Introduction
The postoperative cognitive dysfunction (POCD) is a
common central nervous system complication after anesthesia
(1), which affects many aspects of
cognition, such as memory, attention, information processing and
cognitive flexibility (2,3). POCD tends to occur in elderly patients.
Studies have shown that the probability that patients aged over 65
are subjected to surgery is >50%. Due to the growing aging
problem, the occurrence of POCD has also continuously increase with
the rising number of elderly patients receiving operations
(4). The occurrence of POCD will
prolong the hospital stay of patients, affect the long-term life
quality and even increase the mortality rate, which adds a heavy
burden to the family and society. There are studies suggesting that
POCD is related to the surgery, anesthesia and patients, and more
likely to be affected synthetically by these three factors
(5), but its specific mechanism
remains unclear. Therefore, studying deeply the occurrence
mechanism of POCD will provide new theoretical basis and potential
intervention targets for its prevention and treatment, which is of
great practical significance.
All the information processing in the brain involves
synapses, the nerve impulses will not be transmitted among neurons
without effective synapses, and the cognition and other nervous
processes will also not be achieved. For the normal neural circuit
function, the formation and differentiation of synapses appear to
be particularly important (6). There
is a complex connection between the formation and diverse
differentiation of synapses, and both of them are dependent on the
activity of synapse cell-adhesion molecules (CAMs). The activity of
inhibitory interneurons is under the control of excitatory synapses
and inhibitory synapses formed by glutamatergic neurons and
GABAergic neurons (7,8). When the excitatory signals of pyramidal
cells decrease, the dysfunction of parvalbumin (PV) interneurons
can be induced, weakening the inhibition of pyramidal cells,
resulting in the imbalance of excitatory synapse/inhibitory synapse
transmission and destroying the effective activities of neural
network (9).
CAMs include neurexins (NRXs), neuroligins (NLs),
N-cadherin, synaptic cell adhesion molecule-1 (SynCAM-1), ephrinB,
and ephrinB receptor system (10).
NRXs and NLs are important among them, their specific synapse
functions have been researched most deeply up to now (11). The transsynaptic interaction of
postsynaptic NLs with the presynaptic NRXs holds an important
status in the synaptogenesis and synaptic integrity. NL1 is the CAM
specifically located in the excitatory postsynaptic membrane and
plays a significant role in the formation and function of
excitatory synapses through the transsynaptic combination of
presynaptic neurexin-1β (Nrx1β), which can cause the
differentiation of synapses and regulate the transmission of
neurotransmitter among neurons (12). The decreased interaction between the
two affects the function of excitatory synapses and the
transmission of glutamatergic neurotransmitter and destroys the
effective activities of neural network.
PV interneurons are the inhibitory GABAergic
neurons, accounting for 40–50% in the total GABAergic neurons. They
can release the GABAergic neurotransmitter, transmit the inhibitory
signals to the pyramidal cells and control the excitability of
pyramidal cells (13). Moreover, the
pyramidal cells can also release the glutamatergic
neurotransmitter, transmit the excitatory signals to the PV
interneurons and activate the PV interneurons to release GABA. Such
a process forms a neural circuit that regulates the effective
activities of neural network (14).
Our previous study suggested that the downregulation
of hippocampus NL1 can mediate the recruitment of postsynaptic
glutamate receptors, regulate the release of synapse glutamate to
affect the transmission of excitatory synapses and destroy the
excitatory signals emitted by pyramidal cells to PV interneurons
(unpublished data). This resulted in PV interneuron function
impairment, weakening the inhibition of pyramidal cells, triggering
the imbalance of excitatory synapse/inhibitory synapse transmission
and finally leading to the occurrence of POCD.
Materials and methods
Laboratory animals
A total of 80, 10 month-old C57BL/6 healthy male
mice of clean grade aged 10 weeks were purchased from the
Laboratory Animal Center of Fudan University, and fed in the
specific pathogen-free environment at room temperature of 25±2°C
with free access to food and water. The study was approved by the
Ethics Committee of Nanjing General Hospital (Nanjing, China).
Laboratory animal grouping
Mice were randomly divided into four groups: control
group, control+empty vector group (control+EV), anesthesia
surgery+empty vector group (POCD+EV group) and anesthesia+NL1
overexpression group (POCD+NL1 group), 20 in each group.
Establishment of the animal model
Animals were reared for 14 days to adapt to the
environment. Animals were then randomly divided into 4 groups:
normal control group (n=20, no anesthesia and surgery) and
Control+EV group (n=20 mice, in vivo transfection of empty
vector), POCD+EV group (n=20, anesthesia, surgery and empty vector
transfection) and POCD+NL1 group (n=20, anesthesia, surgery and NL1
expression vector transfection). POCD modeling: anesthesia chamber
was pre-charged with 1.5% isoflurane + 100% oxygen (2 l/min oxygen
flow) for 15 min, and then mice were put into the box to maintain
anesthesia for 30 min. After the mouse right reflex disappeared,
the right lateral position was taken to avoid aspiration and
maintain airway patency. Gas concentration in the anesthesia
chamber was continuously monitored by a continuous monitoring
multi-parameter anesthetic gas monitor, and the vital signs were
checked to maintain the normal anesthetic depth. During the
operation, a 1 cm long incision was made along the midline of the
abdomen to perform an abdominal exploration and ensure that the
direction of the bowel did not change. After exploration, the
muscle fascia and skin were sutured using 5-0 and 4-0 sterile
surgical sutures. The sterile conditions were ensured throughout
the operation. After operation, the mice were returned to the warm
table and kept warm. Mice in normal control group were not treated
except the injection of solvent in an equal volume.
In vivo transfection
A total of 60 POCD mice were divided randomly and
equally into the POCD+EV and POCD+NL1 groups. Nucleic acids (12.5
µg) were diluted into 1 µg/µl with an appropriate amount of
endotoxin-free pure water, and added with 12.5 µl water and 25 µl
10% glucose solution (w/v), and the final volume of 50 µl was mixed
evenly. Twenty-five microliters of Entranster™-in vivo
reagent was diluted with 25 µl of 10% glucose solution to a final
volume of 50 µl. After that, the diluted transfection reagent was
immediately added into the diluted nucleic acid solution and mixed
evenly, obtaining the transfection complex. After being placed at
room temperature for 15 min, the transfection complex was injected
into mice in POCD+NL1 group via the caudal vein. Mice in POCD+EV
group were injected with an equal amount of normal saline.
