treatment. XZ and YD recorded and
analyzed the observational indexes. LG helped with Apgar score
analysis. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Daqing Longnan Hospital (Daqing, China) and informed consents were
signed by the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Estiú MC, Frailuna MA, Otero C, Dericco M,
Williamson C, Marin JJG and Macias RIR: Relationship between early
onset severe intrahepatic cholestasis of pregnancy and higher risk
of meconium-stained fluid. PLoS One. 12:e01765042017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Günaydin B, Bayram M, Altuğ M, Cevher S
and Bozkurt N: Retrospective analysis of maternal, fetal, and
neonatal outcomesof intrahepatic cholestasis of pregnancy at Gazi
University. Turk J Med Sci. 47:583–586. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kong X, Kong Y, Zhang F, Wang T and Zhu X:
Expression and significance of dendritic cells and Th17/Treg in
serum and placental tissues of patients with intrahepatic
cholestasis of pregnancy. J Matern Fetal Neonatal Med. 31:901–906.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tayyar AT, Kozalı S, Yetkin Yildirim G,
Karakus R, Yuksel IT, Erel O, Neselioglu S and Eroglu M: Role of
ischemia-modified albumin in the evaluation of oxidative stress in
intrahepatic cholestasis of pregnancy. J Matern Fetal Neonatal Med.
11:1–5. 2018. View Article : Google Scholar
|
|
5
|
Sharifzadehgan S, Hermann M, Nedellec S,
De Luca D and Benachi A: Intrahepatic cholestasis of pregnancy:
Shorter duration of labor? Eur J Obstet Gynecol Reprod Biol.
225:258–259. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng C, Li WJ, He RH, Sun XW, Wang G and
Wang LQ: Impacts of different methods of conception on the
perinatal outcome of intrahepatic cholestasis of pregnancy in twin
pregnancies. Sci Rep. 8:39852018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wood AM, Livingston EG, Hughes BL and
Kuller JA: Intrahepatic cholestasis of pregnancy: A review of
diagnosis and management. Obstet Gynecol Surv. 73:103–109. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Covach AJ and Rose WN: Intrahepatic
cholestasis of pregnancy refractory to multiple medical therapies
and plasmapheresis. AJP Rep. 7:e223–e225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Floreani A and Gervasi MT: New insights on
intrahepatic cholestasis of pregnancy. Clin Liver Dis. 20:177–189.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koroglu N, Tayyar A, Tola EN, Yetkin
Yildirim G, Temel Yuksel I, Aslan Cetin B, Dag I and Acar DK:
Increased levels of the novel hepatokine fetuin B in patients with
intrahepatic cholestasis of pregnancy. J Matern Fetal Neonatal Med.
12:1–6. 2017.
|
|
11
|
Lin J, Gu W and Hou Y: Diagnosis and
prognosis of early-onset intrahepatic cholestasis of pregnancy: a
prospective study. J Matern Fetal Neonatal Med. 7:1–7. 2017.
|
|
12
|
Mei Y, Gao L, Lin Y, Luo D, Zhou X and He
L: Predictors of adverse perinatal outcomes in intrahepatic
cholestasis of pregnancy with dichorionic diamniotic twin
pregnancies. J Matern Fetal Neonatal Med. 32:472–476. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Adeyemi O, Alvarez-Laviada A, Schultz F,
Ibrahim E, Trauner M, Williamson C, Glukhov AV and Gorelik J:
Ursodeoxycholic acid prevents ventricular conduction slowing and
arrhythmia by restoring T-type calcium current in fetuses during
cholestasis. PLoS One. 12:e01831672017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li L, Chen YH, Yang YY and Cong L: Effect
of intrahepatic cholestasis of pregnancy on neonatal birth weight:
A meta-analysis. J Clin Res Pediatr Endocrinol. 10:38–43. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dadhwal V, Sharma A, Khoiwal K, Deka D,
Sarkar P and Vanamail P: Pregnancy outcomes in HIV-infected women:
Experience from a tertiary care center in India. Int J MCH AIDS.
6:75–81. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maldonado M, Alhousseini A, Awadalla M,
Idler J, Welch R, Puder K, Patwardhan M and Gonik B: Intrahepatic
cholestasis of pregnancy leading to severe vitamin K deficiency and
coagulopathy. Case Rep Obstet Gynecol. 2017:56462472017.PubMed/NCBI
|
|
17
|
Herrera CA, Manuck TA, Stoddard GJ, Varner
MW, Esplin S, Clark EAS, Silver RM and Eller AG: Perinatal outcomes
associated with intrahepatic cholestasis of pregnancy. J Matern
Fetal Neonatal Med. 31:1913–1920. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mattila M, Kemppainen H, Isoniemi H and
Polo-Kantola P: Pregnancy outcomes after liver transplantation in
Finland. Acta Obstet Gynecol Scand. 96:1106–1111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tayyar A, Temel Yuksel I, Koroglu N, Tanay
Tayyar A, Alici Davutoglu E, Akkaya Firat A and Aslan Cetin B:
Maternal copeptin levels in intrahepatic cholestasis of pregnancy.
J Matern Fetal Neonatal Med. 31:2066–2070. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sanhal CY, Daglar K, Kara O, Yılmaz ZV,
Turkmen GG, Erel O, Uygur D and Yucel A: An alternative method for
measuring oxidative stress in intrahepatic cholestasis of
pregnancy: Thiol/disulphide homeostasis. J Matern Fetal Neonatal
Med. 31:1477–1482. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Türkmen GG, Timur H, Yilmaz Z, Kirbas A,
Daglar K, Tokmak A, Uygur D and Danişman N: Effect of intrahepatic
cholestasis of pregnancy on maternal serum screening tests. J
Neonatal Perinatal Med. 9:411–415. 2016. View Article : Google Scholar : PubMed/NCBI
|