Open field test
The open field test is a method used to evaluate the
autonomous behaviors, exploratory behaviors and tension degree of
experimental animals in the new environment, in which the frequency
and duration of certain behaviors of experimental animals in the
new environment are used to reflect their autonomous behaviors and
exploratory behaviors in the unfamiliar environment, and the times
of urination is used to present the tension degree. The open field
test was performed in a quiet environment: Mice were placed in the
center of the box bottom, accompanied by shouting and timing using
the image automatic monitoring system (Jiangsu SANS Biological
Technology Co., Ltd., Jiangsu, China). After observation for a
certain period of time based on experimental requirements
(generally 3–5 min), shouting was terminated. The inner wall and
bottom of the square box were cleaned to prevent the residual
information of animals (such as the urine, feces and odor) in the
last test from affecting the results of the next test. The test was
performed again after mice were replaced.
Fear conditioning test
At day 1 of the test, mice were put into an
experimental box with electric metal fences on the bottom. After
mice adapted to the environment for 3 min, they were stimulated by
the single-frequency sound (3.0 kHz, 65 Db, 30 sec), as well as the
inevitable electric foot-shock (0.7 mA, 2 sec) in the last 2 sec at
the same time. Sound and electric shock were terminated
simultaneously. After the test, mice were kept in the box for 3
min, and then continued to be fed in the cage. The box bottom was
wiped with 75% alcohol after each test. After 24 h, mice with fear
conditioning already established were placed into the original box
without any stimulation. The contextual freezing mainly used to
reflect the hippocampus-dependent memory was recorded within 3 min.
After 2 h, the environment in the box was changed (a white board
was put on the bottom of box to change the color of the box wall),
and mice were placed into it again to adapt to the environment,
after which they were stimulated by the same intensity of sound for
3 min. The cued freezing mainly used to reflect the
non-hippocampus-dependent memory was recorded within 3 min. The
freezing was defined as no other motor behaviors except breathing.
The percentages of time of contextual freezing and cued freezing
were recorded.
Western blot analysis
Detection of NL1, PV and Nrx1β protein expression
levels in hippocampal tissues: At 3 h after fear conditioning test,
mice were decapitated rapidly, and hippocampal tissues were taken
and added with tissue lysis solution, followed by grinding and
homogenization on ice. After centrifugation at 3,000 × g at 4°C for
8 min, the supernatant was taken, and the protein concentration was
detected using the bicinchoninic acid (BCA) method. After the
protein was separated via 12% polyacrylamide gel electrophoresis,
it was transferred onto a polyvinylidene fluoride (PVDF) membrane
using the semi-dry method, and sealed with 5% skim milk powder at
room temperature for 1 h. Then the band was incubated with the
primary antibodies of NL1 (diluted at 1:2,000; cat. no. ab186279),
PV (diluted at 1:1,000; cat. no. ab27853), Nrx1β (diluted at
1:2,000; cat. no. ab2869) and β-actin (diluted at 1:5,000; cat. no.
ab124964) (all from Abcam, Cambridge, UK) at 4°C overnight. The
next day, the membrane was washed with phosphate-buffered saline
with Tween-20 (PBST) 3 times (10 min per time), added with the
secondary antibody (diluted at 1:2,000; cat. no. SA00001-1;
ProteinTech Group, Inc., Chicago, IL, USA) for incubation at room
temperature for 2 h, and washed again with PBST 3 times (10 min per
time). Finally, the ultra-sensitive luminescence solution was
dropwise added, followed by photography using the gel imaging
system and observation of results.
Co-immunoprecipitation
The medium in the culture dish was discarded, the
culture dish was washed with 1X pre-cooled PBS 3 times, and added
with 500 µl IP lysis solution containing protease inhibitor in each
dish, followed by lysis on ice for 30 min. Cells were scraped off
using the cell scraper, blown and beaten repeatedly until they shed
completely followed by centrifugation at 12,000 × g at 4°C for 15
min. The supernatant was transferred into a new Eppendorf (EP)
tube, and the protein concentration was determined using the BCA
method. Whole protein lysis solution (50 µm) was taken as the
input, 500 µg/mg whole protein lysis solution was taken, added with
20 µl Protein G magnetic beads, and rotated at 4°C for 1–2 h. The
tube was put on the magnetic shelf, and the supernatant was
transferred into a new EP tube as the pre-cleaning. IgG (2 µg)
antibody or target antibody NL1 was added into the supernatant, and
the mixture was agitated and kept overnight at 4°C. The next day,
20 µl protein G immunomagnetic beads were added into the above
mixture, and shaken at 4°C for 2 h to couple the antibody with the
magnetic beads. After immunoprecipitation, the mixture was
centrifuged at 3,000 × g at 4°C for 3 min, at which time the beads
were centrifuged to the bottom of tube. Then the beads were washed
with cell lysis solution for 3–4 times, and resuspended with 5X
sodium dodecyl sulphate loading buffer, followed by metal bath at
100°C for 5 min to dissociate the protein in the beads, and
centrifugation at 12,000 × g for 5 min at 4°C. Finally, the
supernatant was transferred into a new EP tube for western blot
analysis.
Immunofluorescence
After dewaxing and rehydration with alcohol,
hippocampal tissue sections of mice were permeabilized with 0.1%
Triton X-100 for 10 min, and sealed with 5% standard bovine serum
albumin (BSA) for 30 min. The postsynaptic density protein 95
(PSD95) primary antibody (diluted at 1:200; Abcam) was added
dropwise onto the section at 4°C overnight. The next day, the
sections were washed with PBST 3 times, and incubated with Alexa
Fluor-labeled fluorescence secondary antibody (diluted at 1:1,000;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at room
temperature for 1 h. After DAPI nuclear staining for 3 min,
sections were observed and photographed under an inverted
fluorescence microscope (DM-6000B; Leica).
Statistical analysis
MedCalc software (Version 18.0.0, Mariakerke,
Belgium) was used for data statistics and processing. Measurement
data are presented as mean ± standard deviation, and
independent-samples t-test was used for the intergroup comparison.
Chi-square test was used for enumeration data. P<0.05 was
considered to indicate a statistically significant difference.
Results
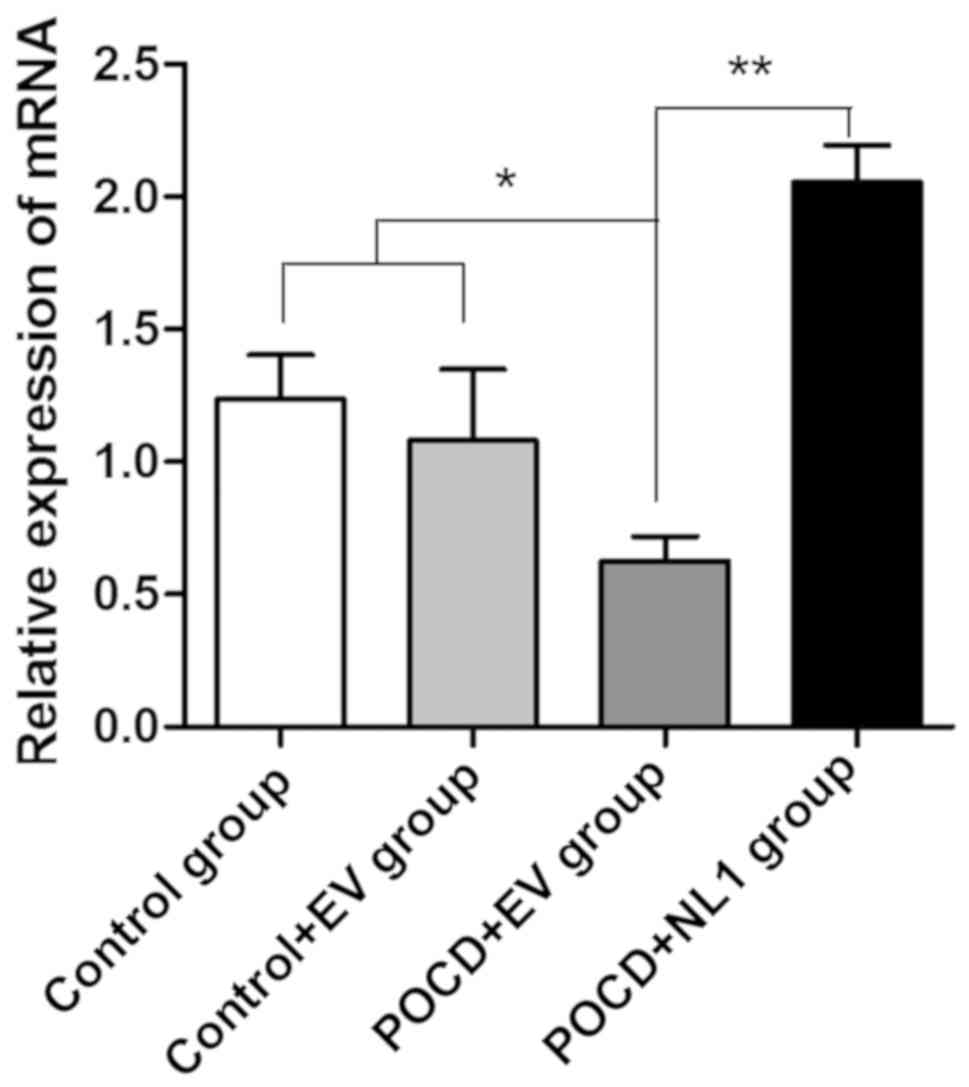
Detection of NL1 messenger ribonucleic
acid (mRNA) expression levels in hippocampal tissues of mice in
four groups
After the experiment, hippocampal tissues were taken
to detect the difference in the NL1 mRNA expression level among the
three groups. As is evident from Fig.
1, levels of NL1 mRNA in the Control+EV and Control groups were
not significantly different (P>0.05). Levels of NL1 in the POCD
group were significantly lower than that in Control group
(P<0.05), which was consistent with the downregulation of NL1
expression in hippocampal tissues of mice after POCD.
Overexpression efficiency in POCD mice was detected after NL1
overexpression in vivo and compared with that in the group
transfected with control plasmid in vivo. Results showed
that the NL1 mRNA expression level was significantly increased
(P<0.05) (Fig. 1).
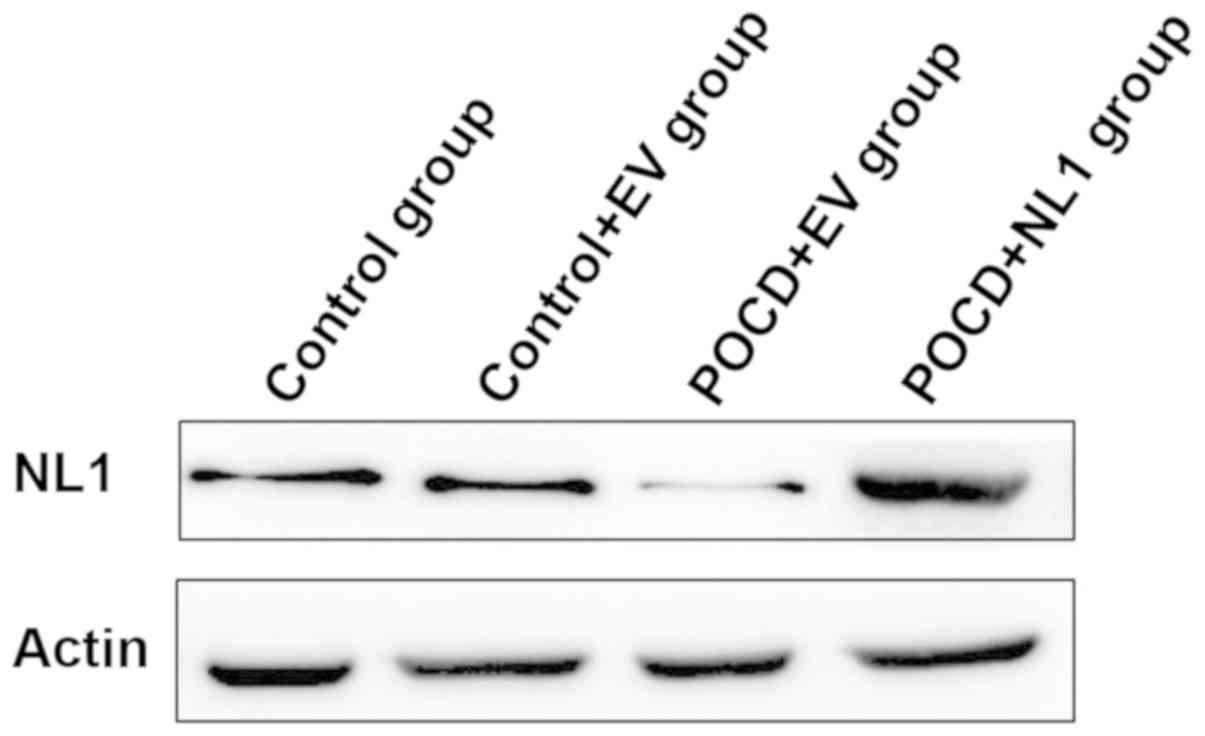
NL1 protein expression level in
hippocampal tissues of mice in POCD group is decreased, while that
in NL1 overexpression group is increased
The NL1 protein expression level was further
detected via western blotting, and quantitative comparison was made
for the gray-scale scanning of band. There was no significant
difference in the NL1 protein level between the Control+EV and
Control groups (P>0.05). It suggests that empty vector has no
effect on gene expression level and can be used in subsequent
experiments. Compared with Control+EV group, the expression level
of NL1 protein in POCD+EV group was significantly downregulated
(P<0.05), while the expression level of NL1 protein in POCD+NL1
group was significantly higher than that in POCD+EV group
(P<0.05) (Fig. 2).
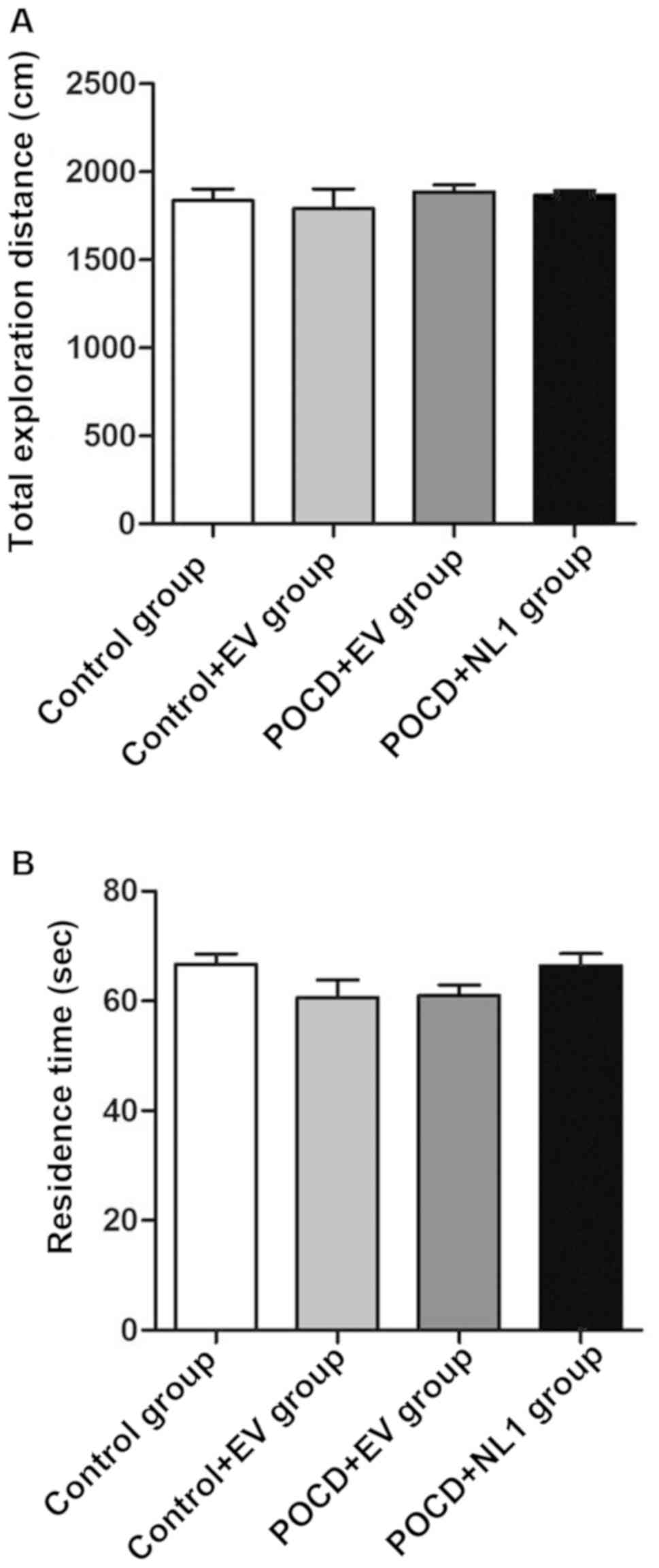
Detection of autonomous behavior and
exploratory behavior of mice in different groups via open field
test
The open field test was used to investigate the
autonomous exploration behavior of animals. There were no
statistically significant differences in the total exploration
distance (Fig. 3A) and residence
time in the central mesh (Fig. 3B)
among the four groups of mice (P>0.05) (Fig. 3).
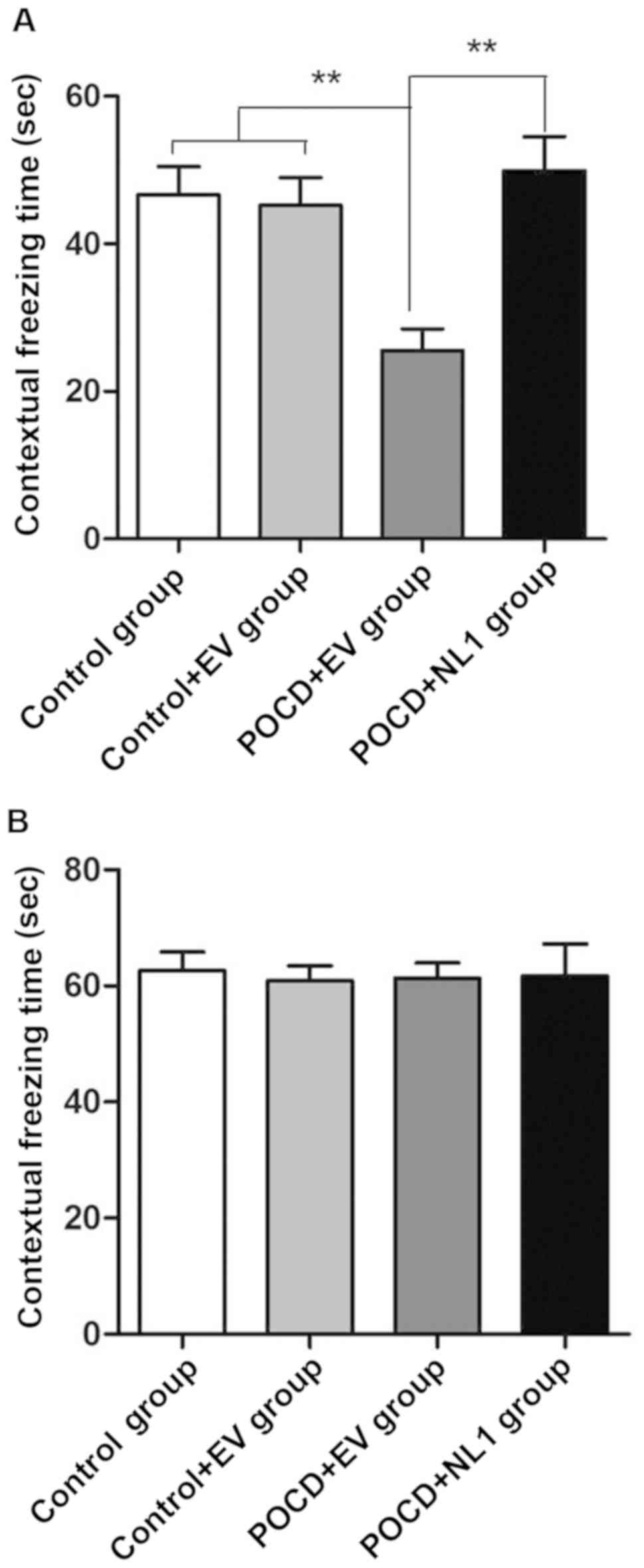
Context-related fear conditioning test
and tone-related fear conditioning test
Control and Control+EV groups had no significant
difference in each index (P>0.05). Compared with Control+EV
group, the percentage of contextual freezing time was obviously
decreased in POCD+EV group compared with that in C+EV group
(P<0.01), and it was obviously increased in POCD+NL1 group
compared with that in POCD+EV group (P<0.01), indicating that
the hippocampus-dependent memory of POCD mice is greatly damaged
and NL1 overexpression can repair such damage (Fig. 4A). However, there was no
statistically significant difference in the cued freezing time
among the three groups in the tone-related fear conditioning test
(P>0.05) (Fig. 4B), suggesting
that anesthesia operation does not affect the
non-hippocampus-dependent memory.
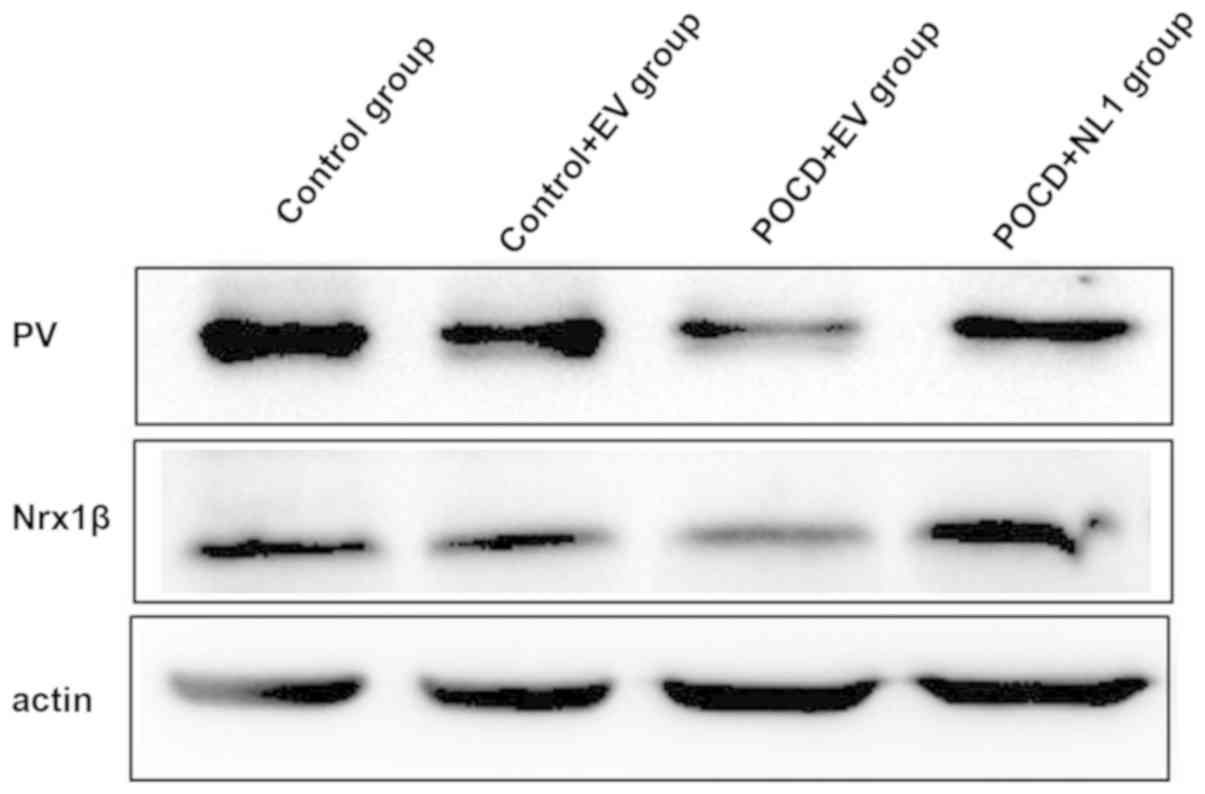
Detection of PV and Nrx1β protein
expression levels in hippocampal tissues of mice in the four
groups
The PV and Nrx1β protein expression levels in
hippocampus were obviously decreased in POCD+EV group compared with
those in C+EV group, and they were obviously increased in POCD+NL1
group compared with those in POCD+EV (Fig. 5).
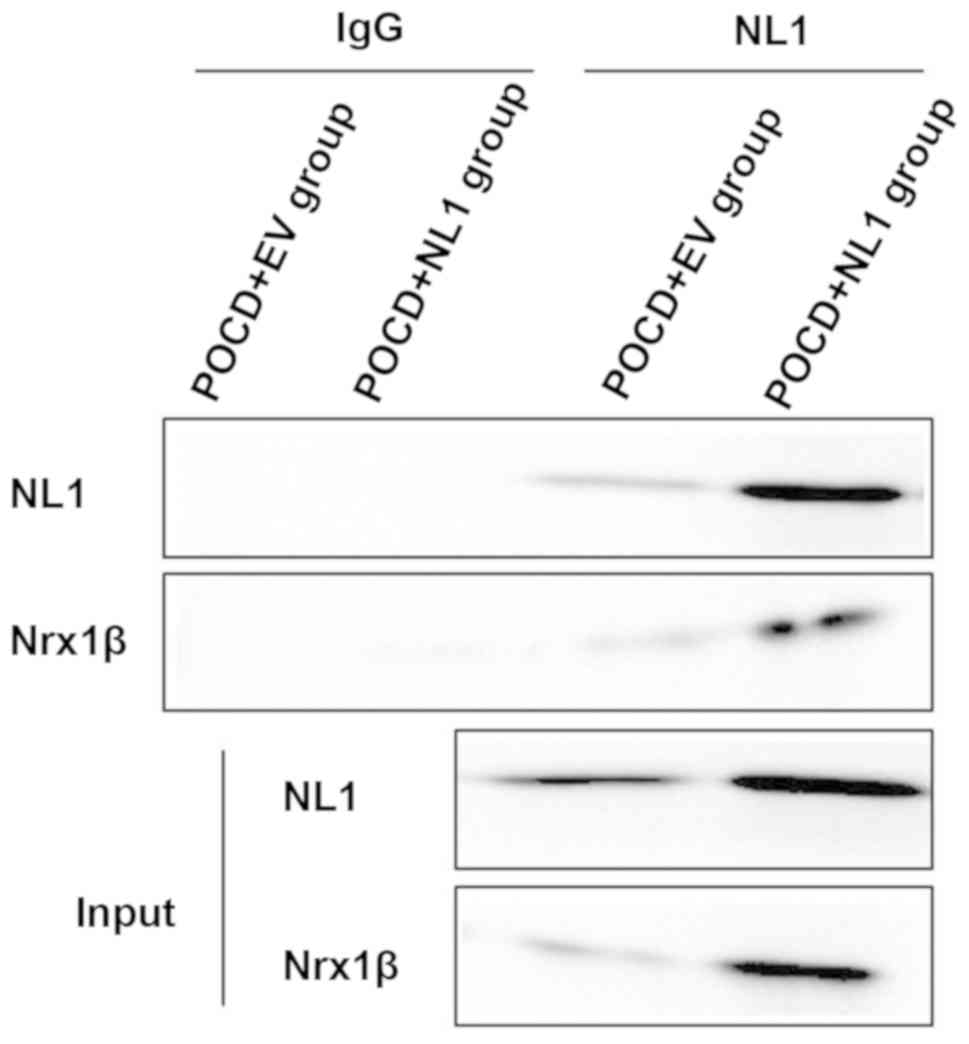
Detection of changes in interaction
between NL1 and Nrx1β via co-immunoprecipitation
NL1 is a kind of CAM specifically located on the
excitatory postsynaptic membrane, which binds to the presynaptic
Nrx1β to play an important role in the formation and function of
excitatory synapses. The enhanced binding of NL1 and Nrx1β was
detected in POCD+NL1 group compared with POCD+EV group (Fig. 6).
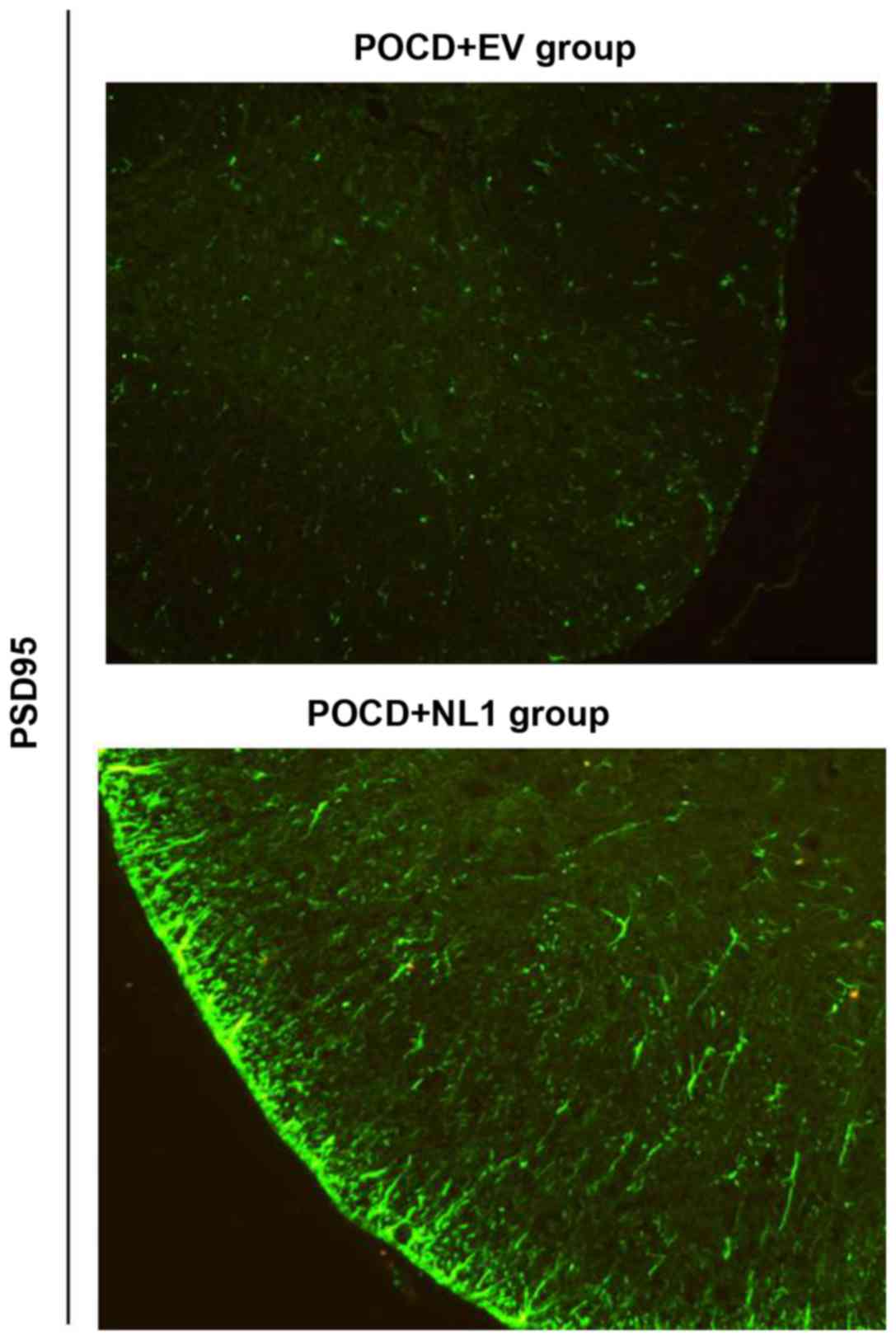
Effect of NL1 overexpression on the
excitability of PV neurons in the mouse model of POCD
PSD95 is an anchorin purified and identified in the
excitatory postsynaptic density. NL1 binds to PSD95 through the PDZ
region in the cytoplasm, and the resulting complex affects the
excitatory synaptic transmission via the direct interaction with
N-methyl-D-aspartic acid receptor (NMDAR) and indirect interaction
with α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
(AMPAR). Changes in the expression of PSD95 were detected to
reflect the excitability of PV neurons after NL1 overexpression.
After NL1 overexpression in POCD mice, the expression level of
PSD95 in tissues was significantly increased, suggesting the
increase of PV nerve excitability (Fig.
7).
Discussion
NL1 binds to PSD95 through the PDZ region in the
cytoplasm, and the resulting complex affects the excitatory
synaptic transmission via direct interaction with NMDAR and
indirect interaction with AMPAR (15). It has been proved in the literature
that the recruitment of hippocampal postsynaptic AMPAR and NMDAR
can be decreased after NL1 knockout, thus reducing the excitatory
synaptic transmission (16). NL1
affects not only the excitatory synaptic transmission through
mediating the recruitment of postsynaptic glutamate receptor, but
also the excitatory synaptic function through regulating the
release of presynaptic glutamate (17). The knockout or decreased expression
of NL1 causes damage to synaptic plasticity, and leads to memory
impairment, which is involved in the occurrence of a variety of
brain dysfunction-related diseases (18).
The downregulation of NL1 in the hippocampus can
mediate the recruitment of postsynaptic glutamate receptor and
regulate the release of presynaptic glutamate, affecting the
excitatory synaptic transmission, and destroying the excitatory
signals sent from pyramidal cells to PV internuncial neurons. As a
result, the function of PV internuncial neurons is damaged and the
inhibition of pyramidal cells declines, thus causing the imbalance
of hippocampal excitatory/inhibitory synaptic transmission, and
leading to POCD (19,20).
In this study, elderly mice were selected as
subjects of study. The mouse model of POCD was constructed via
isoflurane anesthesia and exploratory laparotomy, which is the
commonly used mehod. NL1 was significantly upregulated in the mouse
model of POCD in vivo, and the overexpression efficiency was
verified at both mRNA and protein levels. There were no
statistically significant differences in the movement distance and
activity time in the central region in the open field test among
groups, suggesting that isoflurane anesthesia does not affect the
exercise and exploration abilities of mice. Fear conditioning test
is a kind of sensitive method to study the learning and memory of
small rodents (rats and mice) under fear conditioning. After fear
conditioning training, context-related memory test and tone-related
memory test were performed for experimental animals, respectively,
to display the hippocampus-dependent and non-hippocampus-dependent
learning and memory, respectively. Results in this study manifested
that the percentage of contextual freezing time was obviously
decreased in POCD+EV group compared with that in C+V group, but
there was no statistically significant difference in the cued
freezing time among the three groups in the tone-related fear
conditioning test, indicating that anesthesia operation does not
affect the non-hippocampus-dependent memory. Therefore, it is
speculated that isoflurane anesthesia can lead to the early
hippocampus-dependent cognitive impairment, thus resulting in
POCD.
PV and Nrx1β protein expression levels in
hippocampal tissues of mice in the four groups, and the interaction
between NL1 and Nrx1β were detected via western blotting. Results
demonstrated that Control group and Control+EV group had no
significant difference in each index. Compared with Control+EV
group, the NL1 protein level of POCD group decreased, while the
expression of NL1 protein in POCD+NL1 group was overexpressed at
the same time. Interaction between NL1 and Nrx1β was enhanced.
Furthermore, the expression level of PSD95, a postsynaptic marker
of excitatory synapse, was detected. The expression level of PSD95
in POCD+NL1 group was obviously increased compared with that in
POCD+EV group, indicating that the excitability on PV internuncial
neurons is increased after NL1 overexpression in POCD mice, thus
restoring the hippocampus-dependent memory and cognitive impairment
in POCD.
In conclusion, this study suggests that NL1
overexpression can upregulate the expression levels of PV and Nrx1β
in POCD mice and strengthen the interaction between NL1 and Nrx1β,
thereby further enhancing the excitability on PV internuncial
neurons, which may provide new thoughts for the research on
pathogenesis and prevention and treatment of POCD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MT and YZ drafted the manuscript. MT, YZ, LQ and FL
collected and interpreted the data. FL and LJ revised the
manuscript. LJ, WL and LZ were responsible for the conception and
design of the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Nanjing General Hospital (Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Hanning CD: Postoperative cognitive
dysfunction. Br J Anaesth. 95:82–87. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moller JT, Cluitmans P, Rasmussen LS, Houx
P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD,
et al International Study of Post-Operative Cognitive Dysfunction,
: Long-term postoperative cognitive dysfunction in the elderly
ISPOCD1 study. ISPOCD investigators. Lancet. 351:857–861. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rasmussen LS, Johnson T, Kuipers HM,
Kristensen D, Siersma VD, Vila P, Jolles J, Papaioannou A,
Abildstrom H, Silverstein JH, et al ISPOCD2 (International Study of
Postoperative Cognitive Dysfunction) Investigators, : Does
anaesthesia cause postoperative cognitive dysfunction? A randomised
study of regional versus general anaesthesia in 438 elderly
patients. Acta Anaesthesiol Scand. 47:260–266. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Newman S, Stygall J, Hirani S, Shaefi S
and Maze M: Postoperative cognitive dysfunction after noncardiac
surgery: A systematic review. Anesthesiology. 106:572–590. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krenk L, Rasmussen LS and Kehlet H: New
insights into the pathophysiology of postoperative cognitive
dysfunction. Acta Anaesthesiol Scand. 54:951–956. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Collins S: An introduction to the
information processing components of the brain. Royal Signals and
Radar Establishment, Malvern, UK, 1990. https://apps.dtic.mil/dtic/tr/fulltext/u2/a222656.pdf
|
|
7
|
Godenschwege TA, Kristiansen LV, Uthaman
SB, Hortsch M and Murphey RK: A conserved role for
Drosophila neuroglian and human L1-CAM in central-synapse
formation. Curr Biol. 16:12–23. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim S, Burette A, Chung HS, Kwon SK, Woo
J, Lee HW, Kim K, Kim H, Weinberg RJ and Kim E: NGL family
PSD-95-interacting adhesion molecules regulate excitatory synapse
formation. Nat Neurosci. 9:1294–1301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lepeta K, Lourenco MV, Schweitzer BC,
Martino Adami PV, Banerjee P, Catuara-Solarz S, de La Fuente
Revenga M, Guillem AM, Haidar M, Ijomone OM, et al: Synaptopathies:
Synaptic dysfunction in neurological disorders - A review from
students to students. J Neurochem. 138:785–805. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bush JO and Soriano P: Ephrin-B1 regulates
axon guidance by reverse signaling through a PDZ-dependent
mechanism. Genes Dev. 23:1586–1599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fang M, Wei JL, Tang B, Liu J, Chen L,
Tang ZH, Luo J, Chen GJ and Wang XF: Neuroligin-1 knockdown
suppresses seizure activity by regulating neuronal
hyperexcitability. Mol Neurobiol. 53:270–284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koehnke J, Jin X, Budreck EC, Posy S,
Scheiffele P, Honig B and Shapiro L: Crystal structure of the
extracellular cholinesterase-like domain from neuroligin-2. Proc
Natl Acad Sci USA. 105:1873–1878. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martínez-Guijarro FJ, Blasco-Ibáñez JM and
López-García C: Postnatal increase of GABA- and PV–IR cells in the
cerebral cortex of the lizard Podarcis hispanica. Brain Res.
634:168–172. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dicpinigaitis PV and Dobkin JB:
Antitussive effect of the GABA-agonist baclofen. Chest.
111:996–999. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Budreck EC, Kwon OB, Jung JH, Baudouin S,
Thommen A, Kim HS, Fukazawa Y, Harada H, Tabuchi K, Shigemoto R, et
al: Neuroligin-1 controls synaptic abundance of NMDA-type glutamate
receptors through extracellular coupling. Proc Natl Acad Sci USA.
110:725–730. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chubykin AA, Atasoy D, Etherton MR, Brose
N, Kavalali ET, Gibson JR and Südhof TC: Activity-dependent
validation of excitatory versus inhibitory synapses by neuroligin-1
versus neuroligin-2. Neuron. 54:919–931. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carpentier M, Marcinkiewicz M, Boileau G
and DesGroseillers L: The neuropeptide-degrading enzyme NL1 is
expressed in specific neurons of mouse brain. Peptides.
24:1083–1091. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paraoanu LE, Becker-Roeck M, Christ E and
Layer PG: Expression patterns of neurexin-1 and neuroligins in
brain and retina of the chick embryo: Neuroligin-3 is absent in
retina. Neurosci Lett. 395:114–117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Savory JGA, Hsu B, Laquian IR, Giffin W,
Reich T, Haché RJ and Lefebvre YA: Discrimination between NL1- and
NL2-mediated nuclear localization of the glucocorticoid receptor.
Mol Cell Biol. 19:1025–1037. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cline H: Synaptogenesis: A balancing act
between excitation and inhibition. Curr Biol. 15:R203–R205. 2005.
View Article : Google Scholar : PubMed/NCBI
|